Abstract
Introduction
Opioid withdrawal syndrome is a critical component of opioid abuse and consists of a wide array of symptoms including increases in pain sensitivity (hyperalgesia). A reliable preclinical model of hyperalgesia during opioid withdrawal is needed to evaluate possible interventions to alleviate withdrawal. The following study describes a method for assessing increases in thermal sensitivity on the hotplate in a mouse model of spontaneous morphine withdrawal.
Methods
C57BL/6J mice received 5.5 days of 30, 56, or 100 mg/kg morphine or saline (s.c., twice daily). In Experiment I, thermal sensitivity data were collected at baseline and at 8, 24, 32, 48 hrs and 1 week following the final injection. Thermal sensitivity was assessed by examining latency to respond on a hotplate across a range of temperatures (50, 52, 54, and 56°C). In Experiment II, 0.01 mg/kg buprenorphine was administered 30 min prior to each testing session during the withdrawal period. In Experiment III, jumping during a 30 min period was assessed at baseline and at 0, 8, 24, 32, and 48 hrs following the final morphine injection.
Results
During the withdrawal period, thermal sensitivity increased significantly in all morphine-treated mice as compared to saline-treated mice. Thermal sensitivity was greater in mice treated with 56 mg/kg morphine compared to 30 mg/kg and peaked earlier than in mice treated with 100 mg/kg (32 hrs v 1 wk). The increase in thermal sensitivity following 56 mg/kg morphine was attenuated by a dose of buprenorphine that did not produce antinociception alone (i.e., 0.01 mg/kg). In general, the results of the jumping experiment paralleled those obtained in Experiment I.
Discussion
Response latency on the hotplate is a reliable and sensitive measure of spontaneous morphine withdrawal in mice, making it an ideal behavior for assessing the potential of medications and environmental interventions to alleviate opioid withdrawal.
Keywords: Buprenorphine, hotplate, hyperalgesia, jumping, methods, morphine, mouse, spontaneous withdrawal, thermal sensitivity
1. Introduction
The opioid withdrawal syndrome consists of a constellation of symptoms that appear following the termination of a prolonged period of opioid administration. The presence or desire to avoid these symptoms may even contribute to continued drug taking (Le Moal and Koob, 2007). As such, withdrawal is a critical component of opioid abuse. One of the many symptoms that make up the Clinical Opiate Withdrawal Scale or COWS (Tompkins et al., 2009) is an increase in pain or sensitivity to pain. An increase in pain sensitivity or hyperalgesia during spontaneous withdrawal occurs in pain patients in experimental settings (Lipman and Blumenkopf, 1989) and is reported in case studies, as well (Devulder et al., 1996). Healthy human subjects show hyperalgesia during both spontaneous (Angst et al 2003) and antagonist precipitated withdrawal (Compton et al., 2003; Sun, 1998).
The development of pharmacological and environmental interventions to mitigate hyperalgesia during opioid withdrawal requires reliable preclinical models of this symptom of withdrawal. In 1973, Tilson et al. reported that sensitivity to electric foot shock increases following the cessation of morphine in rats. Since then a modest number of papers have described hyperalgesia in animal models of opioid withdrawal. In rats, hyperalgesia occurs during both precipitated as well as spontaneous morphine withdrawal and is observed with multiple pain assays: hotplate, tail flick, and shock discrimination (Devillers et al., 1995; Dunbar and Pulai 1998; Grilly and Gowans 1986; Jin et al., 2012; Li et al., 2001; Tilson et al., 1973). Hyperalgesia in rats also occurs during withdrawal from fentanyl (Laulin et al., 2002) and heroin (Devillers et al., 1995; Laulin et al., 1998). Beyond rodents, withdrawal hypersensitivity is seen in both dogs (Martin et al., 1987) and cats (Johnson and Duggan, 1981).
Traditionally, opioid withdrawal in mice is measured by the presence of behavioral signs such as jumping, wet dog shakes, piloerection, diarrhea, and ptosis (e.g. Kest et al., 2002; Papaleo and Contarino 2006). To the best of our knowledge only two studies from laboratories other than our own employ a hyperalgesia model for examining opioid withdrawal in mice. These studies examine only a single time point during spontaneous withdrawal (Rubovich et al., 2009) or employ a precipitated, rather than a spontaneous, withdrawal procedure (Crane and Shen 2007).
The current study describes a new method for assessing hyperalgesia in a mouse model of spontaneous morphine withdrawal. We hypothesize that thermal sensitivity on a hotplate will increase during spontaneous withdrawal from a range of morphine does. Further, we hypothesize that buprenorphine treatment during the withdrawal period will attenuate the increase in sensitivity. Buprenorphine, a low efficacy mu agonist, was selected because it is commonly used in agonist replacement therapy for opioid dependence (e.g. Connock et al. 2007 and Kraus et al., 2011), and used to suppress spontaneous opioid withdrawal symptoms during the induction phase of treatment (Strain et al. 2011).
2. METHODS
2.1 Animals
All experiments were conducted in male C57BL/6J mice (Jackson Labs, Raleigh, NC), 10 weeks of age upon delivery. Male C57BL/6J mice were selected to allow comparison with other data collected in our laboratory regarding morphine’s pharmacological effects as well as the extensive literature on the behavioral effects of opioids in C57BL/6 mice. Additionally, in comparison to other inbred strains, C57BL/6J mice are known to be highly sensitive across many behavioral assays. Specifically, they exhibit high sensitivity in measures of acute nociception (Mogil et al., 2000), naloxone precipitated morphine withdrawal (Kest et al. 2002) and morphine self-administration (Elmer et al. 2009).
Mice were individually housed in polycarbonate cages (floor area=335cm2) with continuous access to food and water throughout the study. The colony room was maintained on a 12-hr, reverse, light/dark cycle (lights off at 7:00 am) and all behavioral testing was conducted during the dark cycle, between 9:00 am and 7:00 pm. Mice were habituated to handling and the colony room environment for two weeks prior to any experimental manipulation. Mice were also exposed to the testing environment for at least two days prior to initiation of an experiment and for 1 hr prior to all behavioral testing. Although a criterion was set such that mice <20 g or those that lost >20% of initial body weight would be removed from the study, it was not necessary to remove any mice from the study. Animal protocols were approved by the Institutional Animal Care and Use Committee, and the methods were in accord with the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council, 2011).
2.2 Experimental Procedures
Thermal Sensitivity
Thermal sensitivity was assessed using a hot plate analgesia meter (25.3 × 25.3 cm), Columbus Instruments, Columbus, OH. During each 1-hr hot plate testing period, a temperature-effect curve was determined for each mouse. Sensitivity was evaluated by recording the latency to lick or flutter the hind paw(s), or to jump from the hot plate surface at each of four temperatures presented in the following order: 50, 54, 52, 56°C with 15-min intervals between temperatures. Response latency was measured to the nearest 0.1 sec. To prevent tissue damage, a predetermined cutoff time of 20 sec was defined as the maximal trial duration. Immediately following the termination of a trial, whether due to a mouse’s response or elapsed cutoff time, mice were removed from the hot plate surface. Parameters were selected based on prior work in our laboratory regarding responses on the hot plate (e.g. Fischer et al. 2008; Balter and Dykstra, 2012).
Jumping
To measure jumping, mice were removed from their home cages and placed in a 4L beaker in the center of a Med Associates Inc. activity chamber. Vertical beam breaks, monitored by a computer, were used to count the number of jumps that occurred in a 30-min period.
Pharmacological Procedure
During the saline/morphine administration period, doses of saline, 30 mg/kg, 56 mg/kg or 100 mg/kg of morphine were administered daily for 5.5 days, with injections occurring at 10:00 am and 8:00 pm daily (11 injections total). Morphine sulfate and buprenorphine hydrochloride, provided by the National Institute on Drug Abuse (Bethesda, MD, USA), were both dissolved in 0.9% saline to yield all concentrations. Doses were injected subcutaneously at a volume of 0.1 ml/10 g.
2.3 Experimental Design
Experiment 1: Thermal sensitivity following saline, 30, 56, or 100 mg/kg of morphine
On day one, thermal sensitivity was assessed in all four groups of mice (n=8) at 10:00 am (baseline 1) and at 6:00 pm (baseline 2). A 2-way repeated measures ANOVA revealed no difference between baseline 1 and baseline 2; therefore, baselines were averaged for all analyses and figures. At 10:00 am on day two 30, 56, 100 mg/kg morphine or saline administration began as described above and continued for 5.5 days. Following the last dose of morphine on day seven, thermal sensitivity was assessed six more times: immediately after the final injection (10:00 am on day 7), at 8 hrs (6:00 pm on day 7), at 24 hrs (10:00 am on day 8), at 32 hrs (6:00 pm on day 8), at 48 hrs (10:00 am on day 9) and at 1 week (10:00 am on day 14). This period (days 7–14) was designated as the withdrawal period.
Experiment 2: Buprenorphine and thermal sensitivity
In order to select a dose of buprenorphine that did not produce antinociception on its own, a cumulative dose effect curve (0.01 to 0.32 mg/kg) was obtained for buprenorphine at each of the four temperatures tested during the thermal sensitivity assessment (50, 52, 54 and 56 ±0.1°C). Baseline response latencies on the hot plate were determined twice prior to the beginning of the buprenorphine dose effect curve and spaced 30 min apart. Data from these baselines were averaged to yield one baseline value. Following baseline determination, responding on the hot plate was examined over multiple cycles, and doses of buprenorphine were spaced 30 min apart. Drugs were administered at the start of each cycle and latency on the hot place was determined during the last minute of the cycle. Drug doses were increased cumulatively, with the dose increasing in one-half log unit increments prior to each cycle (0.01, 0.03, 0.1, 0.32 mg/kg). Buprenorphine effects were expressed as a percentage of the maximal possible effect (% MPE) using the following formula:
During the withdrawal experiment, on day one thermal sensitivity was assessed in two groups of mice (n=8) at 10:00 am (baseline 1) and 6:00 pm (baseline 2). A 2-way repeated measures ANOVA revealed no difference between baseline 1 and baseline 2; therefore, baselines were averaged for all analyses and figures. At 10:00 am on day two 56 mg/kg morphine administration began for all mice as described above and continued for 5.5 days. Following the last dose of morphine on day seven, thermal sensitivity was assessed five more times: immediately after the final injection (10:00 am on day 7), at 8 hrs (6:00 pm on day 7), at 24 hrs (10:00 am on day 8), at 32 hrs (6:00 pm on day 8), and at 48 hrs (10:00 am on day 9). A dose of 0.01 mg/kg buprenorphine or saline was administered subcutaneously 30 minutes prior to each testing session on days 7–9. This period (days 7–9) was designated as the withdrawal period.
Experiment 3: Jumping responses following saline, 30, 56, or 100 mg/kg of morphine
On day one, jumping was assessed in all four groups of mice (n=8) at 10:00 am (baseline 1, AM) and at 6:00 pm (baseline 1, PM). One week later on day 8, a second baseline measure (baseline 2, AM and PM) was taken at 10:00 am and 6:00 pm. The second set of baselines (10:00 am and 6:00 pm on day 8) was used for data analysis. At 10:00 am on day nine 30, 56, 100 mg/kg morphine or saline administration began as described above and continued for 5.5 days. Following the last dose of morphine on day 14, thermal sensitivity was assessed five more times: immediately after the final injection (10:00am on day 14), at 8 hrs (6:00 pm on day 14), at 24 hrs (10:00 am on day 15), at 32 hrs (6:00 pm on day 15), at 48 hrs (10:00 am on day 16). This period (days 14–16) was designated as the withdrawal period.
2.4 Data analysis
All data are presented as means (±SEM). In Experiments I and II, response latencies were used to derive a measure of thermal sensitivity, designated as ET10. The ET10 represents the theoretical temperature required to produce a response latency of 10 sec (half the maximal response latency of 20 sec) and was derived using log-linear interpolation. In Experiment III, jumping responses during the withdrawal period are presented and analyzed as jumps during the withdrawal period minus the average number of jumps that occurred during the corresponding baseline period (i.e., Since data for the 0, 24, and 48 hrs withdrawal period fell in the AM, baseline measures from the morning period were used. Likewise since data for the 8 and 36 hrs withdrawal period fell in the PM, baseline measures from the evening period were used.)
Analysis of the latency data used a 3-way repeated measures ANOVA with time and temperature as repeated measures factors and group as an independent factor. ET10 and jumping data were analyzed using a 2-way repeated measures ANOVA with time as the repeated measures factor and group as an independent factor. For the 2- and 3-way ANOVA, an alpha level of significance was set at p<0.01. Following the 3-way ANOVA, appropriate follow-up contrasts and Student’s t-tests were performed using a fully saturated mixed model of the data. The model was a straight model of the means and included random intercepts for each mouse. Following the 2-way ANOVAs, appropriate follow-up contrasts were performed using a model of jumps or ET10 as a function of time and group. The null hypothesis assumed no mean difference in the number of jumps or the ET10 values. Standard error was adjusted for multiple observations within each mouse.
Statistical analyses were conducted with an alpha level of significance set at p<0.001. The alpha level was determined using Bonferoni corrections to account for the large number of comparisons. The ANOVA’s were performed using SPSS for Windows software, version 9.0. All post hoc analysis was performed using SAS for Windows software, version 9.2. Figures were created with GraphPad Prism 5.
3. Results
3.1 Thermal sensitivity following spontaneous withdrawal from 30, 56, or 100 mg/kg morphine
Fig. 1 shows the latency to respond on the hot plate as a function of temperature at baseline, 8, 24, 32, 48 hrs and 1 wk following termination of the 5.5 day treatment period of either 30, 56, or 100 mg/kg morphine or saline. In general, two findings were consistent across all time points. First, latency to respond on the hot plate decreased as a function of temperature. Response latencies in both saline and morphine-treated mice were at or close to the maximal value of 20 sec when the hot plate was set at 50°C; at 52, 54 and 56°C, latencies averaged 12.8, 9.4 and 5.7 sec, respectively. Second, response latencies at the 0 (data not shown), 8, 24, 32 and 48-hr and 1 wk time points for saline-treated mice were never significantly different from baseline, calculated as the average of baseline 1 and 2, indicating that repetition of testing did not produce measurable effects on response latency. In addition, immediately following the final morphine injection (0 hr), response latencies were at the cut off value of 20 sec at all temperatures for morphine-treated mice; consequently these data are not shown. The failure to respond within in the 20 sec maximal trial duration indicates a full antinociceptive response to acute morphine exposure.
Fig 1. Effects of 30, 56 or 100 mg/kg morphine or saline treatment on latency (mean ±SEM) to respond on the hotplate at 50, 52, 54, and 56° C.
Morphine or saline treatment consisted of 5.5 days of twice daily injections (s.c.). Latency on the hotplate was determined at baseline and at 8, 24, 32, 48 hrs, and 1 wk after the final injection. Abscissa: hotplate temperature in ° C. Ordinate: latency to respond in seconds. N=7–8. Statistically significant differences (p<0.001) are indicated as follows: A= 30 mg/kg v. sal, B= 56 mg/kg v sal, C= 100 mg/kg v sal, X= 56 mg/kg v 30 and 100 mg/kg, Y= 100 mg/kg v 30 mg/kg.
A 3-way repeated measures ANOVA revealed main effects of time and temperature F(5, 135) = 33.483, p<0.001 and F(3, 81)=1332.942, p<0.001, respectively. Follow up Student’s t-tests were then used to compare individual groups, time points, and temperatures.
In general, the curves obtained in the morphine-treated mice were displaced downward from those obtained at baseline and from those of saline-treated mice. Significant differences in response latencies were apparent between morphine-treated and saline- treated mice throughout the withdrawal period. Significant differences between the 30 mg/kg morphine- and saline-treated mice were apparent at 32 and 48 hrs (52oC) t621= 3.87, 4.43, p<0.001, respectively. Significant differences between the 56 mg/kg morphine- and saline-treated mice were apparent at 8 hrs (52°C) t621= 3.41, p<0.001; 24 hrs (52 and 54°C) t621= 6.13, 5.25, p<0.001; 32 hrs (50, 52, 54°C) t621=6.12, 4.96, 5.13, p<0.001; 48 hrs (50, 52, 54°C) t621=3.65, 6.787, 3.82, p<0.001; and at 1 wk (50 and 54°C) t621=3.45, 3.55, p<0.001. Significant differences between the 100 mg/kg morphine- and saline-treated mice were apparent at 48 hrs (50 and 52°C) t621= 4.30, 5.21, p<0.001 and at 1 wk (50, 52, 54°C) t621= 6.51, 5.85, 4.37, p<0.001. In addition, the responses of morphine-treated mice were significantly different from baseline at all point where responses were different from those of saline-treated mice. These differences suggest that mice treated with 30, 56, or 100 mg/kg of morphine for 5.5 days and then withdrawn from morphine were more sensitive to the thermal stimulus than mice treated with saline.
It is also important to note significant differences in response latency between different morphine treated groups during the withdrawal period. Response latencies of mice treated with 56 mg/kg morphine were significantly different from those of mice treated with 30 mg/kg morphine at 8hrs and 24hrs (52°C) t621=3.31, 3.70, p<0.001, respectively and at 32 hrs (50°C) t621=5.32, p<0.001. Response latencies in mice treated with 56 mg/kg morphine were also significantly different from response latencies obtained mice treated with 100 mg/kg morphine at 8hrs and 24hrs (52°C) t621=4.08, 3.44, p<0.001, respectively and at 32 hrs (50°C) t621=4.43, p<0.001. Finally, a significant difference in response latencies was apparent between mice treated with 100 mg/kg and 30 mg/kg morphine at 1 wk (50 and 52°C) t621=5.66, 4.02, p<0.001.
Taken together, these data suggest that 5.5 days of morphine treatment was sufficient to produce significant changes in thermal sensitivity compared to both within-subject baselines and saline controls. However, the dose of morphine (30, 56, or 100 mg/kg) affected the extent and time course of this response, with the greatest changes in latency observed following 56 mg/kg morphine and at 32 hrs into the withdrawal period.
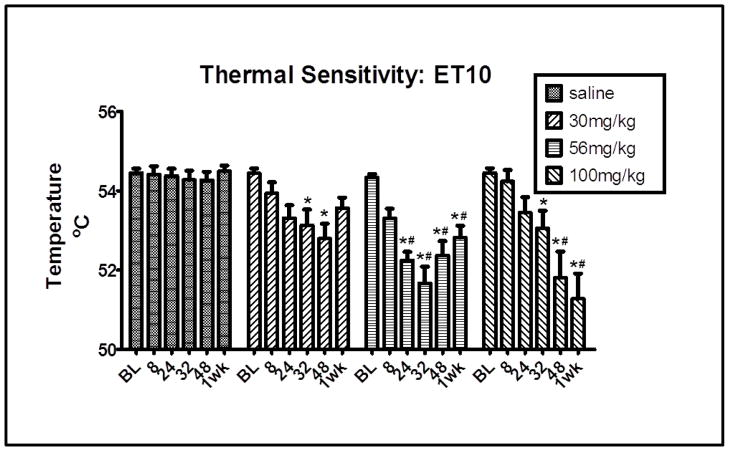
Fig. 2 shows the ET10 value at baseline, 8, 24, 32, 48 hrs and 1 wk following termination of the 5.5-day treatment period with either 30, 56, 100 mg/kg morphine or saline. The ET10 values were derived from the data shown in fig. 1. They represent the theoretical temperature necessary to produce a 10 sec response on the hotplate. A two-way repeated measures ANOVA revealed a main effect of time F(5, 135) = 2.299, p<0.05. Individual groups and time points were compared using appropriate follow up contrasts. For mice treated with 30 mg/kg morphine, a significant difference in ET10 value compared to baseline was apparent at 32 and 48 hrs, t133=3.42, 4.30, p<0.001, respectively. For mice treated with 56 mg/kg morphine, a significant difference in ET10 value compared to baseline was apparent at 24, 32, 48 hrs and 1 wk, t133=5.45, 6.74, 4.97, 3.97, p<0.001, respectively. At each of these time points (24, 32, 48 hrs and 1 wk), the ET10 values of mice treated with 56 mg/kg morphine were also significantly different from those of saline-treated mice, t133=4.46, 5.37, 3.91, 3.52, p<0.001, respectively. For mice treated with 100 mg/kg morphine, a significant difference in ET10 value compared to baseline was apparent at 32, 48 hrs and 1 wk, t133=3.64, 6.89, 8.29, p<0.001, respectively. The ET10 values of mice treated with 100 mg/kg morphine were also significantly different from those of saline-treated mice at 48 hrs and 1 wk, t133=5.16, 6.75, p<0.001, respectively. There were no significant differences between the ET10 values of the groups at baseline or between the ET10 values of saline-treated mice across time. Taken together, these data further support the hypothesis that 5.5 days of morphine treatment significantly increase thermal sensitivity during spontaneous morphine withdrawal.
Fig. 2. ET10 values (mean ±SEM) for mice following 5.5 days of 30, 56 or 100 mg/kg morphine or saline treatment.
ET10 values represent the temperature that would produce a 10 sec response on the hotplate. Response latency on the hotplate was determined at baseline and at 8, 24, 32, 48 hrs and 1 wk after the final injection. N=8. Statistically significant differences are indicated as follows: *= a difference from the group’s baseline, # = a difference between morphine and saline treated mice at a particular time point. p<0.001
3.2 Effects of buprenorphine on thermal sensitivity during spontaneous morphine withdrawal
Buprenorphine is a partial mu-opioid receptor agonist and, like all mu-opioid agonists, it produces antinociception on the hotplate. Consequently, prior to determining whether buprenorphine would attenuate withdrawal induced increases in thermal sensitivity, a dose of buprenorphine that did not produce antinociception on its own was identified.
Fig. 3a presents the dose effect curve of buprenorphine (0.01 mg/kg–0.32 mg/kg) at each of the temperatures used during the thermal sensitivity testing. Based on these data, a dose of 0.01 mg/kg buprenorphine was selected since this dose did not produce measurable antinociception on the hotplate at 50, 52, 54 or 56°C
Fig. 3. The effect of 0.01 mg/kg buprenorphine on withdrawal from 5.5 days of 56 mg/kg morphine.
A. Dose effect curves for buprenorphine (0.01– 0.32 mg/kg) at 50, 52, 54, and 56°C. Mean latencies (±SEM) are presented as % maximum possible effect (%MPE). B. ET10 values (mean ±SEM) for mice treated with 0.01 mg/kg buprenorphine or saline following 5.5 days of 56 mg/kg morphine. ET10 values represent the temperature that would produce a 10 sec response on the hotplate. Response latency on the hotplate was determined at baseline and at 8, 24, 32, and 48 hrs after the final morphine injection. Mice received 0.01 mg/kg buprenorphine (s.c.) 30 min prior to each hotplate test session. N=8. Statistically significant differences are indicated as follows: *= a difference from the group’s baseline, # = a difference between buprenorphine and saline treated mice. p<0.001
Fig. 3b shows the ET10 value at baseline, 8, 24, 32, and 48 hrs following termination of 5.5 days of twice daily morphine. As in Experiment I, ET10 values represent the theoretical temperature necessary to produce a 10 sec response on the hotplate. All mice in this experiment received 56 mg/kg morphine. During the withdrawal period, mice received saline or 0.01 mg/kg buprenorphine treatment 30 min prior to test sessions at 8, 24, 32 and 48 hrs. Immediately following the final morphine injection (0 hr), response latencies were at the cut off value of 20 sec at all temperatures; consequently these data are not shown.
A two-way repeated measures ANOVA revealed a main effect of time as well as a time x group interaction F(4, 56) = 11.978, 3.739, p<0.01, respectively. Individual groups and time points were compared using appropriate follow up contrasts. Significant differences were apparent between the buprenorphine-treated and saline- treated groups at 24 and 32 hours, t56=3.94, 3.56, p<0.001, respectively. Additionally, response latencies of buprenorphine-treated mice showed no difference from baseline through out the withdrawal period (p>0.01). However, significant differences were again apparent between the saline-treated group and baseline at all withdrawal time points (8, 24, 32, and 48 hrs), t56=3.66, 6.35, 6.65, 3.74, p<0.001. These data suggest that buprenorphine can attenuate the decrease in response latency observed during morphine withdrawal.
3.3 Jumping behavior during spontaneous withdrawal from 30, 56, or 100 mg/kg morphine
Experiment III assessed jumping responses during a 30-min period at baselines and at 0, 8, 24, 32, and 48 hrs following termination of the 5.5 day treatment period with either 30, 56, 100 mg/kg morphine or saline (s.c., twice daily). Jumping responses provide a measure of withdrawal for comparison to the thermal sensitivity data.
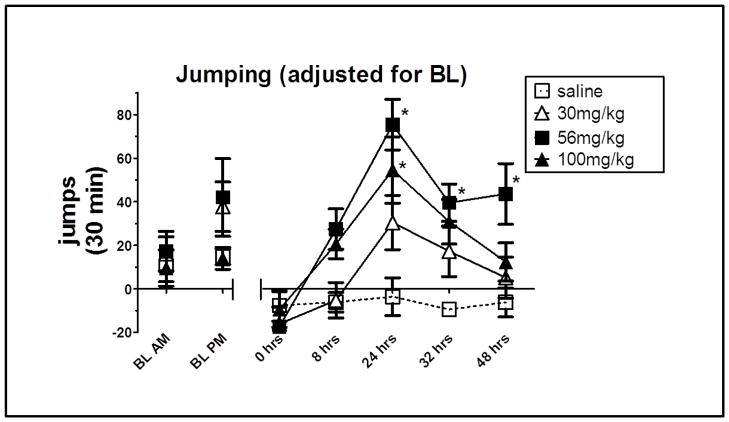
Fig. 4 shows the number of jumps obtained at the morning (10:00 am) and evening (6:00 pm) baselines. Jumping responses during the withdrawal period are presented and analyzed as jumps observed during the withdrawal period minus the average number of jumps that occurred during the corresponding baseline period (i.e., 0, 24, and 48 hrs minus AM baseline; 8 and 36 hrs minus PM baseline). This adjustment for AM and PM baseline measures was included since baseline differences were observed at the two time periods.
Fig. 4. Jumps (mean ±SEM) adjusted for baseline following 30, 56 or 100 mg/kg morphine or saline.
Morphine or saline treatment consisted of 5.5 days of twice daily injections (s.c.). Jumping was determined at baseline and at 0, 8, 24, 32, and 48 hrs after the final injection. Baseline jumps indicate total jumping in 30 min at 10am and 6pm. Jumps at 0, 8, 24, 32, and 48 hrs indicate jumps observed during the 30-min withdrawal period minus the average number of jumps that occurred during the corresponding baseline period. Data obtained for the 0, 24, and 48 hrs withdrawal period fell in the AM; therefore, total jumps were adjusted using baseline measures from the AM period. Data obtained for the 8 and 36 hrs withdrawal period fell in the PM; therefore, total jumps were adjusting using baseline measures from the PM period. N=8. * = a statistically significant difference compared to saline treated mice. p<0.001.
A two-way repeated measures ANOVA revealed a main effect of time as well as a time x group interaction F(4, 108) = 19.57, 2.87, p<0.01, respectively. Individual groups and time points were compared using appropriate follow up contrasts. Significant differences in adjusted jumping between mice treated with 56 mg/kg morphine and saline were apparent at 24, 32 and 48 hrs, t108= 5.81, 3.61, 3.66, p<0.001, respectively. A significant difference was seen in adjusted jumping between mice treated with 100 mg/kg morphine and saline at 24 hrs, t108= 4.43, p<0.001. In addition, immediately following the final morphine injection (0 hr), no jumping was observed in any of the morphine treated mice.
Taken together, these data suggest that 5.5 days of morphine is sufficient to produce significant changes in jumping behavior compared to saline controls. However, as seen in Experiment I, the extent of this response varies with the dose of morphine (30, 56, or 100 mg/kg), with the greatest effects observed following 56 mg/kg.
4. Discussion
The experiments yielded three main findings. First, the results from Experiment I supported the hypothesis that the measurement of changes in thermal sensitivity provides a reliable method for assessing spontaneous withdrawal from morphine in mice. Second, Experiment II demonstrated that buprenorphine could attenuate changes in thermal sensitivity as measured by latency to respond on the hotplate. Third, the results from Experiment III indicated that changes in thermal sensitivity during withdrawal were similar to changes in jumping behavior, a well-established measure of morphine withdrawal. Taken together, these data validate the thermal sensitivity procedure as a method for assessing spontaneous morphine withdrawal.
In the first experiment, an orderly temperature by latency relationship was observed at all time points, with increasing temperatures producing shorter response latencies. Treatment with all three of the morphine doses (30, 56, or 100 mg/kg) produced significant decreases in response latency on the hotplate following the cessation of morphine treatment. The downward displacement of the temperature-response curves was most prominent at 52 and 54°C. At 56°C, response times were so short that changes in response time were difficult to detect. The response latencies of saline-treated control groups were consistent across all time points. This illustrates that neither 1) repeated testing nor 2) time of day measurably affected responding on the hotplate. Finally, across all experimental groups there was little within-group variability as measured by standard error. The observation that mice were more sensitive to a thermal stimulus during morphine withdrawal is consistent with previous research in both humans and animals reporting heightened sensitivity to thermal stimuli following termination of a regimen of morphine administration (Angst et al. 2003; Compton et al. 2003; Dunbar and Pulaj 1998; Rubovitch et al. 2009; Sweitzer et al. 2004).
The effect of dose and the time course of withdrawal are clearly apparent in the ET10 data, where a single latency score was generated for each time point. It is well established that dose of morphine is a factor in the severity of physical dependence (e.g., Papaleo and Contarino, 2006). In the experiment reported here, looking at the totality of the week-long withdrawal period, treatment with 56 mg/kg morphine produced a more pronounced increase in sensitivity than 30 mg/kg morphine; however, the time course during which the behavior was expressed was similar following both 30 and 56 mg/kg. For both groups, thermal sensitivity peaked in the second day following the cessation of morphine administration and showed a return toward baseline levels by one week.
The magnitude of the change in ET10 value in mice treated with 100 mg/kg morphine was similar to that of mice treated with 56 mg/kg; however, the time course of this decrease was shifted temporally. We speculate that treatment with 100 mg/kg morphine produced a more severe withdrawal syndrome and that a change in thermal sensitivity was only apparent as physical dependence eased during the spontaneous withdrawal period. It is possible that other symptoms of withdrawal such as sedation blocked the measurement of increases in thermal sensitivity or that this behavior is only apparent at a certain magnitude of withdrawal severity. Taken together, these data suggest that a change in latency to respond on the hotplate is a sensitive measure of morphine withdrawal; however, time, dose and hotplate temperature are all critical variables to consider when using this measure.
The second experiment demonstrated that changes in thermal sensitivity during withdrawal could be attenuated by treatment with buprenorphine. Buprenorphine was selected because it is commonly used in agonist replacement therapy for opioid dependence (Kraus et al., 2011). Mice received either saline or a non-antinociceptive dose (0.01 mg/kg) of buprenorphine during the withdrawal period, following the cessation of 5.5 days of 56 mg/kg morphine. The response latency of buprenorphine-treated mice was attenuated compared to saline-treated mice at 24 and 32 hrs. Mice that received saline during the withdrawal period showed the same course of withdrawal as mice similarly treated with 56mg/kg morphine in Experiment I.
Experiment III examined jumping behavior as a measure of withdrawal severity. Jumping was selected for comparison because it is a well-established measure of opioid withdrawal (eg. Saelens et al., 1971; Kest et al., 2002; and Papaleo and Contarino, 2006). In the current experiment, withdrawal severity, as measured by number of jumps in a 30-min period replicated the findings of the thermal sensitivity experiments. Termination of treatment with 56 mg/kg morphine produced the most pronounced increase in jumping compared to treatment with 30 mg/kg or 100 mg/kg morphine. Experiment III revealed two major limitations of using jumping to assess withdrawal severity. First, baseline data indicate that time of testing (early or late in the dark-cycle) can affect responding. Second, within-group variability for the jumping response is relatively large. As a result, it is more difficult to determine whether differences between experimental groups are significant when jumping is used to measure withdrawal.
The most notable limitation of the thermal sensitivity procedure examined here is the difficulty in automating the measure since it is time intensive and requires observers who are well trained in the observation of hotplate responses. Nevertheless, the thermal sensitivity procedure could be adjusted for higher throughput screening by examining latencies at a single temperature (52°C) and a single time point (24 or 32 hrs). Additionally, the procedure could be adapted for within subject (baseline v withdrawal period) or between subject (treatment group v untreated withdrawal group) designs.
In summary, the present study supports the use of thermal sensitivity, as measured by changes in response latency on the hotplate, as a reliable method for assessing spontaneous morphine withdrawal in mice. Response latencies on the hotplate show little variability within groups and little effect of repeated testing, maximizing sensitivity to subtle changes in withdrawal severity. The procedure is also well suited for examining withdrawal over longer periods, a distinct advantage over procedures in which withdrawal is precipitated by an antagonist and withdrawal behaviors are observed at a single time point. These characteristics make the thermal sensitivity procedure optimal for assessing the efficacy of medications and environmental interventions for alleviating opioid withdrawal. In fact, our laboratory recently showed that two environmental interventions, i.e., access to a running wheel and group housing, could attenuate the increase in thermal sensitivity observed during spontaneous withdrawal from morphine (Balter and Dykstra, 2012).
Acknowledgments
Supported by NIH grants R01-DA02749 and T32-DA00724.
The authors would like to thank the statistical consulting staff at the Odum Institute, especially Dr. Christopher Wiesen. Additionally we would like to thank Dr. Wendy Mathes and Karl Schmidt for their contributions during the early stages of this project
Footnotes
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angst MS, Koppert W, Pahl I, Clark DJ, Schmeiz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. doi: 10.1016/s0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Balter RE, Dykstra LA. The effect of environmental factors on morphine withdrawal in C57BL/6J mice: running wheel access and group housing. Psychopharmacology. 2012;224(1):91–100. doi: 10.1007/s00213-012-2826-6. [DOI] [PubMed] [Google Scholar]
- Compton P, Athanasos P, Elashoff D. Withdrawal hyperalgesia after acute opioid physical dependence in non addicted humans: a preliminary study. J Pain. 2003;4:511–519. doi: 10.1016/j.jpain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, Fry-Smith A, Day E, Lintzeris N, Roberts T, Burls A, Taylor RS. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. iii–iv. doi: 10.3310/hta11090. Review. [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Naloxone rapidly evokes endogenous kappa opioid receptor-mediated hyperalgesia in naïve mice pretreated briefly with GM1 ganglioside or in chronic morphine-dependent mice. Brain Res. 2007;1167:31–4. doi: 10.1016/j.brainres.2007.06.058. [DOI] [PubMed] [Google Scholar]
- Devillers JP, Boisserie F, Laulin JP, Larcher A, Simonnet G. Simultaneous activation of spinal antiopioid system (neuropeptide FF) and pain facilitatory circuitry by stimulation of opioid receptors in rats. Brain Res. 1995;700:173–181. doi: 10.1016/0006-8993(95)00948-p. [DOI] [PubMed] [Google Scholar]
- Devulder J, Bohyn P, Castille F, De Laat M, Rolly G. A case of uncommon withdrawal symptoms after a short period of spinal morphine administration. Pain. 1996;64(3):589–91. doi: 10.1016/0304-3959(95)00187-5. [DOI] [PubMed] [Google Scholar]
- Dunbar SA, Pulai IJ. Repetitive opioid abstinence causes progressive hyperalgesia sensitive to N-methyl-D-aspartate receptor blockade in the rat. J Pharmacol Exp Ther. 1998;284:678–686. [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Hamilton LR, Wise RA. Qualitative differences between C57BL/6J and DBA/2J mice in morphine potentiation of brain stimulation reward and intravenous self-administration. Psychopharmacology. 2010;208(2):309–21. doi: 10.1007/s00213-009-1732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Zimmerman EI, Picker MJ, Dykstra LA. Morphine in combination with metabotropic glutamate receptor antagonists on schedule-controlled responding and thermal nociception. J Pharmacol Exp Ther. 2008;324(2):732–9. doi: 10.1124/jpet.107.131417. [DOI] [PubMed] [Google Scholar]
- Grilly DM, Gowans GC. Acute morphine dependence: effects observed in shock and light discrimination tasks. Psychopharmacology. 1986;88(4):500–4. doi: 10.1007/BF00178515. [DOI] [PubMed] [Google Scholar]
- Jin H, Li YH, Xu JS, Guo GQ, Chen DL, Bo Y. Lipoxin A4 analog attenuates morphine antinociceptive tolerance, withdrawal-induced hyperalgesia, and glial reaction and cytokine expression in the spinal cord of rat. Neuroscience. 2012;208:1–10. doi: 10.1016/j.neuroscience.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Duggan AW. Tolerance and dependence of dorsal horn neurones of the cat: the role of the opiate receptors of the substantia gelatinosa. Neuropharmacology. 1981;20(11):1033–8. doi: 10.1016/0028-3908(81)90093-9. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni AJ, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002;115:463–469. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Kraus ML, Alford DP, Kotz MM, Levounis P, Mandell TW, Meyer M, Salsitz EA, Wetterau N, Wyatt SA. American Society Of Addiction Medicine: Statement of the American Society Of Addiction Medicine Consensus Panel on the use of buprenorphine in office-based treatment of opioid addiction. J Addict Med. 2011;5(4):254–63. doi: 10.1097/ADM.0b013e3182312983. [DOI] [PubMed] [Google Scholar]
- Laulin JP, Maurette P, Corcuff JB, Rivat C, Chauvin M, Simonnet G. The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance. Anesth Analg. 2002;94(5):1263–9. doi: 10.1097/00000539-200205000-00040. [DOI] [PubMed] [Google Scholar]
- Laulin JP, Larcher A, Célèrier E, Le Moal M, Simonnet G. Long-lasting increased pain sensitivity in rat following exposure to heroin for the first time. Eur J Neurosci. 1998;10(2):782–5. doi: 10.1046/j.1460-9568.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol. 2007;17(6–7):377–93. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Li X, Angst MS, Clark JD. Opioid-induced hyperalgesia and incisional pain. Anesth Analg. 2001;93(1):204–9. doi: 10.1097/00000539-200107000-00040. [DOI] [PubMed] [Google Scholar]
- Lipman JJ, Blumenkopf B. Comparison of subjective and objective analgesic effects of intravenous and intrathecal morphine in chronic pain patients by heat beam dolorimetry. Pain. 1989;39(3):249–56. doi: 10.1016/0304-3959(89)90037-7. [DOI] [PubMed] [Google Scholar]
- Martin WR, Gilbert PE, Jasinski DR, Martin CD. An analysis of naltrexone precipitated abstinence in morphine-dependent chronic spinal dogs. J Pharmacol Exp Ther. 1987;240(2):565–70. [PubMed] [Google Scholar]
- Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24(3):375–89. doi: 10.1016/s0149-7634(00)00015-4. Review. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Contarino A. Gender- and morphine dose-linked expression of spontaneous somatic opiate withdrawal in mice. Behav Brain Res. 2006;170(1):110–8. doi: 10.1016/j.bbr.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Rubovitch V, Pick CG, Sarne Y. Is withdrawal hyperalgesia in morphine-dependent mice a direct effect of a low concentration of the residual drug? Addict Biol. 2009;14(4):438–46. doi: 10.1111/j.1369-1600.2009.00164.x. [DOI] [PubMed] [Google Scholar]
- Saelens JK, Granat FR, Sawyer WK. The mouse jumping test—a simple screening method to estimate the physical dependence capacity of analgesics. Arch Int Pharmacodyn Ther. 1971;190:213–218. [PubMed] [Google Scholar]
- Strain EC, Harrison JA, Bigelow GE. Induction of opioid-dependent individuals onto buprenorphine and buprenorphine/naloxone soluble-films. Clin Pharmacol Ther. 2011;89(3):443–9. doi: 10.1038/clpt.2010.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HL. Naloxone-precipitated acute opioid withdrawal syndrome after epidural morphine. Anesth Analg. 1998;86(3):544–5. doi: 10.1097/00000539-199803000-00019. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Allen Cp, Zissen MH, Kendig JJ. Mechanical allodynia and thermal hyperalgisia upon acute opioid withdrawal in the neonatual rat. Pain. 2004;110:269–280. doi: 10.1016/j.pain.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Tilson HA, Rech RH, Stolman S. Hyperalgesia during Withdrawal as a Means of Measuring the Degree of Dependence in Morphine Dependent Rats. Psychopharmacologia. 1973;28(3):287–300. doi: 10.1007/BF00429309. [DOI] [PubMed] [Google Scholar]
- Tompkins DA, Bigelow GE, Harrison JA, Johnson RE, Fudala PJ, Strain EC. Concurrent validation of the Clinical Opiate Withdrawal Scale (COWS) and single-item indices against the Clinical Institute Narcotic Assessment (CINA) opioid withdrawal instrument. Drug Alcohol Depend. 2009;105(1–2):154–9. doi: 10.1016/j.drugalcdep.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]