Abstract
Atherosclerosis is a chronic inflammatory disease that remains the leading cause of death in the United States. Numerous risk factors for endothelial cell inflammation and the development of atherosclerosis have been identified, including inhalation of ultrafine particles. Recently, engineered nanoparticles (NPs) such as titanium (TiO2) NPs have attracted much attention due to their wide range of applications. However, there are also great concerns surrounding potential adverse health effects in vascular systems. Although TiO2 NPs are known to induce oxidative stress and inflammation, the associated signaling pathways have not been well studied. The focus of this work, therefore, deals with examination of the cellular signaling pathways responsible for TiO2 NP-induced endothelial oxidative stress and inflammation. In this study, primary vascular endothelial cells were treated with TiO2 NPs for 2–16 h at concentrations of 0–50 µg/mL. TiO2 NP exposure increased cellular oxidative stress and DNA binding of NF-κB. Further, phosphorylation of Akt, ERK, JNK and p38 was increased in cells exposed to TiO2 NPs. TiO2 NPs also significantly increased induction of mRNA and protein levels of vascular cell adhesion molecule-1 (VCAM-1) and mRNA levels of monocyte chemoattractant protein-1 (MCP-1). Pretreatment with inhibitors for NF-κB (pyrrolidine dithiocarbamate), oxidative stress (epigallocatechin gallate and apocynin), Akt (LY294002), ERK (PD98059), JNK (SP600125) and p38 (SB203580) significantly attenuated TiO2 NP-induced MCP-1 and VCAM-1 gene expression, as well as activation of NF-κB. These data indicate that TiO2 NPs can induce endothelial inflammatory responses via redox-sensitive cellular signaling pathways.
Keywords: nanoparticle toxicity, titanium dioxide nanoparticles, endothelial cells, adhesion molecules
1. Introduction
Nanotechnology and the production of nano-sized particles have emerged as promising areas of study due to their many applications in industry and medicine (Lam et al., 2006; McIntyre, 2012). Particularly, TiO2 NPs are produced on a large scale and are being employed in a variety of consumer products, such as sunscreens, cosmetics, pharmaceutical additives and food colorants (Nohynek et al., 2007; Skocaj et al., 2011). Although TiO2 is considered to be a safe material, concerns have been raised about the potential adverse health effects in occupational and environmental settings (Elsaesser and Howard, 2012; Linkov et al., 2008). Because of its toxic potential, TiO2 has been classified by the International Agency for Research on Cancer as “possibly carcinogenic to humans” by inhalation (IARC, 2006). Uptake of TiO2 NPs can occur through multiple routes, including inhalation, ingestion and transdermal. Transdermal exposure of TiO2 NPs is linked to the use of sunscreen and cosmetics, although there is no evidence demonstrating that TiO2 can penetrate into normal skin (Schilling et al., 2010). Actually, the major route of human exposure to TiO2 NPs is through its use as a pharmaceutical additive and through food intake, where TiO2 has been widely used as a coloring agent for the food industry. Additionally, studies dealing with oral exposure of TiO2 NPs in mice have demonstrated the presence of particles in distant organs such as the liver, spleen, kidneys and lungs (Wang et al., 2007). These data suggest that TiO2 particles can travel to other tissues and organs following uptake by the gastrointestinal tract, with blood circulation primarily implicated in its biodistribution. A number of studies for TiO2 toxicity in animals have focused on inhalation although potential inhalation exposure to TiO2 NPs occurs mostly in the workplace (Hext et al., 2005; Skocaj et al., 2011). Because of the physicochemical similarities between ambient, nanoscale particles and NPs, there is a strong rationale linking exposure to TiO2 NPs with adverse cardiovascular effects. Past studies have demonstrated that inhaled ambient ultrafine particles can reach deep into the lungs where they can enter the circulatory system, resulting in cardiovascular diseases (Kreyling et al., 2002). Also, multiple studies reporting epidemiological animal data have established a link between ambient particles and the etiology of cardiovascular disease (Floyd et al., 2009; Terzano et al., 2010). Other exposure routes include surgical implant-derived wear debris and intravenously administered contrast agents (Chandran et al., 2011; Umbreit et al., 2012). Although intravenous exposure cases are rare, there is potential concern because a direct injection of TiO2-containing contrast agents into the circulatory system may confer a greater impact on the vascular endothelium due to near-100% bioavailability.
Vascular endothelial cells are potential targets for TiO2 NP toxicity in human exposure. There are several cellular events responsible for the initiation of atherosclerosis in the vascular endothelium, including oxidative stress, inflammation and activation of endothelial cells (Businaro et al., 2012). Previous studies have evaluated endothelial activation and dysfunction in endothelial cells but the intracellular signaling pathways are not fully identified (Montiel-Davalos et al., 2012). The purpose of this study is to determine the intracellular signaling pathways by which TiO2 NPs induce inflammatory responses in vascular endothelial cells. Our data demonstrate that TiO2 NPs increase vascular adhesion molecules such as monocyte chemoattractant protein-1 (MCP-1) and vascular cell adhesion molecule-1 (VCAM-1), and that this is mediated by multiple intracellular signaling pathways including mitogen-activated protein kinases (MAPKs) and phosphatidylinositol-3 kinase (PI3K)/Akt.
2. Materials and Methods
2.1. Materials
Commercial grade uncoated TiO2 NPs (anatase, metal basis, stock# 44689, 99.9% purity, 5 nm) were purchased from Alfa Aesar (Ward Hill, MA). DMSO, apocynin, PD98059, SB203580, SP600125 and LY294002 were purchased from Sigma-Aldrich (St. Louis, MO). The NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) was obtained from Calbiochem (Darmstadt, Germany). Epigallocatechin gallate (EGCG) was purchased from Cayman Chemical (Ann Arbor, MI). VCAM-1, IκBα and p-IκBα antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for extracellular signal-regulated kinase-1/2 (ERK1/2), p42/44, p38 MAPK, c-Jun-N-terminal kinase (JNK) and Akt were obtained from Cell Signaling Technology (Danvers, MA). LC3 antibody was purchased from Novus Biologicals (Littleton, CO).
2.2. Nanoparticle characterization
TiO2 nanoparticle size, surface area and purity were evaluated in comparison to vendor specifications. Elemental analysis of dry TiO2 samples was performed using a Hitachi S-4300 scanning electron microscope (Dallas, TX) and Princeton Gamma-Tech energy dispersive X-ray spectroscopy (EDS) Microanalysis System (Princeton, NJ), primary and aggregate particle sizes were determined using scanning electron microscopy (SEM), and surface area was determined through the Brunauer-Emmett-Teller (BET) method using a Micromeritics TriStar 3000 surface area and pore size analyzer (Norcross, GA), following overnight nitrogen degassing at 120 °C. Characterization results can be found in Table 1 as compared to vendor-supplied values. Suspensions of TiO2 NPs in cell culture media were prepared at a concentration of 5 mg/mL. Nanoparticle dispersion and size characteristics were analyzed by dynamic light scattering (DLS) using a Zetasizer Nano ZS90 particle size analyzer (Malvern Instruments, Westborough, MA), which measures hydrodynamic diameters of nanoparticles (Narband et al., 2009). TiO2 NP suspensions (n=3) were suspended in deionized H2O, sonicated briefly using a probe sonicator (Heat Systems, Inc., Farmingdale, NY) for 15 min to minimize particle aggregates, added to cell culture medium and analyzed via DLS with five measurements per sample. The same dispersion procedure was also used prior to in vitro studies. Sizing data, including mean nanoparticle size (nm) and particle size ranges, was determined using Malvern DTS Software, v. 6.32 (Table 1).
Table 1.
TiO2 Nanoparticle Physicochemical Properties
| Vendor | Vendor Crystal Structure |
Vendor Size (nm) |
Vendor Purity |
Vendor Surface Area (m2/g) |
Analyzed SEM Avg Size and (Size Range) (nm) |
Analyzed DLS Avg Aggregate Size and (Size Range) (nm) |
Analyzed Purity (EDS) |
Analyzed Surface Area (BET) (m2/g) |
|---|---|---|---|---|---|---|---|---|
| Alfa Aesar Stock # 44689 Lot # C22S013 | Anatase | 5 | 99% | 200–220 | 79 (7–232) | 349 (250–396) | 99.7% | 93.9 |
2.3. Primary cell culture and endothelial cell treatments
Primary vascular endothelial cells were isolated from porcine pulmonary arteries and characterized as described previously (Han et al., 2010; Hennig et al., 1984). Cells were cultured in M199 media (Gibco, Grant Island, NY) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad). Cells were grown to confluence and serum starved overnight in medium containing 1% FBS prior to initiation of cell treatments. A stock suspension of 5 mg/mL TiO2 NPs was prepared and dispersed by probe sonication for 15 min. Based on our preliminary studies, we chose to treat cells with TiO2 NPs at 10 and 50 µg/mL, which corresponded to 2 and 10 µg nanoparticles/cm2, respectively. Of particular relevance to the present study, TiO2 NPs have been suggested for use in intravenous applications as contrast agents (Chandran et al., 2011; Umbreit et al., 2012). Due to near-100% bioavailability, potential intravenous applications could allow nanoparticles to achieve significantly higher concentrations in the blood circulation than that from translocation of nanoparticles following occupational and environmental exposure. These nanoparticle concentrations (10 and 50 µg/mL) were selected not only to address potential intravenous and environmental exposure levels but also to correspond with previous studies showing increased expression of inflammatory genes without cell death (Montiel-Davalos et al., 2012; Sanders et al., 2012). Equal volumes of water (up to 1% of media; no hypotonic conditions were produced as shown by autophagy analysis in Fig. 6) were used in place of NP-suspension volumes to serve as controls in cell culture. The TiO2 NP concentrations and treatment intervals employed in these studies did not lead to significant cytotoxicity, as seen by trypan blue exclusion staining (data not shown).
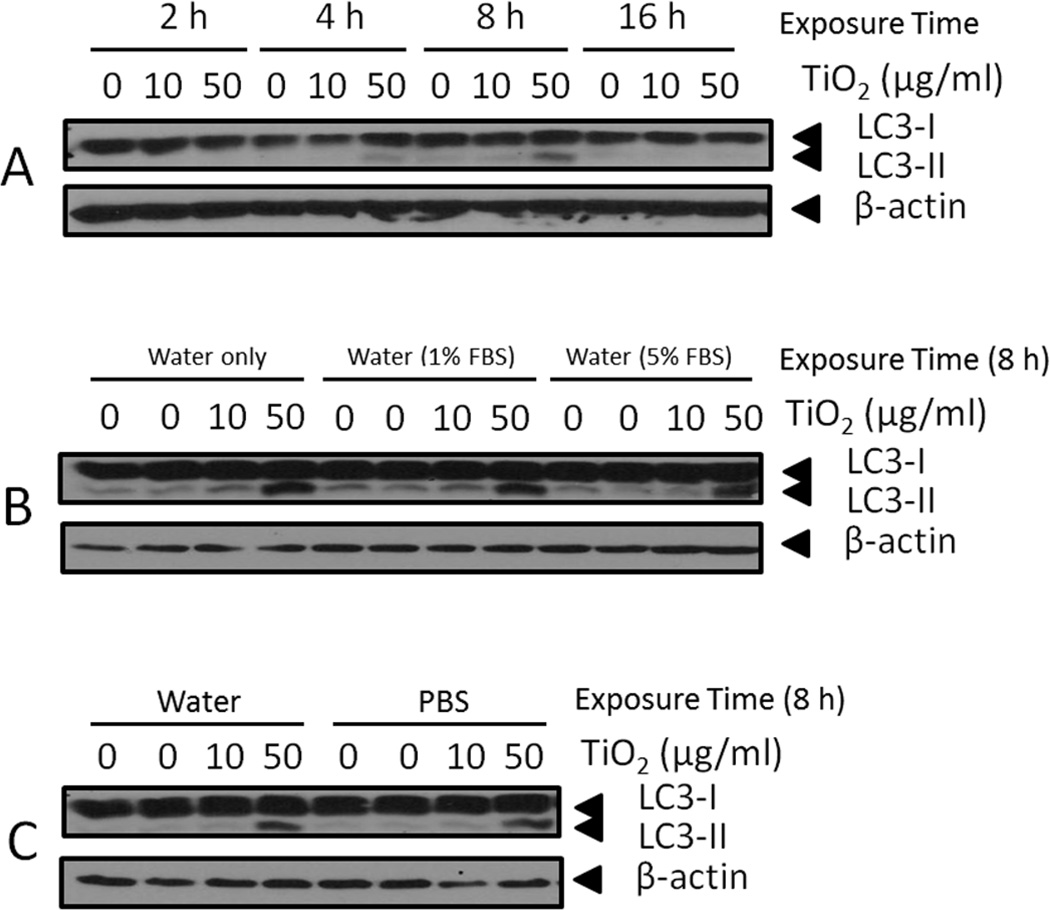
Fig. 6.
Expression of LC3-I/II, an autophagy marker, by endothelial cells. (A) Endothelial cells were treated with 0–50 µg/mL TiO2 NPs at different time points (2–16 h). (B) Endothelial cells were treated with 0–50 µg/mL TiO2 NPs for 8 h using three different NP suspension media: water only, water with 1% FBS and water with 5% FBS. (C) Endothelial cells were treated with 0–50 µg/mL TiO2 NPs for 8 h using two different NP suspension media: water and PBS. Western blots shown here are representative images of three independent blots.
2.4. Assessment of superoxide (O2•−) levels
Endothelial cells were grown to confluence in 8-chamber culture slides (BD Biosciences, Bedford, MA). Following treatment, cells were incubated with a final concentration of 5 µM dihydroethidium (DHE), MitoSOX™ Red mitochondrial superoxide indicator (MitoSOX) or DMSO (blank) in a 5% CO2 incubator for 15 min. Cells were washed 3× with PBS, fixed with 4% formaldehyde, and washed again 3× with PBS. Slides were mounted with ProLong Gold Antifade reagent containing 4’6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA) to visualize the nuclei. Slides were evaluated under a Nikon ECLIPSE TE2000-U fluorescence microscope and the images were captured digitally using a Nikon LH-M100CB-1 camera and NIS-Elements BR 4.00.08 software (Nikon Instruments Inc. Melville, NY).
2.5. Electrophoretic mobility shift assay (EMSA)
Nuclear extracts of endothelial cells were prepared using NE-PER nuclear extraction reagents (Thermo, Rockford, IL). The concentration of nuclear extract was determined using Bradford reagent (Bio-Rad, Richmond, CA). DNA binding activities of NF-κB were determined using a LightShift Chemiluminescent EMSA kit (Pierce, Rockford, IL) according to the manufacturer’s protocol. DNA-binding reactions were performed with a final volume of 20 µL buffer containing 5 µg of nuclear extract, 50 ng/µL Poly (dI·dC) and biotin end-labeled oligonucleotides. Synthetic 5’-biotinylated complementary oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). The oligonucleotides containing the NF-κB consensus sequence (5’-AGTTGAGGGGACTTTCCCAGGC-3’) were described previously (Lim et al., 2007). EMSA gels were quantified by Image J software (NIH, Bethesda, MD).
2.6. Quantitative real-time PCR
Cells were grown in 6-well plates, and total RNA was extracted from the cells using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Reverse transcription was performed using the AMV reverse transcription system (Promega, Madison, WI). The levels of mRNA expression were then assessed by quantitative real-time PCR using a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) and SYBR Green master mix (Applied Biosystems). Data analysis was performed using the relative quantification method (ΔΔCt), in which relative mRNA expression for target mRNAs (i.e., VCAM-1 and MCP-1) was compared to a constitutively expressed gene (i.e., β-actin) in the mRNA samples from untreated and treated cells. Primer sequences for SYBR Green chemistry were designed using the Primer Express Software 3.0 for real-time PCR (Applied Biosystems) and synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). Sequences for porcine VCAM-1, MCP-1 and β-actin were described in previous articles published by our laboratory (Han et al., 2012).
2.7. Western blot analyses
Protein expression was determined using Western blot. Whole cells were lysed in RIPA lysis buffer (Cell Signaling Technology, Danvers, MA) containing protease and phosphatase inhibitor cocktails (Thermo, Waltham, MA). Lysed cells were centrifuged at 12,000 × g for 10 min at 4 °C. Protein levels of the supernatants were determined via BCA assay (Pierce, Rockford, IL). Protein samples (30 µg per treatment) were separated using 10% SDS-PAGE and subsequently were transferred onto nitrocellulose membranes. Membranes were blocked with 5% non-fat milk buffer and incubated overnight at 4 °C with primary antibodies. After washing, membranes were incubated with secondary antibodies conjugated with horseradish peroxidase and visualized using ECL detection reagents (Thermo, Waltham, MA).
2.8. Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). Statistical significance was determined by one-way or two-way ANOVA followed by Student-Newman-Keuls method using Sigma Stat 3.1 software (Systat Software, San Jose, CA). A probability value p < 0.05 was considered statistically significant.
3. Results
3.1. Characterization of TiO2 nanoparticles
Physicochemical characteristics of TiO2 NPs are described in Table 1. TiO2 size analysis using SEM showed average NP and aggregate diameters of 79 nm, ranging from 7 to 232 nm. BET analysis of TiO2 NPs showed a specific surface area of 93.9 m2/g. DLS particle size analysis in cell culture media showed particle aggregates 349 nm in diameter on average, with a range of 250 nm to 396 nm. EDS elemental analysis showed 99.7% TiO2 NP purity, in keeping with manufacturer product information.
3.2. TiO2 NPs increase superoxide production
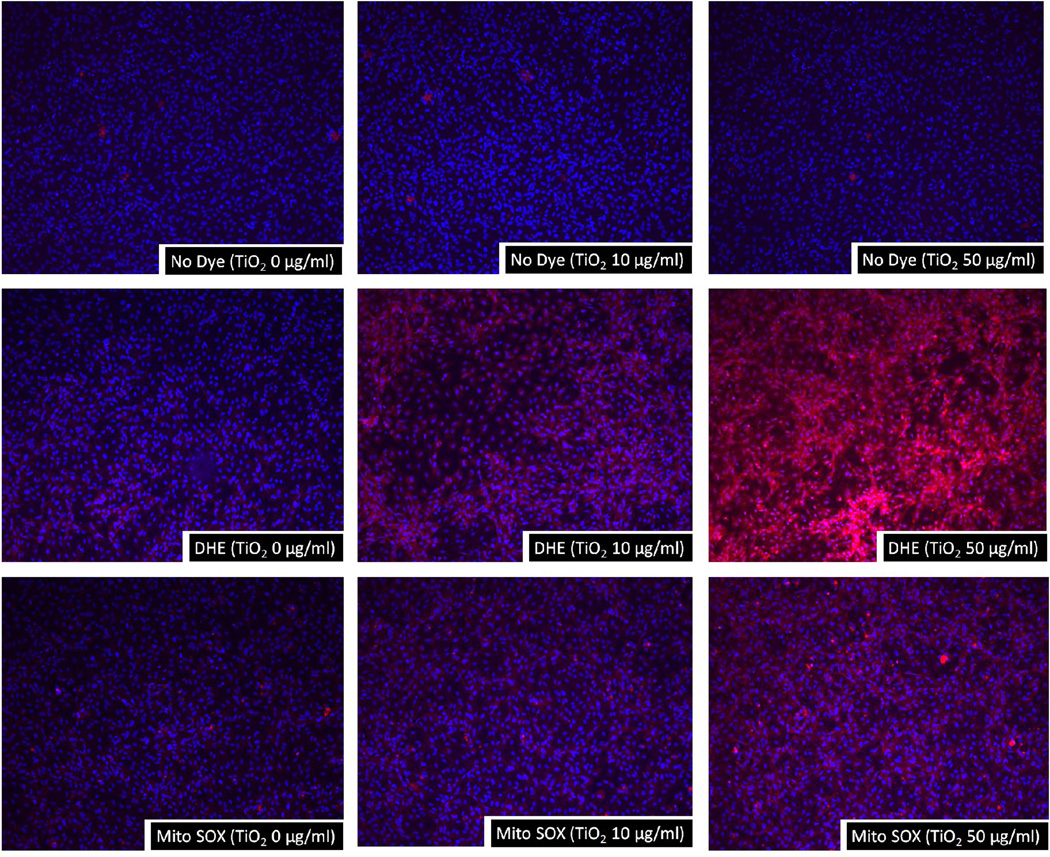
Oxidative stress is a critical event of endothelial inflammation, activation and dysfunction. The flourescent dye DHE is sensitive to reactive oxygen species and, in particular, to the superoxide anion. Once this dye is oxidized by superoxide, it stains cells a bright flourescent red. Our results showed that TiO2 NPs at concentrations of 10–50 µg/mL significantly increased superoxide generation at 1 h post exposure (Fig. 1). In addition, TiO2 NP-induced superoxide production was confirmed by MitoSOX Red fluorescent dye which selectively detects mitochondrial superoxide production. Like DHE staining, 10–50 µg/mL of TiO2 NP exposure markedly increased mitochondrial superoxide production (Fig. 1). Superoxide production was not detected in cells treated with TiO2 NPs (10–50 µg/mL) alone (Fig. 1). No detectable fluorescence was seen in controls without cells but with added TiO2 NPs and fluorescent dye (data not shown).
Fig. 1.
TiO2 NPs increase superoxide production in endothelial cells. Endothelial cells grown on 8-chamber culture slides were treated with TiO2 NPs for 2 h. Cells were then stained with either DHE or MitoSOX, and the intensity of red fluorescence was assessed using a fluorescence microscope. The red areas in the cells represent oxidized DHE and MitoSOX representing the generation of superoxide. The nuclei were stained with DAPI. The images shown here are representatives of three independent experiments.
3.3. Akt and three MAPK pathways are activated by TiO2 NPs in endothelial cells
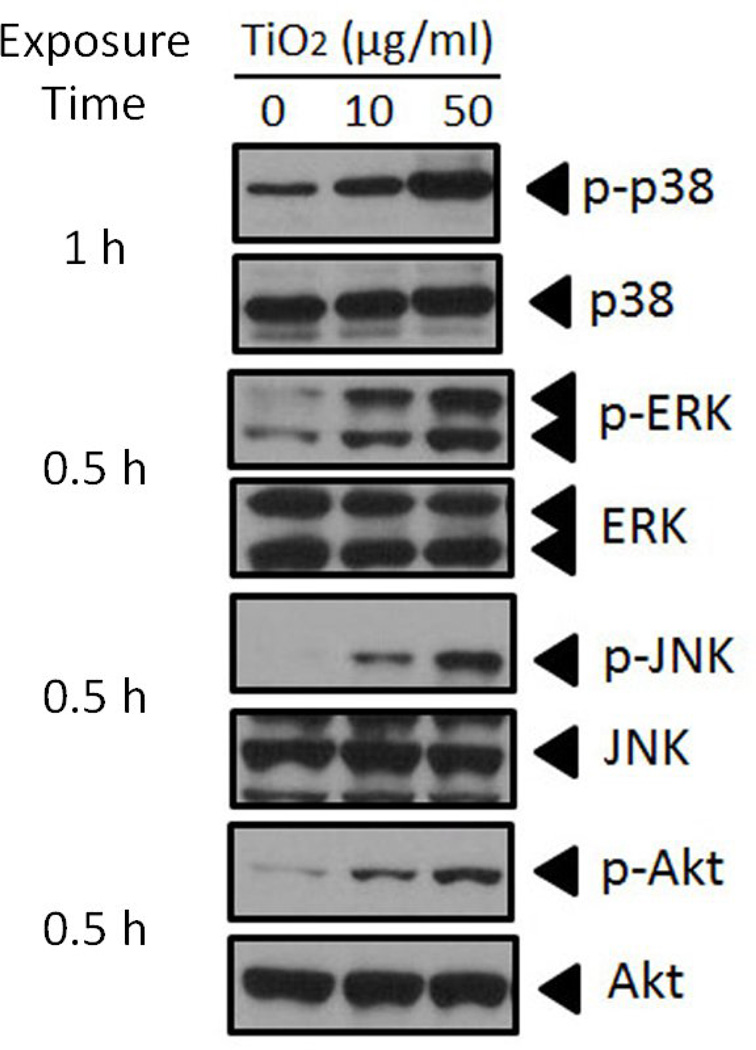
Oxidative stressors are known to activate many intracellular signaling pathways. In order to identity intracellular signaling pathways associated with oxidative stress and subsequent gene expression, we observed the phosphorylation of some key signaling molecules. The PI3K/Akt and MAPK signaling pathways are involved in a wide range of cellular processes, including NF-κB activation, which subsequently can induce cell inflammation in response to extracellular stimuli (Chae et al., 2007; Ozes et al., 1999). We examined potential involvement of the MAPK signaling pathways (i.e., p38, ERK and JNK) in endothelial cells after treatment with various concentrations of TiO2 NPs. Phosphorylation of endogenous p38, ERK and JNK was determined by Western blot analysis. Treatment with both 10 and 50 µg/mL TiO2 NPs markedly increased all three MAPK pathways (Fig. 2). Similar to activation of MAPKs, Akt was phosphorylated at Ser473 by TiO2 NPs (Fig. 2). Phosphorylation of Akt, JNK and ERK peaked as early as 30 min post-TiO2 NP treatment. Phosphorylation of p38 reached a maximum at 1 h post-TiO2 NP exposure.
Fig. 2.
TiO2 NP-induced phosphorylation of MAPKs and PI3K/Akt pathways in endothelial cells. Cells were treated with TiO2 NPs to observe the activation of three types of MAPK signaling pathways, p38, ERK and JNK, as well as the Akt signaling pathway. The images shown here are phosphorylated signaling molecules at the time of maximum induction. The Western blots shown here are representative images of three independent experiments.
3.4. TiO2 NPs activate NF-κB
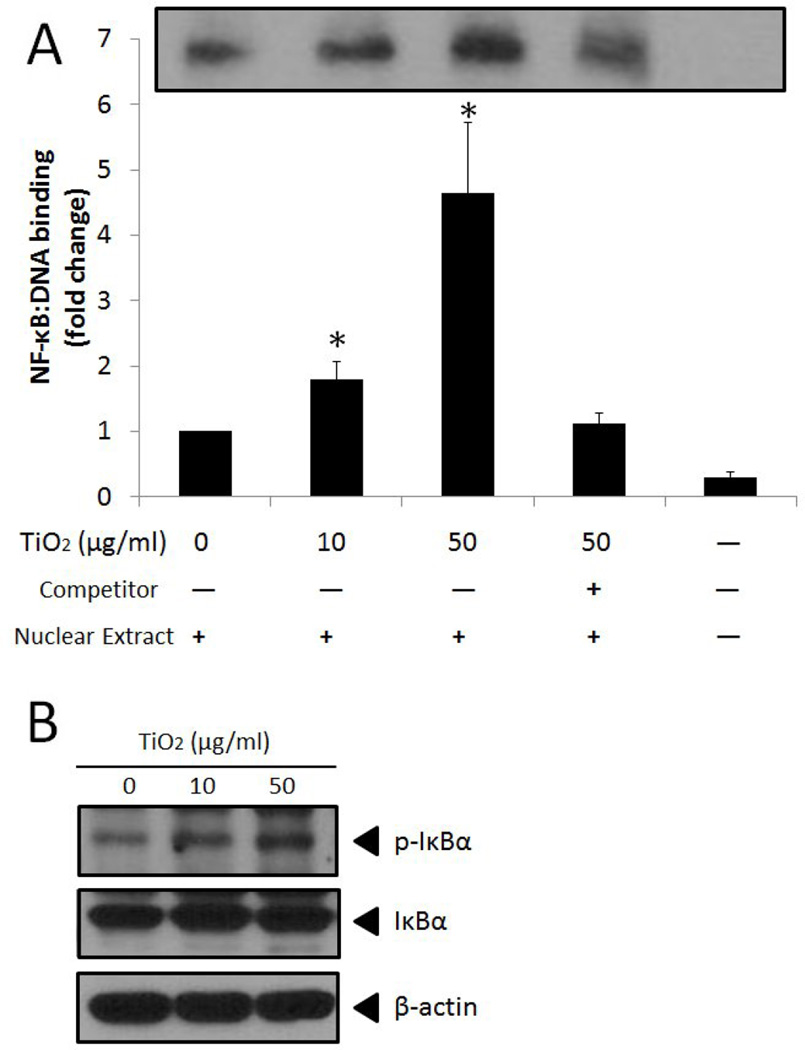
Activation of three MAPKs and PI3K/Akt can lead to activation of the redox-sensitive transcription factor NF-κB that subsequently upregulates genes including VCAM-1 and MCP-1. Our EMSA results showed that TiO2 NPs significantly increased NF-κB DNA binding activity in vascular endothelial cells (Fig. 3A). Competition assays were conducted to ensure the specificity of NF-κB DNA binding (Fig. 3A). Activation of NF-κB was confirmed by phosphorylation of IκBα (Fig. 3B).
Fig. 3.
TiO2 NPs increase activation of NF-κB. (A) NF-κB DNA binding determined by EMSA analysis. (B) Phosphorylation of IκBα analyzed in whole cell lysate by Western blot. Cells were treated with TiO2 NPs (0–50 µg/mL) for 2 h followed by preparation of nuclear extracts for EMSA or whole cell lysates for Western blot. In EMSA, unlabeled competitor sequences were present at a 200-fold molar excess over labeled sequences. Data represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. The EMSA and Western blot shown here is a representative image. *Significantly different compared to control (p < 0.05).
3.5. TiO2 nanoparticles increase gene and protein expression of MCP-1 and VCAM-1
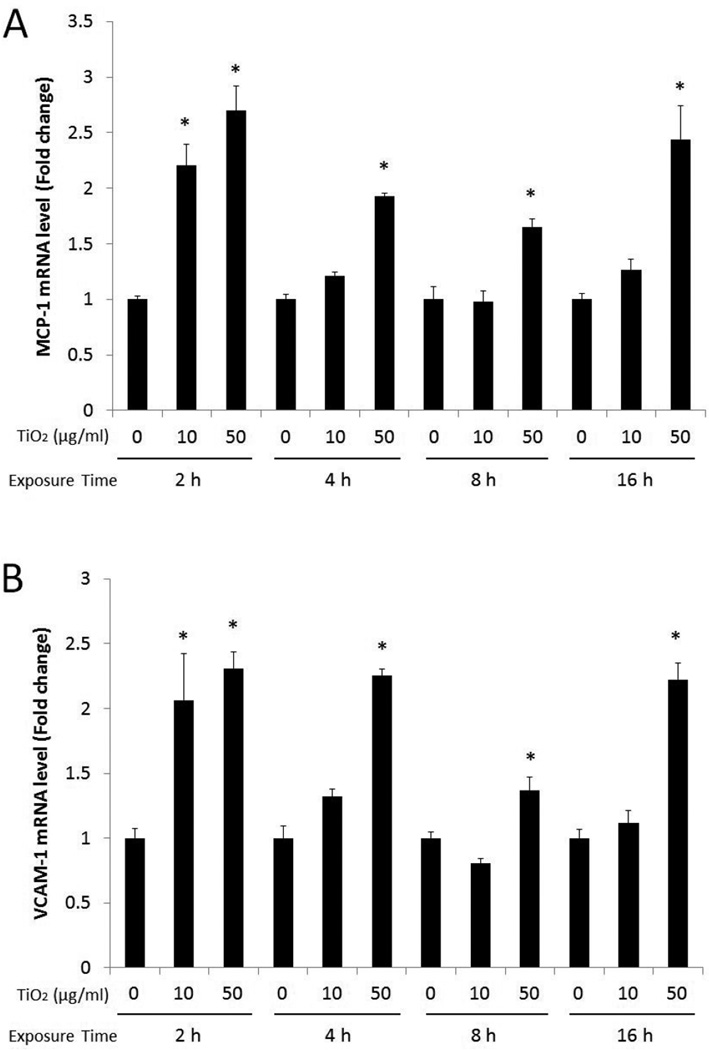
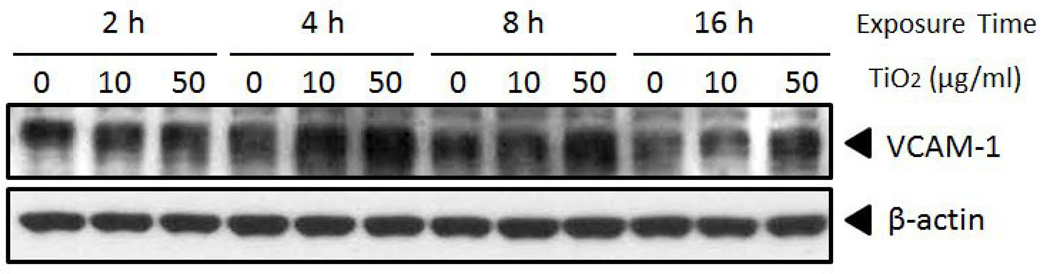
Vascular endothelial cells were treated with TiO2 NPs at concentrations of 0, 10, and 50 µg/mL for 2, 4, 8 and 16 h to determine TiO2 NP-induced expression of inflammatory molecules such as MCP-1 and VCAM-1. TiO2 NPs significantly induced expression of MCP-1 mRNA for all exposure times at a concentration of 50 µg/mL, compared to control (Fig. 4A). In contrast, MCP-1 mRNA was significantly increased only after a 2 h exposure at a concentration of 10 µg/mL TiO2 NPs. Similarly, VCAM-1 mRNA expression was significantly upregulated after cells were treated with TiO2 NPs (50 µg/mL) for all exposure time points, while TiO2 NPs (at 10 µg/mL) significantly increased VCAM-1 mRNA only at 2 h (Fig. 4B). Further, Western blot analysis showed that VCAM-1 protein was markedly increased when cells were treated with TiO2 NPs (10 and 50 µg/mL) for 4 h (Fig. 5). TiO2 NPs at 50 µg/mL maintained significantly higher VCAM-1 protein level up to 16 h. To ensure that the observed inflammatory responses were derived from the NPs and not from surface impurities, PBS-washed TiO2 NPs (using centrifugation, 3000 × g, for 5 min, 3 times) were compared with unwashed TiO2 NPs for VCAM-1 and MCP-1 gene expression. The results showed similar proinflammatory effects by PBS-washed NPs compared to unwashed TiO2 NPs (data not shown).
Fig. 4.
TiO2 NPs increase expression of MCP-1 (A) and VCAM-1 (B) gene. Gene expression levels were measured using quantitative real-time PCR. Primary endothelial cells were treated with 0–50 µg/mL of TiO2 NPs for the indicated times (2–16 h). Results represent the mean ± SEM, with n=3. Experiments were repeated a minimum of three times. *Significantly different compared to control (p < 0.05).
Fig. 5.
TiO2 NPs increase VCAM-1 protein expression in primary endothelial cells. Primary endothelial cells were treated with 0–50 µg/mL of TiO2 NPs for different time points (2–16 h). Western blots show a dose-dependent increase of VCAM-1 by TiO2 NPs. Western blots shown here are representative images of three independent blots.
3.6. TiO2 nanoparticles increase autophagy in endothelial cells
Protein expression of LC3-I and LC3-II was analyzed in order to determine if TiO2 NPs and/or suspension medium increase autophagy levels in porcine endothelial cells. Our data showed that the addition of water (up to 1%) as the nanoparticle suspension medium did not lead to increased endothelial cell autophagy (Fig. 6A). Autophagy was markedly increased, though, when cells were treated with TiO2 NPs (50 µg/mL), where a maximum expression of LC3-II was observed after an 8 h exposure (Fig. 6A). Due to the large surface area associated with NPs, nutrients can adsorb onto NPs in high quantities, which can lead to nutritional deprivation and ultimately to increased autophagy. To address this, we tested if different concentrations of FBS (0, 1 and 5%) in TiO2 suspension media can induce varying levels of autophagy in our cell culture model. A similar level of autophagy was observed only in cells treated with 50 µg/mL TiO2 NPs while other treatment groups did not display autophagy, suggesting nutritional deprivation was not great enough to induce autophagy in our cell culture model (Fig. 6B). Comparison of water and PBS as NP-suspension media revealed no significant difference in the induction of autophagy (Fig. 6C).
3.7. Pharmacological inhibition of NF-κB, oxidative stress, PI3K/Akt and MAPK pathways result in the decreased expression of MCP-1 and VCAM-1
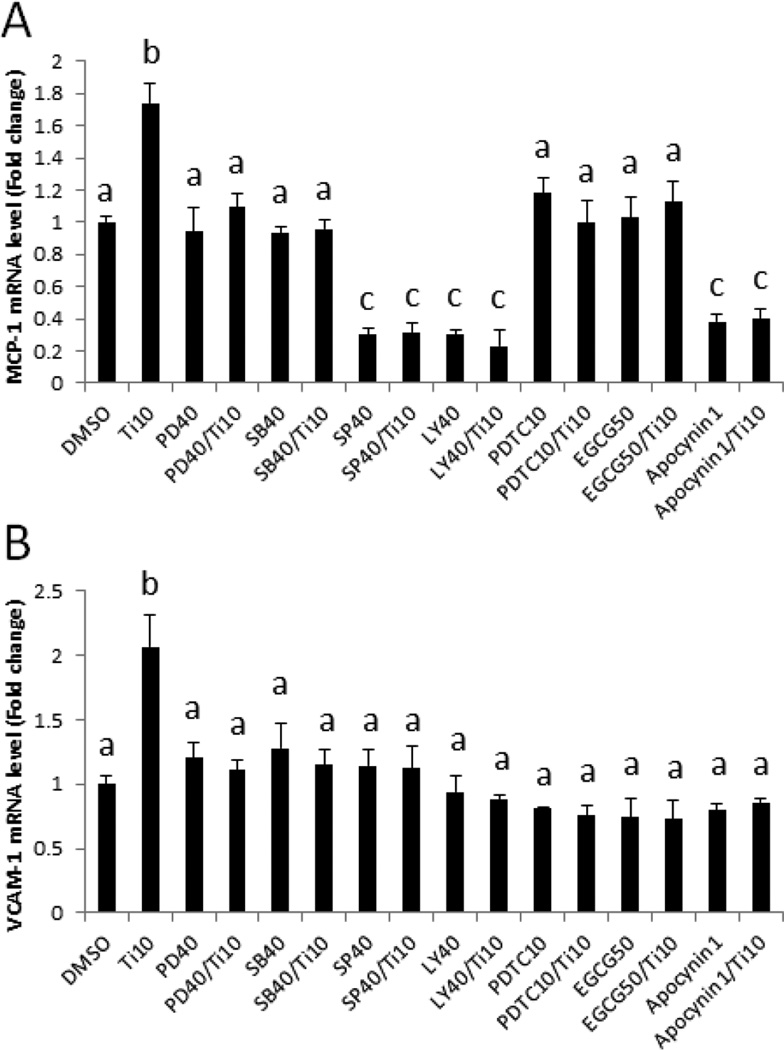
In an effort to further identify signaling pathways associated with TiO2 NP-induced endothelial inflammatory responses, we determined expression of adhesion molecules i.e., MCP-1 and VCAM-1 in cells pretreated with pharmacological inhibitors for three MAPKs, Akt, NF-κB and oxidative stress. Pharmacological inhibitors such as PD98059, SB203580, SP600125 and LY294002 block phosphorylation of ERK, p38, JNK and Akt, respectively. Pretreatment of cells with these inhibitors significantly attenuated TiO2 (10 µg/mL)-induced MCP-1 and VCAM-1 gene expression (Fig. 7A and B), suggesting that all of these signaling molecules can mediate TiO2-induced inflammatory pathways in vascular endothelial cells. Pretreatment of cells with the NF-κB inhibitor PDTC for 30 min followed by TiO2 treatment for 2 h resulted in a significant attenuation of MCP-1 and VCAM-1 gene expression, compare to TiO2 NPs alone (Fig. 7A and B), confirming that NF-κB signaling is a critical mediator of these proinflammatory responses. Similarly, the antioxidant polyphenol EGCG and apocynin significantly down-regulated TiO2 NP-induced expression of these genes (Fig. 7A and B). Treatment with SP600125 (40 µM), LY294002 (40 µM) and apocynin (1 mM) significantly decreased base line MCP-1 mRNA expression but not in VCAM-1 expression. Overall, these data demonstrate that TiO2 NP-induced inflammatory gene expression is mediated by oxidative stress and increased DNA binding of NF-κB.
Fig. 7.
Pharmacological inhibition of NF-κB, oxidative stress, PI3K/Akt and MAPK pathways result in the decreased expression of MCP-1 and VCAM-1. Expression of MCP-1 (A) and VCAM-1 (B) mRNA in endothelial cells. Cells were exposed to TiO2 NPs (10 µg/mL) for 2 h after a 30 min pretreatment with pharmacological inhibitors, i.e., PD98059 (40 µM, ERK inhibitor), SB203580 (40 µM, p38 inhibitor), SP600125 (40 µM, JNK inhibitor) and LY294002 (40 µM, Akt inhibitor). Also, cells were pretreated with the NF-κB inhibitor (PDTC, 10 µM, for 1 h) and antioxidants (EGCG, 50 µM for 3 h and apocynin, 1 mM for 1 h) followed by TiO2 NP (10 µg/mL) exposure for 2 h. mRNA expression levels were measured using quantitative real-time PCR. Results represent the mean ± SEM, with n=3. Different letters correspond to significant differences (p < 0.05) between treatment groups.
4. Discussion
Engineered nanoparticles have been suggested to increase the risk and incidence of cardiovascular diseases, including atherosclerosis (Oberdorster et al., 2005; Shannahan et al., 2012). Occupational and environmental exposure of TiO2 NPs may be a risk factor for increased cardiovascular inflammatory responses in humans. Aerosolized sunscreens may lead to higher inhalation absorption and subsequent translocation of TiO2 NPs into the circulatory system. Also, intravenous exposure of TiO2 NPs for biomedical uses is of special concern due to their higher bioavailability in the human vasculature.
As seen in the current studies and also observed by others, anatase TiO2 NPs tended to agglomerate to sizes ranging from 250 nm to 396 nm in diameter in cell culture media containing 1% fetal bovine serum. In cultured endothelial cells, TiO2 NPs and/or TiO2 NP agglomerates were internalized by cells within one hour, and visualized using a relatively simple fluorescent microscopy method using ARS that labels TiO2 NPs (Thurn et al., 2009). A recent study also highlighted the cellular uptake of TiO2 NPs by cells where nanoparticles were massively internalized into cytoplasmic vacuoles in endothelial cells (Brun et al., 2012); these findings were confirmed using transmission electron microscopy. These previous reports suggest that endothelial cells can internalize both agglomerated and non-agglomerated nanoparticles, although the underlying mechanism associated with nanoparticle uptake is not fully identified.
In the present study, we tested expression of adhesion molecules, particularly VCAM-1 in primary vascular endothelial cells, when treated with anatase TiO2 NPs. The role of endothelial inflammation and expression of adhesion molecules such as MCP-1 and VCAM-1 in early atherosclerotic lesion formation is critical, and studies have found that by blocking VCAM-1 expression, one can reduce monocyte adhesion by 75% (Huo et al., 2000). Results from the current study demonstrated that MCP-1 and VCAM-1 gene expression was significantly higher in cells exposed to TiO2 NPs. Both 10 and 50 µg/mL of TiO2 NPs exhibited the highest gene expression levels after a 2 h exposure. Both MCP-1 and VCAM-1 gene expression were diminished for longer times points when cells were treated with 10 µg/mL of TiO2 NPs, while 50 µg/mL of TiO2 NPs sustained the elevated gene expression up to 16 h. Many mechanisms may be responsible for the decrease of gene expression during the extended exposure time. A recent study demonstrated that TiO2 NPs elicit oxidative damage that can lead to the activation of nuclear factor erythroid 2 related factor 2 (Nrf2) as a protective mechanism (Sun et al., 2012). Nrf2-regulated genes include many antioxidant proteins such as NAD(P)H:quinone oxidoreductase 1 (NQO1) and glutathione S transferase (GST). Enhanced expression of these antioxidant proteins resulted in the reduction of VCAM-1 and MCP-1 gene expression in endothelial cells (Han et al., 2012). In the present study, the cellular defensive system is sufficient to reduce TiO2 NP-induced inflammatory gene expression at a concentration of 10 µg/mL. In contrast, 50 µg/mL of TiO2 NPs was able to overcome the cell’s defensive mechanisms, leading to extended inflammatory gene expression. VCAM-1 protein expression showed similar a pattern to that of mRNA but the maximum expression was observed at 4 h. These data show a potential trend indicating concentration- and time-dependent inflammatory effects associated with TiO2 NP exposure.
Autophagy is a normal cellular process involving the lysosomal degradation of cytoplasmic components and is essential for cell survival and homeostasis (Kroemer et al., 2010). Our data showed that autophagy was increased in cells exposed to 50 µg/mL TiO2 NPs while treatment with 10 µg/mL TiO2 NPs did not increase autophagy. Multiple forms of cellular stress can induce this cellular defensive process and TiO2 NPs additionally were found to increase autophagy in cells (Halamoda Kenzaoui et al., 2012; Zhao et al., 2012). Autophagy is a complex process which is linked to other stress responses. Recent studies have demonstrated that autophagy can negatively regulate NF-κB signaling and gene expression such as MCP-1 (Fujishima et al., 2011; Qing et al., 2007; Yoshizaki et al., 2012). In our study, however, MCP-1 and VCAM-1 gene expression was not significantly affected by autophagy, although limited decrease in expression of these genes was seen 8 h post TiO2 exposure, which occurs concurrently with maximal induction of autophagy. With our current data, it is not clear if TiO2 NP-induced autophagy is a significant factor for the observed gene expression, therefore further studies are needed to elucidate this underlying mechanism.
We also found that TiO2 NPs can increase cardiovascular inflammation by activation of NF-κB and subsequent upregulation of endothelial inflammatory parameters, such as MCP-1 and VCAM-1. Further, mechanisms and signaling pathways associated with TiO2 NP-induced endothelial cell toxicity were studied. TiO2 NPs stimulated production of ROS (e.g., superoxide), which activated four key signaling molecules: three MAPKs (p38, ERK and JNK) and Akt. Activation of such signaling molecules and expression of endothelial inflammatory parameters were dependent on the concentration of TiO2 NPs (0–50 µg/mL). As stated, induction of adhesion molecules including VCAM-1 and MCP-1 is the product of NF-κB activation (Iademarco et al., 1992; Ueda et al., 1997); NF-κB is a critical redox-sensitive transcription factor by which expression of inflammatory genes is regulated by extracellular stimuli in cells (Bubici et al., 2006; Valko et al., 2006). In the present study, we found that TiO2 NPs can activate NF-κB, which is demonstrated by increased DNA binding activity of NF-κB and increased phosphorylation of IκBα. These results subsequently were confirmed in cells pretreated with a specific NF-κB inhibitor (i.e., PDTC) where this inhibitor served to significantly attenuate TiO2 NP-induced VCAM-1 and MCP-1 gene expression. Three MAPK proteins and Akt signaling protein are known to be associated with activation of NF-κB (Valko et al., 2006). Cellular oxidative stress is closely associated with phosphorylation of such signaling molecules and subsequent NF-κB activation. Recent studies have demonstrated that ROS are produced by TiO2 NPs and play an inflammatory role in cells (Halamoda Kenzaoui et al., 2012). Another recent study also implicated ROS in the activation of NF-κB and subsequent induction of inflammatory genes in human endothelial cells treated with silica nanoparticles (Liu and Sun, 2010). Similarly, results in the current study demonstrated that TiO2 NPs induce ROS production; our data show a significant production of superoxide anion after cell exposure to TiO2 NPs. Further, in order to confirm these data, we pretreated cells with antioxidants such as EGCG and apocynin, which resulted in a significant decrease in VCAM-1 and MCP-1 inflammatory gene expression when compared to treatment with TiO2 NPs alone. Mechanisms responsible for TiO2 NP-induced ROS production are not well defined but possibly are linked to the activation of NADPH oxidase (NOX) because apocynin, a specific inhibitor for NOX activation, can attenuate TiO2 NP-induced gene expression for MCP-1 and VCAM-1 (Pietrowski et al., 2011). These results suggest that TiO2 NPs can induce ROS production and that this is a key mechanism associated with TiO2 NP-induced MAPKs, Akt and NF-κB activation.
The mechanisms associated with TiO2 NP-induced inflammation are not simple and likely involve activation of many intracellular signaling molecules. In our studies with endothelial cells, treatment with TiO2 NPs phosphorylated three MAPKs and PI3K/Akt. Phosphorylation of these key signaling molecules can lead to the activation of redox-sensitive transcription factors, such as NF-κB (Valko et al., 2006). Indeed, TiO2 NPs increased phosphorylation of three MAPKs and PI3K/Akt, and DNA binding of NF-κB subsequently led to induction of MCP-1 and VCAM-1. The roles of these three MAPKs and PI3K/Akt were confirmed by pretreating cells with specific MAPK and PI3K/Akt inhibitors, which resulted in a significant decrease in VCAM-1 and MCP-1 gene expression compared to treatment with TiO2 NPs alone. These data suggest a specific role of intracellular signaling pathways in endothelial inflammation following TiO2 NP exposure.
Taken together, data suggest that TiO2 NPs used in our study can increase inflammatory gene expression in vascular endothelial cells via redox-sensitive signaling pathways. This work highlights the toxic effects of anatase TiO2 NPs in the cardiovascular system to better understand potential overall public health concerns associated with metal oxide nanoparticles.
Acknowledgments
Funding Information
This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [grant number P42ES007380] and the University of Kentucky Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Brun E, Carriere M, Mabondzo A. In vitro evidence of dysregulation of blood-brain barrier function after acute and repeated/long-term exposure to TiO(2) nanoparticles. Biomaterials. 2012;33:886–896. doi: 10.1016/j.biomaterials.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- Businaro R, Tagliani A, Buttari B, Profumo E, Ippoliti F, Di Cristofano C, Capoano R, Salvati B, Rigano R. Cellular and molecular players in the atherosclerotic plaque progression. Ann N Y Acad Sci. 2012;1262:134–141. doi: 10.1111/j.1749-6632.2012.06600.x. [DOI] [PubMed] [Google Scholar]
- Chae YJ, Kim CH, Ha TS, Hescheler J, Ahn HY, Sachinidis A. Epigallocatechin-3-O-gallate inhibits the angiotensin II-induced adhesion molecule expression in human umbilical vein endothelial cell via inhibition of MAPK pathways. Cell Physiol Biochem. 2007;20:859–866. doi: 10.1159/000110446. [DOI] [PubMed] [Google Scholar]
- Chandran P, Sasidharan A, Ashokan A, Menon D, Nair S, Koyakutty M. Highly biocompatible TiO(2):Gd(3)(+) nano-contrast agent with enhanced longitudinal relaxivity for targeted cancer imaging. Nanoscale. 2011;3:4150–4161. doi: 10.1039/c1nr10591d. [DOI] [PubMed] [Google Scholar]
- Elsaesser A, Howard CV. Toxicology of nanoparticles. Adv Drug Deliv Rev. 2012;64:129–137. doi: 10.1016/j.addr.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Floyd HS, Chen LC, Vallanat B, Dreher K. Fine ambient air particulate matter exposure induces molecular alterations associated with vascular disease progression within plaques of atherosclerotic susceptible mice. Inhal Toxicol. 2009;21:394–403. doi: 10.1080/08958370802317745. [DOI] [PubMed] [Google Scholar]
- Fujishima Y, Nishiumi S, Masuda A, Inoue J, Nguyen NM, Irino Y, Komatsu M, Tanaka K, Kutsumi H, Azuma T, Yoshida M. Autophagy in the intestinal epithelium reduces endotoxin-induced inflammatory responses by inhibiting NF-kappaB activation. Arch Biochem Biophys. 2011;506:223–235. doi: 10.1016/j.abb.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Halamoda Kenzaoui B, Chapuis Bernasconi C, Guney-Ayra S, Juillerat-Jeanneret L. Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. Biochem J. 2012;441:813–821. doi: 10.1042/BJ20111252. [DOI] [PubMed] [Google Scholar]
- Han SG, Eum SY, Toborek M, Smart E, Hennig B. Polychlorinated biphenyl-induced VCAM-1 expression is attenuated in aortic endothelial cells isolated from caveolin-1 deficient mice. Toxicol Appl Pharmacol. 2010;246:74–82. doi: 10.1016/j.taap.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Han SS, Toborek M, Hennig B. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol Appl Pharmacol. 2012;261:181–188. doi: 10.1016/j.taap.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Shasby DM, Fulton AB, Spector AA. Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis. 1984;4:489–497. doi: 10.1161/01.atv.4.5.489. [DOI] [PubMed] [Google Scholar]
- Hext PM, Tomenson JA, Thompson P. Titanium dioxide: inhalation toxicology and epidemiology. Ann Occup Hyg. 2005;49:461–472. doi: 10.1093/annhyg/mei012. [DOI] [PubMed] [Google Scholar]
- Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res. 2000;87:153–159. doi: 10.1161/01.res.87.2.153. [DOI] [PubMed] [Google Scholar]
- Iademarco MF, McQuillan JJ, Rosen GD, Dean DC. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1) J Biol Chem. 1992;267:16323–16329. [PubMed] [Google Scholar]
- IARC. Carbon black, titanium dioxide and talc IARC monographs on the evaluation of carcinogenic risksto humans. International Agency for Research on Cancer: Lyon. 2006:93. [PMC free article] [PubMed] [Google Scholar]
- Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, Oberdorster G, Ziesenis A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006;36:189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- Lim EJ, Smart EJ, Toborek M, Hennig B. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H3340–H3347. doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- Linkov I, Satterstrom FK, Corey LM. Nanotoxicology and nanomedicine: making hard decisions. Nanomedicine. 2008;4:167–171. doi: 10.1016/j.nano.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Liu X, Sun J. Endothelial cells dysfunction induced by silica nanoparticles through oxidative stress via JNK/P53 and NF-kappaB pathways. Biomaterials. 2010;31:8198–8209. doi: 10.1016/j.biomaterials.2010.07.069. [DOI] [PubMed] [Google Scholar]
- McIntyre RA. Common nano-materials and their use in real world applications. Sci Prog. 2012;95:1–22. doi: 10.3184/003685012X13294715456431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel-Davalos A, Ventura-Gallegos JL, Alfaro-Moreno E, Soria-Castro E, Garcia-Latorre E, Cabanas-Moreno JG, del Pilar Ramos-Godinez M, Lopez-Marure R. TiO(2) nanoparticles induce dysfunction and activation of human endothelial cells. Chem Res Toxicol. 2012;25:920–930. doi: 10.1021/tx200551u. [DOI] [PubMed] [Google Scholar]
- Narband N, Uppal M, Dunnill CW, Hyett G, Wilson M, Parkin IP. The interaction between gold nanoparticles and cationic and anionic dyes: enhanced UV-visible absorption. Phys Chem Chem Phys. 2009;11:10513–10518. doi: 10.1039/b909714g. [DOI] [PubMed] [Google Scholar]
- Nohynek GJ, Lademann J, Ribaud C, Roberts MS. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;37:251–277. doi: 10.1080/10408440601177780. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Pietrowski E, Bender B, Huppert J, White R, Luhmann HJ, Kuhlmann CR. Pro-inflammatory effects of interleukin-17A on vascular smooth muscle cells involve NAD(P)H- oxidase derived reactive oxygen species. J Vasc Res. 2011;48:52–58. doi: 10.1159/000317400. [DOI] [PubMed] [Google Scholar]
- Qing G, Yan P, Qu Z, Liu H, Xiao G. Hsp90 regulates processing of NF-kappa B2 p100 involving protection of NF-kappa B-inducing kinase (NIK) from autophagy-mediated degradation. Cell Res. 2007;17:520–530. doi: 10.1038/cr.2007.47. [DOI] [PubMed] [Google Scholar]
- Sanders K, Degn LL, Mundy WR, Zucker RM, Dreher K, Zhao B, Roberts JE, Boyes WK. In vitro phototoxicity and hazard identification of nano-scale titanium dioxide. Toxicol Appl Pharmacol. 2012;258:226–236. doi: 10.1016/j.taap.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Schilling K, Bradford B, Castelli D, Dufour E, Nash JF, Pape W, Schulte S, Tooley I, van den Bosch J, Schellauf F. Human safety review of "nano" titanium dioxide and zinc oxide. Photochem Photobiol Sci. 2010;9:495–509. doi: 10.1039/b9pp00180h. [DOI] [PubMed] [Google Scholar]
- Shannahan JH, Kodavanti UP, Brown JM. Manufactured and airborne nanoparticle cardiopulmonary interactions: a review of mechanisms and the possible contribution of mast cells. Inhal Toxicol. 2012;24:320–339. doi: 10.3109/08958378.2012.668229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skocaj M, Filipic M, Petkovic J, Novak S. Titanium dioxide in our everyday life; is it safe? Radiol Oncol. 2011;45:227–247. doi: 10.2478/v10019-011-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Tan D, Zhou Q, Liu X, Cheng Z, Liu G, Zhu M, Sang X, Gui S, Cheng J, Hu R, Tang M, Hong F. Oxidative damage of lung and its protective mechanism in mice caused by long-term exposure to titanium dioxide nanoparticles. J Biomed Mater Res A. 2012;100:2554–2562. doi: 10.1002/jbm.a.34190. [DOI] [PubMed] [Google Scholar]
- Terzano C, Di Stefano F, Conti V, Graziani E, Petroianni A. Air pollution ultrafine particles: toxicity beyond the lung. Eur Rev Med Pharmacol Sci. 2010;14:809–821. [PubMed] [Google Scholar]
- Thurn KT, Paunesku T, Wu A, Brown EM, Lai B, Vogt S, Maser J, Aslam M, Dravid V, Bergan R, Woloschak GE. Labeling TiO2 nanoparticles with dyes for optical fluorescence microscopy and determination of TiO2-DNA nanoconjugate stability. Small. 2009;5:1318–1325. doi: 10.1002/smll.200801458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J Biol Chem. 1997;272:31092–31099. doi: 10.1074/jbc.272.49.31092. [DOI] [PubMed] [Google Scholar]
- Umbreit TH, Francke-Carroll S, Weaver JL, Miller TJ, Goering PL, Sadrieh N, Stratmeyer ME. Tissue distribution and histopathological effects of titanium dioxide nanoparticles after intravenous or subcutaneous injection in mice. J Appl Toxicol. 2012;32:350–357. doi: 10.1002/jat.1700. [DOI] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, Li Y, Jiao F, Zhao Y, Chai Z. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168:176–185. doi: 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Yoshizaki T, Kusunoki C, Kondo M, Yasuda M, Kume S, Morino K, Sekine O, Ugi S, Uzu T, Nishio Y, Kashiwagi A, Maegawa H. Autophagy regulates inflammation in adipocytes. Biochem Biophys Res Commun. 2012;417:352–357. doi: 10.1016/j.bbrc.2011.11.114. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Howe JL, Yu Z, Leong DT, Chu JJ, Loo JS, Ng KW. Exposure to Titanium Dioxide Nanoparticles Induces Autophagy in Primary Human Keratinocytes. Small. 2012 doi: 10.1002/smll.201201363. [DOI] [PubMed] [Google Scholar]