Abstract
Adult mice with a Leydig cell specific deletion of MAPK kinase (MEK) 1 and 2 (Mek1f/f;Mek2−/−;Cre+) mice display Leydig cell hypoplasia and hypergonadotropic hypogonadism. We used radioimmunoassays and quantitative PCR to evaluate the function and expression of the Leydig cell genes involved in the conversion of cholesterol to testosterone (Star, Cyp11a1, Hsd3b6, Cyp17a1 and Hsd17b3), androgen metabolism (Srda1 and Dhrs9), and four transcription factors (Creb1, Nr5a1, Nr4a1 and Nr0b1) that regulate the expression of steroidogenic genes. We show that Star, Hsd3b6, Cyp17a1 and Hsd17b3 are downregulated in Ledyig cells of adult Mek1f/f;Mek2−/−;Cre+ mice whereas Srda1 and Dhrs9 are upregulated and Creb1, Nr5a1, Nr4a1 and Nr0b1 are unchanged or upregulated. Functionally, all the downregulated genes but none of the upregulated genes contribute to the decrease in testosterone synthesis in Leydig cells of adult Mek1f/f;Mek2−/−;Cre+ mice because they produce low testosterone and dihydrotestosterone when stimulated with hCG or when incubated with testosterone precursors such as progesterone or androstenedione.
Keywords: Leydig cells, MAPK kinase (MEK), MAP kinase (ERK), Luteinizing hormone, Steroidogenesis, Androgens
1.1 Introduction
Adult Leydig cells arise from postnatal stem Leydig cells several days after birth and they proliferate and differentiate to produce testosterone, a hormone that is essential for puberty, spermatogenesis and the maintenance of secondary sexual characteristics (Ge and Hardy, 2010; Stanley et al., 2012; Stanley et al., 2011; Teerds, 2010).
The proliferation and differentiation of postnatal Leydig cells are dependent on luteinizing hormone (LH) and its cognate receptor (LHR). High levels of LH or mutations of the LHR that lead to constitutive activation result in hyperandrogenemia and Leydig cell hyperplasia or Leydig cell adenomas, whereas LH deficiency, or mutations that inactivate the LHR result in Leydig cell hypoplasia and hypoandrogenemia (Baker and O'Shaughnessy, 2001; Bernichtein et al., 2008; Dufau and Tsai-Morris, 2007; Huhtaniemi, 2010; Huhtaniemi et al., 2005; Lei et al., 2001; O'Shaughnessy et al., 1998; O'Shaughnessy et al., 2009; Rulli and Huhtaniemi, 2005; Segaloff, 2009; Themmen, 2005; Zhang et al., 2001).
The Leydig cell ERK1/2 cascade is one of the signaling pathways activated by the agonist-engaged LHR (Hirakawa and Ascoli, 2003; Shiraishi and Ascoli, 2006) and the involvement of ERK1/2 on the proliferation of postnatal Leydig cells has been recently documented in vitro (Shiraishi and Ascoli, 2007), and in vivo (Yamashita et al., 2011). For the in vivo studies we crossed Mek1f/f;Mek2−/− and Cyp17iCre mice to generate a mouse model with a Leydig cell specific deletion of Mek1 in the context of a global deletion of Mek2 (Mek1f/f;Mek2−/−;Cre+). The postnatal Leydig cells of Mek1f/f;Mek2−/−;Cre+ mice lack MEK2, express substantially reduced levels of MEK1 and normal levels of ERK1/2 (Yamashita et al., 2011). As expected from the deletion of MEK1/2, the phosphorylation of ERK1/2 stimulated by hCG, cAMP analogs, EGF or Kit ligand is substantially reduced or absent in the postnatal Leydig cells of Mek1f/f;Mek2−/−;Cre+ mice (Yamashita et al., 2011). Adult Mek1f/f;Mek2−/−;Cre+ mice develop Leydig cell hypoplasia, hypergonadotropic hypogonadism and experience an age-dependent decrease in fertility (Yamashita et al., 2011). Leydig cells isolated from adult Mek1f/f;Mek2−/−;Cre+ mice also displayed a reduced ability to synthesize testosterone when incubated with hCG, a permeable cAMP analog, 22-hydroxycholesterol or pregnenolone (Yamashita et al., 2011), thus suggesting that the ERK1/2 cascade also regulates the steroidogenic potential of Leydig cells.
The regulation of the acute stimulation of Leydig cell steroidogenesis by the ERK1/2 cascade has been studied in some detail in vitro. All investigators agree that pharmacological inhibitors of the ERK1/2 pathway inhibit the acute stimulation of steroidogenesis by cAMP or trophic hormones (Evaul and Hammes, 2008; Gyles et al., 2001; Manna et al., 2006; Manna et al., 2007; Martinelle et al., 2004) but there is no consensus on the mechanisms involved. Some reported that this is due to an inhibition of the expression and/or phosphorylation of StAR (Evaul and Hammes, 2008; Gyles et al., 2001; Martinelle et al., 2004) whereas others reported that pharmacological inhibitors of ERK1/2 increased the expression and phosphorylation of StAR while inhibiting the acute stimulation of steroiodogenesis by more complex mechanisms involving changes in the expression of a orphan nuclear receptor (Nr0b1) and the scavenger receptor class B type 1 (Manna et al., 2006; Manna et al., 2007).
In contrast, to the studies summarized above, the effects of the ERK1/2 cascade on the expression of the other genes involved in androgen biosynthesis in Leydig cells has not been explored. Here we report that Leydig cells from adult Mek1f/f;Mek2−/−;Cre+ mice have decreased expression of many of the genes involved in testosterone synthesis and enhanced expression of two genes coding for enzymes that metabolize androgens. Our results are the first to document the involvement of the ERK1/2 as a coordinate regulator of the expression of androgenic genes in Leydig cells in vivo. They also show that this decline cannot be attributed to a decrease in the expression of several transcription factors known to coordinately regulate the expression of steroidogenic enzymes in Leydig cells.
1.2 Materials and Methods
1.2.1 Animals
Mek1f/f;Mek2−/−;Cre+ mice were generated as described earlier (Yamashita et al., 2011). The mouse colony was maintained by breeding Mek1f/f;Mek2−/−;Cre− males with Mek1f/f;Mek2−/−;Cre+ females so that littermates could be used as experimental and control animals. Mice were housed and bred under standard conditions with food and water ad libitum and were maintained on a 12 h dark/light cycle. Genotyping was performed using tail genomic DNA followed by PCR amplification as described earlier (Yamashita et al., 2011). All animal procedures were approved by the Institutional Animal Care and Use Committee for the University of Iowa.
1.2.2 Decapsulated testes incubations
Testes were weighed, decapsulated, placed in 12 × 75 mm polystyrene tubes with 500 µl of cold DMEM/F12 without phenol red but containing 10 mM HEPES, 50 µg/ml gentamicin, 1mg/ml BSA, pH 7.4 and incubated at 34°C for 30 min. The medium was aspirated and the testes were then incubated for 4 h at 34°C in 1 ml of fresh medium containing vehicle only or 100 ng/ml hCG. Alternatively they were incubated in 1 ml of fresh DMEM/F12 medium containing 10 µl of ethanol, 10 µM androstenedione or 10 µM progesterone (each added as 10 µl aliquots of 100-fold concentrated solutions). Media were collected, centrifuged and stored at −80°C until used for radioimmunoassay.
1.2.3 Isolation of Leydig cells
Purified Leydig cells were prepared from the testes of a single mouse by centrifuging a mechanically-dispersed population of interstitial cells (O'Shaughnessy et al., 2002) through a Percoll gradient (Schumacher et al., 1978). The testes from one mouse were decapsulated and placed in a culture dish containing cold DMEM/F12 supplemented with 10 mM HEPES, 50 µg/ml gentamicin, 1 mg/ml BSA, pH 7.4. Under a dissecting microscope, tubules were teased using fine forceps (O'Shaughnessy et al., 2002). The tubules were discarded and the resulting cell suspension was filtered through a 70 µm Nylon cell strainer. Filtered cells were collected by centrifugation and resuspended in 2 ml of Dulbecco’s Phosphate Buffered Saline (D-PBS) containing 20 mM HEPES and 0.7 mg/ml BSA, pH 7.4. Leydig cells were then purified using a discontinuous four-layer Percoll (GE Healthcare Life Sciences, Piscataway, NJ, USA) density gradient modified from a previously published protocol (Schumacher et al., 1978) as follows. Two ml of the interstitial cell suspension (obtained from a single mouse, see above) were layered on top of a discontinuous density gradient (prepared in a 15 ml conical tube) containing 3 ml of 53%, 1 ml of 40%, 4 ml of 37% and 2 ml of 20% Percoll (all prepared in the D-PBS solution with BSA listed above). The gradient was centrifuged at 800 × g for 20 min at room temperature (Schumacher et al., 1978) and the Leydig cells, which migrated to the 53% Percoll fraction, were diluted 2 fold with the D-PBS solution with BSA and recovered by centrifuged at 150 × g for 15 min at room temperature. The yield of Leydig cells/mouse was 2–3 × 105 and 5–6 × 105 for the 21 and 50 day-old mice, respectively.
For steroid assays, the Leydig cell fraction obtained from a single mouse was resuspended in 500 µl DMEM/F12 without phenol red containing 10 mM HEPES, 50 µg/ml gentamicin, 1 mg/ml BSA, pH 7.4 (Assay medium) and 250 µl aliquots were incubated in a shaking water bath at 34C for 4h in medium containing vehicle only or 100 ng/ml hCG. Alternatively they were incubated with vehicle only (ethanol), 10 µM androstenedione, or 10 µM progesterone. At the end of the incubation the cells were collected by centrifugation, and the media were collected and stored at −80°C until used for radioimmunoassay. For Western blots, Leydig cells (or the 37% Percoll fraction containing germ cells) purified from the testes of one mouse were washed twice in a buffer containing 150 mM NaCl and 20 mM HEPES (pH 7.4), lysed and analyzed as described below. For real-time PCR assays (qPCR), the Leydig or germ cells purified from the testes of one mouse were centrifuged at 1200 × g for 10 min, resuspended in 600 µl of RLT Lysis buffer (QIAGEN Inc., Valencia, MO, USA) containing 10% of β-mercaptoethanol and stored at −80°C until RNA extractions were performed as described in Section 1.2.6.
1.2.4 Cellular lysates and Western blot analysis
Leydig or germ cells purified from the testes of one mouse were lysed in 20–40 µl of RIPA buffer (150 mM NaCl, 50 mM Tris, 1 mM EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM Na3VO4 and 1 mM NaF, pH 7.4) supplemented with a commercial mixture of protease inhibitors (Roche, Indianapolis, IN, USA). Homogenates were kept in ice for 30 min, with occasional mechanical disruption using a pipet, followed by a centrifugation at 13,000 × g for 10 min. Supernatants were collected and assayed for protein content using BCA protein assay kit from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Cellular lysates were used immediately or stored at −80°C until used. Western blots were done as described earlier (Shiraishi and Ascoli, 2007) using 2–15 µg of lysate protein. The solutions used to block and wash the membranes, and perform the primary and secondary antibody incubations contained either 5% milk or 1–5% bovine serum albumin depending on the antigen being detected. The length (1–18 h) and temperature (4°C or ambient) of the incubation and the dilutions of the primary antibodies also varied depending on the antigen being detected. The secondary antibody dilution (1/3,000) and length and temperature of incubation (1h at room temperature) were constant, however. The secondary anti-mouse or anti-rabbit antibodies coupled to horseradish peroxidase were from Bio-Rad Laboratories (catalog # 170–6515). The immune complexes in the Western blots were eventually visualized using ECL (Pierce Chemical) and exposed to film. The source of antibodies was as follows: VASA (Abcam # 13840), GAPDH (Cell Signaling # 2118), and 3βHSD which was kindly donated by the late Anita Payne.
1.2.5 Radioimmunoassay
The antibodies to testosterone and progesterone were kindly donated by Dr. Gordon Niswender. The dihydrotestosterone antibody (#20-DR14) was purchased from Fitzgerald Industries International (Acton, Ma, USA). The radioimmunoassays were performed and validated using conventional procedures as described earlier (Lacroix et al., 1979; Yamashita et al., 2011). The intra-assay coefficients of variation were 1.5, 1.5 and 3% for progesterone, testosterone and dihydrotestosterone, respectively. The inter-assay coefficients of variation were 8.4, 10.1 and 2.1% for progesterone, testosterone and dihydrotestosterone, respectively. Under the conditions used for the testosterone radioimmunoassays there was no interference of the androstenedione or progesterone added to the medium.
1.2.6 RNA extraction, reverse transcription and real-time PCR
Total RNA was prepared from purified mouse Leydig cells obtained from one mouse (see above) using the QIAGEN RNeasy mini kit (QIAGEN Inc., Valencia, MO, USA). Equal amounts of purified Leydig cell RNA (200 ng) were reversed transcribed using dN6 random primers (Applied Biosystems, Carlsbad, CA, USA) and M-MLV Reverse Transcriptase (Promega, Madison, WI, USA) as described elsewhere (Frungieri et al., 2002).
Real time PCR reactions were performed in a 25 µl volume using 300 nM of each primer and 1X iQ SYBR Green Super Mix (Bio-Rad Laboratories Inc., Hercules, CA, USA) and fluorescence was detected on the CFX96 Real Time PCR Detection System (Bio-Rad Laboratories Inc.). The conditions for the qPCR were optimized for each gene and the target gene expression was normalized to an internal control, glyceraldehyde-3-phosphate dehydrogenase (Gapdh). The sequence for the Cyp11a1 primers was: forward = CCCGGAGCGTTCCTT; reverse = CCAATGGGCCTCTGATAATACTG; the sequence for the Nr5a1 primers was: forward = GCCCTGTTGGATTACACCTTG; reverse = GTTGCCAAATGCTTGTGGTA; an the sequence for the Creb1 primers was: forward = AGCCGGGTACTACCATTCTAC; reverse = GCAGCTTGAACAACAACTTGG. The sequences from the other primers used were derived from the indicated references: Gapdh (Donadeu and Ascoli, 2005), Star, Hsd3b6, Cyp17a1, Hsd17b3, Dhrs9, Srd5a1 (O'Shaughnessy et al., 2002), Nr4a1(Martin et al., 2008), Nr0b1 (Ludbrook et al., 2012) and VASA (Kim et al., 2011). The relative expression of the different target genes in the Leydig cells from Mek1f/f;Mek2−/−;Cre+ and Mek1f/f;Mek2−/−;Cre− mice shown in Figures 2 and 3 were calculated by the method of Pfaffl (Pfaffl, 2001). For each gene analyzed, all data are expressed relative to the expression of the same gene in the Leydig cells of 21 day old Mek1f/f;Mek2−/−;Cre− mice.
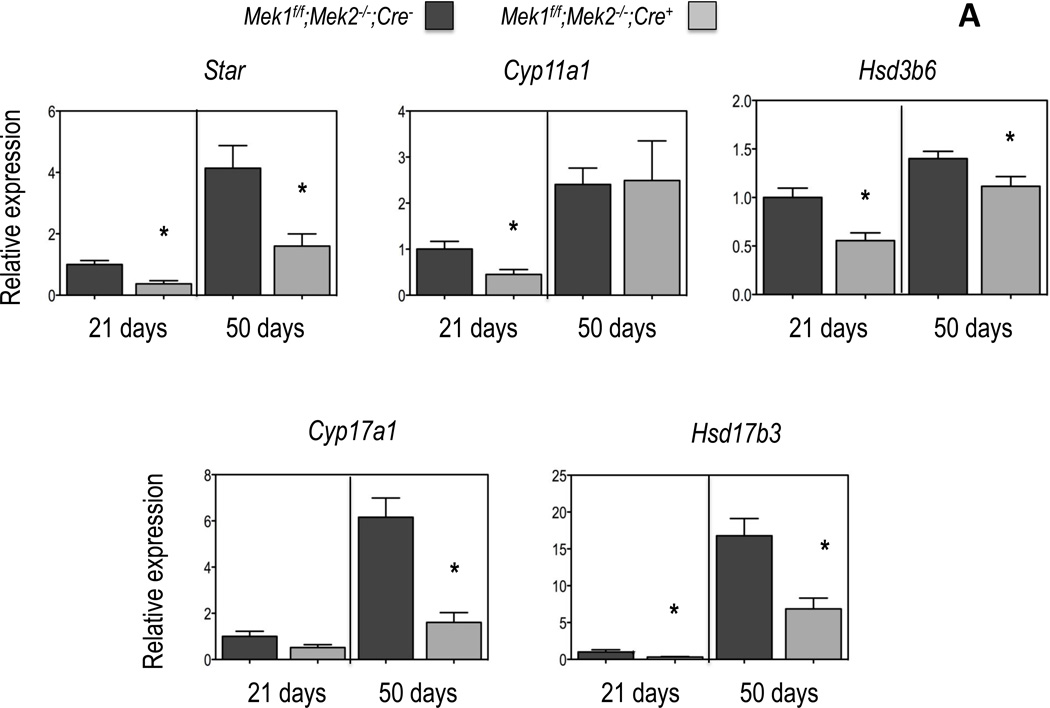
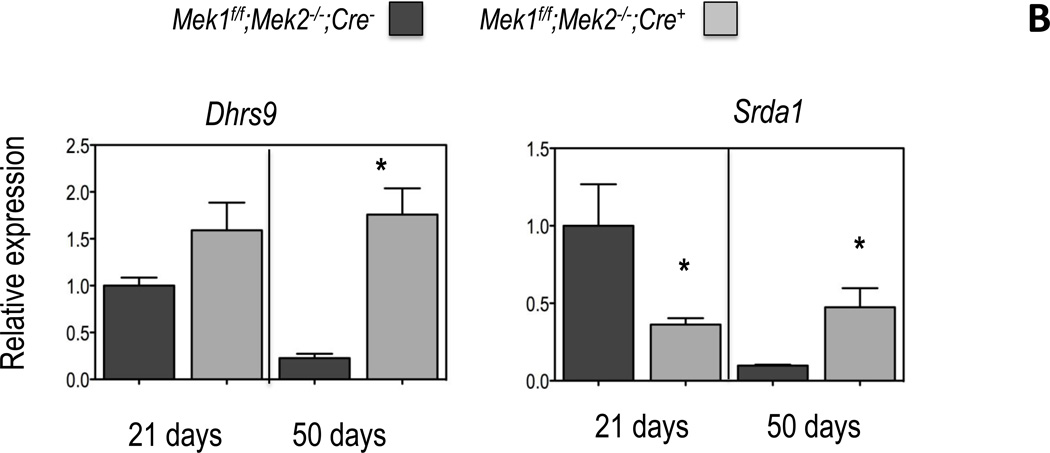
Figure 2. Expression of genes involved in testosterone biosynthesis and metabolism in Leydig cells of Mek1f/f;Mek2−/−;Cre− and Mek1f/f;Mek2−/−;Cre+ mice.
Gene expression was measured by real time PCR using equal amounts of RNA isolated from freshly purified Leydig cells (see Figure 1) obtained from the testes of individual mice of the indicated genotype and age as detailed in Materials and Methods. All data are corrected for the expression of Gapdh and expressed relative to the expression of the indicated genes in the Leydig cells of the 21 day old Mek1f/f;Mek2−/−;Cre− mice. (A) Enzymes involved in testosterone synthesis; (B) enzymes involved in testosterone metabolism. Results are the mean ± SEM of at least 5 mice. Asterisks indicate statistically different results (p < 0.05) between the two genotypes of age-matched mice
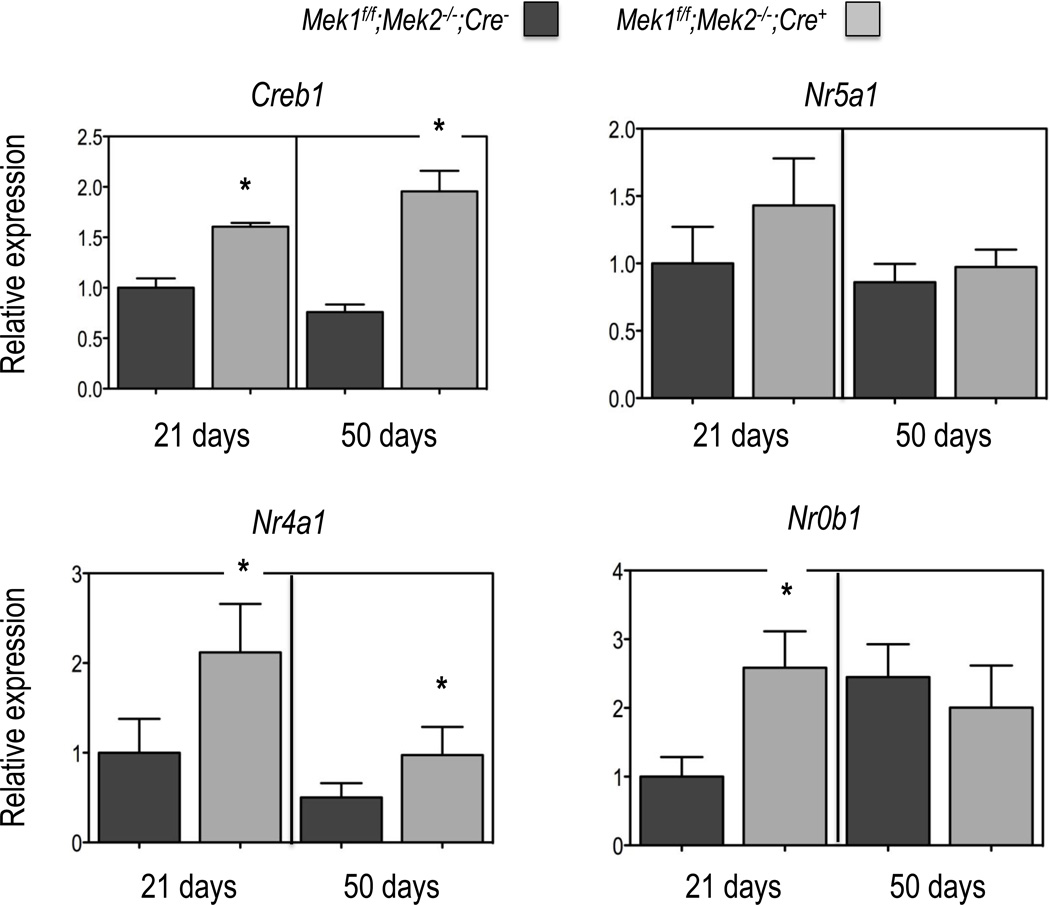
Figure 3. Expression of selected transcription factors in Leydig cells of Mek1f/f;Mek2−/−;Cre− and Mek1f/f;Mek2−/−;Cre+ mice.
Gene expression was measured by real time PCR using equal amounts of RNA isolated from freshly purified Leydig cells (see Figure 1) obtained from the testes of individual mice of the indicated genotype and age as detailed in Materials and Methods. All data are corrected for the expression of Gapdh and expressed relative to the expression of the indicated genes in the Leydig cells of the 21 day old Mek1f/f;Mek2−/−;Cre− mice. Results are the mean ± SEM of at least 5 mice. Asterisks indicate statistically different results (p < 0.05) between the two genotypes of age-matched mice.
1.2.7 Statistical analyses
Statistical analyses were performed using Student’s t test. Statistical significance was considered at p < 0.05.
1.3 Results
1.3.1 Effects of a Leydig cell specific deletion of Mek1 and Mek2 on steroidogenic enzyme and transcription factor expression in postnatal Leydig cells
To analyze the expression of steroidogenic genes and the intrinsic steroidogenic capacity of Leydig cells we purified a mechanically-dispersed population of interstitial cells (O'Shaughnessy et al., 2002) obtained from individual 21 or 50 day-old mice using a modified Percoll gradient protocol (Schumacher et al., 1978) as detailed in Materials and Methods. Since the testes of Mek1f/f;Mek2−/−;Cre+ mice have fewer Leydig cells than those of Mek1f/f;Mek2−/−;Cre− mice (see (Yamashita et al., 2011)) the use of isolated Leydig cells is necessary because the data so obtained is normalized to the number of Leydig cells. This normalization ensures that the functional differences observed are due to changes in the properties of the Leydig cells rather than to changes in the number of Leydig cells/testes. We also chose 21 and 50 day old mice because serum LH and testosterone are at prepuberal and adult levels, respectively (O'Shaughnessy et al., 2002; Wu et al., 2010).
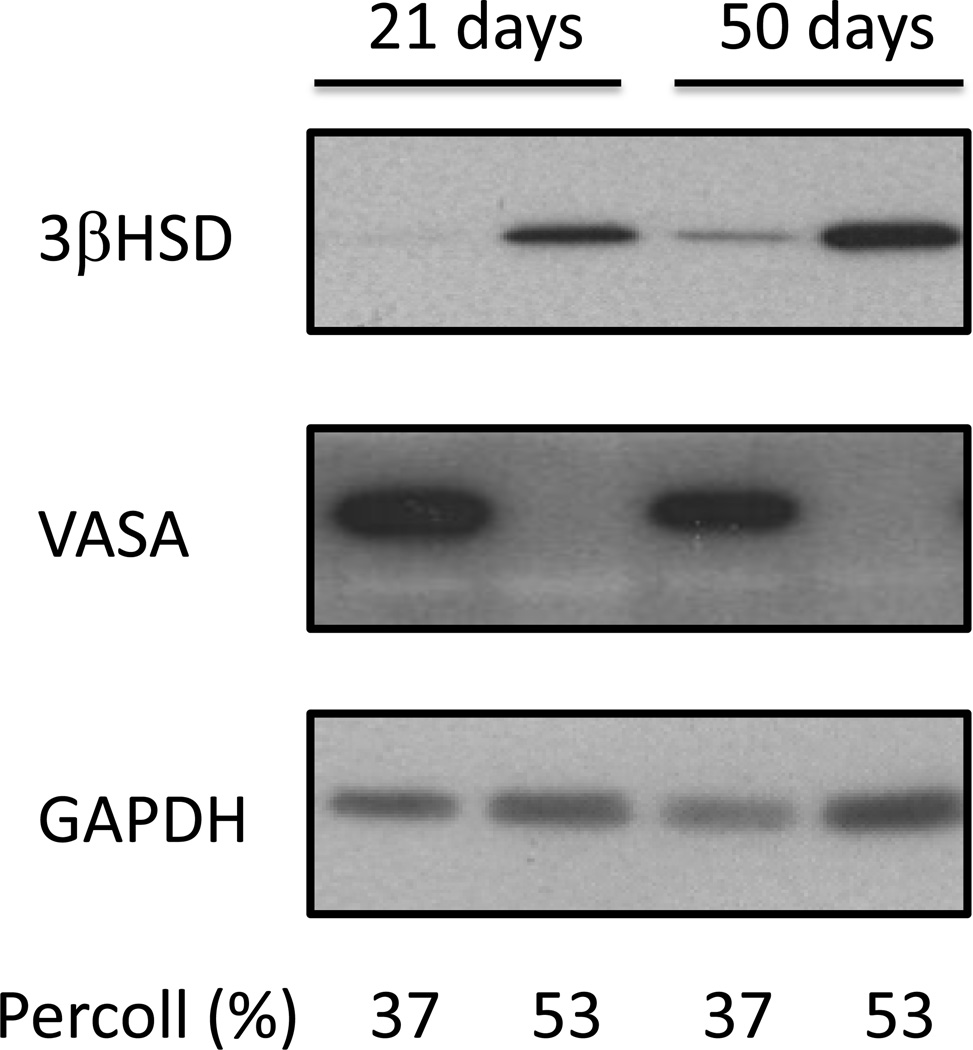
Figure 1 shows that we can isolate Leydig cells that are relatively free of germ cells as judged by the Western blot detection of VASA, an abundant germ cell marker. Some Leydig cells may contaminate the germ cell fraction but using Western blots we cannot detect an abundant germ cell marker (VASA) in the Leydig cell fraction prepared from either 21- or 50-day old mice. VASA was also barely detectable in the Leydig cell fraction using quantitative PCR. Under identical assay conditions, the Ct values for VASA expression were ~35 and ~25 cycles in the Leydig cell and germ cell fractions of 50 day-old mice, respectively. In contrast, the Ct values for all the steroidogenic enzymes shown in Figure 2 were in the range of 21–25 cycles in the Leydig cell fraction of 50 day-old mice.
Figure 1. A germ cell-free Leydig cell fraction can be readily obtained from the testes of a single mouse by Percoll gradient centrifugation.
Mechanically dispersed interstitial cells from the testes of individual Mek1f/f;Mek2−/−;Cre− mice of the indicated ages were fractionated on Percoll gradients as described in Materials and Methods. The cells migrating into the 37% or 53% Percoll solutions were collected, lysed and analyzed for VASA (a germ cell marker), 3βHSD (a Leydig cell marker) or GAPDH (loading control) using Western blots. DMRT-1, a marker for Sertoli cells and spermatogonia was also undetectable in the 53% Percoll fraction (data not shown). The results of a representative experiment are shown.
An analysis of the expression of the five genes involved in the conversion of cholesterol to testosterone in Leydig cells was next performed using cells freshly isolated and purified as described above. The previously reported age- and LH-dependent increase in the expression of the five genes in question (Ge and Hardy, 1998; O'Shaughnessy et al., 2002; Wu et al., 2010) was readily apparent in the Leydig cells of Mek1f/f;Mek2−/−;Cre− mice (Figure 2A). Importantly, the expression of these five genes is decreased in the Leydig cells of Mek1f/f;Mek2−/−;Cre+ mice. The timing of the decreased expression varies, however. Star, Hsd3b6 and Hsd17b3 are decreased in the Leydig cells of 21- and 50-day old mice. Cyp11a1 is decreased only on 21-day old mice whereas Cyp17a1 is decreased only on 50-day old mice.
We next analyzed the expression of two Leydig cell genes involved in androgen metabolism. Dhrs9 codes for 3α–hydroxysteroid dehydrogenase the enzyme that converts androstenedione to androsterone and dihydrotestosterone to 3α-DIOL; Srda1 codes for 5α-reductase, a Leydig cell enzyme that converts testosterone to dihydrotestosterone1. In agreement with the results of others (Ge and Hardy, 1998; O'Shaughnessy et al., 2002; Wu et al., 2010) we also found and age-related decrease in the expression of Dhrs9 and Srda1 in the Leydig cells of Mek1f/f;Mek2−/−;Cre− mice (Figure 2B). Changes in the expression of Dhrs9 and Srda1 in the Leydig cells of Mek1f/f;Mek2−/−;Cre+ mice are complex. Dhrs9 expression is unchanged at 21 days of age and increased at 50 days of age whereas Srda1 expression is decreased at 21 days of age and increased at 50 days of age (Fig 2B).
Last, we also measured the expression of Creb1, Nr5a1, Nr4a1 and Nr0b1 in Leydig cells (Figure 3). Three of these transcription factors, Creb1, Nr5a1 and Nr4a1 stimulate the expression of many of the genes needed to convert cholesterol to testosterone whereas Nr0b1 can act as a negative regulator of the transcriptional activity of several Leydig cell nuclear receptors (Martin and Tremblay, 2010). The expression of these transcription factors was relatively constant from 21 to 50 days of age. The expression of Nr5a1 was the same in both mouse genotypes at both ages but Creb1, Nr4a1 and Nr0b1 expression was increased in the Leydig cells of Mek1f/f;Mek2−/−;Cre+ mice at both ages (Creb1 and Nr4a1) or at 21 days of age (Nr0b1). The expression of Nr5a2, another important Leydig cell transcription factor (Martin and Tremblay, 2010) was too low to be accurately measured.
1.3.2 Effects of a Leydig cell specific deletion of Mek1 and Mek2 on androgen synthesis and metabolism in postnatal Leydig cells
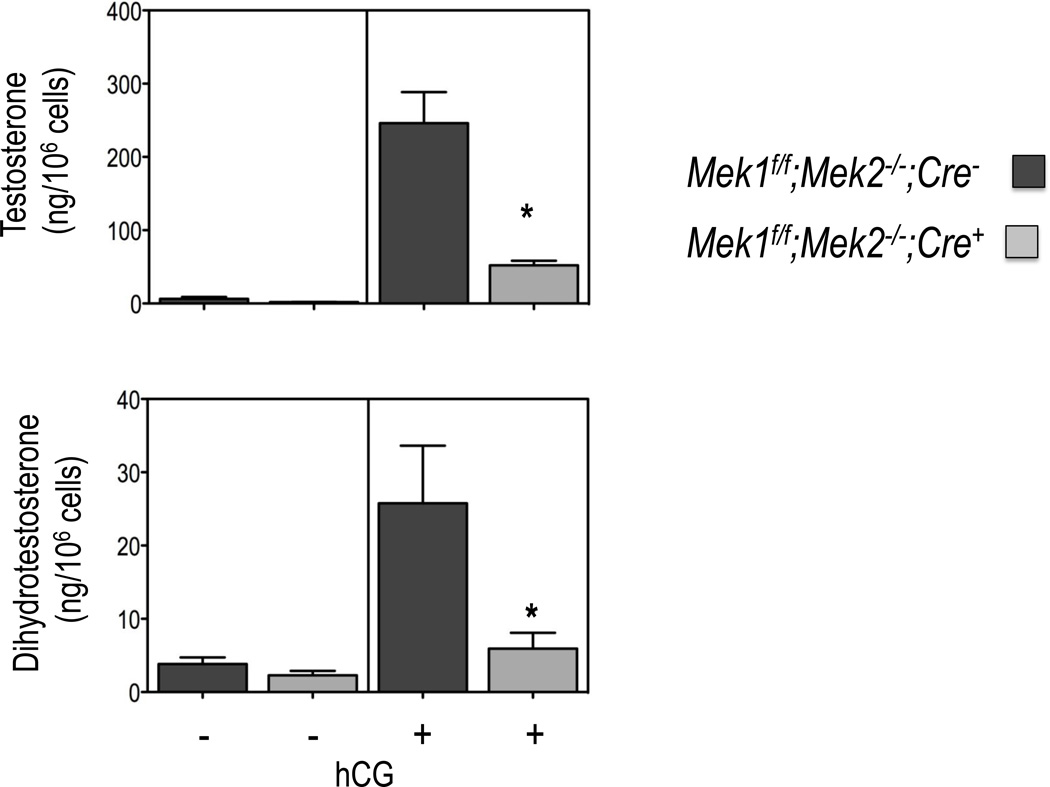
Because of the observed changes in the expression of the genes involved in testosterone synthesis and metabolism we also examined testosterone and dihydrotestosterone synthesis in Leydig cells purified from 50-day old mice. As documented in Figure 4, the hCG-provoked accumulation of testosterone and dihydrotestosterone are both decreased to about the same extent in freshly purified Leydig cells from adult Mek1f/f;Mek2−/−;Cre+ mice. The basal levels of testosterone are indistinguishable in the Leydig cells of the two mouse genotypes.
Figure 4. Testosterone and dihydrotestosterone synthesis in Leydig cells of Mek1f/f;Mek2−/−;Cre− and Mek1f/f;Mek2−/−;Cre+ mice.
Testosterone and dihydrotestosterone levels were measured in purified Leydig cells from 50 day old mice of the indicated genotype incubated without or with 100 ng/ml hCG for 4h at 34C. Results are the mean ± SEM of 3–4 mice. Asterisks indicate statistically different results (p < 0.05) between the two genotypes.
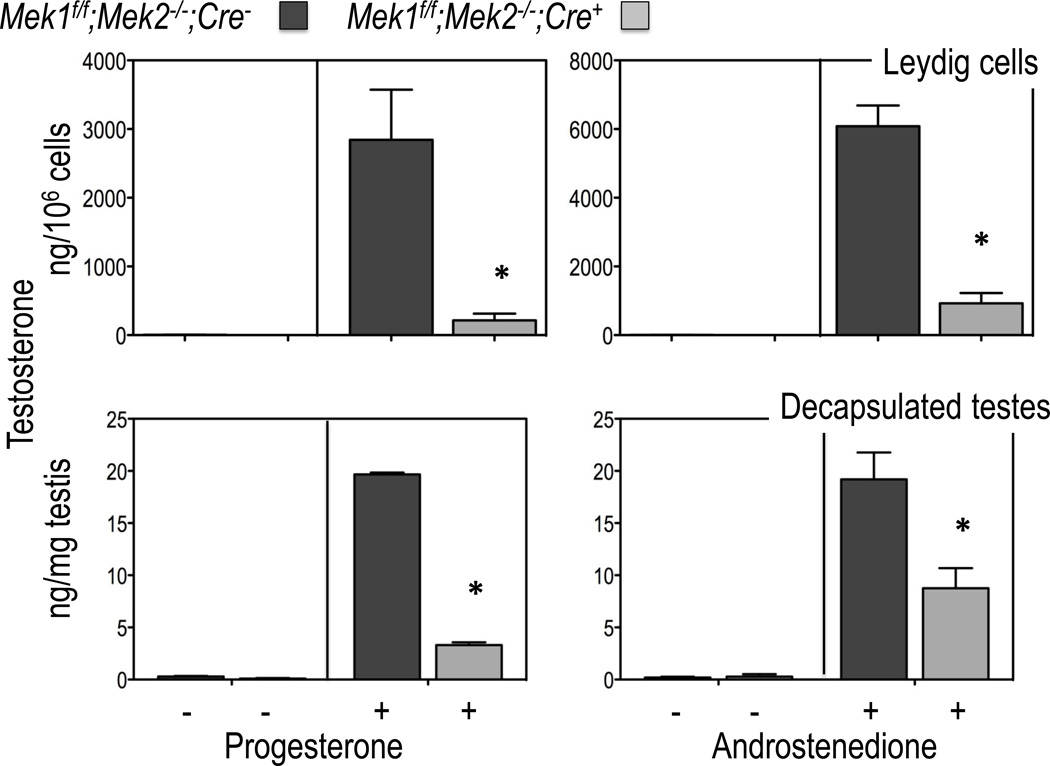
The decreased levels of androgens detected in the Leydig cells of 50 day old Mek1f/f;Mek2−/−;Cre+ mice are accompanied by an increase in the accumulation of progesterone and pregnenolone (not shown) suggesting that the reduced expression of Hsd3b6, Cyp17a1 and Hsd17b3 are particularly important determinants of the reduced levels of androgens produced by Leydig cells isolated from 50 day old mice. This conclusion is supported by the data presented in Figure 5A showing that the conversion of progesterone to testosterone (which requires expression of Cyp17a1 and Hsd17b31), and the conversion of androstenedione to testosterone (which requires only the expression of Hsd17b31), are substantially lower in the purified Leydig cells from 50-day old Mek1f/f;Mek2−/−;Cre+ mice than in those of Mek1f/f;Mek2−/−;Cre− mice. To better relate these changes to the phenotype of the Mek1f/f;Mek2−/−;Cre+ and Mek1f/f;Mek2−/−;Cre− mice we also measured the conversion of progesterone to testosterone and androstenedione to testosterone in decapsulated testes instead of purified Leydig cells. The results obtained with decapsulated testes (Figure 5B) and Leydig cells (Figure 5A) are very similar. Since the number of Leydig cells is 2–4 times lower in the testes of Mek1f/f;Mek2−/−;Cre+ mice (Yamashita et al., 2011) these results in Figure 5 suggest that the reduction in the expression of Cyp17a1 and/or Hsd17b3 in the remaining Leydig cells of the testes of Mek1f/f;Mek2−/−;Cre+ mice is quantitatively more important than the reduction in the number of Leydig cells per testes.
Figure 5. Testosterone synthesis in decapsulated testes or Leydig cells of Mek1f/f;Mek2−/−;Cre− and Mek1f/f;Mek2−/−;Cre+ mice incubated with testosterone precursors.
Testosterone levels were measured in purified Leydig cells or decasulated testes from 50 day old mice of the indicated genotype incubated without or with 10 µM progesterone or androstenedione for 4h at 34C. Results are the mean ± SEM of at least 5 mice. Asterisks indicate statistically different results (p < 0.05) between the two genotypes.
1.4 Discussion
In a previous publication (Yamashita et al., 2011) we showed that primary cultures of Leydig cells of 50-day old Mek1f/f;Mek2−/−;Cre+ mice, display a normal cAMP response to hCG but the synthesis of testosterone is greatly decreased when stimulated with hCG, cAMP analogs, 22-hydroxycholesterol or pregnenolone. Thus, the reduced steroidogenic capacity of Leydig cells of Mek1f/f;Mek2−/−;Cre+ mice cannot be explained by a decrease in the density of the LH receptor or the ability of LH/CG to stimulate cAMP accumulation, it can only be explained by a decrease in the expression and/or activity of one or more steroidogenic enzymes. The experiments presented here were thus designed to identify the steroidogenic enzymes that are decreased in the Leydig cells of Mek1f/f;Mek2−/−;Cre+ mice.
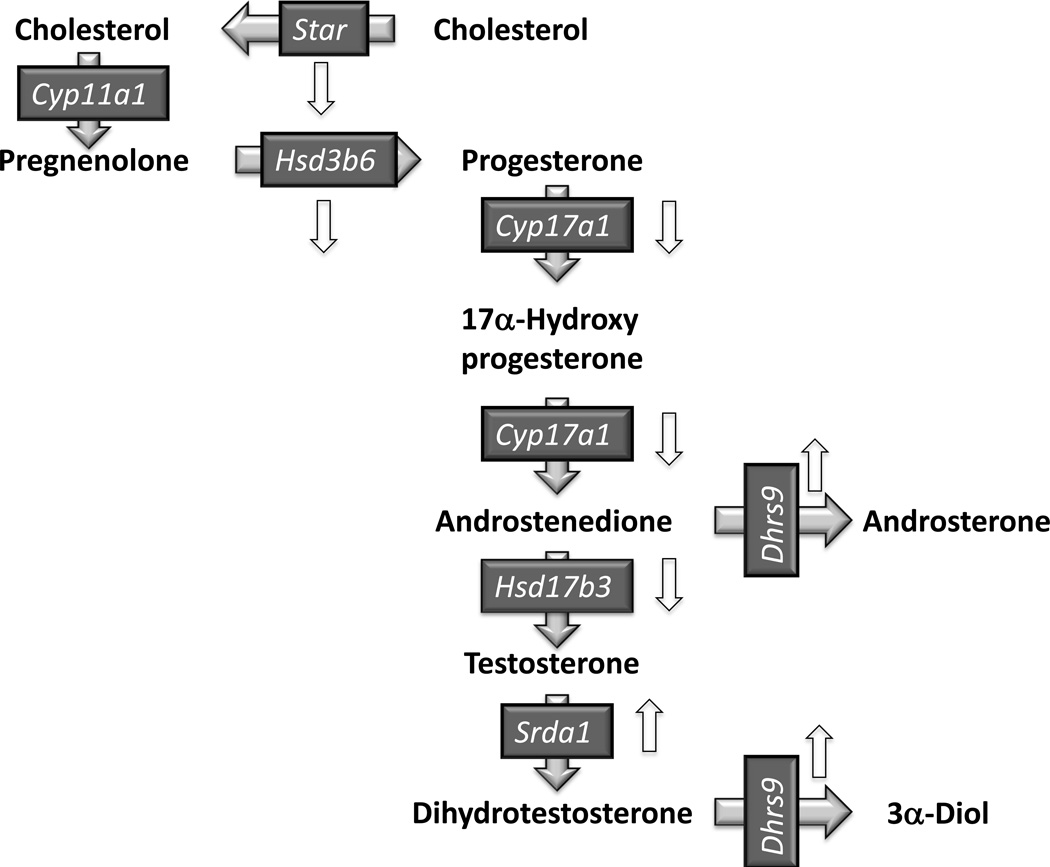
Pharmacological inhibition of the ERK1/2 cascade in vitro impairs the acute stimulation of steroidogenesis in Leydig cells by decreasing the availability of cholesterol and/or the transport of cholesterol to the mitochondria (Brion et al., 2011; Evaul and Hammes, 2008; Gyles et al., 2001; Manna et al., 2006; Manna et al., 2007; Martinelle et al., 2004). The potential involvement of the ERK1/2 pathway on the steroidogenic capacity (i.e., expression of steroidogenic genes) of Leydig cells remains largely underexplored, however. There are several reports on the effects of the ERK1/2 cascade on the expression and phosphorylation of StAR in vitro but the results are conflicting (Brion et al., 2011; Evaul and Hammes, 2008; Gyles et al., 2001; Manna et al., 2006; Manna et al., 2007; Martinelle et al., 2004). Pharmacological inhibitors of ERK1/2 have also been reported to affect the expression or activity of certain transcription factors that affect steroidogenic genes (Gyles et al., 2001; Manna et al., 2006; Manna et al., 2007). With the exception of StAR (see above), however, the effects of these inhibitors on the expression of the genes involved in androgen synthesis in Leydig cells have not been explored. Lastly, there are also several reports on the effects of ERK1/2 on the expression of steroidogenic genes in other tissues but these cannot always be extrapolated to Leydig cells because they can be variable from one cell type to another (reviewed by Manna and Stocco, 2011). Thus, the results presented herein showing that the Leydig cells of adult Mek1f/f;Mek2−/−;Cre+ mice have decreased expression of most androgenic genes (down arrows in Figure 6) and have reduced ability to synthesize testosterone are novel. In fact our data show that the reduced expression of Hsd17b3 is particularly important to the decreased androgenic capacity of the Leydig cells of adult Mek1f/f;Mek2−/−;Cre+ mice. These cells synthesize very low levels of testosterone even when given androstenedione as a precursor.
Figure 6. Pathways for testosterone synthesis and metabolism in Leydig cells.
Gene symbols are shown for the protein involved in the transport of cholesterol to the inner mitochondrial membrane (Star), the four enzymes that convert cholesterol to testosterone (Cyp11a1, Hsd3b6, Cyp17a1, and Hsd17b3) and the two enzymes that metabolize androgens, Srda1 and Dhrs9 in Leydig cells (Payne, 2007). Only the Δ4 pathway for testosterone synthesis is shown because this is the predominant pathway in murine Leydig cells (Payne, 2007). The up and down white arrows highlight genes that are up or down regulated in the Leydig cells of 50 day-old Mek1f/f;Mek2−/−;Cre+ mice.
In contrast to the decreased expression of enzymes involved in androgen biosynthesis, the expression of two Leydig cell androgen-metabolizing genes, Dhrs9 and Srda1 is increased in adult Mek1f/f;Mek2−/−;Cre+ mice (up arrows in Fig 6). The increased expression of Srda1 is unlikely to contribute to the decreased levels of testosterone in the Leydig cells of Mek1f/f;Mek2−/−;Cre+ mice because dihydrotestosterone is also low in these cells. These results suggest that the conversion of testosterone to dihydrotestosterone in Leydig cells is more likely dictated by the levels of testosterone rather than by the expression of Srda1. We restricted our studies to Srda1 because Srda2 is expressed in other cells in the testis, but not in Leydig cells (Ge and Hardy, 1998; Pratis et al., 2000). Lastly, we cannot elaborate on the functional significance of the increase in Dhrs9 expression because of the lack of commercially available reagents to measure the products of the action of 3α-hydroxysteroid dehydrogenase, the enzyme encoded by the Dhrs9 gene. An increase in the expression of Dhrs9 in the Leydig cells of Mek1f/f;Mek2−/−;Cre+ mice could underestimate the extent of conversion of androstenedione to testosterone shown in Fig 5, however. If there is increased conversion of the exogenous androstenedione to androsterone this could reduce the amount of androstenedione available for conversion to testosterone.
The decrease expression of androgenic genes in the Leydig cells of adult Mek1f/f;Mek2−/−;Cre+ mice suggest that the ERK1/2 cascade regulates the expression and/or activity of a transcription factor (or factors) that coordinately regulate the expression of these androgenic genes. Since this is a property of several transcription factors such as Creb1, Nr4a1, Nr0b1 and Nr5a1 expressed in Leydig cells (Martin and Tremblay, 2010), we measured their expression as well. Our data show that the decrease in androgenic gene expression in the Leydig cells of Mek1f/f;Mek2−/−;Cre+ mice cannot be explained by a decrease in the expression of Creb1, Nr4a1 or Nr5a1 or by an increase in the expression of Nr0b1. It is possible that the deletion of Mek1/2 in Leydig cells could affect the activities of CREB (the product of the Creb1 gene), SF-1, (the product of the Nr5a1 gene) or Nur77, (the product of the Nr4a1 gene) through post-translational modifications. Indeed, acute pharmacological inhibition of ERK1/2 impairs the cAMP-dependent phosphorylation and transcriptional activity of SF-1 on the Star promoter in MA-10 cells (Gyles et al., 2001). This interesting finding does not imply that the ERK1/2-mediated phosphorylation of SF-1 has the same effect on other androgenic gene promoters, however, because the effects of SF-1 phosphorylation on its transcriptional activity are promoter-dependent (Hoivik et al., 2010; Schimmer and White, 2010). Similarly, acute pharmacological inhibition of ERK1/2 in MA-10 has been reported to decrease the expression of Nr0b1 (Manna et al., 2007) which acts as a negative regulator of the transcriptional activity of several nuclear receptors (Martin and Tremblay, 2010). In contrast, our data show an increase in the expression of Nr0b1 in the Leydig cells of Mek1f/f;Mek2−/−;Cre+ mice but only at 21 days of age. Lastly, our studies on MA-10 cells (not presented) show that acute pharmacological inhibition of MEK1/2 with UO126 does not affect the ability of hCG to increase the phosphorylation of CREB. Clearly more studies are needed to identify the transcription factors that mediate the effects of ERK1/2 on androgenic gene expression. This will require additional approaches that are unbiased and discovery-based.
An important question in the characterization of the Mek1f/f;Mek2−/−;Cre+ mice (or other mice with reduced androgen levels) is whether the hypoandrogenism is caused by a decrease in the number of Leydig cells, a change in their functional properties or a combination of both. Since Leydig cells are routinely identified using approaches that rely on the immunological detection of markers such as Cyp17a1, 3βHSD or Cyp11a1 (Cacciola et al., 2008; Coonce et al., 2009; Hu et al., 2010; Yamashita et al., 2011; Zhang et al., 2001) it is difficult to know if a change in marker intensity is due to a change in the number of cells expressing the marker, to a change in the level of expression of the marker without a change in the actual number of cells or to a combination of these two. We have previously reported that the density of Leydig cells/testes is decreased in the Mek1f/f;Mek2−/−;Cre+ mice when Leydig cells are identified in histological sections of testes using Cyp11a1 as a marker (Yamashita et al., 2011). Since we show herein that the expression of Cyp11a1 in Leydig cells of Mek1f/f;Mek2−/−;Cre+ is reduced at some but not all ages it is possible that the previously described reduction in Leydig cell density was influenced by a decrease in the expression of Cyp11a1 in the Leydig cells. When we counted the total interstitial cell population instead of the Cyp11a1 positive interstitial cells, however, the results were similar (Yamashita et al., 2011). Thus, our conclusion that the ERK1/2 cascade is an important determinant of Leydig cell proliferation and/or survival remains sound (Yamashita et al., 2011).
In summary, the experiments presented here show that the Leydig cells of Mek1f/f;Mek2−/−;Cre+ mice have a coordinate reduction in the expression of many of the genes that participate in androgen synthesis and that the net result of these changes is a decrease in the capacity of Leydig cells from adult animals to synthesize androgens. We can also conclude that the reduction of steroidogenic gene expression in Leydig cells and the reduction in the number of Leydig cells/testis contribute to the decrease in androgen levels in adult Mek1f/f;Mek2−/−;Cre+ mice.
1.5 Conclusions
The Leydig-cell specific deletion of Mek1/2 leads to a coordinate decrease in the expression of Star, Hsd3b6, Cyp17a1 and Hsd17b3 and an increase in Srda1 and Dhrs9 without changes in the expression of several transcription factors that coordinately regulate the expression of steroidogenic genes in Leydig cells. The decrease in the expression of Hsd17b3 seems to be particularly important to the reduced androgenic potential of Leydig cells with reduced expression of Mek1/2 because they synthesize low amounts of testosterone even when supplied with androstenedione, the substrate for 17βHSD3, the product of the Hsd17b3 gene.
Highlights.
Deletion of Mek1/2 decreases expression of androgenic genes in Leydig cells.
Deletion of Mek1/2 increases expression of androgen metabolism genes in Leydig cells.
Deletion of Mek1/2 decreases androgen synthesis in Leydig cells.
Acknowledgments
Supported by a grant from the NIH (CA-40629) to M.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Figure 6, used in the Discussion, shows the pathway for androgen biosynthesis and metabolism in Leydig cells but it may be useful to look at it while reading the Results section.
References
- 1.Baker PJ, O'Shaughnessy PJ. Role of gonadotropins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction. 2001;122:227–234. doi: 10.1530/rep.0.1220227. [DOI] [PubMed] [Google Scholar]
- 2.Bernichtein S, Alevizaki M, Huhtaniemi I. Is the adrenal cortex a target for gonadotropins? Trends in Endocrinology and Metabolism. 2008;19:231–238. doi: 10.1016/j.tem.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Brion L, Maloberti PM, Gomez NV, Poderoso C, Gorostizaga AB, Mori Sequeiros Garcia MM, Acquier AB, Cooke M, Mendez CF, Podesta EJ, Paz C. MAPK phosphatase-1 (MKP-1) expression is up-regulated by hCG/cAMP and modulates steroidogenesis in MA-10 Leydig cells. Endocrinology. 2011;152:2665–2677. doi: 10.1210/en.2011-0021. [DOI] [PubMed] [Google Scholar]
- 4.Cacciola G, Chioccarelli T, Mackie K, Meccariello R, Ledent C, Fasano S, Pierantoni R, Cobellis G. Expression of type-1 cannabinoid receptor during rat postnatal testicular development: possible involvement in adult leydig cell differentiation. Biology of reproduction. 2008;79:758–765. doi: 10.1095/biolreprod.108.070128. [DOI] [PubMed] [Google Scholar]
- 5.Coonce MM, Rabideau AC, McGee S, Smith K, Narayan P. Impact of a constitutively active luteinizing hormone receptor on testicular gene expression and postnatal Leydig cell development. Molecular and Cellular Endocrinology. 2009;298:33–41. doi: 10.1016/j.mce.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donadeu FX, Ascoli M. The Differential Effects of the Gonadotropin Receptors on Aromatase Expression in Primary Cultures of Immature Rat Granulosa Cells Are Highly Dependent on the Density of Receptors Expressed and the Activation of the Inositol Phosphate Cascade. Endocrinology. 2005;146:3907–3916. doi: 10.1210/en.2005-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufau ML, Tsai-Morris CH. The luteinizing hormone receptor. In: Payne AH, Hardy MP, editors. The Leydig cell in health and disease. Totowa, NJ: Humana Press; 2007. pp. 227–252. [Google Scholar]
- 8.Evaul K, Hammes SR. Cross-talk between G Protein-coupled and Epidermal Growth Factor Receptors Regulates Gonadotropin-mediated Steroidogenesis in Leydig Cells. Journal of Biological Chemistry. 2008;283:27525–27533. doi: 10.1074/jbc.M803867200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frungieri MB, Weidinger S, Meineke V, Kohn FM, Mayerhofer A. Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPARgamma : Possible relevance to human fibrotic disorders. Proc Natl Acad Sci U S A. 2002;99:15072–15077. doi: 10.1073/pnas.232422999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge R, Hardy MP. Regulation of Leydig cells during pubertal development. In: Payne AHa.H, MP, editors. The Leydig cell in health and disease. Totowa, New Jersey: Humana Press; 2010. pp. 55–70. [Google Scholar]
- 11.Ge R-S, Hardy MP. Variation in the End Products of Androgen Biosynthesis and Metabolism during Postnatal Differentiation of Rat Leydig Cells. Endocrinology. 1998;139:3787–3795. doi: 10.1210/endo.139.9.6183. [DOI] [PubMed] [Google Scholar]
- 12.Gyles SL, Burns CJ, Whitehouse BJ, Sugden D, Marsh PJ, Persaud SJ, Jones PM. ERKs regulate cyclic AMP-induced steroid synthesis through transcription of the steroidogenic acute regulatory (StAR) gene. J Biol Chem. 2001;276:34888–34895. doi: 10.1074/jbc.M102063200. [DOI] [PubMed] [Google Scholar]
- 13.Hirakawa T, Ascoli M. The lutropin/choriogonadotropin receptor (LHR)-induced phosphorylation of the extracellular signal regulated kinases (ERKs) in Leydig cells is mediated by a protein kinase A-dependent activation of Ras. Molecular Endocrinology. 2003;17:2189–2200. doi: 10.1210/me.2003-0205. [DOI] [PubMed] [Google Scholar]
- 14.Hoivik EA, Lewis AE, Aumo L, Bakke M. Molecular aspects of steroidogenic factor 1 (SF-1) Mol Cell Endocrinol. 2010;315:27–39. doi: 10.1016/j.mce.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Hu G-X, Lin H, Chen G-R, Chen B-B, Lian Q-Q, Hardy DO, Zirkin BR, Ge R-S. Deletion of the Igf1 Gene: Suppressive Effects on Adult Leydig Cell Development. J Androl. 2010;31:379–387. doi: 10.2164/jandrol.109.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huhtaniemi I. Are gonadotrophins tumorigenic--A critical review of clinical and experimental data. Molecular and Cellular Endocrinology. 2010;329:56–61. doi: 10.1016/j.mce.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 17.Huhtaniemi IT, Rullin S, Ahtiainen P, Poutanen M. Multiple sites of tumorigenesis in transgenic mice overproducing hCG. Molecular and Cellular Endocrinology. 2005;234:117–126. doi: 10.1016/j.mce.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Kim B, Kim Y, Sakuma R, Hui CC, Ruther U, Jorgensen JS. Primordial germ cell proliferation is impaired in Fused Toes mutant embryos. Dev Biol. 2011;349:417–426. doi: 10.1016/j.ydbio.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Lacroix A, Ascoli M, Puett D, McKenna TJ. Steroidogenesis in hCG-responsive Leydig cell tumor variants. Journal of Steroid Biochemistry. 1979;10:669–675. doi: 10.1016/0022-4731(79)90520-x. [DOI] [PubMed] [Google Scholar]
- 20.Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, Rao CV. Targeted Disruption of Luteinizing Hormone/Human Chorionic Gonadotropin Receptor Gene. Molecular Endocrinology. 2001;15:184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- 21.Ludbrook LM, Bernard P, Bagheri-Fam S, Ryan J, Sekido R, Wilhelm D, Lovell-Badge R, Harley VR. Excess DAX1 leads to XY ovotesticular disorder of sex development (DSD) in mice by inhibiting steroidogenic factor-1 (SF1) activation of the testis enhancer of SRY-box-9 (Sox9) Endocrinology. 2012;153:1948–1958. doi: 10.1210/en.2011-1428. [DOI] [PubMed] [Google Scholar]
- 22.Manna PR, Chandrala SP, King SR, Jo Y, Counis R, Huhtaniemi IT, Stocco DM. Molecular mechanisms of insulin-like growth factor-I mediated regulation of the steroidogenic acute regulatory protein in mouse leydig cells. Mol Endocrinol. 2006;20:362–378. doi: 10.1210/me.2004-0526. [DOI] [PubMed] [Google Scholar]
- 23.Manna PR, Jo Y, Stocco DM. Regulation of Leydig cell steroidogenesis by extracellular signal-regulated kinase 1/2: role of protein kinase A and protein kinase C signaling. J Endocrinol. 2007;193:53–63. doi: 10.1677/JOE-06-0201. [DOI] [PubMed] [Google Scholar]
- 24.Manna PR, Stocco DM. The role of specific mitogen-activated protein kinase signaling cascades in the regulation of steroidogenesis. Journal of signal transduction. 2011;2011:821615. doi: 10.1155/2011/821615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin LJ, Boucher N, Brousseau C, Tremblay JJ. The orphan nuclear receptor NUR77 regulates hormone-induced StAR transcription in Leydig cells through cooperation with Ca2+/calmodulin-dependent protein kinase I. Mol Endocrinol. 2008;22:2021–2037. doi: 10.1210/me.2007-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin LJ, Tremblay JJ. Nuclear Receptors in Leydig Cell Gene Expression and Function. Biology of Reproduction. 2010;83:3–14. doi: 10.1095/biolreprod.110.083824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinelle N, Holst M, Soder O, Svechnikov K. Extracellular Signal-Regulated Kinases Are Involved in the Acute Activation of Steroidogenesis in Immature Rat Leydig Cells by Human Chorionic Gonadotropin. Endocrinology. 2004;145:4629–4634. doi: 10.1210/en.2004-0496. [DOI] [PubMed] [Google Scholar]
- 28.O'Shaughnessy PJ, Baker P, Sohnius U, Haavisto A-M, Charlton HM, Huhtaniemi I. Fetal Development of Leydig Cell Activity in the Mouse Is Independent of Pituitary Gonadotroph Function. Endocrinology. 1998;139:1141–1146. doi: 10.1210/endo.139.3.5788. [DOI] [PubMed] [Google Scholar]
- 29.O'Shaughnessy PJ, Morris ID, Huhtaniemi I, Baker PJ, Abel MH. Role of androgen and gonadotrophins in the development and function of the Sertoli cells and Leydig cells: Data from mutant and genetically modified mice. Molecular and Cellular Endocrinology. 2009;306:2–8. doi: 10.1016/j.mce.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 30.O'Shaughnessy PJ, Willerton L, Baker PJ. Changes in Leydig Cell Gene Expression During Development in the Mouse. Biology of Reproduction. 2002;66:966–975. doi: 10.1095/biolreprod66.4.966. [DOI] [PubMed] [Google Scholar]
- 31.Payne AH. Steroidogenic enzymes in Leydig cells. In: Payne AH, Hardy MH, editors. The Leydig cell in Health and Disease. Totowa, NJ: 2007. pp. 157–171. [Google Scholar]
- 32.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pratis K, O'Donnell L, Ooi GT, McLachlan RI, Robertson DM. Enzyme assay for 5alpha-reductase type 2 activity in the presence of 5alpha-reductase type 1 activity in rat testis. The Journal of steroid biochemistry and molecular biology. 2000;75:75–82. doi: 10.1016/s0960-0760(00)00139-4. [DOI] [PubMed] [Google Scholar]
- 34.Rulli SB, Huhtaniemi I. What have gonadotrophin overexpressing transgenic mice taught us about gonadal function? Reproduction. 2005;130:283–291. doi: 10.1530/rep.1.00661. [DOI] [PubMed] [Google Scholar]
- 35.Schimmer BP, White PC. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol. 2010;24:1322–1337. doi: 10.1210/me.2009-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schumacher M, Schäfer G, Holstein AF, Hilz H. Rapid isolation of mouse Leydig cells by centrifugation in percoll density gradients with complete retention of morphological and biochemical integrity. FEBS Letters. 1978;91:333–338. doi: 10.1016/0014-5793(78)81204-6. [DOI] [PubMed] [Google Scholar]
- 37.Segaloff DL. Diseases Associated with Mutations of the Human Lutropin Receptor. In: Ya-Xiong T, editor. Progress in Molecular Biology and Translational Science. Academic Press; 2009. pp. 97–114. [DOI] [PubMed] [Google Scholar]
- 38.Shiraishi K, Ascoli M. Activation of the lutropin/choriogonadotropin receptor (LHR) in MA-10 cells stimulates tyrosine kinase cascades that activate Ras and the extracellular signal regulated kinases (ERK1/2) Endocrinology. 2006;147:3419–3427. doi: 10.1210/en.2005-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiraishi K, Ascoli M. Lutropin/choriogonadotropin (LH/CG) stimulate the proliferation of primary cultures of rat Leydig cells through a pathway that involves activation of the ERK1/2 cascade. Endocrinology. 2007;148:3214–3225. doi: 10.1210/en.2007-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanley E, Lin CY, Jin S, Liu J, Sottas CM, Ge R, Zirkin BR, Chen H. Identification, Proliferation, and Differentiation of Adult Leydig Stem Cells. Endocrinology. 2012;153:5002–5010. doi: 10.1210/en.2012-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanley EL, Johnston DS, Fan J, Papadopoulos V, Chen H, Ge RS, Zirkin BR, Jelinsky SA. Stem Leydig cell differentiation: gene expression during development of the adult rat population of Leydig cells. Biology of reproduction. 2011;85:1161–1166. doi: 10.1095/biolreprod.111.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teerds KaR, E . Dynamics of Leydig cell regeneration after EDS: a model for postnatal Leydig cell development. In: Payne AH, Hardy MP, editors. The Leydig cell in health and disease. Totowa, New Jersey: Humana Press; 2010. pp. 91–116. [Google Scholar]
- 43.Themmen APN. An update of the pathophysiology of human gonadotrophin subunit and receptor gene mutations and polymorphisms. Reproduction. 2005;130:263–274. doi: 10.1530/rep.1.00663. [DOI] [PubMed] [Google Scholar]
- 44.Wu X, Arumugam R, Zhang N, Lee M. Androgen profiles during pubertal Leydig cell development in the mouse. Reproduction. 2010;140:113–121. doi: 10.1530/REP-09-0349. [DOI] [PubMed] [Google Scholar]
- 45.Yamashita S, Tai P, Charron J, Ko C, Ascoli M. The Leydig Cell MEK/ERK Pathway Is Critical for Maintaining a Functional Population of Adult Leydig Cells and for Fertility. Molecular Endocrinology. 2011;25:1211–1222. doi: 10.1210/me.2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang F-P, Poutanen M, Wilbertz J, Huhtaniemi I. Normal Prenatal but Arrested Postnatal Sexual Development of Luteinizing Hormone Receptor Knockout (LuRKO) Mice. Molecular Endocrinology. 2001;15:172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]