Abstract
Serpiginous choroiditis (SC) is a posterior uveitis displaying a geographic pattern of choroiditis, extending from the juxtapapillary choroid and intermittently spreading centrifugally. The choroiditis involves the overlying retinal pigment epithelium, and the outer retina. This intraocular inflammation typically involves both eyes in otherwise healthy, middle-aged individuals with no familial or ethnic predilection. Pathogenesis is unclear; however, based on limited histopathologic studies, favorable response to immunosuppressive agents, and the absence of association with systemic or local infectious or noninfectious diseases, an organ-specific autoimmune inflammation seems likely to be the underlying process. Patients, particularly from tuberculosis-endemic regions, may present with fundus changes simulating SC, but show evidence of active tuberculosis and/or the presence of mycobacterial DNA in the aqueous humor. This has been referred to as serpiginous-like choroiditis, but we prefer the description multifocal serpiginoid choroiditis (MSC). We present the distinguishing features of SC and infectious multifocal serpiginoid choroiditis simulating SC. The distinction is crucial to avoid unnecessarily treating SC with antimicrobial agents. Advances in diagnostic and imaging modalities can help differentiate SC from MSC. Novel local and systemic treatment approaches improve the outcome and preserve vision in SC.
Keywords: serpiginous choroiditis, serpiginous-like choroiditis, multifocal serpiginoid choroiditis, tuberculosis, herpes virus
I. Introduction
Serpiginous choroiditis (SC) is a descriptive term for an intraocular inflammatory disease characterized by a geographic pattern of choroiditis that typically extends from the peripapillary area and affects the overlying retinal pigment epithelium (RPE) and the outer retina.84 This recurrent and progressive choroidal inflammation usually involves both eyes and can cause irreversible damage to the photoreceptors with permanent vision loss if the process involves the fovea.2 Although likely there is an underlying autoimmune pathogenesis, the specific trigger for this localized ocular immune process remains unknown.102,185 Recent studies using polymerase chain reaction (PCR) and interferon gamma release assay suggest that microorganisms may be the inciting agents, 13,14,65,105,106,133,135 either through active proliferation or through induction of an immune response against the microbes. Such an immune response may also target the uvea and retina through a process of molecular mimicry. In most patients, however, diagnostic studies seeking an infectious cause remain inconclusive; thus, the intraocular inflammation is treated with corticosteroids and immunosuppresants without the use of anti-microbial agents, on the assumption that this choroidal inflammation is driven by an underlying, organ-specific, autoimmune disorder. 4,146,153,159,162,178,180
Since the last major review of SC,102 multiple breakthroughs have occurred with respect to the pathogenesis, diagnosis, and treatment of this disease. These can be divided into four groups: 1. etiology and pathogenesis of SC; 2. novel imaging modalities and their potential diagnostic and prognostic importance; 3. new treatment approaches, both systemic and local; and 4. the importance of differentiating idiopathic, presumed immune-mediated SC from infectious serpiginoid choroiditis.137,160 In this review, the idiopathic noninfectious choroiditis presenting with fundus changes of geographic choroiditis that extend from the juxtapapillary choroid is described as serpiginous choroiditis, whereas the infectious choroiditis presenting with fundus findings that simulate SC is described as multifocal serpiginoid choroiditis (MSC),137 a designation that better reflects the clinical features of infectious choroiditis and is preferable to the previously used serpiginous-like choroiditis.176
Although pathogenesis, etiology, and differential diagnosis of the SC and the mimicking conditions remain a challenge, PCR studies to detect microbial DNA reveal that Mycobacterium tuberculosis (MTB) and Herpes zoster infection play a role in the subset of patients with MSC.”137 Differentiation of SC from the mimicking choroiditis is vital for proper management and assessment of prognosis. We attempt to clarify the distinction between SC and infectious choroiditis mimicking SC and propose an approach to the diagnosis and management of SC.
II. Serpiginous choroiditis and synonyms
In 1900, Jonathan Hutchinson (Fig. 1), an English surgeon, dermatologist, and ophthalmologist, first described SC as a unique pattern of choroidal inflammation characterized by a creeping progression with active borders that, when healed, had the appearance of “the borders of a continent in a map.”77 He clearly and precisely illustrated the pattern of SC in patients with variable general health backgrounds, including those with no known underlying disorder, patients with syphilis, and a patient with cervical lymphadenopathy and pulmonary lesions likely caused by MTB. He even noted the involvement of “internal parts of choroid and pigment layer.” 77 In 1970, Gass coined serpiginous choroiditis to describe this entity with recurrences that usually begins in the peripapillary area and spread centrifugally over a period of months or years in a serpiginous or jigsaw puzzle-like distribution.51 Because of the variation in clinical presentation, this choroiditis was also described by other names, including peripapillary chorioretinitis,52 helicoid peripapillary choroidal degeneration,46 geographic choroiditis,17 geographic choroidopathy,68 and geographic helicoid peripapillary choroidopathy.149 All of these entities have fundus appearances virtually identical to SC and should be considered one clinical entity. Over time, the description serpiginous choroiditis has been commonly accepted to describe this posterior uveitis. Despite our expanded understanding of the presentation, natural course, and prognosis of SC, the pathogenesis of the choroiditis remains enigmatic.
Figure 1.

Jonathan Hutchinson (1828-1913), an English surgeon, ophthalmologist, dermatologist, and pathologist, described serpiginous choroiditis in a healthy individual, in a patient with tuberculosis lymphadenopathy, and in a patient with syphilis.
III. Clinical features
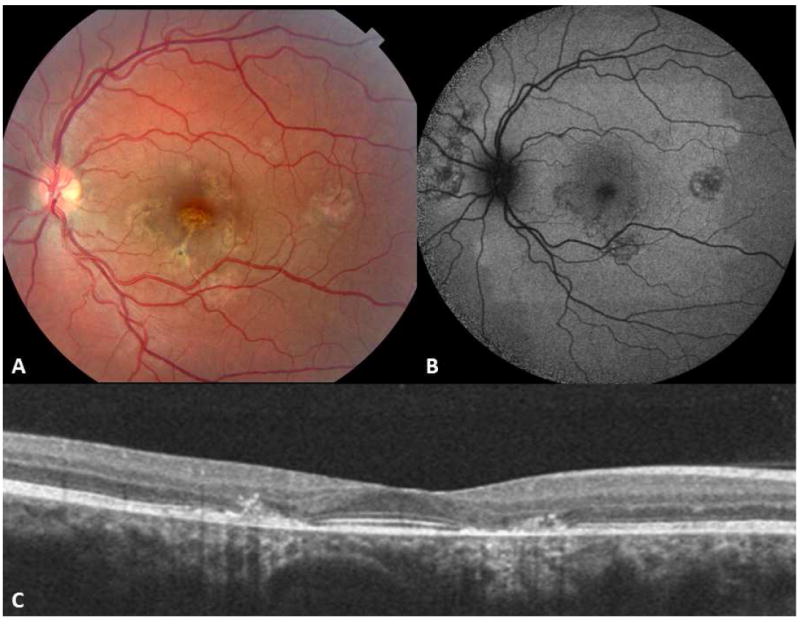
SC may manifest with variable features, although a creeping pattern of choroiditis, extending from the juxtapapillary area, with grayish yellow discoloration, minimal to no inflammatory cell infiltration in the vitreous, and recurrences of the lesions at the margins of the healed scars, is most commonly encountered (Fig. 2). 28,52,102 New lesions of SC present insidiously and are usually symptomatic (Figs. 3 and 4).94 Patients may initially complain of blurred vision, difficulty with reading, metamorphopsia, paracentral scotomas or other visual field defects, or floaters.2,3,69,94 Visual acuity is typically 20/40 or less; however, it may range from 20/20 to counting fingers at 1 to 3 feet. External and slit-lamp examinations usually show a quiet eye. Anterior chamber and vitreous reaction, if present, is low-grade.31 Intraocular pressure remains normal. 69 The new lesions are characterized by well-circumscribed patches of grayish-white or grayish-yellow discoloration at the level of the deep retina and RPE. Such active lesions usually arise from the margins of healed lesions (Fig. 4). These display a geographic pattern of choroidal atrophy associated with RPE changes that extends from the peripapillary area (Fig. 5). Retina and RPE outside the margins of active or healed lesions appear normal. Examination of the fellow eye may show similar atrophic lesions in the juxtapapillary choroid.
Figure 2.
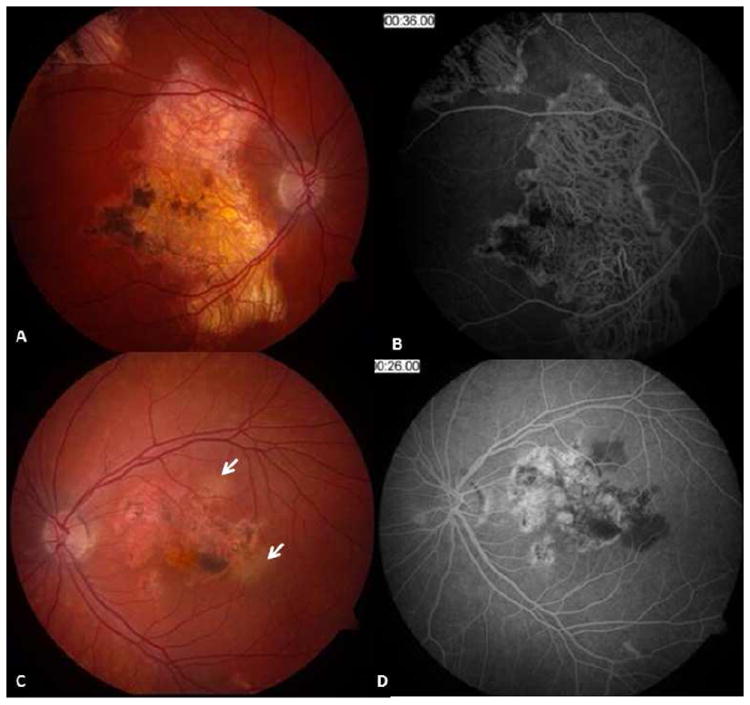
Macular serpiginous choroiditis in a 26-yr-old Caucasian man. Fundus photographs of the right (A) and left (C) eyes display geographic atrophy patches accompanied with pigment clumping. Mid-phase fluorescein angiogram of the right eye (B) shows geographic atrophy involving the retinal pigment epithelium and choroid with hyperpigmentation along the margins and at the fovea. Note border staining from choriocapillaris of adjacent choroid. Two patches of choroiditis reactivation are present at the temporal and superior margins of the macular lesion in the left eye (C - arrows). Early phase fluorescein angiogram (D) reveals blocked fluorescence corresponding to the active choroiditis patches in the left eye. (Images courtesy of Kumar Rao, MD, Saint Louis, MO)
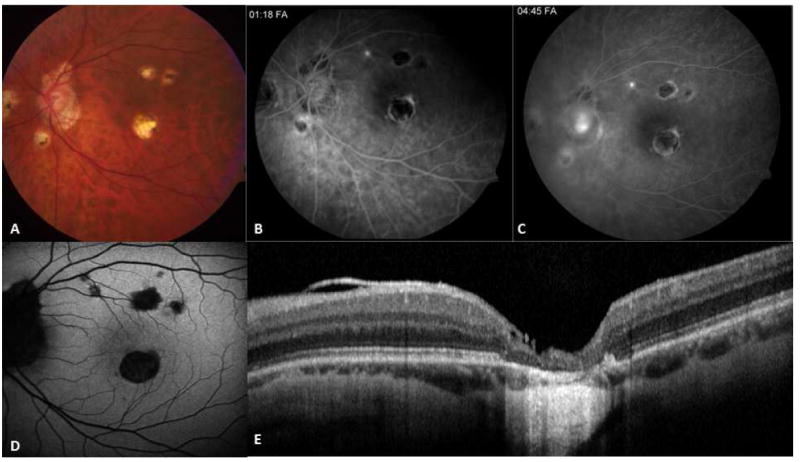
Figure 3.
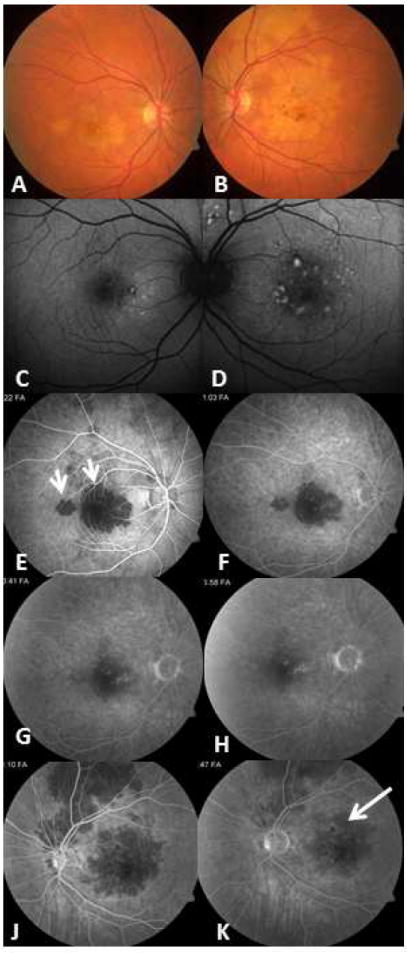
67-yr-old Caucasian woman with bilateral active serpiginous choroiditis lesions (A, B). In the early phase, while retinal pigment epithelium is not damaged, lesions are underrepresented in fundus autofluorescence (C, D); however, fluorescein angiography (E-K) better revealed the extent of hypofluorescence corresponding to the choriocapillaris nonperfusion patches. Short arrows indicate hypofluorescent patches in the early phase (E). The initial hypo-perfused patches (short arrows in E) progressively stain and reveal more prominent hyperfluorescence in the borders in later frames. The right eye presents as macular serpiginous choroiditis with isolated patches not connected to the optic disc. Left eye demonstrates serpiginous choroiditis lesions superior to the optic disc and a separate macular lesion. Early hypofluorescent patches (J) of the active choroiditis stain progressively and become relatively hyperfluorescent in late phase angiogram (K-long arrow).
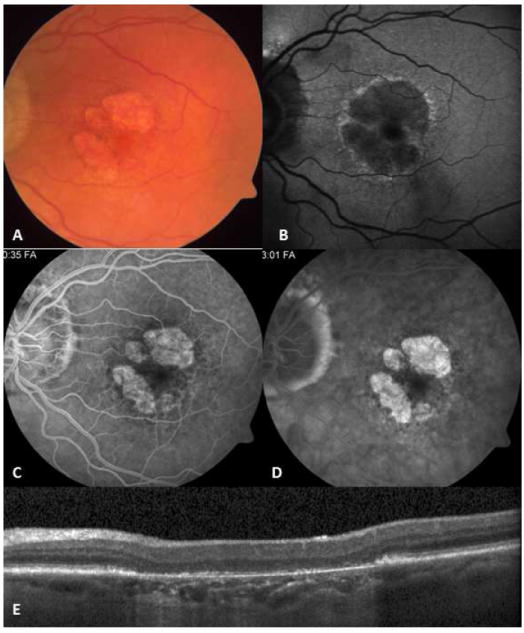
Figure 4.
Fundus photograph (A) and early (B) and mid-phase (C) fluorescein angiograms from a 42-yr-old Caucasian woman with serpiginous choroiditis reveal choroiditis reactivation in the fovea. Vision has acutely declined to 5/200. A, while healed scars originating from the peripapillary area are characterized with RPE and choroidal atrophy, and pigment clumping, active choroiditis (short arrow) demonstrates gray-yellow discoloration of the retina. In fluorescein angiography, active choroiditis appears as a hypofluorescent patch with progressive marginal hyperfluorescence from the leaking vessels (long arrow). Healed lesions are surrounded by marginal staining (B,C), which is more prominent in late phases of the angiogram (arrowhead). (Images courtesy of Kumar Rao, MD, Saint Louis, MO)
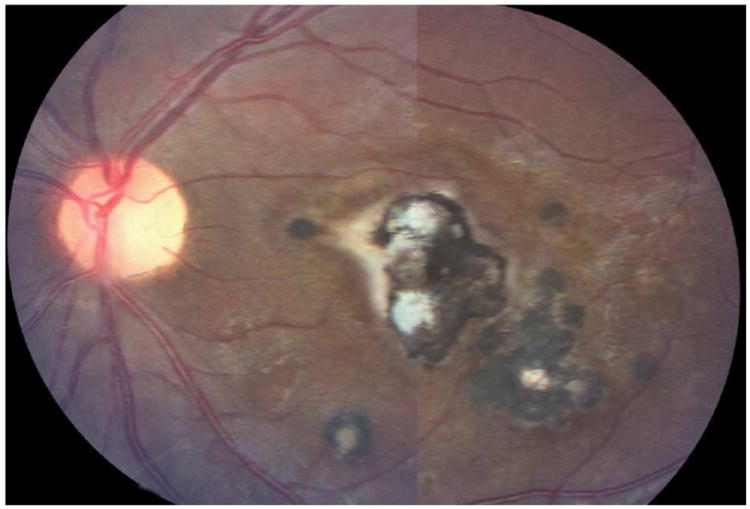
Figure 5.
76-yr-old Caucasian woman with serpiginous choroiditis. Fundus photographs show progressive expansion of the geographic choroiditis lesions. (This patient has been reported in part in reference 176.)
Over weeks to months, the grayish-white lesions are replaced with mottled RPE, with or without pigment epithelial hyperplasia and fibrosis. If left untreated, the signs of activity may last for up to 9 months. 94 The simultaneous presence of active and healed lesions is strong evidence for the diagnosis. 31,68 The healed inactive chorioretinal lesions appear as geographic atrophic areas, with pseudopodial extensions and well-demarcated sharp borders, with or without pigment epithelial hyperplasia at the borders.94
The natural course in untreated eyes is variable, but usually consists of multiple recurrences of choroidal inflammation and progression over a period of months to years that may eventually involve the fovea (Figs. 4 and 5).94 The frequency of recurrences depends directly on the length of the follow up. Laatikainen and Erkkilä followed 15 patients with SC for a period of 1-10 years. Choroiditis recurred over a span of 3 months to 4 years in 8 patients.94 About 50% of patients with SC are expected to have at least one recurrence in 5 years.182 In the absence of anti-inflammatory treatment, the choroiditis ends after 20 years or more, leaving extensive chorioretinal scarring.
Serpiginous choroiditis is typically bilateral but asymmetric. At the time of presentation, the symptoms may be restricted to one eye, and the second eye may have previously undetected healed lesions from earlier bouts of choroiditis or may be normal with later development of choroiditis.108 The average time between presentation in one eye and onset in the second eye is about 5 years.31 Unilateral cases are more commonly reported from areas where tuberculosis is endemic;60 and such cases might represent tuberculosis-related choroiditis misclassified as SC.
Macular involvement occurs in up to 88% of untreated patients with SC.182 Also, in long-standing, untreated cases, the lesions extend to the equator. Some patients present with exclusive macular involvement; such cases may be sub-classified as macular SC. Rarely, there is peripheral, multifocal, and unilateral involvement; 31,52 however, it is unclear whether these lesions were in fact from SC or whether they were MSC caused by infectious agents.
Macular serpiginous choroiditis
In about one third of patients the lesions arise initially or exclusively at the macula (Fig. 3-A). Such a pattern of choroiditis is recognized as macular SC, 69,108,147 and presents with floaters, flashing lights, scotomas, and blurred vision.108,147 Diagnosis can be challenging because new lesions usually lack the characteristic geographic atrophic scars,108 and the juxtapapillary choroid may not be involved. The angiographic features of macular SC, however, are identical to those of classic SC.69,108 The macular variant of SC may warrant extensive investigations to rule out infectious etiology, including an aqueous or vitreous tap to detect herpes virus or MTB by PCR. Similar-looking entities, such as age-related macular degeneration, idiopathic subretinal neovascular membranes, retinal pigment epithelitis, persistent placoid maculopathy, and acute posterior multifocal placoid pigment epitheliopathy (APMPPE) are in the differential.56,57,69,108
Macular SC lesions bear a less favorable visual prognosis.108 Choroidal neovascularization (CNV) develops more frequently. Three of seven patients with macular SC in one series developed CNV.108 Since these patients usually have symptoms early in the course of the disease, they often receive prompt treatment, resulting in less extensive involvement of the posterior retina and choroid over the long term.2
IV. Epidemiology
The actual prevalence of SC in the general population is unclear, but uveitis series from general ophthalmology practices, as well as from referral uveitis clinics, show that it is rare.115 In a series of 213 uveitis patients seen by general ophthalmologists in the Los Angeles area, there was not a single case of SC.115 In the same geographic area, however, SC was reported in 2.7% of patients seen in a uveitis referral center.71 This difference may reflect not only rarity, but also the possibility that early SC is underdiagnosed by general ophthalmologists. The reported prevalence of SC also varies geographically. A referral-based epidemiologic study found SC represented 18.9% of posterior uveitis cases in India.20 In this series, the SC is reported as “peripapillary geographic helicoid choroiditis.”20 In contrast, the reported prevalence of SC in areas where tuberculosis is not endemic generally varies from 1.6% to 5.3% of posterior uveitides (Table 1). The higher prevalence of SC in India may reflect the inclusion of tuberculous choroiditis, which can mimic SC. Although initial reports described SC in Caucasians, the disease has been observed in Asian, African American, Middle Eastern, Asian Indian, and Spanish patients.20,60,95,157,161,181,186
Table 1.
Reported prevalence of serpiginous choroiditis from various countries. Reports are from general ophthalmology practice, referral uveitis practice, and population-based studies.
| Study by (year) | Country | Type of study | Number of cases | Number of posterior uveitis | Number of patients with SC | Percentage in total patients | Percentage in posterior uveitis patients |
|---|---|---|---|---|---|---|---|
| Henderly, et al (1987) 71 | USA | Referral - based | 445 | 230 | 12 | 2.7% | 5.2% |
| Smit, et al (1993) 158 | Netherlands | Referral-based | 750 | 178 | 4 | 0.5% | 2% |
| McCannel, et al (1995) 115 | USA | General practice | 213 | 16 | 0 | 0% | 0% |
| Rodriguez, et al (1996) 144 | USA | Referral-based | 1237 | 260 | 4 | 0.32% | 1.6% |
| Biswas, et al (1996) 20 | India | Referral-based | 1273 | 366 | 69 | 5.4% | 18.89% |
| Bodaghi, et al (2001) 22 | France | Referral based | 927 | 200 | 4 | 0.4% | 2% |
| Soheilian, et al (2002) 161 | Iran | Referral-based | 544 | 101 | 4 | 0.7% | 4% |
| Oruc, et al (2003)126 | USA | Referral-based | 853 | 414 | 16 | 1.9% | 3.9% |
| Wakayabayashi, et al (2003) 181 | Japan | Referral-based | 189 | 59 | 2 | 1% | 3.4% |
| Yang, et al (2005) 186 | China | Referral-based | 1752 | 119 | 5 | 0.3% | 4.2% |
| Khairallah, et al (2007) 90 | Tunisia | Referral-based | 472 | 133 | 7 | 1.5% | 5.3% |
| Rathinam, et al (2011) 139 | India | Population-based | 5500* | 11 | 1 | 0.02% | 9.1% |
total number of study population
SC= Serpiginous choroiditis
Since SC as a separate uveitis entity was only recognized in the 1970s, it is not surprising that it may have been misclassified as idiopathic posterior uveitis or as other uveitis entities in the past.71 In a university uveitis clinic-based study in Rome, Pivetti-Pezzi and colleagues reviewed 1417 endogenous uveitis cases between 1986 and 1993 and found 14 with SC (1%).134 In two earlier series from the same institution, not a single case of SC had been recognized in 1380 uveitis patients seen from 1968 to 1977 and 865 seen from 1978 to1985.
Affected individuals are typically middle aged, although the age range is 11 to 70 years.2,31 While the mean age at the time of initial diagnosis of SC in Caucasians is reported to be from 43.4 to 59.1 years,17,22,44,54,69 in parts of the world where tuberculosis is endemic, the mean age is 30.3 years2 which may reflect tuberculosis-related choroiditis cases misclassified as SC. Most series reveal slightly increased prevalence among men compared to women.2,68,69,149
V. Histopathologic features
Current knowledge about the pathogenesis of SC is limited, in part perhaps because of the rarity of histopathologic studies documenting SC. While no active lesions have come to histopathology, findings in clinically inactive lesions in long-standing SC are a moderate mononuclear inflammatory cell infiltration of the choroid with focal aggregation of lymphocytes.185 Fibrotic choroidal lesions are surrounded with variable degrees of RPE hyperplasia and defects in Bruch membrane with atrophic choriocapillaris, RPE, and photoreceptors.24,52 Choroidal vessels in unaffected areas are unremarkable, and a few retinal vessels show moderate lymphocytic infiltration.
VI. Etiology/pathogenesis
The etiology of SC has always been a subject of speculation, with degenerative, autoimmune, and/or infectious processes are considered (Table 2).17,78,113 Recurrent choroiditis with creeping serpentine progression occurs in healthy individuals with no ocular symptoms, as well as in patients with clinical or laboratory evidence of ocular or systemic infections.52,77,94 A unifying, underlying ocular or systemic pathogenesis is lacking. Patients with SC are typically in good general health. Anecdotal association with conditions such as diabetes mellitus,182 systemic lupus erythematosus,48 Crohn disease,173 hypertension,108,182 non-Hogdkin lymphoma,140 auto immune thrombocytopenic purpura,118 celiac disease, 118 extrapyramidal dystonia,142 lung carcinoma,102 antiphospholipid antibody syndrome,168 and vitamin A deficiency108 appears to be co-incidental.43,118,142,173 Additionally, while fluorescein and indocyanine green angiography of active lesions demonstrate predominant choriocapillaris involvement,24,54,55 there is no systemic vasculitis.44 Moreover, SC has no known association with occupational or environmental exposure to toxic substances.
Table 2.
The two major categories of choroiditis presenting with recurrent serpentine/geographic lesions
| 1. Non-infectious |
|
| 2. Infectious | Multifocal serpiginoid choroiditis/ Serpiginous-like choroiditis resulting from infection of
|
The fundus changes seen in SC and the preferential involvement of middle-aged individuals prompt consideration of a degenerative process as the underlying mechanism,46, 113 but degenerations are generally associated with accumulation of acellular material and loss of tissue, which is not supported by clinical, imaging, and histopathologic studies of SC.51,95 The asymmetric development in the two eyes, lack of familial aggregation, and late onset militate against a hereditary dystrophic process.113, 185
Angiographic studies reveal that choriocapillaris occlusion is a major feature of SC.31,94 The contribution of vascular endothelial cell injury to the choriocapillaris 24 in patients with SC was studied by measuring levels of Von Willebrand factor in eight patients with SC.92 The mean activity was about twice as high in patients with SC compared to the disease-free, age- and sex-matched control group. The authors suggested SC may represent an occlusive vascular phenomenon involving choroidal circulation in some patients.92 Meanwhile, scleroderma, polymyalgia rheumatica, and Raynaud’s phenomenon, which are typically associated with elevated levels of Von Willebrand factor, are not associated with SC.
A. Autoimmune
The higher frequency of HLA-B7, HLA-A2, HLA-B8 and HLA-Dw3 in patients with SC compared to the general population may suggest an underlying, genetically predisposed autoimmune process 18,44,118,94 There is no consistent association of these HLA frequencies and any systemic autoimmune disorders in SC patients. SC patients manifest auto-reactivity of circulating lymphocytes to retinal S antigen.26 Broekhuyse and colleagues investigated immune responsiveness to retinal S antigen and opsin by in vitro lymphocyte proliferation, leukocyte migration inhibition, and enzyme-linked immunosorbent assays in SC, APMPPE, and retinitis pigmentosa. They found immune reactivity to retinal S antigen, but not to opsin in SC, in contrast to APMPPE or retinitis pigmentosa.26 This may imply an organ-specific autoimmune process directed at the outer retina, the site richly endowed with S antigen, with secondary choroid and RPE involvement.26 Nevertheless, clinical observation and fluorescein and indocyanine green studies revealed that in active lesions, the inflammatory processes are mainly localized to the choriocapillaris and RPE cells. Moreover, such an anti-S antigen immune response could be an epiphenomenon from extensive damage to the retina by SC, unlike APMPPE and retinitis pigmentosa. The favorable response of SC inflammation to immunosuppressive agents supports a substantial contribution of immune processes in pathogenesis, but the triggering event for this localized inflammatory response remains unclear. While the triggering event for the initiation or perpetuation of the choroidal and RPE inflammation is still obscure, it is thus justified to consider SC as an “idiopathic” intraocular inflammation with a possible organ-specific autoimmune process.
B. Infectious
Microbial infections have been suspected as possible inciting agents since the initial report by Hutchinson, who associated SC with both syphilis and tuberculosis. 77, 183 Subsequently, there have extensive attempts to detect infectious agents in SC. In a series of fifteen patients, Erkkilä and Laatikainen44 conducted a long list of laboratory tests looking for infectious disease. They detected no antibodies against Toxoplasma gondii, ornithosis, or Mycoplasma pneumonia. Also, antibody titers against vaccina virus, herpes simplex virus (HSV), herpes zoster virus (VZV), cytomegalovirus (CMV), adenovirus, influenza virus A and B, parainfluenza 1, 2, and 3, mumps virus, respiratory syncitial virus, rubella, rubeola, reovirus, polio virus 1, 2, and 3, and coxsackie virus A7, A9 and B5 were negative. Seven of fifteen patients had elevated antistreptolysin titers, but none had a detectable focal streptococcal infection. In the same series, nine patients had a positive tuberculin skin test (TST). Although they suggested mycobacterial infection was a possible etiologic agent,44 such positive skin tests could be from latent tuberculosis, unrelated to the SC. None of their patients had evidence of active mycobacterial infection.44 Proposed infectious etiologies for SC can be grouped as bacterial, viral, protozoal, and fungal. 106,113
1. Bacteria
Although serpiginous or multifocal serpiginoid lesions have been reported in a few patients with syphilis,50,72 presumed or definite MTB is the most often considered underlying bacterial infection. Following Hutchinson’s report of SC in a presumed tuberculosis patient,71 the association between a geographic pattern of choroiditis and tuberculosis was emphasized by Witmer in 1952 and by Schlaegel in 1969.150 In 1970, Maumenee reported a high incidence of sensitivity to TST in patients with geographic choroiditis.113
Of the 9 patients with positive TST mentioned above, two had a history of pulmonary TB, and two had a history of TB in their family members. 44, 94 However, anti-tuberculosis treatment failed to halt the progression of the lesions, suggesting that active mycobacterial infection was not the etiology of the choroiditis.87 Likewise, other series from areas where tuberculosis is not endemic found that most patients with SC had no evidence of active or prior tuberculosis infection.31, 171 Furthermore, in such patients, tuberculosis reactivation did not occur following the immunosuppressive treatment. In non-tuberculosis-endemic regions, MTB is unlikely to be the cause SC. Moreover, the epidemiologic, clinical, and prognostic features of patients with SC reported in non-endemic areas differ significantly from those where MTC is endemic.171
2. Viruses
Herpes viruses are proposed etiologic agents of SC.52,54,55,135,143 Gass described a patient who presented with Herpes zoster ophthalmicus who showed fundus changes of SC and suggested VZV as the etiologic agent. 52 Interestingly, a recent PCR study from a tuberculosis-endemic country found VZV and HSV DNA in the aqueous humor of patients with SC.135 In this study, aqueous humor samples from nine patients with recurrent active SC were studied for VZV, HSV, and CMV by conventional virus cytology, culture, and PCR techniques.135 All were macular SC, with or without associated peripapillary lesions, with a negative TST and a normal chest X-ray. While culture and cytology were negative for HSV, VZV, and CMV, the PCR results, confirmed with gel electrophoresis and DNA sequencing, showed the presence of the VZV genome in five patients and the HSV genome in one. Three were negative for either virus.135 The authors suggested that choroidal inflammation may be triggered or perpetuated by herpes virus infection.135 Although it is unclear whether antiviral treatment can halt choroiditis progression or recurrence in such herpes virus-related SC, they recommend performing herpes virus PCR studies in those cases of SC with rapid progression associated with vitritis that present with lesions in the macula.135 Unlike idiopathic SC, these virus-associated cases have multifocal lesions, primarily involving the macula, with vitritis and anterior chamber cellular reaction. In contrast, in another PCR study on a long-standing, documented SC eye obtained at autopsy, testing was negative for HSV, VZV, EBV, CMV, and human herpes virus-8.6 Although anti-hepatitis A antibodies were detected in one series from Finland, this was attributed to the high prevalence of such antibodies in the population.44
3. Protozoa
A few investigators consider Toxoplasma gondii a possible etiologic agent for SC.18,44,106 Chorioretinal scars of toxoplasma retinochoroiditis may resemble old, inactive SC lesions.52, 106 While active lesions usually arise from the borders of old scars in both conditions, toxoplasma retinochoroiditis-unlike SC-produces primarily retinal lesions with accompanying vitritis. In some studies of SC, testing for anti-toxoplasma antibodies gave negative results.33,94,146 Moreover, detection of anti-toxoplasma immunoglobulin G in a patient with typical serpiginous lesions, but without vitreous inflammation, may merely indicate a previous encounter with the organism.17
4. Fungi
Intraocular fungal infections have a fulminant course and are unlikely to be confused with SC. A recent PCR study, however, has raised the possibility that Candida sp may cause SC.133 In four of five patients with SC, Candida DNA, Candida antigens, and high serum titers of antibodies against Candida species were detected.133 This has not been confirmed by other investigators.
Although various infectious agents are proposed as potential etiologic agents for SC and MSC, these are two distinct entities with different clinical manifestations and management. SC presents with lesions that originate from the peripapillary region and extend from borders of pre-existing lesions with finger-like projections. The active lesions in SC respond to immunosuppressive agents. In patients with presumed or PCR-proven infectious multifocal serpiginoid choroiditis, the changes consist of multifocal active or healed choroiditis lesions displaying irregular margins that are usually associated with a vitreous cellular inflammatory reaction. These multifocal lesions require treatment with anti-microbial agents, with or without systemic corticosteroids (Table 3).
Table 3.
Serpiginous choroiditis: proposed etiologies.
| Proposed etiology | In favor | Against |
|---|---|---|
|
| ||
| Autoimmune | Negative infectious disease work up in most patients with typical presentation of the disease Association with HLA-B7 and -A2 Association with HLA-B8 and -Dw3 Favorable control of inflammation with immunosuppression Anecdotal association with autoimmune disease (celiac disease, Crohn’s disease, polyarthritis nodosa, autoimmune thrombotic thrombocytopenic purpura) Histopathology reports |
Only a few patients show systemic autoimmune disease |
|
| ||
| Infection | ||
| Bacterial | ||
| Tuberculosis | Serpiginous-like lesions in patients with latent systemic tuberculosis Serpiginous-like feature in patients with PCR evidence for mycobacterium tuberculosis |
Clinical features of the typical SC differs from tuberculosis-related multifocal serpiginoid choroiditis/serpiginous-like lesions; the latter presents with multifocal choroidal lesions Vitritis and in some patients, anterior chamber reaction Standard immunosuppressive treatment does not lead to manifest systemic tuberculosis Response to immunosuppressive agents and absence of response to anti-tuberculosis agents |
| Syphilis | Syphilis could present with any chorioretinitis feature Serpiginous-like choroiditis lesions reported in patients with syphilis |
Syphilis serology is negative in all typical cases of SC |
| Viral | ||
| Varicella zoster or Herpes simplex | Serpiginous choroiditis reported in patients with herpes zoster ophthalmicus PCR-proven presence of herpes zoster or herpes simplex virus DNA in aqueous humor samples from patients with multifocal serpiginous-like choroiditis |
Diagnostic work up for the viral infection is negative in typical patients Serpiginous choroiditis does not respond to antiviral agents Standard immunosuppressive treatment is usually sufficient to control the inflammation |
|
| ||
| vascular | Elevated factor VIII/von Willebrand factor | Other diseases with elevated factor VIII/von Willebrand factor level do not show serpiginous choroiditis features |
|
| ||
| Degenerative/dystrophy | Chronic and progressive course Occurrence in middle age |
SC is an inflammatory condition treatment consists of immunosuppression |
PCR= polymerase chain reaction; RPE= retinal pigment epithelium; SC= serpiginous choroiditis
VII. Laboratory tests and imaging studies
In typical SC, the fundus appearance may suffice for clinical diagnosis, but laboratory investigations are recommended to rule out infectious causes, particularly those with a fundus appearance simulating SC, before initiating immunosuppressive treatment. Moreover, various imaging studies are usually needed in cases of atypical presentation of SC to determine activity and to monitor disease progression (Table 4). These imaging studies can help evaluate response to treatment and detect complications such as choroidal neovascularization.
Table 4.
Clinical characteristics and imaging aspects of new active choroiditis lesions versus choroiditis reactivation versus healed lesions in serpiginous choroiditis
| New active choroiditis | Choroiditis reactivation | Healed | |
|---|---|---|---|
|
| |||
| Symptoms and signs | Recent decrease in visual acuity | Recent decrease in visual acuity | Stable scotoma |
| Central or paracentral scotoma | New scotoma or expansion of existing scotoma | ||
| Increased floaters | |||
| Increased floaters | |||
|
| |||
| Fundus appearance | Gray-white discoloration of the retina in peripapillary area or in the macula | Gray-white discoloration of the retina in the margins of healed lesions | Geographic atrophy of the retina, RPE, and choroid with sharp margins |
| Absent or mild vitreous cellular reaction | Absent or mild vitritis | Jigsaw pattern of the atrophic lesions with tongue like projections | |
| With gradual clearing of the lesion from the center, the peripheral edges of the lesion often maintain grayish-white active appearance | Gradual clearing from the center while the margins show signs of activation | Pigment clumping, more prominent at the margins | |
|
| |||
| Fluorescein angiography | Early phase angiogram: hypofluorescent patch with poorly defined borders | Early phase angiogram: new hypofluorescent patch at the margins of healed scar | Early phase angiogram: hypofluorescent patch |
| Mid-phase angiogram: gradual increase in fluorescence at the borders; hyperfluorescence | Mid-phase angiogram: Hyperfluorescence at the borders. Healed areas start to stain in the margins | Mid-phase angiogram: hypofluorescent patches with staining at borders | |
| Late phase angiogram: borders show hyperfluorescence; hyperfluorescence may spread centrally to form a uniform or spotty appearance in the entire lesion | Late phase angiogram: uniform or spotty hyperfluorescence. | Late phase angiogram: hypofluorescent patch with sharp staining of the margins | |
|
| |||
| Indocyanine green angiography | Subclinical lesion (before clinical manifestation): hypofluorescent patch | Lesions usually appear at the margin of healed lesions Subclinical lesion: hypofluorescent patch |
Early and late hypofluorescence with well-defined margins |
| Active lesion: two patterns: either 1) early and late hypoautofluorescence with ill-defined margins; or 2) early hypoautofluorescence with increased fluorescence toward the late phases with faint edges. |
Active areas: two patterns: either 1) early and late hypoautofluorescence with ill-defined margins; or 2) early hypoautofluorescence with increased fluorescence toward the late phases with faint edges. |
||
| Sub-healing lesions: early hypofluorescence with late hyperfluorescence | Sub-healing lesions: early hypofluorescence with late hyperfluorescence | ||
|
| |||
| Optical coherence tomography | Slightly increased retinal thickness (retinal edema) | Slightly increased retinal thickness (retinal edema) | Thin retina Loss of outer retina architecture (IS/OS and ELM loss) |
| Outer retina and choroid hyperreflectivity | Outer retina and choroid hyperreflectivity | Outer retina and choroid hyperreflectivity | |
|
| |||
| Fundus autofluorescence | First 1-2 days after clinical presentation: no fundus autofluorescence abnormalities. | Lesions usually appear at the margin of healed lesions | Uniform hypoautofluorescent patch with sharp margins |
| 1-2 days after acute lesion manifests clinically: hyper- and hypoautofluorescence patches with sharp margins | Active areas appear similar to fresh active choroiditis lesions next to hypoautofluorescent patches of healed lesions | ||
| 3-5 days after active lesion manifests to 2 weeks: hyperautofluorescence patches with hypoautofluorescent margin | |||
| After 2 weeks: granular/speckled hyperautofluorescence | |||
ELM=external limiting membrane; IS/OS=inner segment/outer segment junction; RPE=retinal pigment epithelium
A. Laboratory tests
The diagnosis of SC requires ruling out SC-mimicking conditions, primarily tuberculosis, syphilis, and herpetic infections. In patients with typical SC who are from an area where tuberculosis is not endemic and who have no prior history of contact with tuberculosis, a negative TST, negative syphilis serology, and normal chest x-ray are usually sufficient. Noninfectious choroiditis typically extends from the juxtapapillary area, without significant vitritis or anterior chamber reaction. In contrast, atypical features, such as multifocal choroidal lesions, peripapillary choroid sparing, and significant anterior chamber or vitreous cellular reaction, mandate further investigations. In such patients, tuberculosis work up, syphilis serology, and anterior chamber or vitreous fluid analysis by PCR for MTB, VZV, and HSV may be required.
B. Imaging and other ancillary diagnostic tools
Fluorescein and indocyanine green angiography help support the diagnosis and management of SC and the early detection of complications, including subretinal neovascular membrane formation.54,55,78 Recently introduced imaging modalities, such as optical coherence tomography (OCT), fundus autofluorescence imaging, and microperimetry, have gained importance in delineating RPE changes, retinal damage, and the extent of retinal/choroidal involvement. Table 4 summarizes typical clinical and imaging features of SC in new lesions, recurrences, and healed lesions.
1. Fluorescein angiography
Active SC lesions display hypofluorescent patches with irregular, poorly-defined borders during the early phase of fluorescein angiography (FA) (Figs. 3-E,4-B). This hypofluorescence probably results from choriocapillaris hypoperfusion and blocked fluorescence as a result of edematous RPE and retina.31,108,111,148,149 In the mid-phase angiogram, a prominent hyperfluorescence may appear at the borders of the lesion (Fig. 4-C). This is due to leakage from the bordering choriocapillaris. Focal areas of retinal vasculopathy may occasionally light up as retinal vessel wall staining. 47,52 In the late phases of FA, the entire active lesion demonstrates a fairly uniform or spotty hyperfluorescence caused by leaking from the large choroidal vessels (Fig. 3-H).68,111,149
On the other hand, healed lesions show angiographic evidence of destruction of the RPE and choriocapillaris.52 In clear contrast to the lesions of active choroiditis, these healed lesions demonstrate hypofluorescence with sharp margins in the early and mid-phase angiograms as a result of choriocapillaris damage and blockage from RPE hyperplasia (Fig. 2-B). In late phases, healed lesions show a variable amount of staining at the borders from leakage of the large choroidal vessels and surrounding choriocapillaris. 17,52,68,94,148,149 The presence and severity of the pigment epithelial atrophy and hyperplasia, subretinal fibrosis, and choroidal atrophy modify the FA appearance of the healed lesions. Fluorescein angiography is also helpful for detection of choroidal neovascularization, a complication of SC.
2. Indocyanine green angiography
According to Giovannini et al.54,55 and supported by others, 11,24,34,148,165 SC lesions can be reclassified into four stages rather than just active and healed lesions. The indocyanine green angiography (ICGA)-based classification includes a subclinical or choroidal phase, an active phase, a sub-healing phase; and an inactive healed phase.
In the subclinical or choroidal phase, in which the inflammation is limited to the choriocapillaris and has not yet involved the overlying RPE and retina, fundus examination and FA show no signs of choroiditis; however, ICGA reveals choriocapillaris nonperfusion as hypofluorescent patches. These hypofluorescent patches progress to active and visible choroiditis with an overlying yellowish-white retinal lesion54,55 that may show two patterns in ICGA. The more common pattern is characterized by early and late hypofluorescent patches with ill-defined margins that are larger than reciprocal FA lesions. Margins of active lesions may show hyperfluorescence during the late phases. The less common pattern is early hypofluorescence with faint edges and late leakage and hyperfluorescence. With anti-inflammatory treatment, the size and intensity of ICGA lesions decrease.54,55 In sub-healing choroidal lesions, choroidal permeability alteration, usually adjacent to a recently healed active patch, can only be detected by ICGA as a slight, late hyperfluorescence.54,55 Healed lesions show early and late hypofluorescence with well-defined margins. These hypofluorescent patches indicate irreversible loss of choriocapillaris and consequent choroidal atrophy 24 (Fig. 6) and may be non-uniform because of the altered pigmentation of the choroid and RPE and the variable preservation of the larger choroidal vessels.54,55. The practical role of this ICGA classification in differential diagnosis and management of SC and mimicking conditions is unclear.
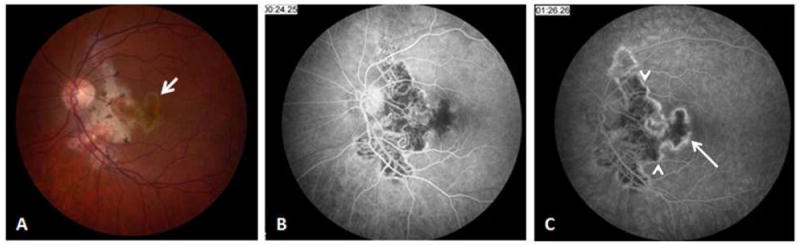
Figure 6.
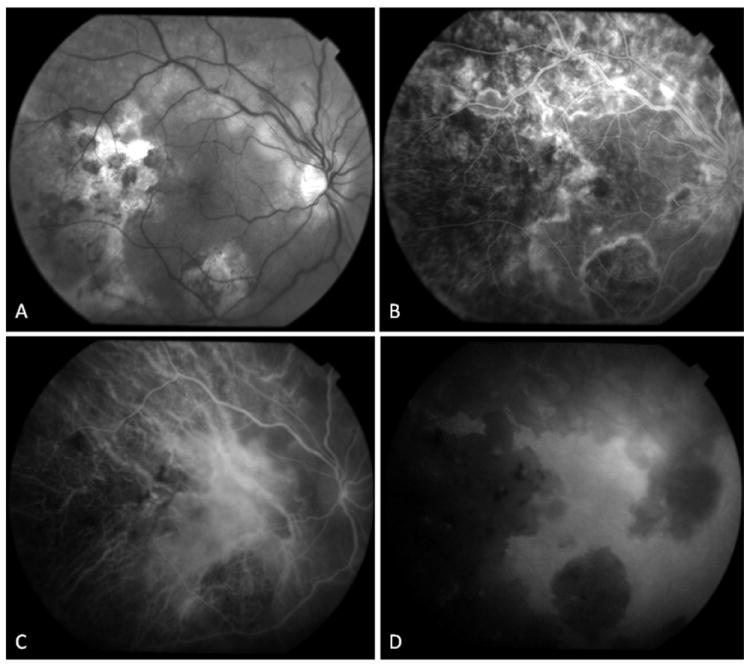
34-yr old Afghani woman with history of bilateral choroiditis that progressed rapidly after treatment with oral corticosteroids. PPD was positive and chest x-ray was negative. (A) Red free fundus photography of the right eye reveals geographic areas of choroidal atrophy, pigment clumping, and cystoid macular edema. (B) In fluorescein angiography, border staining delineates atrophic areas with some blockage from pigment proliferation. Early accumulation of dye in cystoid spaces is evident. (C, D) Mid-phase and late phase indocyanine green angiography reveals choriocapillaris loss in areas of healed lesions with preservation of large choroidal vessels. Choroidal circulation in non-involved areas is normal.
3. Optical coherence tomography
Characteristic OCT features of active SC lesions include photoreceptor layer disruption associated with outer retinal and choriocapillaris hyperreflectivity.27,49 In active choroiditis, the outer retina shows a uniform increased reflectivity, but the inner retina is usually spared. Outer retina may appear hyperreflective in healed lesions also, but in contrast to the active phase, outer retinal hyperreflectivity in healed lesions originates from RPE proliferation and migration. Consequently, outer retinal hyperreflectivity in healed lesions is more granular and non-uniform. Retinal and RPE inflammation in active choroiditis is often associated with limited subretinal fluid overlying the area of choroiditis. Although active and healed lesions share similarities in OCT features,14 they are usually differentiable based on the findings mentioned above. Also, retinal thickness is normal or slightly increased in the active choroiditis phase,49,136,174 but in a healed lesion, it is mildly attenuated because of outer retinal atrophy.27
Choroid may also appear hyper-reflective on OCT. This increased choroidal reflectivity is described as a “waterfall effect” and is attributed to inflammatory cell infiltration of the choroid.49 The choroidal hyperreflectivity in healed SC lesions is attributed to enhanced light transmission through overlying RPE atrophy. Choroidal visualization is limited in contemporary time-domain and spectral-domain OCT methods. Novel enhanced depth spectral domain-OCT imaging may provide more details on the thickness and reflectivity of the choroid in active SC.
Given the higher resolution and point-to-point registration of the OCT scans to the retinal image in spectral domain-OCT (SD-OCT), this modality reveals detailed retina and RPE structure and may prove useful in detection of choroiditis activity and follow-up examinations.136 Conventional SD-OCT features of SC are as mentioned above. En-face imaging of the retina in active phase of choroiditis showed the exact location of SC lesions in relation to other retinal structures. 174 As in conventional OCT B-scans, inner retinal layers appeared normal in en-face SD-OCT images.174
Active and healed SC and MSC share similar features in SD-OCT imaging. In contrast, SD-OCT may provide valuable information for differentiating active SC lesions from the lesions seen in multifocal choroiditis. While disturbances are usually limited to the outer retina in SC, active multifocal choroiditis lesions show inner retinal hyperreflectivity, indicating a full-thickness retinal inflammation174After the resolution of active choroiditis, healed multifocal choroiditis lesions display outer retinal hyperreflectivity (Fig. 7-E).
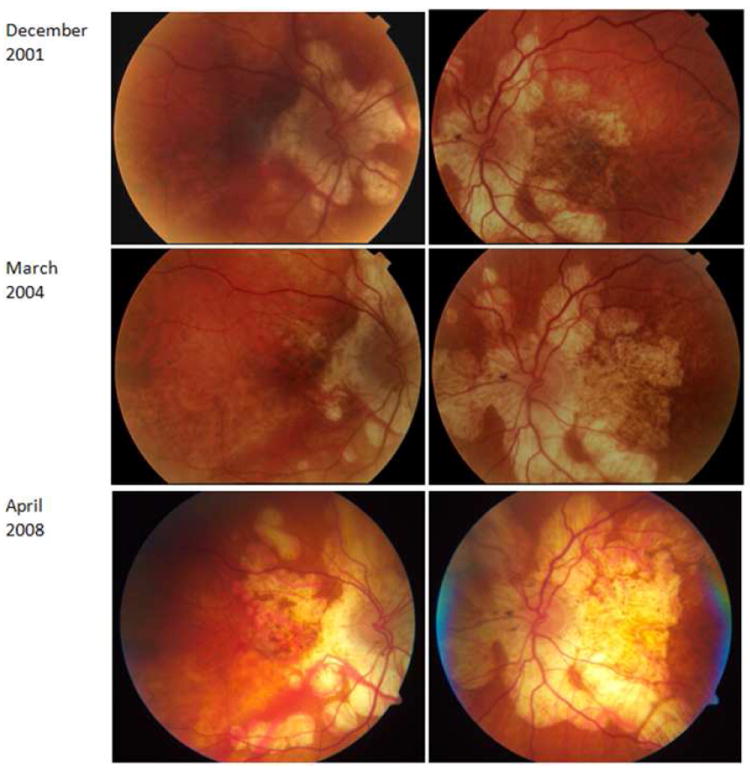
Figure 7.
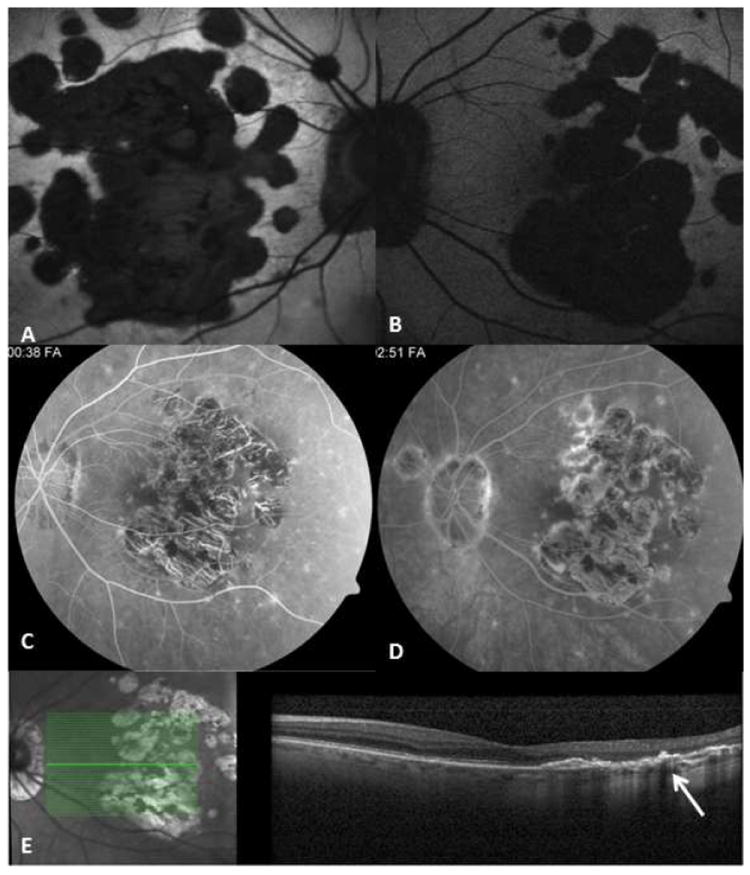
75-yr-old Caucasian woman with a documented history of contact with a patient with active pulmonary tuberculosis. She converted to PPD positive, and for this, she received anti-tuberculosis treatment. However, the number of drugs taken and the duration of treatment are unknown. Right and left eyes (A, B) fundus autofluorescent images; the autofluorescence is lost uniformly in atrophic areas. Note multifocal nature of the lesions that have coalesced in the center to form a larger patch. Satellite lesions are present. C and D correspond to mid-phase and late-phase fluorescein angiography and reveal a late staining of the exposed scleral bed and lesion borders. Optical coherence tomography scan (E) demonstrates loss of outer retina with pigment epithelial hypertrophy (arrow).
While physical examination, FA, and ICGA are traditionally used for diagnosis and follow-up of SC patients, the non-invasive OCT provides additional details on the development and extent of complications such as cystoid macular edema, subretinal fluid, epiretinal membrane, and subretinal neovascularization. 49,136,174
4. Fundus autofluorescence
Fundus autofluorescence (FAF) signals are mainly derived from lipofuscin accumulation within the RPE cells and indirectly reveal changes in the RPE layer. The FAF imaging is sufficiently sensitive to detect early damage to the RPE in acute and chronic episodes of SC.14,27 In the early stages, new lesions show a slight hypoautofluorescence, probably due to the masking effects of edematous outer retina on normal RPE autofluorescence. Within two to five days after the appearance of the active lesion, autofluorescence increases gradually, and hyperautofluorescent areas admixed with hypoautofluorescent areas appear (Figs. 3-C and D). These may correspond to the accumulation of lipofuscin in the stressed RPE. The brief latency between the appearance of whitish-yellow patches of choroiditis and the development of hyperautofluorescence is attributed to the time needed for the RPE cells to accumulate sufficient fluorophores.27 A weak hyperfluorescence halo that appears around the borders of some active lesions originates from thickening of the normal surrounding RPE cells pushed by edema of the involved RPE (Fig. 3-C).27 Within a few days, a hypoautofluorescent rim surrounds the hyperautofluorescent patches of the active lesions. This rim is the result of the corresponding RPE depigmentation seen on ophthalmoscopy.27 Within weeks, central hyperautofluorescence turns from a granular to a speckled pattern. With progression of the disease, and entering into the healing phase, fundus autofluorescence of the involved area decreases gradually until undetectable.27 This progressive shift from hyper- to hypofluorescence reflects progressive atrophy and degeneration of the RPE cells in the healed lesions.
With each exacerbation, new areas of hyperautofluorescence appear at the borders of hypoautofluorescence areas.187 Fundus autofluorescence images correspond well with the area of involved RPE and provide a precise delineation of the RPE alteration, allowing clinicians to evaluate the relationship of the lesions to the fovea. Notably, the multifocality of the lesions in MSC is better appreciated in FAF images (Figs. 7A and 7B and Fig. 9). Noninvasive FAF provides a unique tool to monitor progression and remission.27
Figure 9.
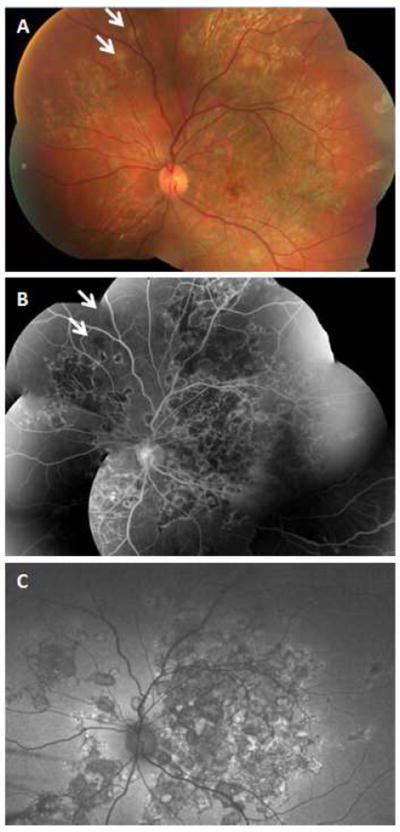
32-yr-old Asian Indian man with unilateral choroiditis that started in the macula and progressed rapidly following treatment with high dose oral corticosteroids. Tuberculosis work-up revealed positive QuantiFERON Gold test. Choroiditis subsided following institution of anti-tuberculosis treatment. Montage fundus photography (A) shows extensive healed lesions with pigmentary change in the posterior pole and in the periphery. Even though tuberculosis-associated multifocal serpiginoid choroiditis lesions are not connected to the peripapillary area at early presentation, advanced cases may present with extensive multifocal lesions involving the peripapillary area as well as the peripheral retina. There are two active patches of choroiditis in the supranasal periphery (arrow). Montage fluorescein angiogram (B) and wide-field autofluorescence image (C) reveal vividly the multifocal nature of the lesions.
The autofluorescence of the lesions may be influenced by treatment. Lesions treated early with corticosteroids reveal less hypofluorescence compared to those not treated during their active stage, suggesting that the anti-inflammatory treatment reduces damage to the RPE.27
5. Perimetry/microperimetry
Visual acuity hardly indicates the full visual impact of SC lesions, which may extensively affect extrafoveal areas with resulting visual field defects that correspond to the active choroiditis and inactive atrophic areas. Dense scotomas related to both central and peripheral lesions have been well documented using Goldmann perimetry and Amsler grid testing.17,132,182 Visual field studies also confirmed the preservation of the inner retinal layers, including the nerve fiber layer, in the presence of severe outer retinal destruction. Even in the presence of serpiginous atrophic scars in the papillomacular bundle, the absence of foveal involvement leaves the central vision unaffected.149
If foveal function is compromised and the patient has unstable or extrafoveal fixation, conventional perimetry is not accurate enough to record the full functional impact of the inflammation and resultant scarring.132 Microperimetry, with point-to-point quantification of retinal sensitivity and projection onto the corresponding retinal areas in fundus photography, precisely maps dense scotomas related to active lesions and atrophic areas.132 These scotomas are surrounded by a relative loss of retinal sensitivity in about one third of the involved eyes.132 Relative scotomas detected in about one third of these patients are attributed to subclinical lesions. Such lesions are not apparent on fundus examination, and FA and may correspond to the hypofluorescent patches of subclinical lesions in ICGA studies.122 The presence of such areas may indicate wider distribution of choriocapillaris circulation abnormalities and the propensity for development of new lesions in areas beyond apparent active or healed lesions.132
6. Retinal electrophysiology and other tests
Most retinal electrophysiology measures mass responses elicited from the entire retina and focal, non-extensive serpiginous lesions have a near normal full field electroretinogram.17,31,52,68,182 Nevertheless, extensive involvement and destruction of the retina in long-standing, untreated cases of SC result in a subnormal electroretinogram. 17,31,68 Electro-oculograms may also show subnormal values in patients with extensive lesions. 17,31,94
Color vision may be normal or subnormal, depending upon the extent of foveal involvement. Patients with foveal involvement and subnormal color vision do not show any differentiating pattern.17
Ultra-widefield digital retinal imaging systems provide a view of the posterior segment out to anterior equator (Fig. 8A).155 These systems incorporate color fundus imaging, fluorescein angiography, and fundus autofluorescence imaging.
Figure 8.
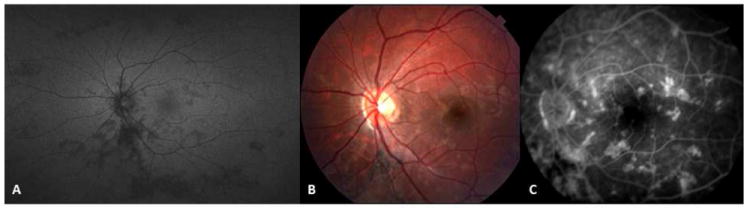
A 14-yr-old Caucasian woman with relentless placoid chorioretinitis (ampiginous choroiditis). (A) wide-field fundus autofluorescence image demonstrates multifocal inactive lesions distributed in the posterior pole and in the periphery of the left eye. Fundus photography (B) and late-phase fluorescein angiogram (C) reveal multifocal well-demarcated posterior pole lesions with late staining.
In a given patient presenting with serpiginous or serpiginoid choroiditis, initial imaging should include color photography to document new and healed lesions and to allow comparison with those obtained at follow-up examinations. In addition, we recommend FA to reveal active progressing choroiditis at the margins of pre-existing changes and to differentiate active and healed lesions. ICGA, which demonstrates choriocapillaris involvement in SC, may not be required because the practical value of ICGA findings is not clear. In contrast, OCT specifically delineates the extent of damage to retina, RPE and inner choroid.
We use SD-OCT in all patients for the detection of complications such as cystoid macular edema, epiretinal membrane, and subretinal neovascularization. Outer retinal involvement versus full thickness retinal involvement, as revealed by SD-OCT, may be a differentiating feature for SC and multifocal choroiditis. FAF is useful for delineating multifocal lesions, as seen in MSC.176 Moreover, FAF reveals the extent of inflammatory insult to RPE during the active and healed stages of the disease. FAF provides additional information regarding the extent of permanent damage to RPE as shown by the loss of autofluorescence. Microperimetry and multifocal ERG are functional tests, and selected cases may benefit from documentation of the size and degree of retinal damage but are not routinely performed.
VIII. Differential diagnosis
The diagnosis of SC with typical fundus changes, particularly from those regions where tuberculosis is not endemic, remains clinical and relatively straightforward. But SC with atypical clinical findings should be differentiated from the infectious choroiditis with clinical features that simulate SC. Mycobacterium tuberculosis, herpes viruses, and treponema pallidum are the main infectious causes of choroiditis that may present with fundus features mimicking SC. Various Idiopathic choroiditis and degenerative macular diseases may also manifest retinal and choroidal features simulating SC.
A. Multifocal serpiginoid choroiditis; Serpiginous-like choroiditis
1. Tuberculous choroiditis
This entity is characterized by multifocal choroidal lesions of varying size and shape. Each lesion mimics the fundus changes and angiographic features of SC. Such MSC lesions are seen in patients with tuberculosis; however, they can be observed in herpetic viral infections and syphilis. The serpiginous–like/multifocal serpiginoid choroiditis is a feature of intraocular tuberculosis and this infectious uveitis presents as posterior uveitis. 23,39,62,63,66,70 The uveitis associated with intraocular tuberculosis may manifest as choroidal/subretinal granuloma, focal retinitis and retinal vasculitis, multifocal choroiditis, miliary choroidal lesions, and choroiditis mimicking SC. 23,39,62,63,66,117,120,156
Using molecular methods, Gupta et al demonstrated MTB DNA in the ocular fluids of a group of patients with features of MSC. All such PCR-positive cases were from tuberculosis endemic countries.65 Despite some similarities between tuberculosis-associated MSC and non-tuberculous idiopathic SC, the former can be differentiated from idiopathic noninfectious SC (Table 5).65,104,176, 189 Patients with PCR-supported or presumed tuberculosis-related serpiginous-like choroiditis are primarily from countries where tuberculosis is endemic, emigrated from such countries to non-endemic regions, or have a history of contact with active pulmonary tuberculosis. 65,66,76,175Unlike in typical SC, the ocular involvement in tuberculosis-related MSC is mostly unilateral, with multifocal, irregular, serpiginoid lesions involving the posterior pole, mid-periphery, and periphery, (Fig, 9) but usually sparing the juxtapapillary area (Fig, 7).180 However, peripapillary choroid may be involved in advanced cases of multifocal serpiginoid choroiditis (Fig, 9). The multifocality and sparing of the juxtapapillary choroid is detected better with a fundus autofluorescence (Figs. 7 A, B). In contrast to patients with the typical (noninfectious) SC, those with tuberculosis-related MSC have a prominent inflammatory cellular reaction in the vitreous, usually with a cellular reaction in the anterior chamber. The progressive course of the choroiditis and the development of new lesions usually stop with anti-tuberculosis treatment. In contrast to tuberculosis-associated MSC, patients with typical SC are usually from areas where tuberculosis is not endemic. TST/interferon gamma release assay (IGRA) and chest x-ray are negative. They are successfully managed with corticosteroids and other immunosuppressive agents without antimicrobials. SC is rarely multifocal, primarily involves the posterior pole--especially around the optic disc--and extends contiguously in a centrifugal pattern.175
Table 5.
Differentiating features of serpiginous choroiditis and tuberculosis-associated multifocal serpiginoid choroiditis (serpiginouslike choroiditis)
| Serpiginous choroiditis | Tuberculosis multifocal serpiginoid choroiditis | |
|---|---|---|
| History | Born and raised in non-endemic area No history of contact with tuberculosis |
Lived in endemic area History of contact with tuberculosis patient |
| Clinical features | Not multifocal Bilateral Absence of vitritis and anterior chamber reaction or low level of inflammatory reaction Lesions starting from peripapillary area No response to antituberculosis treatment Pigment clumping is located in the borders of healed lesions |
Multifocal lesions Usually unilateral Considerable vitreous/anterior chamber inflammatory reaction Lesions starting from macula without peripapillary involvement in the early phases Improved with antituberculosis treatment Pigment clumping usually at the center of lesions |
| laboratory studies | Negative tuberculin skin test Normal chest x-ray Negative IGRA |
Tuberculin skin test usually positive Chest x-ray usually negative IGRA usually positive |
IGRA= Interferon gamma release assay
Angiographic features of both typical SC and presumed tuberculosis-associated serpiginous-like choroiditis lesions appear similar, with early blockage and late staining of the active lesions and central hypofluorescence/peripheral hyperfluorescence of inactive lesions (Figs. 3 and 6). 10,176 The difference in distribution of the lesions is more striking in fundus autofluorescence. In tuberculosis-associated serpiginous-like choroiditis, hypoautofluorescence patches related to RPE defects are multiple and discrete with an irregular geographic or serpentine pattern or like pieces of a puzzle. But in SC, there is a large geographic loss of RPE surrounded by hypertrophic/hyperplastic RPE. Spectral domain-OCT reveals outer retinal disruption and increased outer retinal and choroidal reflectivity in the involved areas in both conditions. 175
The definite diagnosis of tuberculosis-related uveitis is made when MTB is cultured from intraocular fluid samples or detected with staining methods, but this is rare. 25 In recent years, PCR-based detection of MTB DNA in ocular fluids has provided evidence supporting the diagnosis of tuberculosis-related uveitis simulating SC.61,65,66,167 Nonetheless, obtaining tissue samples from the eye for microbiologic or molecular studies is not always possible and may be associated with vision-threatening complications. Thus, ophthalmologists mainly rely on a combination of history, clinical findings, and investigations such as chest x-ray, TST/IGRA to arrive at an etiology of MTB uveitis.1,65,67,66,177 The sensitivity and specificity of chest x-ray, chest CT-scan, TST, or a combination of the three are, however, limited. 116,177
Interferon gamma release assay is more specific than the TST in identifying MTB infection/encounter, but not more sensitive. 13 IGRA is useful when there is a history of Bacillus Calmette-Gurein (BCG) vaccination, because, unlike TST, IGRA is negative in such patients.13 However, IGRA, like TST, cannot distinguish latent TB from active infection, and the advantage of IGRA over TST is minimal for use as a screening tool in developed countries with a low incidence of tuberculosis.9 In addition, IGRA use in immunosuppressed patients and those receiving immunosuppressive treatment and during the follow-up period of anti-tuberculosis treatment is not well studied. 9,13 A negative IGRA does not exclude a diagnosis of tuberculosis and should be interpreted with caution. On the other hand, a positive IGRA is suggestive of tuberculosis encounter (latent tuberculosis) but does not necessarily signify the presence of an active infection requiring anti-tuberculosis medication.9 Despite their shortcomings, both TST and IGRA improve the diagnosis of intraocular tuberculosis. 9,13,104,169 A positive IGRA should prompt further clinical and laboratory investigations for systemic tuberculosis, and in selected cases, the analysis of intraocular fluids by PCR.104 A therapeutic trial of anti-tuberculosis treatment may even be warranted when there is a strong clinical suspicion of tuberculosis, even in the absence of positive TST/IGRA.66, 114
Tuberculosis-related multifocal serpiginoid choroiditis is not usually accompanied by signs of active or healed pulmonary tuberculosis on chest X-ray. 65, 66, 104 All patients with MTB-associated SC in our series had a highly positive TST with normal chest radiographs, and there was no evidence of tuberculosis elsewhere.176 Moreover, while a high resolution chest CT scan increases the probability of detecting pulmonary tuberculosis, novel imaging approaches, like PET scans, may prove more sensitive in detecting hilar adenopathy.40,41
The pathogenesis of tuberculosis-associated MSC remains unclear. This choroiditis may represent a reactivation of dormant foci of mycobacteria in RPE cells.138 The RPE cells may serve as an ocular sanctuary for the dormant MTB bacilli and there are similarities between these cells and alveolar macrophages, where the bacteria are known to remain dormant.138
Continued or even paradoxical worsening of choroiditis after anti-tuberculous therapy is attributed to an enhanced host immune response to released tubercular antigens in a Jarisch-Herxheimer-like mechanism.64 In a series of tuberculosis-related MSC, 14% showed continued progression of the choroiditis despite standard anti-tuberculosis treatment. These cases were only controlled after administration of high-dose systemic corticosteroids or the addition of immunomodulatory agents to the anti-tuberculosis regimen,64 indicating a prominent role of immune reaction to the mycobacteria. Similar immune reactivation is reported in tuberculosis meningitis and pericarditis, requiring corticosteroids. 85,131
In a recent report of 70 patients diagnosed with SC in India, only 5 (7.1%) had evidence of MTB infection based on TST.2 Such report indicates that anti-tuberculosis medications should be used judiciously even in tuberculosis-endemic regions considering the potential side-effects of these agents.2,14,104,170
2. Herpetic choroiditis
Herpetic infection may manifest with features simulating SC.105 Priya and colleagues analyzed aqueous humor samples from nine patients who presented with features simulating SC with an anterior chamber cellular reaction, Using PCR, they detected herpes viral DNA.135 In patients with atypical features of SC and rapid progression, especially if the macula is the primary site of involvement, further investigation may be warranted, including aqueous humor analysis by PCR for herpes viruses. Empirical coverage with acyclovir while on high-dose corticosteroids and immunomodulatory agents may also be prudent.
3. Syphilitic choroiditis
Syphilis chorioretinitis and its sequelae are seen in secondary and tertiary syphilis. Posterior placoid chorioretinitis 172 described in late latent syphilis may mimic SC; 50,77 however, a cellular inflammatory reaction is more prominent in syphilis, and the lesions tend to be multifocal. 172 The Centers for Disease Control and Prevention (CDC) still recommend initial screening with non-treponemal tests (such as VDRL and RPR) and confirmation of the positive or borderline results with treponemal tests (like FTA-ABS or MHA-TP). But since ocular syphilis mainly presents during the late phases of the disease, when non-treponemal tests show only 70% sensitivity to detect the infected cases, most uveitis experts order a combination of non-treponemal and treponemal tests. 29
B. Idiopathic multifocal choroiditis
Idiopathic multifocal choroiditis (MFC) might be confused with an atypical form of SC or MSC. Lesion size and distribution, clinical course, and patient age help to differentiate these entities.42,88 The ophthalmic hallmarks of MFC include the presence of variable numbers of 200-1000 μm, yellow-white chorioretinal lesions distributed in the posterior pole and the equatorial area (Fig. 10). Healed lesions demonstrate a well-demarcated, punched-out appearance, surrounded by pigment clumping (Fig. 10). Vitreous and anterior chamber inflammatory reactions may be prominent in MFC patients, who are typically young myopic women. Similarly, punctate inner choroidopathy (PIC) usually involves young myopic women; however, the lesions in PIC are smaller (100-300 μm) 53 than those of MFC and SC. New lesions may appear during the follow-up period, and cystoid macular edema and CNV are the main causes of visual morbidity in MFC. Fluorescein angiographic features of the active lesions are similar to those of SC, with early hypofluorescence and late hyperfluorescence. Healed lesions similarly show early hypofluorescence with late marginal staining and blocking effects from pigment clumps. Spectral domain OCT is a helpful diagnostic modality to differentiate this entity from SC. While SC lesions show outer retinal reflectivity, MFC manifests as full-thickness, hyperreflective lesions.174
Figure 10.
A 24-yr-old Caucasian woman with idiopathic multifocal choroiditis. Left eye color photograph (A) and autofluorescent image (B) demonstrate multiple, round, atrophic lesions associated with pigment alterations in the posterior pole. The size and distribution of the lesions help in differentiation from larger and usually confluent patches seen in serpiginous choroiditis. Spectral domain-optical coherence tomography scan (C) reveals outer retinal destruction and pigment migration to the outer retina corresponding to healed chorioretinal lesions.
C. Acute posterior multifocal placoid pigment epitheliopathy
This acute, self-limited, multifocal and usually bilateral disease of RPE affects young adults and resolves spontaneously. 81,103,112,141 Acute lesions may appear identical to new active SC lesions, with deep yellowish patches at the level of RPE. But the lesions of APMPPE usually resolve in about 2 weeks with minimal RPE change. Additionally, APMPPE is generally considered a non-recurrent condition with a favorable visual prognosis. Rare recurrences are usually multifocal (like the original disease) and don’t arise from the margins of healed lesions with pigment alterations (if such pigmentary changes do exist).103 A diagnosis of APMPPE should always be questioned in patients with unilateral, recurrent, or progressive lesions without significant visual recovery. Ryan and Maumenee reported a unilateral case in their series of APMPPE patients who progressed during the follow-up period, eventually diagnosed as helicoid peripapillary chorioretinal atrophy.141.
D. Ampiginous choroiditis and relentless placoid chorioretinitis
Recurrent APMPPE that resembled SC in its FA features, its resultant pigmentary disturbances, and its recurrent course but tended to involve the peripheral retina and choroid has frequently been included in SC series.24,60,102,103,182 Nussenblatt designated these cases “ampiginous choroiditis” to indicate that their clinical features were similar to both APMPPE and SC.102,122 The ampiginous choroiditis lesions, as in APMPPE, develop at the level of RPE and choriocapillaris, but unlike in APMPPE, pigment disturbances remain after the lesions resolve.122 Also, the lesions involve both the posterior pole and the periphery.102 Given the similar clinical features and courses of ampiginous choroiditis and SC, Nussenblatt considers ampiginous choroiditis as a variant of SC.102 Jones and colleagues reported six patients with clinical, fluorescein and indocyanine green angiography findings of both APMPPE and serpiginous choroidopathy and called them “relentless placoid chorioretinitis;”82 however, the clinical course and the number and location of the lesions in these patients were not typical for SC. They develop 50 or more separate lesions scattered in the macula and periphery with the simultaneous presence of both active and healing lesions.82 The lesions appeared at the level of RPE and deep retina and tended to recur and to involve large areas. The patients, however, maintained good central vision (Fig. 8). 81,122 Although the choroiditis was called “relentless” because of the continuous appearance of new lesions,82 the patients were not initially treated with sufficient doses of immunosuppressive agents, and the disease process in most was controlled only after an increase in their dose of corticosteroids.82 The authors considered relentless placoid chorioretinitis “a variant of SC or new entity” 82,125 Since the terms “ampiginous choroiditis” and “relentless placoid chorioretinopathy” have been used to describe patients with similar clinical features and prognosis, we and others102 believe both entities represent the same condition. 81,122,125
In an OCT study of patients with ampiginous choroiditis, the inner and outer retinal layers showed hyperreflectivity.12 In contrast, SC lesions demonstrated only outer retinal hyperreflectivity, which persisted even in healed lesions. While the FAF appearance of active ampiginous choroiditis lesions is not described, old healed lesions show diffuse hypoautofluorescence from widespread atrophy and focal areas of RPE hypertrophy.187 Serpiginous choroiditis and ampiginous choroiditis patients are treated similarly with immunosuppressive agents.82,188 In regions where tuberculosis is endemic, ampiginous choroiditis/relentless placoid chorioretinitis cases should be investigated to rule out intraocular tuberculosis.
E. Miscellaneous entities
Choroid and RPE express limited morphologic changes in response to pathologic insults. It is not unusual to encounter posterior uveitis syndromes manifesting as geographic-like changes involving the choroid and RPE, with retinal atrophy with hypo- and hyperpigmentation. Ocular sarcoidosis with inflammatory changes and SC-like angiographic features has been reported. 43,45,113 Posterior scleritis may also present with fundus changes simulating SC; however, the presence of pain and characteristic ultrasonography features in the former condition help differentiate the two conditions.163 Systemic Hodgkin and non-Hodgkin lymphomas 140,141 and choroiditis in the context of Crohn disease183 may simulate SC. Moreover, SC may simulate various pathologies causing peripapillary and macular atrophy and pigment alteration and neovascularization, such as presumed ocular histoplasmosis syndrome (Fig. 11), age-related macular degeneration (AMD) (Fig. 12), angioid streaks, idiopathic choroidal neovascularization, or toxoplasma retinochoroiditis (Fig. 13).52,56 Acute choroiditis, with or without accompanying healed lesions, may be mistaken for subretinal exudation overlying a choroidal neovascular membrane. Also, inactive healed atrophic patches may resemble the geographic atrophy caused by non-exudative AMD. However, clinical and angiographic features are helpful in differentiating SC from exudative and non-exudative AMD.
Figure 11.
50-yr-old Caucasian man with presumed ocular histoplasmosis syndrome (POHS). Fundus photograph (A) and mid- and late angiograms (B, C) reveal multifocal punched-out atrophic lesions accompanied with peripapillary atrophy. Lesions are more prominent in fundus autofluorescence than in color fundus image (D). Spectral domain optical coherence tomography scan passing through the foveal lesion (E) shows a full-thickness destruction of the retina and choroid.
Figure 12.
Nonexudative, age-related macular degeneration with geographic atrophy may resemble inactive serpiginous choroiditis lesion (A). In fundus autofluorescence imaging (B) a hyperautofluorescent halo surrounds the hypofluorescent lesion. Early window defect (C) due to atrophic retinal pigment epithelial (RPE) layer and late staining (D) of the atrophic bed with sharp margins are similar to angiographic features of inactive serpiginous choroiditis lesions. Optical coherence tomography scan (E) reveals atrophic RPE and increased backscattering from the choroid; however, outer retina retains normal reflectivity.
Figure 13.
Toxoplasma chorioretinitis. Note a large geographic lesion within macula with satellite lesions.
IX. Complications
Involvement of the fovea is the main vision-threatening concern in SC.87 Central vision is usually affected when the foveal and parafoveal areas are directly involved by the lesions or when the extrafoveal lesions are complicated by CNV, which develops in up to 35% of eyes with SC, usually at the surrounding boundaries of healed lesions. 21,32,80,94,96,99,109,132 Although the exact pathogenesis of CNV in SC is unknown, possibilities include ischemic injury to the choroid, Bruch membrane, and outer retina secondary to the choriocapillaris inflammation have been proposed.127 CNV development is not necessarily related to the disease activity and can be seen in both active and healed lesions.21,94 Prompt anti-inflammatory treatment may lower CNV incidence. 15,36 Meanwhile, all SC patients should be instructed to perform daily self-monitoring with Amsler grids for earlier detection of CNV lesions.69, 80 Although fundus examination, angiographic studies, and OCT imaging features of CNV in the context of SC are similar to those of CNV in AMD, the disease course and the accompanying features are helpful in differential diagnosis.
Retinal vasculitis and ischemia are not constant features in SC, although there are reports of vascular occlusions and secondary neovascularization.18,21,47,94,111 Such neovascularization might lead to retinal and vitreous hemorrhage. Optic nerve head neovascularization and combined retinal artery and vein occlusion also occur in SC.47,111,184 Strict inflammatory control may decrease the incidence of such complications or even lead to spontaneous regression of the neovascularization.52,94,96,184
Exacerbations of choroiditis might be accompanied by serous retinal detachment 31,52,68,75,80,109,151 or pigment epithelial detachment75, 184in the absence of CNV. These lesions usually resolve when the choroiditis subsides. Review of FA pictures as well as serial OCT examinations are crucial to rule out the presence of CNV, macular holes, and cystoid macular edema.59,166
X. Management
Appropriate treatment depends on the extent of choroiditis, its proximity to the fovea, and the side effect profile of the therapeutic agents. Before starting treatment, systemic and local infection should be ruled out. Published series and recommendations based on the consensus of a panel of uveitis experts suggest that treatment with systemic corticosteroids combined with immunosuppressive agents preserves central vision when used early in the disease course.79 The treatment approaches are systemic, local, or a combination of the two. In the absence of prospective, randomized trials, optimal treatment of SC remains controversial.2,15,31,32,73,74,94,97,100,122,146,161,162
A. Systemic treatment
Based on the presumed autoimmune pathogenesis of SC, the main approach has been the use of immunosuppressive agents. While initial high-dose corticosteroids usually limit acute choroiditis, long-term treatment to prevent recurrences relies on immunomodulatory agents. More potent immunomodulatory regimens have yielded lower rates of recurrence and eventual preservation of vision, although their side effects can be significant. 5,8,58 In some patients recurrences resolve without treatment. Some have proposed initial treatment with corticosteroids without immunomodulatory agents32,72,79,152; but, since the SC is almost always a bilateral disease with a prolonged inflammatory course, initiating treatment with a combination of corticosteroids and immunomodulatory agents results in a better long-term control 179 and has been recommended by a panel of uveitis experts.79 Meanwhile, there is no uniform agreement about the proper time for discontinuing immunosuppressive medication. Similarly, the optimal agents for induction of lengthy remission and complete elimination of recurrences with an acceptable initial or maintenance regimen are unclear.72
1. Corticosteroids
Corticosteroids alone or in combination with other immunosuppressive agents are the mainstay of therapy for SC. 69,149,182 Although moderate doses of corticosteroids (equal to 40 mg/day of prednisone) were initially used, such treatment did not change the natural course of SC lesions.31,68, 111,95,149 Prompt treatment with 60-80 mg oral prednisone or intravenous pulse methyl prednisone (1 gm/day for 3 days) can limit choroiditis progression and prevent visual loss. 69,75,110,123
Hoyng and colleagues described two patients with recurrent SC involving the macula who were treated promptly with 80 mg/day oral prednisone. 75 By day 14, visual acuity improved and FA revealed regression of the lesion. After gradual taper, the patients were maintained at a low dose (5 mg/day) for 6 months before weaning off the prednisone. Neither developed chorioretinal scar or recurrences within 2 and 5 years after treatment. They concluded that the isolated macular lesions might be accompanied by a transient occlusion of the choriocapillaris that is amenable to early anti-inflammatory treatment. A clinical response to high-dose prednisone (1 mg/kg) is usually evident within 24-36 hours,123 and complete resolution takes 2-8 weeks.69,75 By the time the lesions are resolved, the initial high dose should be followed by a gradual taper after1-3 months. While prednisone is the main corticosteroid medication used in the treatment of SC patients in the United States, betamethasone and methylprednisolone have been employed successfully.123Corticosteroids might prevent recurrences if given at high enough doses for a prolonged period; however, there are significant complications of long-term corticosteroid use, and recurrences may arise shortly after tapering or discontinuation.32,79
2. Immunomodulatory agents
An immunomodulatory agent is usually required to prevent recurrence of the choroiditis and to avoid or minimize corticosteroid side effects.5,32,178 Christmas et al showed that all seven SC patients who were treated with either corticosteroid or observation developed recurrences, whereas, only two of six patients treated with immunomodulatory drugs (azathioprine, cyclosporine, and/or mycophenolate mofetil) relapsed.32 No consensus exists as to the best immunosuppressive agent for the treatment of SC.5 Immunosuppressive agents that have been used in the management of SC include T cell inhibitors, antimetabolites, alkylating agents, and biological agents.
a. T cell inhibitor
Cyclosporine has been used in the treatment of SC. Although initial reports on cyclosporine monotherapy were disappointing,97, 100 later studies noted favorable long-term control of inflammation and low complication rate with cyclosporine, used either alone or in combination with other immunosuppressive agents.11,32,72,151,152
b. Antimetabolites
Azathioprine and mycophenolate mofetil are two antimetabolites used in SC patients. Azathioprine is a purine analog that has been effective for suppression of inflammatory bouts and induction of long-term remission in patients with SC.32,37,72,178 Azathioprine and cyclosporine synergistically modulate the immune response.164
Although published experiences in the use of mycophenolate mofetil for SC are limited, 18,154, its safety profile makes it an ideal immunomodulatory agent for long-term use. Gastrointestinal disturbances are common, and patients should be monitored with complete blood count and liver function tests every 6 weeks.
c. Alkylating agents
If the choroiditis cannot be controlled adequately with the above agents, and in order to induce long-term remission, alkylating agents can be employed.5,7,8, 58,146 Cyclosporine and chlorambucil may induce a sustained remission in vision threatening and refractory cases. 8,58
Although cyclophosphamide use is associated with increased risk of bladder malignancies,8,79 overall cancer-related mortality does not increase significantly in patients receiving this alkylating agent.89 Considering the acceptable success rate with less toxic medications, alkylating agents should only be employed for recalcitrant cases that have shown multiple recurrences and progression while under treatment.5
d. Biological agents
Alternative treatment with biological agents such as interferon alpha-2a is a consideration for severe cases that required continued therapy with alkylating agents.159 In a prospective non-comparative case series, eight eyes of five patients with SC were treated with interferon alpha-2a.159 Prednisone was also given initially, then tapered and discontinued. All active lesions, including complicating subfoveal CNV in one eye, resolved within 6 months. None experienced recurrences. Acuity improved in four of eight eyes (50%); and the other four eyes maintained (50%) useful vision during the 16- to 48-month follow-up.159 Complete blood count, liver function tests, blood urea nitrogen, and creatinine should be checked initially and every 6 weeks thereafter.124
Anti-tumor necrosis factor (TNF) agents
Cordero-Coma reported a patient with fundus features mimicking SC and a negative work-up for tuberculosis in whom treatment with anti-TNF medication led to disseminated tuberculosis.35 On the other hand, Seve et al reported that anti-TNF treatment helped control inflammation in their patient with a fundus appearance of typical SC.154
B. Local treatment
Topical administration of corticosteroid may be necessary in patients with anterior segment inflammation. Intravenous,123 peribulbar,123 subtenon,69 and intravitreal 4,86,129 corticosteroid injections have also been used with variable success. Intravitreal injection of triamcinolone acetonide was first used in patients for whom systemic corticosteroids were contraindicated. 4,86,129 Considering successful inflammation control and the low rate of reported complications, Wadhwa and colleagues conducted a prospective, non-randomized, interventional case series that included eight patients with unilateral macular SC who opted for intravitreal injection of 4 mg/0.1 ml triamcinolone acetonide.180 The choroidal inflammation subsided within 2 weeks in all eight patients, and there was no remission during 6 months of follow-up.180 The intravitreal corticosteroid injection might also have beneficial effects on CNV development in inflammatory conditions; however, the side effect profile, including cataract progression, increased intraocular pressure, and a small possibility of endophthalmitis may limit its use.
Sustained-release intravitreal implants provide a theoretically ideal treatment mode for SC. Seth and Gaudio used an intravitreal injection of triamcinolone to treat active SC threatening the fovea, resulting in immediate control of the choroiditis.153 An intraocular fluocinolone acetonide implant was then placed, and the choroiditis remained inactive during the follow-up period of 14 months.
C. Combined systemic and local treatment
Local drug delivery of corticosteroids in SC patients who are managed with systemic corticosteroids and or additional immunosuppressive agents can reduce intraocular inflammation and minimize the required dose of systemic corticosteroids or immunomodulatory agents. It can also aid in the rapid taper of the systemic agents. Such local added treatment includes intravitreal and subtenon injection of corticosteroids.
Multifocal Serpiginoid Choroiditis encompasses different infectious choroiditis entities that require specific anti-microbial treatment. Tuberculosis-related MSC is treated with a multidrug regimen, initially consisting of four drugs for 2-3 months, followed by a course of two antibiotics lasting up to 12 months. Ideally, the treatment should be instituted and monitored in collaboration with infectious disease experts. Although the optimal treatment for herpes-related multifocal serpiginoid choroiditis has not been studied, the treatment regimen used for herpetic retinitis, consisting of high dose intravenous acyclovir or a similar antiviral agent for a week or ten days followed by a longer course of oral antivirals, would seem appropriate for viral MSC. Syphilitic serpiginoid choroiditis should be treated as neurosyphilis.
D. Management of complications
Timely diagnosis and appropriate inflammation control in SC is the key in preventing visual loss from foveal involvement, choroidal neovascular membrane development, and cystoid macular edema. Prompt anti-inflammatory treatment can prevent or decrease the extent of chorioretinal scar formation.73,75 The incidence of CNV is significantly lower in patients treated in the early phase of the disease with a proper anti-inflammatory regimen.15,36,90
Laser photocoagulation has failed to prevent the extension of SC involving the fovea,51; but direct laser application is still a legitimate treatment option in SC cases complicated with extrafoveal CNV. 80,159 Repeated application of the laser to recurrent CNV appears to have the same initial favorable result in obliterating the neovascular membrane and resorbing the subretinal fluid and hemorrhage,185 but recurrence of CNV is not totally preventable.94 Indocyanine green-mediated photothrombosis combined with intravitreal triamcinolone injection was employed successfully for peripapillary CNV in the context of SC.119 Within 12 weeks, the patient’s visual acuity improved from 20/100 to 20/20, and OCT confirmed a reduction of retinal elevation and subretinal fluid.119 Photodynamic therapy with verteporfin has also been used to treat CNV associated with SC101,127 however, repeated treatments may be necessary.
The food and drug administration (FDA) of United States has approved the use of ranibizumab, a fragment of humanized monoclonal antibodies, to treat CNV associated with AMD by pharmacologic inhibition of vascular endothelial growth factor (VEGF). Favorable visual outcome has been reported with intravitreal injection of ranibizumab and bevacizumab, a full-length anti-VEGF antibody, to treat CNV secondary to inflammatory ocular diseases. 16,38,93,107,109,145,171 Regression of neovascular membrane was apparent shortly after injection, and visual acuity was improved or stabilized. Choroidal neovascularization secondary to SC responds well to the conventional dosage of intravitreal ranibizumab (0.5 mg/0.05ml) and bevacizumab (1.25 mg/0.05 ml or 2.5 mg/0.1 ml). 16,19,83,145,162 The antiangiogenic effects are transient, and repeated injections are needed in most cases.83 Although the data on appropriate dosing and frequency of anti-VEGF injection in patients with SC is limited, the same protocol used in the management of multifocal choroiditis complicated with CNV can be used in SC patients presenting with CNV.128 Three monthly intravitreal bevacizumab injections followed by careful monthly examination to detect early recurrence and as-needed retreatment has been associated with beneficial effects in treatment of subfoveal CNV in patients with multifocal choroiditis.128 Posterior subtenon injection of triamcinolone acetonide and anecortave acetate, an angiostatic corticosteroid, has also been used successfully to treat CNV.121
XI. Prognosis
The long, drawn-out chronic inflammatory nature of SC, with unpredictable episodes of activity and inactivity over several years, makes proper study of the natural course of the disease difficult.2 Generally, the visual prognosis for patients with SC is considered guarded. Mean final visual acuity is reported at 20/125, ranging from 20/20 to no light perception.32,87,98 The natural course of classic SC is of progressive choroiditis with recurrences and involvement of new areas; therefore, an untreated patient will generally experience extensive chorioretinal atrophic changes and lose visual acuity.31 Hence, the prognosis of the disease is mainly correlated with the extent of inflammatory control and the prevention of recurrences.32,130 Macular forms of the disease are usually associated with a worse visual prognosis. 32, 108 Extension of choroiditis to the fovea and subfoveal CNV are the main causes of central vision loss.32 Meanwhile, immunomodulatory agents have improved the prognosis by reducing the recurrence rate. The number of inflammatory recurrences in patients treated with immunomodulatory agents combined with corticosteroids is significantly lower than in those who were followed without treatment or treated with corticosteroids alone.32 Most recurrences develop when the corticosteroid dose is tapered or discontinued. Close monitoring during corticosteroid tapering and institution of proper immunomodulatory treatment is mandatory for preservation of vision.
XII. Conclusion
Serpiginous choroiditis is a recurring, asymmetric, bilateral, and progressive inflammation of the inner choroid involving the choriocapillaris and RPE. Available evidence suggests that SC is primarily an idiopathic or autoimmune process. A considerable subset of patients with atypical features mimicking SC has been delineated. Such cases are labeled as MSC, and represent infectious choroiditis secondary to MTB or herpes family virus infection.
Spectral domain OCT and novel imaging techniques such as FAF have improved diagnostic accuracy, management, follow-up, and determination of the visual prognosis. The main treatment goal is to suppress active inflammation and reduce the number of recurrences. A combined regimen of oral corticosteroids and immunomodulatory agents appears to be the most effective management. Local treatment with corticosteroids and antiangiogenic agents are effective adjunctive treatment options for SC and its complications.
XIII. Literature search method
We searched English literature in Medline for the following keywords: serpiginous choroiditis, serpiginous choroidopathy, multifocal serpiginoid choroiditis, serpiginous-like choroiditis, serpiginous-like choroidopathy, helicoid choroidopathy, helicoid choroiditis, geographic choroiditis, geographic choroidopathy, macular serpiginous choroiditis, and macular serpiginous choroidopathy. All retrieved articles were read in full text, and related references were searched for pertinent publications.
Acknowledgments
Supported in part by NIH Core Grant EY03040.
Footnotes
Financial disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrams J, Schlaegel TF., Jr The tuberculin skin test in the diagnosis of tuberculous uveitis. Am J Ophthalmol. 1983;96(3):295–8. doi: 10.1016/s0002-9394(14)77817-1. [DOI] [PubMed] [Google Scholar]
- 2.Abrez H, Biswas J, Sudharshan S. Clinical profile, treatment, and visual outcome of serpiginous choroiditis. Ocul Immunol Inflamm. 2007;15(4):325–35. doi: 10.1080/09273940701375162. [DOI] [PubMed] [Google Scholar]
- 3.Abu el-Asrar AM. Serpiginous (geographical) choroiditis. Int Ophthalmol Clin. 1995;35(2):87–91. doi: 10.1097/00004397-199503520-00008. [DOI] [PubMed] [Google Scholar]
- 4.Adigüzel U, Sari A, Ozmen C, Oz O. Intravitreal triamcinolone acetonide treatment for serpiginous choroiditis. Ocul Immunol Inflamm. 2006;14(6):375–8. doi: 10.1080/09273940601025974. [DOI] [PubMed] [Google Scholar]
- 5.Akpek EK, Baltatzis S, Yang J, Foster CS. Long-term immunosuppressive treatment of serpiginous choroiditis. Ocul Immunol Inflamm. 2001;9(3):147–67. doi: 10.1076/ocii.9.3.153.3962. [DOI] [PubMed] [Google Scholar]
- 6.Akpek EK, Chan CC, Shen D, Green WR. Lack of herpes virus DNA in choroidal tissues of a patient with serpiginous choroiditis. Ophthalmology. 2004;111(11):2071–5. doi: 10.1016/j.ophtha.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Akpek EK, Ilhan-Sarac O. New treatments for serpiginous choroiditis. Curr Opin Ophthalmol. 2003;14(3):128–31. doi: 10.1097/00055735-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Akpek EK, Jabs DA, Tessler HH, et al. Successful treatment of serpiginous choroiditis with alkylating agents. Ophthalmology. 2002;109(8):1506–13. doi: 10.1016/s0161-6420(02)01097-7. [DOI] [PubMed] [Google Scholar]
- 9.Albini TA, Karakousis PC, Rao NA. Interferon-gamma release assays in the diagnosis of tuberculous uveitis. Am J Ophthalmol. 2008;141(4):486–8. doi: 10.1016/j.ajo.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Al-Mezaine HS, Al-Muammar A, Kangave D, Abu El-Asrar AM. Clinical and optical coherence tomographic findings and outcome of treatment in patients with presumed tuberculous uveitis. Int Ophthalmol. 2008;28(6):413–23. doi: 10.1007/s10792-007-9170-6. [DOI] [PubMed] [Google Scholar]
- 11.Altan-Yaycioglu R, Akova YA, Akca S, Yilmaz G. Inflammation of the posterior uvea: findings on fundus fluorescein and indocyanine green angiography. Ocul Immunol Inflamm. 2006;14(3):171–9. doi: 10.1080/09273940600660524. [DOI] [PubMed] [Google Scholar]
- 12.Amer R, Florescu T. Optical coherence tomography in relentless placoid chorioretinitis. Clin Experiment Ophthalmol. 2008;36(4):388–90. doi: 10.1111/j.1442-9071.2008.01773.x. [DOI] [PubMed] [Google Scholar]
- 13.Ang M, Htoon HM, Chee SP. Diagnosis of tuberculous uveitis: clinical application of an interferon-gamma release assay. Ophthalmology. 2009;116(7):1391–6. doi: 10.1016/j.ophtha.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Arantes TE, Matos K, Garcia CR, et al. Fundus autofluorescence and spectral domain optical coherence tomography in recurrent serpiginous choroiditis: case report. Ocul Immunol Inflamm. 2011;19(1):39–41. doi: 10.3109/09273948.2010.515373. [DOI] [PubMed] [Google Scholar]
- 15.Araujo AA, Wells AP, Dick AD, Forrester JV. Early treatment with cyclosporin in serpiginous choroidopathy maintains remission and good visual outcome. Br J Ophthalmol. 2000;84(9):979–82. doi: 10.1136/bjo.84.9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arevalo JF, Adan A, Berrocal MH, et al. Intravitreal bevacizumab for inflammatory choroidal neovascularization: results from the Pan-American Collaborative Retina Study Group at 24 months. Retina. 2011;31(2):347–63. doi: 10.1097/IAE.0b013e3181ed8cec. [DOI] [PubMed] [Google Scholar]
- 17.Baarsma GS, Deutman AF. Serpiginous (geographic) choroiditis. Doc Ophthalmol. 1976;40(2):269–85. doi: 10.1007/BF00155041. [DOI] [PubMed] [Google Scholar]
- 18.Baglivo E, Boudjema S, Pieh C, et al. Vascular occlusion in serpiginous choroidopathy. Br J Ophthalmol. 2005;89(3):387–8. doi: 10.1136/bjo.2004.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battaglia Parodi M, Iacono P, et al. Antivascular endothelial growth factors for inflammatory chorioretinal disorders. Dev Ophthalmol. 2010;41:84–95. doi: 10.1159/000320011. [DOI] [PubMed] [Google Scholar]
- 20.Biswas J, Narain S, Das D, Ganesh SK. Pattern of uveitis in a referral uveitis clinic in India. Int Ophthalmol. 1996;20(4):223–8. doi: 10.1007/BF00175264. [DOI] [PubMed] [Google Scholar]
- 21.Blumenkranz MS, Gass JD, Clarkson JG. Atypical serpiginous choroiditis. Arch Ophthalmol. 1982;100(11):1773–5. doi: 10.1001/archopht.1982.01030040753008. [DOI] [PubMed] [Google Scholar]
- 22.Bodaghi B, Cassoux N, Wechsler B, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore) 2001;80(4):263–70. doi: 10.1097/00005792-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Bodaghi B, LeHoang P. Ocular tuberculosis. Curr Opinion Ophthalmol. 2000;11(6):443–8. doi: 10.1097/00055735-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Bouchenaki N, Cimino L, Auer C, et al. Assessment and classification of choroidal vasculitis in posterior uveitis using indocyanine green angiography. Klin Monbl Augenheilkd. 2002;219(4):243–9. doi: 10.1055/s-2002-30661. [DOI] [PubMed] [Google Scholar]
- 25.Bouza E, Merino P, Muñoz P, et al. Ocular tuberculosis. A prospective study in a general hospital. Medicine (Baltimore) 1997;76(1):47–61. doi: 10.1097/00005792-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Broekhuyse RM, van Herck M, Pinckers AJ, et al. Immune responsiveness to retinal S-antigen and opsin in serpiginous choroiditis and other retinal diseases. Doc Ophthalmol. 1988;69(1):83–93. doi: 10.1007/BF00154420. [DOI] [PubMed] [Google Scholar]
- 27.Cardillo Piccolino F, Grosso A, Savini E. Fundus autofluorescence in serpiginous choroiditis. Graefes Arch Clin Exp Ophthalmol. 2009;247(2):179–85. doi: 10.1007/s00417-008-0951-z. [DOI] [PubMed] [Google Scholar]
- 28.Carr RE, Noble KG. Disorders of the fundus 8. Geographic (serpiginous) choroidopathy. Ophthalmology. 1980;87(10):1065–8. doi: 10.1016/s0161-6420(80)35125-7. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC) Discordant results from reverse sequence syphilis screening -- five laboratories, United States, 2006-2010. MMWR Morb Mortal Wkly Rep. 2011;60(50):133–7. [PubMed] [Google Scholar]
- 30.Chen E. Relentless placoid chorioretinitis. Ophthalmic Surg Lasers Imaging. 2009;40(1):87–8. doi: 10.3928/15428877-20090101-03. [DOI] [PubMed] [Google Scholar]
- 31.Chisholm IH, Gass JD, Hutton WL. The late stage of serpiginous (geographic) choroiditis. Am J Ophthalmol. 1976;82(3):343–51. doi: 10.1016/0002-9394(76)90482-7. [DOI] [PubMed] [Google Scholar]
- 32.Christmas NJ, Oh KT, Oh DM, Folk JC. Long-term follow-up of patients with serpinginous choroiditis. Retina. 2002;22(5):550–6. doi: 10.1097/00006982-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Ciulla TA, Gragoudas ES. Serpiginous choroiditis. Int Ophthalmol Clin. 1996;36(1):135–43. doi: 10.1097/00004397-199603610-00014. [DOI] [PubMed] [Google Scholar]
- 34.Cohen SY, Dubois L, Quentel G, Gaudric A. Is indocyanine green angiography still relevant? Retina. 2011;31(2):209–21. doi: 10.1097/IAE.0b013e31820a69db. [DOI] [PubMed] [Google Scholar]
- 35.Cordero-Coma M, Benito MF, Hernández AM, et al. Serpiginous choroiditis. Ophthalmology. 2008;115(9):1633. doi: 10.1016/j.ophtha.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Dees C, Arnold JJ, Forrester JV, Dick AD. Immunosuppressive treatment of choroidal neovascularisation associated with endogenous posterior uveitis. Arch Ophthalmol. 1998;116(11):1456–61. doi: 10.1001/archopht.116.11.1456. [DOI] [PubMed] [Google Scholar]
- 37.Depla JA, Van Calster J. Serpiginous choroiditis treated in a stepladder approach. Bull Soc Belge Ophtalmol. 2007;306:9–13. [PubMed] [Google Scholar]
- 38.Doctor PP, Bhat P, Sayed R, Foster CS. Intravitreal bevacizumab for uveitic choroidal neovascularization. Ocul Immunol Inflamm. 2009;17(2):118–26. doi: 10.1080/09273940802650406. [DOI] [PubMed] [Google Scholar]
- 39.Donahue HC. Ophthalmologic experience in a tuberculosis sanatorium. Am J Ophthalmol. 1967;64(4):742–8. doi: 10.1016/0002-9394(67)92860-7. [DOI] [PubMed] [Google Scholar]
- 40.Doycheva D, Deuter C, Hetzel J, et al. The use of positron emission tomography/CT in the diagnosis of tuberculosis-associated uveitis. Br J Ophthalmol. 2011;95(9):1290–4. doi: 10.1136/bjo.2010.182659. [DOI] [PubMed] [Google Scholar]
- 41.Doycheva D, Pfannenberg C, Hetzel J, et al. Presumed tuberculosis-induced retinal vasculitis, diagnosed with positron emission tomography (18F-FDG-PET/CT), aspiration biopsy, and culture. Ocul Immunol Inflamm. 2010;18(3):194–9. doi: 10.3109/09273948.2010.483318. [DOI] [PubMed] [Google Scholar]
- 42.Dreyer RF, Gass JD. Multifocal choroiditis and panuveitis. A syndrome that mimics ocular histoplasmosis. Arch Ophthalmol. 1984;94(12):1776–84. doi: 10.1001/archopht.1984.01040031440019. [DOI] [PubMed] [Google Scholar]
- 43.Edelsten C, Stanford MR, Graham EM. Serpiginous choroiditis: an unusual presentation of ocular sarcoidosis. Br J Ophthalmol. 1994;78(1):70–1. doi: 10.1136/bjo.78.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erkkilä H, Laatikainen L, Jokinen E. Immunological studies on serpiginous choroiditis. Graefes Arch Clin Exp Ophthalmol. 1982;219(3):131–4. doi: 10.1007/BF02152297. [DOI] [PubMed] [Google Scholar]
- 45.Fiore PM, Friedman AH. Unusual chorioretinal degeneration associated with sarcoidosis. Am J Ophthalmol. 1988;106(4):490–1. doi: 10.1016/0002-9394(88)90891-4. [DOI] [PubMed] [Google Scholar]
- 46.Franceschetti A. A curious affection of the fundus oculi: helicoid peripapillar chorioretinal degeneration. Its relation to pigmentary paravenous chorioretinal degeneration. Doc Ophthalmol. 1962;16:81–110. doi: 10.1007/BF00146721. [DOI] [PubMed] [Google Scholar]
- 47.Friberg TR. Serpiginous choroiditis with branch vein occlusion and bilateral periphlebitis. Case report. Arch Ophthalmol. 1988;106(5):585–6. doi: 10.1001/archopht.1988.01060130635012. [DOI] [PubMed] [Google Scholar]
- 48.Fuentes-Paez G, Celis-Sanchez J, Torres J, et al. Serpiginous choroiditis in a patient with systemic lupus erythematosus. Lupus. 2005;14(11):928–9. doi: 10.1191/0961203305lu2233xx. [DOI] [PubMed] [Google Scholar]
- 49.Gallagher MJ, Yilmaz T, Cervantes-Castañeda RA, Foster CS. The characteristic features of optical coherence tomography in posterior uveitis. Br J Ophthalmol. 2007;91(12):1680–5. doi: 10.1136/bjo.2007.124099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990 Oct;97(10):1288–97. doi: 10.1016/s0161-6420(90)32418-1. [DOI] [PubMed] [Google Scholar]
- 51.Gass JDM. Stereoscopic Atlas of Macular Diseases: A Funduscopic and Angiographic Presentation. St Louis: CV Mosby Co; 1970. p. 66. [Google Scholar]
- 52.Gass JDM. Stereoscopic Atlas of Macular Diseases: A Funduscopic and Angiographic Presentation. St Louis: CV Mosby Co; 1997. pp. 158–165. [Google Scholar]
- 53.Gerstenblith AT, Thorne JE, Sobrin L, et al. Punctate inner choroidopathy: a survey analysis of 77 persons. Ophthalmology. 2007;114(6):1201–4. doi: 10.1016/j.ophtha.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 54.Giovannini A, Mariotti C, Ripa E, Scassellati-Sforzolini B. Indocyanine green angiographic findings in serpiginous choroidopathy. Br J Ophthalmol. 1996;80(6):476–40. doi: 10.1136/bjo.80.6.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giovannini A, Ripa E, Scassellati-Sforzolini B, et al. Indocyanine green angiography in serpiginous choroidopathy. Eur J Ophthalmol. 1996;6(3):299–306. doi: 10.1177/112067219600600314. [DOI] [PubMed] [Google Scholar]
- 56.Golchet PR, Jampol LM, Wilson D, et al. Persistent placoid maculopathy: a new clinical entity. Ophthalmology. 2007;114(8):1470–40. doi: 10.1016/j.ophtha.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 57.Golchet PR, Jampol LM, Wilson D, et al. Persistent placoid maculopathy: a new clinical entity. Trans Am Ophthalmol Soc. 2006;104:108–20. [PMC free article] [PubMed] [Google Scholar]
- 58.Goldstein DA, Fontanilla FA, Kaul S, et al. Long-term follow-up of patients treated with short-term high-dose chlorambucil for sight-threatening ocular inflammation. Ophthalmology. 2002;109(2):370–7. doi: 10.1016/s0161-6420(01)00942-3. [DOI] [PubMed] [Google Scholar]
- 59.Gregory ME, Bhatt U, Benskin S, Banerjee S. Bilateral full thickness macular holes in association with serpiginous choroiditis. Ocul Immunol Inflamm. 2009;17(5):328–9. doi: 10.3109/09273940903105128. [DOI] [PubMed] [Google Scholar]
- 60.Gupta V, Agarwal A, Gupta A, et al. Clinical characteristics of serpiginous choroidopathy in North India. Am J Ophthalmol. 2002;134(1):47–56. doi: 10.1016/s0002-9394(02)01501-5. [DOI] [PubMed] [Google Scholar]
- 61.Gupta V, Arora S, Gupta A, et al. Management of presumed intraocular tuberculosis: possible role of the polymerase chain reaction. Acta Ophthalmol Scand. 1998;76(6):679–82. doi: 10.1034/j.1600-0420.1998.760609.x. [DOI] [PubMed] [Google Scholar]
- 62.Gupta A, Bansal R, Gupta V, et al. Ocular signs predictive of tubercular uveitis. Am J Ophthalmol. 2010;149(4):562–70. doi: 10.1016/j.ajo.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 63.Gupta A, Gupta V. Tubercular posterior uveitis. Int Ophthalmol Clin. 2005;45(2):71–88. doi: 10.1097/01.iio.0000155934.52589.e3. [DOI] [PubMed] [Google Scholar]
- 64.Gupta V, Bansal R, Gupta A. Continuous progression of tubercular serpiginous-like choroiditis after initiating antituberculosis treatment. Am J Ophthalmol. 2011;152(5):857–63. doi: 10.1016/j.ajo.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Gupta V, Gupta A, Arora S, et al. Presumed tubercular serpiginous like choroiditis: clinical presentations and management. Ophthalmology. 2003;110(9):1744–9. doi: 10.1016/S0161-6420(03)00619-5. [DOI] [PubMed] [Google Scholar]
- 66.Gupta V, Gupta A, Rao NA. Intraocular tuberculosis--an update. Surv Ophthalmol. 2007;52(6):561–87. doi: 10.1016/j.survophthal.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Hamade IH, Tabbara KF. Complications of presumed ocular tuberculosis. Acta Ophthalmol. 2010;88(8):905–9. doi: 10.1111/j.1755-3768.2009.01579.x. [DOI] [PubMed] [Google Scholar]
- 68.Hamilton AM, Bird AC. Geographical choroidopathy. Br J Ophthalmol. 1974;58(9):784–97. doi: 10.1136/bjo.58.9.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hardy RA, Schatz H. Macular geographic helicoid choroidopathy. Arch Ophthalmol. 1987;105(9):1237–42. doi: 10.1001/archopht.1987.01060090095036. [DOI] [PubMed] [Google Scholar]
- 70.Helm CJ, Holland GN. Ocular tuberculosis. Surv Ophthalmol. 1993;38(3):229–56. doi: 10.1016/0039-6257(93)90076-j. [DOI] [PubMed] [Google Scholar]
- 71.Henderly DE, Genstler AJ, Smith RE, Rao NA. Changing patterns of uveitis. Am J Ophthalmol. 1987;103(2):131–6. doi: 10.1016/s0002-9394(14)74217-5. [DOI] [PubMed] [Google Scholar]
- 72.Hooper PL, Kaplan HJ. Triple agent immunosuppression in serpiginous choroiditis. Ophthalmology. 1991;98(6):944–51. doi: 10.1016/s0161-6420(91)32198-5. discussion 951-2. [DOI] [PubMed] [Google Scholar]
- 73.Hooper PL, Secchi AG, Kaplan HJ. Serpiginous choroiditis. In: Pepose JS, Holland GN, Wilhelmus KR, editors. Ocular Infection and Immunity. St Louis: Mosby; 1996. pp. 1262–85. [Google Scholar]
- 74.Hoyng C, Pinckers A, Deutman A. Juvenile atrophy of pigment epithelium and choriocapillaris. Graefes Arch Clin Exp Ophthalmol. 1992;230(3):230–2. doi: 10.1007/BF00176294. [DOI] [PubMed] [Google Scholar]
- 75.Hoyng C, Tilanus M, Deutman A. Atypical central lesions in serpiginous choroiditis treated with oral prednisone. Graefes Arch Clin Exp Ophthalmol. 1998;236(2):154–6. doi: 10.1007/s004170050057. [DOI] [PubMed] [Google Scholar]
- 76.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993;17(6):968–75. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 77.Hutchinson J. Serpiginous choroiditis in scrofulous subjects: choroidal lupus. Arch Surg (Lond) 1900;11:126–35. [Google Scholar]
- 78.Hyvarinen L, Maumenee AE, George T, Weinstein GW. Fluorescein angiography of the choriocapillaris. Am J Ophthalmol. 1969;67(5):647–66. doi: 10.1016/s0002-9394(69)90987-8. [DOI] [PubMed] [Google Scholar]
- 79.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 80.Jampol LM, Orth D, Daily MJ, Rabb MF. Subretinal neovascularization with geographic (serpiginous) choroiditis. Am J Ophthalmol. 1979;88(4):683–9. doi: 10.1016/0002-9394(79)90665-2. [DOI] [PubMed] [Google Scholar]
- 81.Jyotirmay B, Jafferji SS, Sudharshan S, Kalpana B. Clinical profile, treatment, and visual outcome of ampiginous choroiditis. Ocul Immunol Inflamm. 2010;18(1):41–51. doi: 10.3109/09273940903402637. [DOI] [PubMed] [Google Scholar]
- 82.Jones BE, Jampol LM, Yannuzzi LA, et al. Relentless placoid chorioretinitis: A new entity or an unusual variant of serpiginous chorioretinitis? Arch Ophthalmol. 2000;118(7):931–8. [PubMed] [Google Scholar]
- 83.Julián K, Terrada C, Fardeau C, et al. Intravitreal bevacizumab as first local treatment for uveitis-related choroidal neovascularization: long-term results. Acta Ophthalmol. 2011;89(2):179–84. doi: 10.1111/j.1755-3768.2010.02046.x. [DOI] [PubMed] [Google Scholar]
- 84.Jumper JM, McDonald R, Johnson RN, et al. Serpiginous choroiditis. In: Ryan SJ, editor. Retina. 4. 2 chapter 105. St Louis: Elsevier Mosby; 2006. pp. 1811–20. [Google Scholar]
- 85.Kadhiravan T, Deepanjali S. Role of corticosteroids in the treatment of tuberculosis: an evidence-based update. Indian J Chest Dis Allied Sci. 2010;52(3):147–8. [PubMed] [Google Scholar]
- 86.Karacorlu S, Ozdemir H, Karacorlu M. Intravitreal triamcinolone acetonide in serpiginous choroiditis. Jpn J Ophthalmol. 2006;50(3):290–1. doi: 10.1007/s10384-005-0298-5. [DOI] [PubMed] [Google Scholar]
- 87.Karagiannis DA, Sampat FV, Dowler J. Serpiginous choroidopathy with bilateral foveal sparing and good visual acuity after 18 years of disease. Retina. 2007;27(7):989–90. doi: 10.1097/IAE.0b013e3180592b8e. [DOI] [PubMed] [Google Scholar]
- 88.Kedhar SR, Thorne JE, Wittenberg S, et al. Multifocal choroiditis with panuveitis and punctate inner choroidopathy: comparison of clinical characteristics at presentation. Retina. 2007;27(9):1174–9. doi: 10.1097/IAE.0b013e318068de72. [DOI] [PubMed] [Google Scholar]
- 89.Kempen JH, Daniel E, Dunn JP, et al. Overall and cancer related mortality among patients with ocular inflammation treated with immunosuppressive drugs: retrospective cohort study. BMJ. 2009;339:b2480. doi: 10.1136/bmj.b2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khairallah M, Yahia SB, Letaief M, et al. A prospective evaluation of factors associated with chorioretinitis in patients with West Nile virus infection. Ocul Immunol Inflamm. 2007;15(6):435–9. doi: 10.1080/09273940701798488. [DOI] [PubMed] [Google Scholar]
- 91.Khalifa YM, Monahan PM, Acharya NR. Ampiginous choroiditis following quadrivalent human papilloma virus vaccine. Br J Ophthalmol. 2010;94(1):137–9. doi: 10.1136/bjo.2009.159293. [DOI] [PubMed] [Google Scholar]
- 92.King DG, Grizzard WS, Sever RJ, Espinoza L. Serpiginous choroidopathy associated with elevated factor VIII - von Willebrand factor antigen. Retina. 1990;10(2):97–101. doi: 10.1097/00006982-199004000-00001. [DOI] [PubMed] [Google Scholar]
- 93.Kramer M, Axer-Siegel R, Jaouni T, Reich E, et al. Bevacizumab for choroidal neovascularization related to inflammatory diseases. Retina. 2010;30(6):938–44. doi: 10.1097/IAE.0b013e3181c96a00. [DOI] [PubMed] [Google Scholar]
- 94.Laatikainen L, Erkkilä H. A follow-up study on serpiginous choroiditis. Acta Ophthalmol (Copenh) 1981;59(5):707–18. doi: 10.1111/j.1755-3768.1981.tb08737.x. [DOI] [PubMed] [Google Scholar]
- 95.Laatikainen L, Erkkilä H. Serpiginous choroiditis. Br J Ophthalmol. 1974;58(9):777–83. doi: 10.1136/bjo.58.9.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laatikainen L, Erkkilä H. Subretinal and disc neovascularisation in serpiginous choroiditis. Br J Ophthalmol. 1982;66(5):326–31. doi: 10.1136/bjo.66.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Laatikainen L, Tarkkanen A. Failure of cyclosporin A in serpiginous choroiditis. J Ocul Ther Surg. 1984;3(6):280–2. [Google Scholar]
- 98.Lampariello DA. Geographic (serpiginous) choroiditis. J Am Optom Assoc. 1992;63(2):112–6. [PubMed] [Google Scholar]
- 99.Lee DK, Suhler EB, Augustin W, Buggage RR. Serpiginous choroidopathy presenting as choroidal neovascularisation. Br J Ophthalmol. 2003;87(9):1184–5. doi: 10.1136/bjo.87.9.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leznoff A, Shea M, Binkley KE, et al. Cyclosporine in the treatment of nonmicrobial inflammatory ophthalmic disease. Can J Ophthalmol. 1992;27(6):302–6. [PubMed] [Google Scholar]
- 101.Lim JI, Flaxel CJ, LaBree L. Photodynamic therapy for choroidal neovascularisation secondary to inflammatory chorioretinal disease. Ann Acad Med Singapore. 2006;35(3):198–202. [PubMed] [Google Scholar]
- 102.Lim WK, Buggage RR, Nussenblatt RB. Serpiginous choroiditis. Surv Ophthalmol. 2005;50(3):231–44. doi: 10.1016/j.survophthal.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 103.Lyness AL, Bird AC. Recurrences of acute posterior multifocal placoid pigment epitheliopathy. Am J Ophthalmol. 1984;98(2):203–7. doi: 10.1016/0002-9394(87)90355-2. [DOI] [PubMed] [Google Scholar]
- 104.Mackensen F, Becker MD, Wiehler U, et al. QuantiFERON TB-Gold--a new test strengthening long-suspected tuberculous involvement in serpiginous-like choroiditis. Am J Ophthalmol. 2008;141(5):761–6. doi: 10.1016/j.ajo.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 105.Madhavan HN, Priya K, Biswas J. Current perspectives of herpes viral retinitis and choroiditis. Indian J Pathol Microbiol. 2004;47(4):447–68. [PubMed] [Google Scholar]
- 106.Mahendradas P, Kamath G, Mahalakshmi B, Shetty KB. Serpiginous choroiditis-like picture due to ocular toxoplasmosis. Ocul Immunol Inflamm. 2007;15(2):127–30. doi: 10.1080/09273940701244202. [DOI] [PubMed] [Google Scholar]
- 107.Mansour AM, Arevalo JF, Ziemssen F, et al. Long-term visual outcomes of intravitreal bevacizumab in inflammatory ocular neovascularization. Am J Ophthalmol. 2009;148(2):310.e2–316.e2. doi: 10.1016/j.ajo.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 108.Mansour AM, Jampol LM, Packo KH, Hrisomalos NF. Macular serpiginous choroiditis. Retina. 1988;8(2):125–31. doi: 10.1097/00006982-198808020-00008. [DOI] [PubMed] [Google Scholar]
- 109.Mansour AM, Mackensen F, Arevalo JF, et al. Intravitreal bevacizumab in inflammatory ocular neovascularization. Am J Ophthalmol. 2008;141(3):410–6. doi: 10.1016/j.ajo.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 110.Markomichelakis NN, Halkiadakis I, Papaeythymiou-Orchan S, et al. Intravenous pulse methylprednisolone therapy for acute treatment of serpiginous choroiditis. Ocul Immunol Inflamm. 2006;14(1):29–33. doi: 10.1080/09273940500227192. [DOI] [PubMed] [Google Scholar]
- 111.Masi RJ, O’Connor GR, Kimura SJ. Anterior uveitis in geographic or serpiginous choroiditis. Am J Ophthalmol. 1978;86(2):228–32. doi: 10.1016/s0002-9394(14)76817-5. [DOI] [PubMed] [Google Scholar]
- 112.Matsumoto Y, Haen SP, Spaide RF. The white dot syndromes. Compr Ophthalmol Update. 2007;8(4):179–200. discussion 203-4. [PubMed] [Google Scholar]
- 113.Maumenee AE. Clinical entities in “uveitis”. An approach to the study of intraocular inflammation. Am J Ophthalmol. 1970;69(1):1–27. doi: 10.1016/0002-9394(70)91852-0. [DOI] [PubMed] [Google Scholar]
- 114.Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1–25. [PubMed] [Google Scholar]
- 115.McCannel CA, Holland GN, Helm CJ, et al. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol. 1996;121(1):35–41. doi: 10.1016/s0002-9394(14)70532-x. [DOI] [PubMed] [Google Scholar]
- 116.Morimura Y, Okada AA, Kawahara S, et al. Tuberculin skin testing in uveitis patients and treatment of presumed intraocular tuberculosis in Japan. Ophthalmology. 2002;109(5):851–7. doi: 10.1016/s0161-6420(02)00973-9. [DOI] [PubMed] [Google Scholar]
- 117.Morinelli EN, Duger PU, Riffenburgh R, Rao NA. Infectious multifocal choroiditis in patients with acquired immune deficiency syndrome. Ophthalmology. 1993;100(7):1014–21. doi: 10.1016/s0161-6420(93)31543-5. [DOI] [PubMed] [Google Scholar]
- 118.Mulder CJ, Pena AS, Jansen J, Oosterhuis JA. Celiac disease and geographic (serpiginous) choroidopathy with occurrence of thrombocytopenic purpura. Arch Intern Med. 1983;143(4):842. [PubMed] [Google Scholar]
- 119.Navajas EV, Costa RA, Farah ME, et al. Indocyanine green-mediated photothrombosis combined with intravitreal triamcinolone for the treatment of choroidal neovascularization in serpiginous choroiditis. Eye (Lond) 2003;17(5):563–6. doi: 10.1038/sj.eye.6700457. [DOI] [PubMed] [Google Scholar]
- 120.Nayak S, Basu S, Singh MK. Presumed tubercular retinal vasculitis with serpiginous-like choroiditis in the other eye. Ocul Immunol Inflamm. 2011;19(5):361–2. doi: 10.3109/09273948.2011.590917. [DOI] [PubMed] [Google Scholar]
- 121.Nóbrega MJ, Rosa EL, Belfort R, Jr, Farah ME. Anecortave may help choroidal neovascularization regression in serpiginous choroiditis. Can J Ophthalmol. 2007;42(1):151–2. [PubMed] [Google Scholar]
- 122.Nussenblatt RB. Serpiginous choroiditis. In: Nussenlatt RB, Whitcup SM, editors. Uveitis, Fundamentals and Clinical Practice. 4. Edinburgh: Mosby/Elsevier; 2010. pp. 373–382. [Google Scholar]
- 123.Nussenblatt RB, Hooper PL, Kaplan HJ. Triple agent immunosuppression in serpiginous choroiditis. Ophthalmology. 1991;98(6):944–51. doi: 10.1016/s0161-6420(91)32198-5. in discussion of discussion 951-2. [DOI] [PubMed] [Google Scholar]
- 124.Onal S, Kazokoglu H, Koc A, et al. Long-term efficacy and safety of low-dose and dose-escalating interferon alfa-2a therapy in refractory Behçet uveitis. Arch Ophthalmol. 2011;129(3):288–94. doi: 10.1001/archophthalmol.2011.3. [DOI] [PubMed] [Google Scholar]
- 125.Orihara T, Wakabayashi T, Okada AA, et al. A young Japanese man with relentless placoid chorioretinitis. Jpn J Ophthalmol. 2005;49(6):479–42. doi: 10.1007/s10384-005-0241-9. [DOI] [PubMed] [Google Scholar]
- 126.Oruc S, Kaplan AD, Galen M, Kaplan HJ. Uveitis referral pattern in a Midwest University Eye Center. Ocul Immunol Inflamm. 2003;11(4):287–98. doi: 10.1076/ocii.11.4.287.18270. [DOI] [PubMed] [Google Scholar]
- 127.Park SP, Ko DA, Chung H, Yu HG. Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization in serpiginous choroiditis. Ophthalmic Surg Lasers Imaging. 2006;37(5):425–8. doi: 10.3928/15428877-20060901-12. [DOI] [PubMed] [Google Scholar]
- 128.Parodi MB, Iacono P, Kontadakis DS, et al. Bevacizumab vs photodynamic therapy for choroidal neovascularization in multifocal choroiditis. Arch Ophthalmol. 2010;128(9):1100–3. doi: 10.1001/archophthalmol.2010.205. [DOI] [PubMed] [Google Scholar]
- 129.Pathengay A. Intravitreal triamcinolone acetonide in serpiginous choroidopathy. Indian J Ophthalmol. 2005;47(1):77–9. doi: 10.4103/0301-4738.15295. [DOI] [PubMed] [Google Scholar]
- 130.Patorgis CJ. Serpiginous (geographic) choroiditis. J Am Optom Assoc. 1983;54(6):529–32. [PubMed] [Google Scholar]
- 131.Pepper DJ, Marais S, Maartens G, et al. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis. 2009;48(11):e96–107. doi: 10.1086/598988. [DOI] [PubMed] [Google Scholar]
- 132.Pilotto E, Vujosevic S, Grgic VA, et al. Retinal function in patients with serpiginous choroiditis: a microperimetry study. Graefes Arch Clin Exp Ophthalmol. 2010;248(9):1331–7. doi: 10.1007/s00417-010-1405-y. [DOI] [PubMed] [Google Scholar]
- 133.Pisa D, Ramos M, García P, et al. Fungal infection in patients with serpiginous choroiditis or acute zonal occult outer retinopathy. J Clin Microbiol. 2008;41(1):130–5. doi: 10.1128/JCM.02605-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pivetti-Pezzi P, Accorinti M, La Cava M, et al. Endogenous uveitis: an analysis of 1,417 cases. Ophthalmologica. 1996;210(4):234–8. doi: 10.1159/000310715. [DOI] [PubMed] [Google Scholar]
- 135.Priya K, Madhavan HN, Reiser BJ, et al. Association of herpesviruses in the aqueous humor of patients with serpiginous choroiditis: a polymerase chain reaction-based study. Ocul Immunol Inflamm. 2002;10(4):247–61. doi: 10.1076/ocii.10.4.253.15585. [DOI] [PubMed] [Google Scholar]
- 136.Punjabi OS, Rich R, Davis JL, et al. Imaging serpiginous choroidopathy with spectral domain optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2008;39(4 Suppl):S95–8. doi: 10.3928/15428877-20080715-05. [DOI] [PubMed] [Google Scholar]
- 137.Rao NA. Stephan Foster: Curbside consultation in uveitis: 49 clinical questions. 1. Thorofare, NJ: SLACK; When should I be suspicious of tuberculosis and what testing is useful? pp. 59–61. [Google Scholar]
- 138.Rao NA, Saraswathy S, Smith RE. Tuberculous uveitis: distribution of Mycobacterium tuberculosis in the retinal pigment epithelium. Arch Ophthalmol. 2006;124(12):1777–9. doi: 10.1001/archopht.124.12.1777. [DOI] [PubMed] [Google Scholar]
- 139.Rathinam SR, Krishnadas R, Ramakrishnan R, Thulasiraj RD, et al. Population-based prevalence of uveitis in Southern India. Br J Ophthalmol. 2011;95(4):413–7. doi: 10.1136/bjo.2010.182311. [DOI] [PubMed] [Google Scholar]
- 140.Rattray KM, Cole MD, Smith SR. Systemic non-Hodgkin’s lymphoma presenting as a serpiginous choroidopathy: report of a case and review of the literature. Eye. 2000;14:706–10. doi: 10.1038/eye.2000.188. [DOI] [PubMed] [Google Scholar]
- 141.Ryan SJ, Maumenee AE. Acute posterior multifocal placoid pigment epitheliopathy. Am J Ophthalmol. 1972;74(6):1066–74. doi: 10.1016/0002-9394(72)90722-2. [DOI] [PubMed] [Google Scholar]
- 142.Richardson RR, Cooper IS, Smith JL. Serpiginous choroiditis and unilateral extrapyramidal dystonia. Ann Ophthalmol. 1981;13(1):15–9. [PubMed] [Google Scholar]
- 143.Rodman J, Pizzimenti J. Serpiginous choroiditis in a herpes-positive patient. Optom Vis Sci. 2011;88(6):776–80. doi: 10.1097/OPX.0b013e3182139357. [DOI] [PubMed] [Google Scholar]
- 144.Rodriguez A, Calonge M, Pedroza-Seres M, et al. Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol. 1996;114(5):593–9. doi: 10.1001/archopht.1996.01100130585016. [DOI] [PubMed] [Google Scholar]
- 145.Rouvas A, Petrou P, Douvali M, et al. Intravitreal ranibizumab for the treatment of inflammatory choroidal neovascularization. Retina. 2011;31(5):871–9. doi: 10.1097/IAE.0b013e3182003ca8. [DOI] [PubMed] [Google Scholar]
- 146.Sahin OG. Long-term cyclophosphamide treatment in a case with serpiginous choroiditis. Case Report Ophthalmol. 2010;1(2):71–6. doi: 10.1159/000321401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sahu DK, Rawoof A, Sujatha B. Macular serpiginous choroiditis. Indian J Ophthalmol. 2002;50(3):189–96. [PubMed] [Google Scholar]
- 148.Salati C, Pantelis V, Lafaut BA, et al. A 8 months indocyanine angiographic follow-up of a patient with serpiginous choroidopathy. Bull Soc Belge Ophtalmol. 1997;265:29–33. [PubMed] [Google Scholar]
- 149.Schatz M, Maumenee AE, Patz A. Geographic helicoid peripapillary choroidopathy: clinical presentation and fluorescein angiographic findings. Trans Am Acad Ophthalmol Otolaryngol. 1974;78:747–61. [Google Scholar]
- 150.Schlaegel TF. Metastatic nonsuppurative uveitis. In: Schlaegel TF, editor. Essentials of Uveitis. Boston: Little Brown & Co; 1969. pp. 77–107. [Google Scholar]
- 151.Secchi AG, De Rosa C, Pivetti-Pezzi P, et al. Open noncontrolled multicenter long-term trial with ciclosporin in endogenous non-infectious uveitis. Ophthalmologica. 1991;202(4):217–24. doi: 10.1159/000310206. [DOI] [PubMed] [Google Scholar]
- 152.Secchi AG, Tognon MS, Maselli C. Cyclosporine-A in the treatment of serpiginous choroiditis. Int Ophthalmol. 1990;14(5-6):395–9. doi: 10.1007/BF00163565. [DOI] [PubMed] [Google Scholar]
- 153.Seth RK, Gaudio PA. Treatment of serpiginous choroiditis with intravitreous fluocinolone acetonide implant. Ocul Immunol Inflamm. 2008;16(3):103–5. doi: 10.1080/09273940802023778. [DOI] [PubMed] [Google Scholar]
- 154.Seve P, Mennesson E, Grange JD, et al. Infliximab in serpiginous choroiditis. Acta Ophthalmol. 2010;88(8):e342–3. doi: 10.1111/j.1755-3768.2009.01738.x. [DOI] [PubMed] [Google Scholar]
- 155.Shah SP, Jain A, Coffee RE, McCannel TA. Optos Panoramic 200MA ultrawide-field imaging of peripheral RPE adenoma. Semin Ophthalmol. 2009;24(1):37–9. doi: 10.1080/08820530802520210. [DOI] [PubMed] [Google Scholar]
- 156.Shiono T, Abe S, Horiuchi T. A case of miliary tuberculosis with disseminated choroidal haemorrhages. Br J Ophthalmol. 1990;74(5):317–9. doi: 10.1136/bjo.74.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Singh R, Gupta V, Gupta A. Pattern of uveitis in a referral eye clinic in north India. Indian J Ophthalmol. 2004;52(2):121–5. [PubMed] [Google Scholar]
- 158.Smit RL, Baarsma GS, de Vries J. Classification of 750 consecutive uveitis patients in the Rotterdam Eye Hospital. Int Ophthalmol. 1993;17(2):71–6. doi: 10.1007/BF00942778. [DOI] [PubMed] [Google Scholar]
- 159.Sobaci G, Bayraktar Z, Bayer A. Interferon alpha-2a treatment for serpiginous choroiditis. Ocul Immunol Inflamm. 2005;13(1):59–66. doi: 10.1080/09273940490518865. [DOI] [PubMed] [Google Scholar]
- 160.Sobrin L, Kim EC, Christen W, et al. Infliximab therapy for the treatment of refractory ocular inflammatory disease. Arch Ophthalmol. 2007;125:895–900. doi: 10.1001/archopht.125.7.895. [DOI] [PubMed] [Google Scholar]
- 161.Soheilian M, Heidari K, Yazdani S, et al. Patterns of uveitis in a tertiary eye care center in Iran. Ocul Immunol Inflamm. 2004;12(4):297–310. doi: 10.1080/092739490500174. [DOI] [PubMed] [Google Scholar]
- 162.Song MH, Roh YJ. Intravitreal ranibizumab for choroidal neovascularisation in serpiginous choroiditis. Eye (Lond) 2009;23(9):1873–5. doi: 10.1038/eye.2008.346. [DOI] [PubMed] [Google Scholar]
- 163.Sonika, Narang S, Kochhar S, et al. Posterior scleritis mimicking macular serpiginous choroiditis. Indian J Ophthalmol. 2003;51(4):351–3. [PubMed] [Google Scholar]
- 164.Squifflet JP, Sutherland DE, Rynasiewicz JJ, et al. Combined immunosuppressive therapy with cyclosporin A and azathioprine. A synergistic effect in three of four experimental models. Transplantation. 1982;34(6):315–8. doi: 10.1097/00007890-198212000-00001. [DOI] [PubMed] [Google Scholar]
- 165.Squirrell DM, Bhola RM, Talbot JF. Indocyanine green angiographic findings in serpiginous choroidopathy: evidence of a widespread choriocapillaris defect of the peripapillary area and posterior pole. Eye (Lond) 2001;15(Pt 3):336–8. doi: 10.1038/eye.2001.110. [DOI] [PubMed] [Google Scholar]
- 166.Steinmetz RL, Fitzke FW, Bird AC. Treatment of cystoid macular edema with acetazolamide in a patient with serpiginous choroidopathy. Retina. 1991;11(4):412–5. [PubMed] [Google Scholar]
- 167.Tabbara KF. Tuberculosis. Curr Opin Ophthalmol. 2007;18(6):493–501. doi: 10.1097/ICU.0b013e3282f06d2e. [DOI] [PubMed] [Google Scholar]
- 168.Tang J, Fillmore G, Nussenblatt RB. Antiphospholipid antibody syndrome mimicking serpiginous choroidopathy. Ocul Immunol Inflamm. 2009;17(4):278–81. doi: 10.1080/09273940902989340. [DOI] [PubMed] [Google Scholar]
- 169.Thompson MJ, Albert DM. Ocular tuberculosis. Arch Ophthalmol. 2005;123:844–9. doi: 10.1001/archopht.123.6.844. [DOI] [PubMed] [Google Scholar]
- 170.Tostmann A, Boeree MJ, Aarnoutse RE, et al. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23(2):192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 171.Tran TH, Fardeau C, Terrada C, et al. Intravitreal bevacizumab for refractory choroidal neovascularization (CNV) secondary to uveitis. Graefes Arch Clin Exp Ophthalmol. 2008;241(12):1685–92. doi: 10.1007/s00417-008-0906-4. [DOI] [PubMed] [Google Scholar]
- 172.Tsimpida M, Low LC, Posner E, et al. Acute syphilitic posterior placoid chorioretinitis in late latent syphilis. Int J STD AIDS. 2009;20(3):207–8. doi: 10.1258/ijsa.2008.008283. [DOI] [PubMed] [Google Scholar]
- 173.Ugarte M, Wearne IM. Serpiginous choroidopathy: an unusual association with Crohn’s disease. Clin Experiment Ophthalmol. 2002;30(6):437–9. doi: 10.1046/j.1442-9071.2002.00580.x. [DOI] [PubMed] [Google Scholar]
- 174.van Velthoven ME, Ongkosuwito JV, Verbraak FD, et al. Combined en-face optical coherence tomography and confocal ophthalmoscopy findings in active multifocal and serpiginous chorioretinitis. Am J Ophthalmol. 2006;141(5):972–5. doi: 10.1016/j.ajo.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 175.Varma D, Anand S, Reddy AR, et al. Tuberculosis: an under-diagnosed aetiological agent in uveitis with an effective treatment. Eye (Lond) 2006;20(9):1068–73. doi: 10.1038/sj.eye.6702093. [DOI] [PubMed] [Google Scholar]
- 176.Vasconcelos-Santos DV, Rao PK, Davies JB, et al. Clinical features of tuberculous serpiginouslike choroiditis in contrast to classic serpiginous choroiditis. Arch Ophthalmol. 2010;128(7):847–8. doi: 10.1001/archophthalmol.2010.116. [DOI] [PubMed] [Google Scholar]
- 177.Vasconcelos-Santos DV, Zierhut M, Rao NA. Strengths and weaknesses of diagnostic tools for tuberculous uveitis. Ocul Immunol Inflamm. 2009;17(5):351–5. doi: 10.3109/09273940903168688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vianna RN, Ozdal PC, Deschênes J, Burnier MN., Jr Combination of azathioprine and corticosteroids in the treatment of serpiginous choroiditis. Can J Ophthalmol. 2006;41(2):183–9. doi: 10.1139/I06-006. [DOI] [PubMed] [Google Scholar]
- 179.Vonmoos F, Messerli J, Moser HR, et al. Immunosuppressive therapy in serpiginous choroiditis--case report and brief review of the literature. Klin Monbl Augenheilkd. 2001;218(5):394–7. doi: 10.1055/s-2001-15910. [DOI] [PubMed] [Google Scholar]
- 180.Wadhwa N, Garg SP, Mehrotra A. Prospective evaluation of intravitreal triamcinolone acetonide in serpiginous choroiditis. Ophthalmologica. 2010;224(3):183–7. doi: 10.1159/000252981. [DOI] [PubMed] [Google Scholar]
- 181.Wakabayashi T, Morimura Y, Miyamoto Y, Okada AA. Changing patterns of intraocular inflammatory disease in Japan. Ocul Immunol Inflamm. 2003;11(4):277–86. doi: 10.1076/ocii.11.4.277.18260. [DOI] [PubMed] [Google Scholar]
- 182.Weiss H, Annesley WH, Shields JA, et al. The clinical course of serpiginous choroidopathy. Am J Ophthalmol. 1979;87:133–42. doi: 10.1016/0002-9394(79)90131-4. [DOI] [PubMed] [Google Scholar]
- 183.Wenick AS, Odel JG. Serpiginous choroiditis in any other name. Arch Ophthalmol. 2005;123:1616–8. doi: 10.1001/archopht.123.11.1616. [DOI] [PubMed] [Google Scholar]
- 184.Wojno T, Meredith TA. Unusual findings in serpiginous choroiditis. Am J Ophthalmol. 1982;94(5):650–5. doi: 10.1016/0002-9394(82)90010-1. [DOI] [PubMed] [Google Scholar]
- 185.Wu JS, Lewis H, Fine SL, et al. Clinicopathologic findings in a patient with serpiginous choroiditis and treated choroidal neovascularization. Retina. 1989;9(4):292–301. doi: 10.1097/00006982-198909040-00010. [DOI] [PubMed] [Google Scholar]
- 186.Yang P, Zhang Z, Zhou H, et al. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res. 2005;30(11):943–8. doi: 10.1080/02713680500263606. [DOI] [PubMed] [Google Scholar]
- 187.Yeh S, Forooghian F, Wong WT, et al. Fundus autofluorescence imaging of the white dot syndromes. Arch Ophthalmol. 2010;128(1):41–56. doi: 10.1001/archophthalmol.2009.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yeh S, Lew JC, Wong WT, Nussenblatt RB. Relentless placoid chorioretinitis associated with central nervous system lesions treated with mycophenolate mofetil. Arch Ophthalmol. 2009;127(3):341–3. doi: 10.1001/archophthalmol.2009.12. [DOI] [PubMed] [Google Scholar]
- 189.Znaor L, Medic A, Karaman K, Perkovic D. Serpiginous-like choroiditis as sign of intraocular tuberculosis. Med Sci Monit. 2011;17(7):CS88–90. doi: 10.12659/MSM.881839. [DOI] [PMC free article] [PubMed] [Google Scholar]


