Abstract
Cell-cell fusion is critical for the conception, development and physiology of multicellular organisms. Although cellular fusogenic proteins and the actin cytoskeleton are implicated in cell-cell fusion, whether and how they coordinate to promote plasma membrane fusion remain unclear. Here, we reconstituted a high-efficiency, inducible cell-fusion culture system in the normally non-fusing Drosophila S2R+ cells. Both fusogenic proteins and actin cytoskeletal rearrangements were necessary for cell fusion, and, in combination, were sufficient to impart fusion competence. Localized actin polymerization triggered by specific cell-cell or cell-matrix adhesion molecules propelled invasive cell membrane protrusions, which, in turn, promoted fusogenic protein engagement and plasma membrane fusion. This de novo cell-fusion culture system reveals a general role for actin-propelled invasive membrane protrusions in driving fusogenic protein engagement during cell-cell fusion.
Cell-cell fusion occurs in many biological processes such as fertilization, myogenesis, placenta formation, bone remodeling and immune response (1–3). While transmembrane fusogenic proteins are implicated in fusing multiple cell types in C. elegans (4), actin polymerization is implicated in fusing muscle cells in Drosophila, zebrafish and mice (5–7). Whether and how fusogenic proteins and the actin cytoskeleton coordinate during cell-cell fusion remains unknown. We addressed these questions by reconstituting cell fusion de novo in the otherwise non-fusing S2R+ cells, a hemocyte-like cell line derived from Drosophila embryos (8).
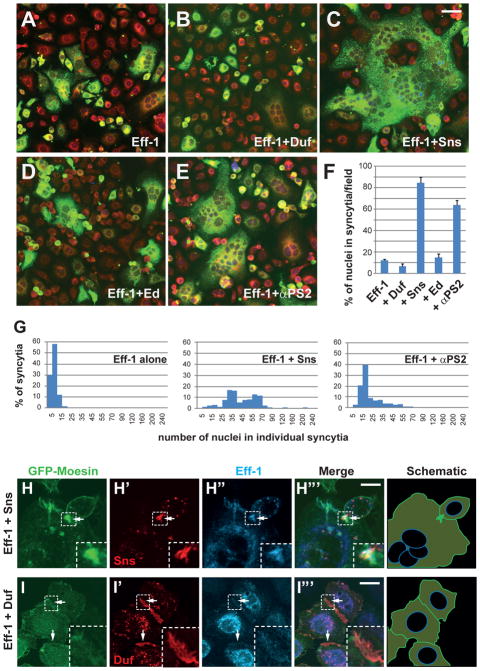
Transfecting known components of Drosophila myoblast fusion including cell adhesion molecules (9, 10) and actin cytoskeletal regulators (11–14) failed to induce S2R+ cell fusion, despite causing extensive cell adhesion and F-actin enrichment at cell-cell contact sites (fig. S1, A to C). Expressing a C. elegans fusogenic protein Eff-1 (15, 16) induced low-level S2R+ cell fusion (Fig. 1, A and F). Multinucleate syncytia were observed 24 hrs after Eff-1 transfection, and by 72 hrs post-transfection, ~12% (12.1 ± 1.1%) Eff-1-positive cells were in multinucleate syncytia, with each syncytium containing a median number of 8 nuclei (Fig. 1, F and G). These Eff-1-induced multinucleate syncytia resulted from cell fusion (fig. S2, A to B‴), and Eff-1 was required in both fusion partners (fig. S2C), similar to that reported in the moth Sf9 cells (16).
Fig. 1. Co-expression of Drosophila adhesion molecules and the C. elegans fusogenic protein Eff-1 induces high efficiency cell fusion in Drosophila S2R+ cells.
(A to G) Expression of Sns or integrin significantly enhances Eff-1-mediated cell fusion. Cells were transfected with Eff-1-V5 alone (A) or co-transfected with Eff-1-V5 and an adhesion molecule – Duf (B), Sns (C), Ed (D) or αPS2 (E), and stained with anti-V5 (green) and a membrane dye (red). (F) Quantification of the fusion index as the percentage of nuclei in multinucleate syncytia (containing ≥3 nuclei) vs. the total number of nuclei in transfected cells. Error bars: standard deviations. N = 30 randomly chosen 40x microscopic field. (G) Distribution of nuclei number in multinucleate syncytia. (H and I) Sns and Duf trigger distinct actin cytoskeletal rearrangement at cell-cell contact sites. Cells were co-transfected with Eff-1-HA, GFP-Moesin (33), and Sns-V5 (H) or Duf-V5 (I), and stained with anti-V5 (red) and anti-HA (cyan). F-actin was visualized by GFP-Moesin (green). Boxed areas magnified in insets and schematic drawings of F-actin shown on the right. Arrow in H: an F-actin-enriched focus associated with Sns and Eff-1 accumulation at a cell-cell contact site. Arrows in I: cell-cell contact sites enriched with Duf, but not F-actin. Scale bars: 40 μm (A to E); 10 μm (H and I).
Because close membrane apposition is a prerequisite for membrane fusion, we asked whether Eff-1-induced fusion could be enhanced by co-expressing cell adhesion molecules. Dumbfounded (Duf) and Sticks and stones (Sns) are Ig domain-containing transmembrane proteins required for Drosophila myoblast fusion (9, 10), but are not normally expressed in S2R+ cells (fig. S1D). Exogenous Duf, but not Sns, promotes homophilic cell adhesion in cultured Drosophila cells (17–19), and so does Echinoid (Ed), an Ig-containing transmembrane protein not implicated in myoblast fusion (20, 21). Among the three proteins, only Sns enhanced Eff-1-mediated fusion (Fig. 1, B, C, D and F), suggesting that membrane apposition mediated by cell adhesion per se is not sufficient to promote Eff-1-mediated fusion. Nearly 90% (86.3 ± 2.9%) of the Sns-Eff-1 co-expressing cells were in multinucleate syncytia (Fig. 1C), representing a seven-fold increase over Eff-1-induced fusion (Fig. 1F). These large syncytia contained up to 220 nuclei/cell with a median number of 44 nuclei/cell (Fig. 1G). Live imaging confirmed that Sns-Eff-1-induced syncytial formation resulted from cell fusion (fig. S3, A and B; movies S1 and S2). Besides Sns, overexpressing an α subunit (αPS2) of the cell-matrix adhesion molecule integrin (22), which has been implicated in multiple types of cell fusion events (23–26), enhanced Eff-1-mediated fusion by five-fold (63.9 ± 4.3%) with a median number of 20 nuclei/cell (Fig. 1, E, F and G). The dramatic enhancement of Eff-1-mediated cell fusion by Sns and integrin, neither of which mediates homophilic cell adhesion nor interacts with Eff-1 more strongly than Duf (fig. S4), prompted us to examine the cellular mechanisms underlying their fusion-enhancing activity.
In Drosophila, Sns and Duf trigger distinct actin cytoskeletal changes during myoblast fusion – Sns organizes an F-actin-enriched invasive podosome-like structure (PLS) in the fusion competent myoblast (27, 28), while Duf promotes the formation of a thin sheath of actin underlying the apposing founder cell membrane (27). Their differential activity in remodeling the actin cytoskeleton was recapitulated in S2R+ cells, as F-actin-enriched foci were observed at cell-cell contact sites marked by the accumulation of Eff-1 and the cell adhesion molecule in Sns-Eff-1-expressing (Fig. 1H; fig. S5A), but not Duf-Eff-1-expressing cells (Fig. 1I; fig. S5B). Live imaging revealed that these F-actin foci corresponded to sites of fusion (Fig. 2A; fig. S6, A and B; movies S3, S4 and S5). Using a cell-mixing fusion assay, we showed that Sns was only required in one of the two fusion partners to promote efficient cell fusion (fig. S7).
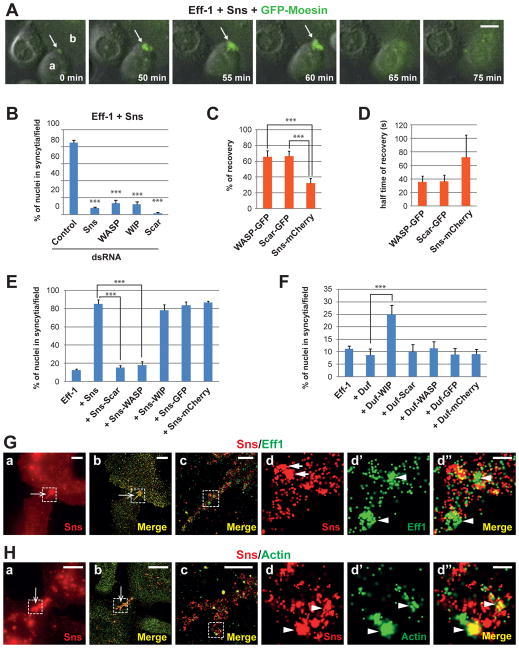
Fig. 2. Actin-propelled invasive finger-like protrusions promote high efficiency cell fusion in Sns-Eff-1-expressing S2R+ cells.
(A) A dynamic F-actin focus mediating cell fusion. Cells co-expressing Eff-1, Sns and GFP-Moesin were subjected to time-lapse imaging. Stills from a representative movie (movie S3) are shown. 0 min represents 3:00:51 in movie S3. Arrow indicates the F-actin focus at the protruding tip of cell “a”, which was about to fuse with cell “b”. (B) Arp2/3-mediated actin polymerization is required for Sns-enhanced cell fusion. Fusion indexes resulting from RNAi knockdown of Sns, WASP, WIP or Scar in cells co-expressing Sns and Eff-1. Statistical significance was determined using the two-tailed student’s t test (***p < 0.001) by comparing control vs. RNAi samples. (C and D) FRAP assays of WASP, Scar and Sns at sites of fusion. Sns-mCherry and WASP-GFP (or Scar-GFP) foci were photobleached and monitored for fluorescence recovery in cells co-expressing Eff-1, Sns-mCherry and WASP-GFP (or Scar-GFP). Error bars: standard deviations. N = 4. (E and F) Effects of chimeric proteins of cell adhesion molecules and Arp2/3 regulators on cell fusion. Cells were transfected with Eff-1 alone or co-transfected with Eff-1 and indicated plasmids. Fusion indexes were quantified as described. (G and H) Sns and Eff-1 accumulate in distinct clusters on the cell membrane along the finger-like protrusions. Cells co-transfected with Sns-V5 and Eff-1-HA were stained with anti-V5 (red), anti-HA (green in G) and anti-Actin (green in H), and subjected to STORM analysis. Images were acquired by either widefield microscopy (a) or STORM (b to d″). Boxed area in (a) and (b) enlarged in (c), and that in (c) enlarged in (d to d″). Arrow in (a) and (b) indicates finger-like protrusions extending from a cell to its fusion partner. (G) Distinct clusters of Sns and Eff-1 indicated by arrows in (d) and arrowheads in (d′ and d″), respectively. (H) Arrowheads in (d to d″) indicate the frequently overlapping signals of Sns and Actin. Scale bars: 10 μm (A); in (G) and (H): 20 μm (a and b), 500 nm (c), 100 nm (d to d″).
Because the actin nucleation-promoting factors (NPFs) of the Arp2/3 complex, WASP (11–13) and Scar/WAVE (14), are required for Sns-induced PLS formation in Drosophila myoblast fusion (27), we investigated whether WASP and Scar are required for Sns-Eff-1-induced cell fusion. RNAi knockdown of WASP, its binding partner WASP-interacting protein (WIP) (11, 12), or Scar abolished Sns-induced F-actin foci (fig. S8) and eliminated Sns-enhanced cell fusion (Fig. 2B). FRAP analysis revealed more dynamic exchanges of WASP and Scar at sites of fusion compared with Sns (Fig. 2, C and D; fig. S9; movies S6 and S7), suggesting that Sns provides a relatively stable organizing center at these sites to recruit WASP and Scar. Thus, dynamic actin cytoskeletal rearrangement is required for Sns-Eff-1-induced cell fusion. RNAi knockdown of the P40 subunit of the Arp2/3 complex in moth Sf9 cells also decreased Eff-1-induced fusion (7.2 ± 1.2% compared with 16.7 ± 6.1%; fig. S10), demonstrating that Arp2/3-mediated actin polymerization is generally required for fusion in different cell types.
To examine whether Arp2/3-mediated actin polymerization is sufficient to enhance Eff-1-mediated fusion, we fused WIP, WASP or Scar to the C-terminus of Duf or Sns and co-expressed each chimeric protein with Eff-1 in S2R+ cells. Attaching WIP to Sns did not affect Sns’ ability to organize actin polymerization at cell-cell contact sites (fig. S11A) or enhance fusion (Fig. 2E). Attaching WIP to Duf induced the formation of F-actin-enriched hair-like protrusions at cell-cell contact sites (fig. S11B) and converted Duf into a fusion-promoting molecule (Fig. 2F), suggesting that WIP-mediated actin cytoskeletal rearrangement is sufficient to enhance Eff-1-mediated cell fusion. Unexpectedly, attaching WASP or Scar directly to Duf did not enhance Eff-1-mediated fusion (Fig. 2F), and attaching these NPFs to Sns abolished Sns’ fusion-enhancing activity (Fig. 2E) due to mislocalization of the chimeric proteins. Unlike Duf-WIP or Sns-WIP, which was correctly targeted to cell membrane (fig. S11, A and B), Duf-WASP, Duf-Scar, Sns-WASP and Sns-Scar were localized in the cytoplasm (fig. S11, C and D), where they induced actin comet tails propelling rapid movement of vesicles containing Eff-1 and the correspondent chimeric protein (fig. S11, C and D; movies S8 and S9). Thus, localized Arp2/3-mediated actin polymerization at cell-cell contact sites promotes Eff-1-mediated fusion.
Because Arp2/3 nucleates a branched actin network that drives membrane protrusions, we predicted that the F-actin foci at cell-cell contact sites may be invasive. To test this, we conducted super-resolution TIRF-based stochastic optical reconstruction microscopy (STORM) (29) and ultrastructural electron microscopy (EM). Both analyses revealed a tightly packed group of finger-like protrusions extending from one cell into its fusion partner in Sns-Eff-1-, but not Duf-Eff-1-, expressing cells (Fig. 2, G and H, b and c; fig. S12; Fig. 4, A and A′), consistent with the reported ultrastructure of the invasive PLS in Drosophila myoblast fusion (27, 30, 31). These fingers contained distinct clusters of Sns and Eff-1 (Fig. 2G, d to d″) and frequently overlapping Sns and actin signals (Fig. 2H, d to d″). Strikingly, segments of electron-dense “ladders” were present between the apposing membranes along the invasive fingers (Fig. 4A′ and A″). These electron-dense “ladders” grossly resembled the electron-dense spikes formed by the virus-packaged Eff-1 on the viral envelope (32) (albeit thinner than the latter), and spatially corresponded to clusters of Eff-1 on the invasive fingers observed by STORM (Fig. 2G, d′). Indeed, immunogold labeling confirmed the presence of Eff-1 on the membranes along the invasive fingers (fig. S13, B and B′). Thus, in Sns-Eff-1-induced cell fusion, Arp2/3-mediated actin polymerization generates finger-like membrane protrusions that promote Eff-1 engagement across the apposing cell membranes.
Fig. 4. Invasive finger-like membrane protrusions promote fusogenic protein engagement.
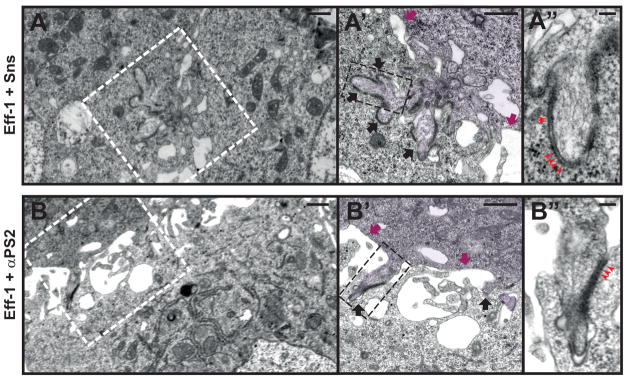
(A-A″) Electron micrographs of Sns-Eff-1-expressing cells. Boxed area in (A) enlarged in (A′), and that in (A′) enlarged in (A″). (A) A low magnification view of two adherent cells. (A′) Cell on the right (pseudo-colored purple) extended a group of finger-like protrusions (black arrows) to invade the cell on the left. Segments of electron-dense materials were present on the membranes along the protrusive fingers, but absent elsewhere on the cell membrane (magenta arrows). (A″) At a higher magnification, the electron-dense materials appeared like ladders between the two apposing cell membranes (red arrowheads). (B-B″) Electron micrographs of αPS2-Eff-1-expressing cells. Boxed area in (B) enlarged in (B′), and that in (B′) enlarged in (B″). (B) A low magnification view of the two adherent cells. (B′) Cell on the top (pseudo-colored purple) extended individual finger-like protrusions to invade the cell at the bottom. The invasive fingers (black arrows) were scattered along the broad cell-cell contact zone, and were associated with segments of electron-dense materials, which were absent elsewhere on the cell membranes (magenta arrows). (B″) At a higher magnification, the electron-dense materials also showed a ladder-like appearance (red arrowheads) between the two apposing membranes, as in Sns-Eff-1-expressing cells (A″). Scale bar: 500 nm (A, A′, B, and B′); 100 nm (A″ and B″).
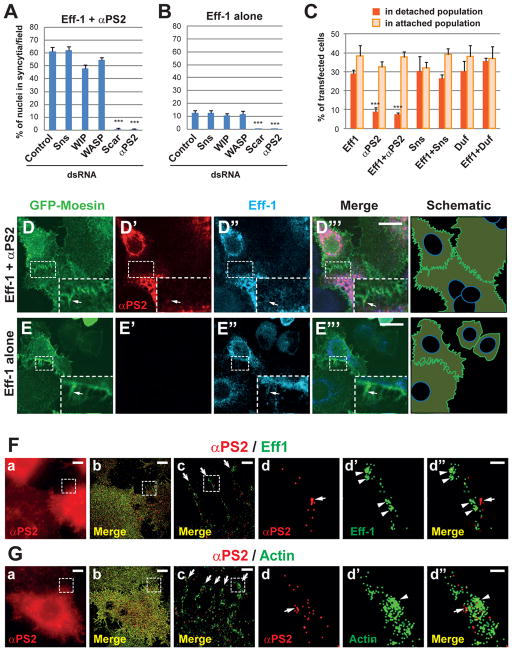
To determine whether actin-propelled membrane protrusions are generally involved in cell-cell fusion, we investigated how integrin modulated Eff-1-mediated fusion. Loss- and gain-of-αPS2 function abolished or enhanced Eff-1-mediated cell fusion commensurate with the strength of cell-matrix adhesion (Fig. 1, E and F; Fig. 3, A to C). Unlike Sns-Eff-1-induced fusion, αPS2-Eff-1-induced fusion required Scar, but not the WASP-WIP complex (Fig. 3A; fig. S14A), which is normally recruited to sites of myoblast fusion by Sns in Drosophila (11,12). As a consequence, αPS2-Eff-1-expressing cells formed numerous F-actin-containing hair-like projections, instead of dense F-actin foci, along the broad cell-cell contact zone (Fig. 3D; movie S10). Notably, cells expressing Eff-1 alone also occasionally formed multiple hair-like projections along cell-cell contact zones (Fig. 3E), and RNAi of Scar, but not WASP or WIP, abolished the basal level of Eff-1-mediated cell fusion (Fig. 3B; fig. S14B). STORM and EM analyses revealed individual finger-like protrusions sparsely localized at the cell periphery and along the cell-cell contact zone of αPS2-Eff-1-expressing cells (Fig. 3F and G, b and c; Fig. 4B), corresponding to the hair-like projections visualized by confocal microscopy (Fig. 3D). These finger-like protrusions contained Eff-1 clusters (Fig. 3F, d and d″) and were anchored at their basal side by exogenous αPS2 (Fig. 3, F and G, d to d″). Like in Sns-Eff-1-expressing cells (Fig. 4A″), the invasive fingers in αPS2-Eff-1-expressing cells contained segments of electron-dense “ladders” (Fig. 4B″), corresponding to the Eff-1 clusters observed by STORM (Fig. 3F, d′ and d″). Thus, despite the differences in the requirement of actin regulators (Scar vs. WASP-Scar) and the overall morphology of F-actin enrichment (hairs vs. foci), αPS2-Eff-1- and Sns-Eff-1-expressing cells use similar invasive finger-like membrane protrusions to promote fusogenic protein engagement during cell fusion.
Fig. 3. F-actin-enriched hair-like projections promote cell fusion in αPS2-Eff-1-expressing S2R+ cells.
A) Scar, but not WASP-WIP, is required for αPS2-Eff-1-induced cell fusion. Fusion indexes resulting from RNAi knockdown of Sns, WASP, WIP, Scar or αPS2 in αPS2-Eff-1-expressing cells. (B) Cell fusion induced by Eff-1 alone is mediated by endogenous Scar and αPS2. Fusion indexes resulting from RNAi knockdown of indicated genes in Eff-1-expressing cells. (C) Cells overexpressing αPS2 show stronger adhesion to the culture dish. Cells expressing indicated proteins were subjected to adhesion assays (see Materials and Methods). αPS2 expression reduced the percentage of transfected cells in the detached population. (D and E) F-actin-enriched hair-like projections along the cell-cell contact zone of αPS2-Eff-1- and Eff-1-expressing cells. Cells were transfected with αPS2-Eff-1 (D) or Eff-1 alone (E) and GFP-Moesin (D and E), and stained with anti-V5 (red; αPS2-V5) and anti-HA (cyan; Eff-1-HA). Boxed areas magnified in insets, and schematic drawings of F-actin shown on the right. Inset in (D) shows F-actin-enriched hair-like projections at the cell periphery of αPS2-Eff-1-expressing cells. Arrow in (D′) indicates a small punctum of αPS2 within a hair-like projection. Inset in (E) shows the occasionally observed F-actin-enriched projections (one indicated by arrow) in Eff-1-expressing cells. (F and G) Distinct distribution of αPS2 and Eff-1 on the membrane along the F-actin-enriched finger-like protrusions. Cells co-transfected with αPS2-V5 and Eff-1-HA were stained with anti-V5 (red), anti-HA (green in F) and anti-Actin (green in G), and subjected to STORM analysis. Images were acquired by either widefield microscopy (a) or STORM (b to d″). Boxed area in (a) and (b) enlarged in (c), and that in (c) enlarged in (d to d″). Arrows in (c) indicate individual finger-like protrusions. Arrow in (d and d″) and arrowheads in (d′ and d″) indicate distinct domains of αPS2 and Eff-1 (or Actin). Scale bars: 10 μm (D and E); in F and G: 20 μm (a and b), 500 nm (c), 100 nm (d to d″).
In summary, reconstitution of high efficiency cell-cell fusion in a non-fusing cell line reveals two fundamental principles underlying cell-cell fusion. First, a transmembrane fusogenic protein is indispensable for cell-cell fusion, because fusion does not occur without a fusogenic protein irrespective of actin cytoskeletal remodeling. Second, the actin cytoskeleton provides an active driving force for cell-cell fusion by generating membrane protrusions that are necessary and sufficient to promote fusion mediated by fusogenic proteins. Membrane protrusions induced by different adhesion molecules share common characteristics of invasiveness and engagement of fusogenic proteins and represent a general mechanism underlying cell-cell fusion.
Supplementary Material
Summary.
Interplay between cellular fusogenic proteins and actin-propelled invasive membrane protrusions revealed by an inducible cell-fusion system.
Acknowledgments
We thank P. Beachy for the S2R+ cell line and G. Seydoux for the C. elegans cDNA; M. Delannoy at Johns Hopkins Microscope Facility for advice on cell cuture TEM and immunoEM; J. Reidler, J. Hill and J. DeWitt at Nikon Instruments Inc. for advice on STORM; S. Craig, J. Nathans, E. Olson, D. Pan, D. Robinson, G. Seydoux, J. Yang, and members of the Chen lab for disccusions and critical reading of the manuscript. K.S. was a postdoctoral fellow of the American Heart Association. Supported by the National Institutes of Health (R01 GM098816) and the Packard Foundation (E.H.C.).
Footnotes
Materials and Methods
Captions for Movies S1 to S10
References and Notes
- 1.Chen EH, Olson EN. Unveiling the mechanisms of cell-cell fusion. Science. 2005;308:369. doi: 10.1126/science.1104799. [DOI] [PubMed] [Google Scholar]
- 2.Ogle BM, Cascalho M, Platt JL. Biological implications of cell fusion. Nat Rev Mol Cell Biol. 2005;6:567. doi: 10.1038/nrm1678. [DOI] [PubMed] [Google Scholar]
- 3.Oren-Suissa M, Podbilewicz B. Cell fusion during development. Trends Cell Biol. 2007;17:537. doi: 10.1016/j.tcb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Avinoam O, Podbilewicz B. Eukaryotic cell-cell fusion families. Curr Top Membr. 2011;68:209. doi: 10.1016/B978-0-12-385891-7.00009-X. [DOI] [PubMed] [Google Scholar]
- 5.Rochlin K, Yu S, Roy S, Baylies MK. Myoblast fusion: When it takes more to make one. Dev Biol. 2010;341:66. doi: 10.1016/j.ydbio.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen EH. Invasive podosomes and myoblast fusion. Curr Top Membr. 2011;68:235. doi: 10.1016/B978-0-12-385891-7.00010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abmayr SM, Pavlath GK. Myoblast fusion: lessons from flies and mice. Development. 2012;139:641. doi: 10.1242/dev.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanagawa S, Lee JS, Ishimoto A. Identification and characterization of a novel line of Drosophila Schneider S2 cells that respond to wingless signaling. J Biol Chem. 1998;273:32353. doi: 10.1074/jbc.273.48.32353. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Gomez M, Coutts N, Price A, Taylor MV, Bate M. Drosophila dumbfounded: a myoblast attractant essential for fusion. Cell. 2000;102:189. doi: 10.1016/s0092-8674(00)00024-6. [DOI] [PubMed] [Google Scholar]
- 10.Bour BA, Chakravarti M, West JM, Abmayr SM. Drosophila SNS, a member of the immunoglobulin superfamily that is essential for myoblast fusion. Genes Dev. 2000;14:1498. [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, et al. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell. 2007;12:571. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Massarwa R, Carmon S, Shilo BZ, Schejter ED. WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev Cell. 2007;12:557. doi: 10.1016/j.devcel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Schafer G, et al. The Wiskott-Aldrich syndrome protein (WASP) is essential for myoblast fusion in Drosophila. Dev Biol. 2007;304:664. doi: 10.1016/j.ydbio.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Richardson BE, Beckett K, Nowak SJ, Baylies MK. SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development. 2007;134:4357. doi: 10.1242/dev.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohler WA, et al. The type I membrane protein EFF-1 is essential for developmental cell fusion. Dev Cell. 2002;2:355. doi: 10.1016/s1534-5807(02)00129-6. [DOI] [PubMed] [Google Scholar]
- 16.Podbilewicz B, et al. The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Dev Cell. 2006;11:471. doi: 10.1016/j.devcel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Dworak HA, Charles MA, Pellerano LB, Sink H. Characterization of Drosophila hibris, a gene related to human nephrin. Development. 2001;128:4265. doi: 10.1242/dev.128.21.4265. [DOI] [PubMed] [Google Scholar]
- 18.Galletta BJ, Chakravarti M, Banerjee R, Abmayr SM. SNS: Adhesive properties, localization requirements and ectodomain dependence in S2 cells and embryonic myoblasts. Mech Dev. 2004;121:1455. doi: 10.1016/j.mod.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Chen EH, Pryce BA, Tzeng JA, Gonzalez GA, Olson EN. Control of myoblast fusion by a guanine nucleotide exchange factor, loner, and its effector ARF6. Cell. 2003;114:751. doi: 10.1016/s0092-8674(03)00720-7. [DOI] [PubMed] [Google Scholar]
- 20.Islam R, Wei SY, Chiu WH, Hortsch M, Hsu JC. Neuroglian activates Echinoid to antagonize the Drosophila EGF receptor signaling pathway. Development. 2003;130:2051. doi: 10.1242/dev.00415. [DOI] [PubMed] [Google Scholar]
- 21.Johnston CA, Hirono K, Prehoda KE, Doe CQ. Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell. 2009;138:1150. doi: 10.1016/j.cell.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotwals PJ, Paine-Saunders SE, Stark KA, Hynes RO. Drosophila integrins and their ligands. Curr Opin Cell Biol. 1994;6:734. doi: 10.1016/0955-0674(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 23.Schwander M, et al. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4:673. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 24.Fenichel P, Durand-Clement M. Role of integrins during fertilization in mammals. Hum Reprod. 1998;13(Suppl 4):31. doi: 10.1093/humrep/13.suppl_4.31. [DOI] [PubMed] [Google Scholar]
- 25.McNally AK, Anderson JM. Beta1 and beta2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation. Am J Pathol. 2002;160:621. doi: 10.1016/s0002-9440(10)64882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabata N, et al. Expression of fusion regulatory proteins (FRPs) on human peripheral blood monocytes. Induction of homotypic cell aggregation and formation of multinucleated giant cells by anti-FRP-1 monoclonal antibodies. J Immunol. 1994;153:3256. [PubMed] [Google Scholar]
- 27.Sens KL, et al. An invasive podosome-like structure promotes fusion pore formation during myoblast fusion. J Cell Biol. 2010;191:1013. doi: 10.1083/jcb.201006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haralalka S, et al. Asymmetric Mbc, active Rac1 and F-actin foci in the fusion-competent myoblasts during myoblast fusion in Drosophila. Development. 2011;138:1551. doi: 10.1242/dev.057653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat Methods. 2006;3:793. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin P, et al. Competition between Blown Fuse and WASP for WIP Binding Regulates the Dynamics of WASP-Dependent Actin Polymerization In Vivo. Dev Cell. 2011;20:623. doi: 10.1016/j.devcel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan R, et al. Group I PAKs function downstream of Rac to promote podosome invasion during myoblast fusion in vivo. J Cell Biol. 2012;199:169. doi: 10.1083/jcb.201204065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avinoam O, et al. Conserved eukaryotic fusogens can fuse viral envelopes to cells. Science. 2011;332:589. doi: 10.1126/science.1202333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards KA, Demsky M, Montague RA, Weymouth N, Kiehart DP. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev Biol. 1997;191:103. doi: 10.1006/dbio.1997.8707. [DOI] [PubMed] [Google Scholar]
- 34.Kim DH, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Chen EH. Ultrastructural Analysis of Myoblast Fusion in Drosophila. In: Chen EH, editor. Cell Fusion: Overviews and Methods. Humana Press; NJ: 2008. pp. 275–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCartney BM, Fehon RG. Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophila homologues of moesin and the neurofibromatosis 2 tumor suppressor, merlin. J Cell Biol. 1996;133:843. doi: 10.1083/jcb.133.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.