Abstract
Since its description by Paul Grammont from Dijon, France, several tens of thousands of reverse total shoulder arthroplasties (RTSA) have been performed for diverse conditions. The purpose of this analysis is to identify the complications of this procedure in the literature and in clinical practice. A total of 240 papers concerning RTSA published between 1996 and 2012 have been identified. Over 80 papers describe complications associated with this type of implant. A list of prostheses satisfying European and US standards, CE and FDA approved, has been produced on the basis of information provided by the manufacturers. Data from the literature do not support a meta-analysis. The inventory of best practices shows excellent results in the short and medium term in specific indications, while the number of complications varies between 10 and 65 % in long-term series. Complications can be classified into (A) non-specific including infections (superficial and deep), phlebitis, haematoma, neurological complications of the suprascapular, radial and axillary nerves and (B) specific complications associated with RTSA including (1) on the glenoid side: intraoperative fracture of the glenoid and acromion, late fracture of the scapula, impingement at the scapular neck (notching), glenoid loosening, dissociation of the glenoid component (snatching of the glenosphere) and fractures of the glenoid baseplate; (2) on the humeral side: metaphyseal deterioration, humeral loosening, instability of the shoulder, stiffness with limitation of external and/or internal rotation; and (3) muscular complications with fatty degeneration of the deltoid. Additionally we have identified specific situations related to the type of implant such as the disassembly of the humeral or the glenoid component, dissociation of the polyethylene humeral plate, dissociation of the metaphysis and osteolysis of the tuberosities. The integration of results from different clinical series is difficult because of the lack of a database and the multitude of implants used.
Introduction
In 1993 Paul Grammont, Pierre Trouilloud and Emmanuel Baulot from Dijon, France described the original concept of the modern reverse arthroplasty showing that if the rotator cuff cannot be restored, functional recovery of the shoulder can be obtained with a total shoulder prosthesis, medialising the glenohumeral centre of rotation and elongating the remaining deltoid muscle. The early results were published by Baulot et al. in the Belgian Journal of Orthopaedics [1]. Previous concepts published by the Dijon school included a humero-acromial prosthesis (Acromion) (Fig. 1) and a first-generation reverse implant called the “Trumpet” prosthesis (Fig. 2) that were abandoned. The original Delta prosthesis (Fig. 3) was designed by Grammont, Trouilloud, Baulot and Capon with Michel Colombier, a mechanical engineer from Medinov, a French company that developed this gamut of implants. Medinov was later purchased by Landanger and became a part of DePuy in 1999. DePuy introduced the Delta prosthesis in the USA by 2004 and by that time seven manufacturers were producing different reverse shoulder models [2]. Currently there are more than 20 companies marketing reverse shoulder implants and other manufacturers are developing new designs. The differences between the implants are related to the glenoid fixation (oval or circular glenoid baseplate, long or short central peg, locked or unlocked fixation screws); and to the humeral stem and metaphysis (big or small metaphyseal component, cemented or cementless). An important difference relates to the proximal humeral cut that ranges from 125 to 155° in respect of the cervical-diaphyseal angle and between the surface treatments of the different implants. A common fact related to reverse total shoulder arthroplasty (RTSA) is that all the manufacturers are continuously searching for ways to improve and modify the current implants. The object of this review paper is to identify the different complications related directly to this type of surgery (Table 1).
Fig. 1.

The Acromion prosthesis was designed for treating cuff tear arthropathy associated with superior migration and escape when the coraco-acromial arch was compromised and the humeral head was in contact with the undersurface of the acromion
Fig. 2.
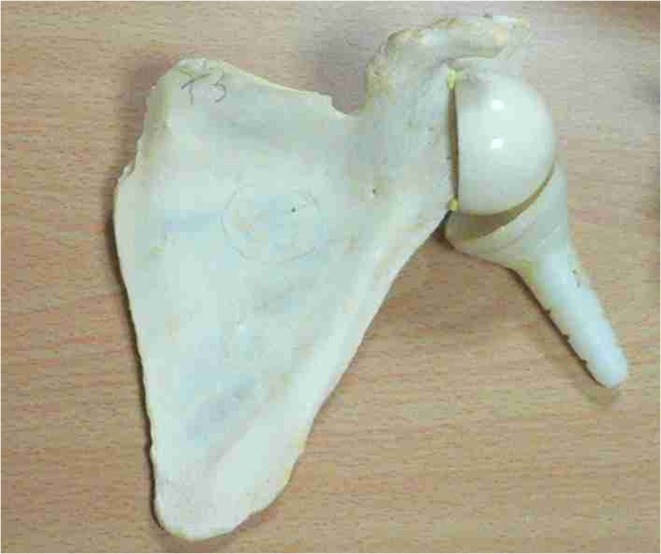
The Trumpet prosthesis was designed by the Dijon team as the first reverse shoulder implant with a glenosphere extending from the scapula and medialising the lever arm of the deltoid muscle
Fig. 3.

The first Delta prosthesis was designed in 1991 and it was very similar to the implant currently being used
Table 1.
Classification of complications reported with RTSA
| Non-specific situations |
| - Superficial and deep infections |
| - Haematoma |
| - Neurological complications (peripheral nerve palsy) |
| ◦ NB: different reports concerning the suprascapular nerve, the radial nerve, the axillary nerve, etc. |
| - Phlebitis |
| Specific situations related to reverse arthroplasty |
| On the glenoid side |
| - Intraoperative glenoid fracture |
| - Intraoperative acromial fracture |
| - Late scapular fracture (acromion, spina scapulae, body) |
| - Scapular notching |
| - Glenoid loosening by dissociation, screw breakage or snatching |
| On the humeral side |
| - Humeral polyethylene liner deterioration |
| - Humeral loosening |
| - Shoulder Instability with or without dislocation |
| - Stiffness with limited internal or external rotation |
| Muscular complications |
| - Shoulder lengthening with deltoid fatty infiltration |
| Brand-related specific problems |
| - Dismantling of the humeral component |
| - Dismantling of the glenoid component |
| - Polyethylene dissociation from the humeral component |
| - Dissociation of the humeral baseplate from the stem |
| - Tuberosity lysis related to a voluminous proximal humeral metaphysis |
Indications
Indications for RTSA include degenerative arthritis associated with irreparable cuff tears (cuff tear arthropathy) and irreparable cuff tears with loss of function in the elderly (pseudoparalytic shoulder) [3–9], aseptic necrosis of the humeral head in the elderly [1], shoulder reconstruction in rheumatoid arthritis [5, 10], chronic shoulder dislocations in the elderly [11], reconstruction surgery for tumour [12, 13], revision surgery after failed anatomical or resurfacing arthroplasty [14–19], failed rotator cuff repair with superior escape and reconstruction for comminuted fractures of the proximal humerus in the elderly [20, 21]. Any procedure addressing shoulder reconstruction with RTSA demands the existence of a functional deltoid muscle that makes shoulder mobility possible with this type of implant [20]. The major indication for using RTSA is arthritis associated with massive rotator cuff tears which accounts for approximately 90 % of the cases in different series [2, 5, 6, 8, 9, 22]. The patient’s age is important for the indication, as the implant longevity is not well understood; therefore, most authors do not advocate use of this type of implant for patients under 65 years of age [1, 5, 9, 23–26]. The use of reverse arthroplasty changes the anatomy of the shoulder, translates the centre of rotation, lengthens the arm and provides a better lever for the deltoid muscle (Fig. 4). The proprioception is changed [27].
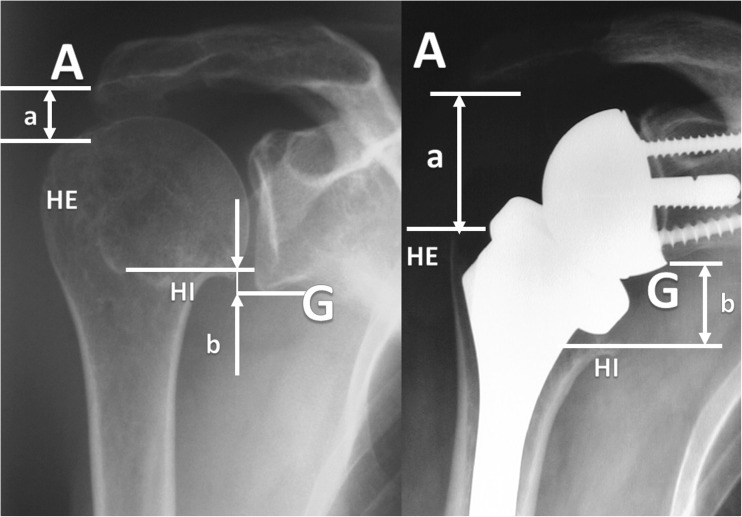
Fig. 4.
Reverse shoulder arthroplasty changes the anatomy of the shoulder and lengthens the humero-acromial space (A). The distance between the inferior part of the humeral head (HI) and the inferior glenoid (G) turns from a negative value to positive
Revision shoulder arthroplasty using a reverse implant is associated with higher rates of complications, such as infection, neurological injury and intraoperative fractures. In addition to these problems, the situation may be complicated by significant proximal humeral deficiency, specifically loss of the proximal shaft, metaphysis and tuberosities [9, 16, 18, 28, 29].
The research tools
The assessment of the results with this type of arthroplasty was made using general and specific tools. The general tools for the clinical assessment of patients treated with RTSA was made with different validated scores and tools such as the simple shoulder test (SST), the Constant and Murley score, the American Shoulder and Elbow Surgeons (ASES) score and the University of California, Los Angeles (UCLA) score. A specific tool for the assessment of glenoid bone loss when a reverse shoulder implant shows notching and lysis of the inferior pillar of the scapula was described by Cécile Nérot and François Sirveaux from France, cited by Lévigne et al. [30].
Approach to the complications with a reverse shoulder implant
The literature relating to RTSA has been abundant lately and includes more than 50 new papers per year. The increase has been exponential during the last 24 months recorded by our research of the literature and there are several papers dealing with complications and eventful outcomes.
Case series recording how an implant performed in vivo bring valuable information about the incidence of complications. The results depend on the type of implant, on the indication (primary, revision or trauma) and on the surgical performance.
Case series with recent implants that are supposed to perform better by addressing specific causes of failures of the previous implants (i.e. notching or restricted rotation).
Case reports describing different type of events leading to failure of RTSA.
Research papers dealing with tests and trials in order to explain a specific type of failure.
Expert opinion papers dealing with the surgical approach, anatomical features or technical details in RTSA that may provide better results.
Instructional lectures and textbook chapters presenting an overview of the authors’ experience with such events.
Thorough mastery of the procedure of implantation for different indications seems to be crucial as the complications are more frequent during the first implantations, as suggested by Kempton and colleagues. From a series of 200 reverse arthroplasties performed in 191 patients by a single surgeon including 40 revisions the local complication rate was higher in the first 40 shoulders (23.1 %) versus the last 160 shoulders (6.5 %). The authors conclude that the early complication-based learning curve for RTSA is approximately 40 cases. There were more complications and more neuropathies in revision versus primary reverse arthroplasties [31].
Non-specific complications
Superficial and deep infections are well described in a study published by Zavala and co-workers in 2011 [32]. In a retrospective study the authors identified eight cases of deep infection after 138 implantations of an RTSA prosthesis. Six infections occurred in patients who had had previous shoulder surgery. The bacterial organism was identified in six patients. Three patients had diabetes mellitus. Six patients were managed with irrigation and debridement, while two patients required resection arthroplasty. Patients managed with debridement, intravenous antibiotics and retention of components demonstrated good pain relief and function, without evidence of radiographic loosening. Resection resulted in pain relief but poor functional outcomes.
Haematoma is a common complication of different surgical procedures. It is to be treated in a standardised manner with drainage and prevention of infection.
Neurological complications after RTSA implantation were studied by Lädermann and colleagues [33]. This study focused on the clinical, radiographic, preoperative and post-operative electromyographic evaluation, with measurement of arm lengthening, according to a previously validated protocol. A series of 41 patients (42 shoulders) underwent reverse shoulder arthroplasty, while 19 patients had anatomical primary shoulder arthroplasty. Electromyography showed subclinical electromyographic changes in nine shoulders of the reverse arthroplasty group, involving mainly the axillary nerve; eight resolved in less than six months. In the anatomical shoulder arthroplasty group, a brachial plexus lesion was seen in one case. The prevalence of acute post-operative nerve injury was significantly more frequent in the reverse shoulder arthroplasty group (p = 0.002), with a 10.9 times higher risk (95 % confidence interval 1.5–78.5). The mean lengthening (and standard deviation) of the arm after reverse shoulder arthroplasty was 2.7 ± 1.8 cm (range 0–5.9 cm) compared with the normal, contralateral side. The authors conclude that a peripheral neurological lesion following RTSA is relatively common, but usually transient. Arm lengthening with this procedure may be responsible for the nerve injuries.
Phlebitis and thrombosis are cited in diverse types of shoulder arthroplasty.
Specific problems related to the implant
The Delta reverse shoulder was the oldest and the most widely used implant in its three consecutive versions (Delta 1, Delta 2 and Delta Xtend), which is probably why problems with this type of prosthesis are frequently reported in the literature [3, 8, 28, 34–38], but other implants were recorded with similar complications [7, 8, 16, 37, 39–41].
Internal glenoid notching may appear very early; follow-ups at six months showed 53 % in a case series of 45 patients by Boileau et al. [9] and 67 % in a case series of 77 patients by Sirveaux et al. [3].
Imbalance of the muscles around the reconstructed joint may be responsible for instability or dislocation of the prosthetic joint [33, 39, 40]. Muscular weakness may result in instability or dislocations of the reverse shoulder [11, 39, 42].
Limited external rotation may be related to suprascapular nerve injury that could occur during the glenoid preparation [33, 40], while weak internal rotation may arise from injury to the subscapularis muscle or from the prosthetic design. The prosthetic offset and placement of the humeral baseplate in a neutral or eccentric position play a role in the mobility of the shoulder with a reverse implant [43, 44].
De Wilde and Walch published a report of three cases showing mechanical dismantling of the reverse Delta prosthesis either by unscrewing of the humeral lengthener or by metallic failure of the medial epiphysis. They conclude that the problems were related to the design of the implant or to surgical errors during the implantation procedure [38]. We saw this type of complication in two cases, one of which followed reconstruction after trauma (Fig. 5).
Middernacht and colleagues determined the incidence of glenosphere disengagement and clinical outcomes in a comparative series of 479 RTSA (468 Delta III and 11 Aequalis) with a minimum follow-up of 12 months. Disengagement of the glenosphere occurred in 16 of 479 shoulders (3.2 %). In 13 patients, the disengagement was partial and was not associated with a poor functional outcome with this short-term follow-up. In three patients, the disengagement led to a fracture of the central screw and complete disengagement of the glenosphere from the baseplate requiring revision that some of the patients refused. Partial disengagement was noted in 45.4 % of the Aequalis prostheses and in 1.7 % of the 468 Delta prostheses (1.7 %). Three total disengagements with central screw breakage occurred in Delta III prostheses [45].
Crosby et al. reviewed a series of 400 RSA performed over a 4.5-year period looking for fractures of the scapula [46]. They found 22 cases of scapular fractures and identified three fracture patterns. Small avulsion fractures of the anterior acromion were classified as type I (2 %); fractures through the anterior acromion just posterior to the acromioclavicular joint were classified as type II (2.5 %); and fractures of the posterior acromion or scapular spine were classified as type III (1.0 %). The average time for these fractures to appear was ten months. Type I healed without surgery, while types II and III required surgical fixation. Type II fractures are treated with acromioclavicular joint resection if stable and by open reduction and internal fixation if the fracture is unstable.
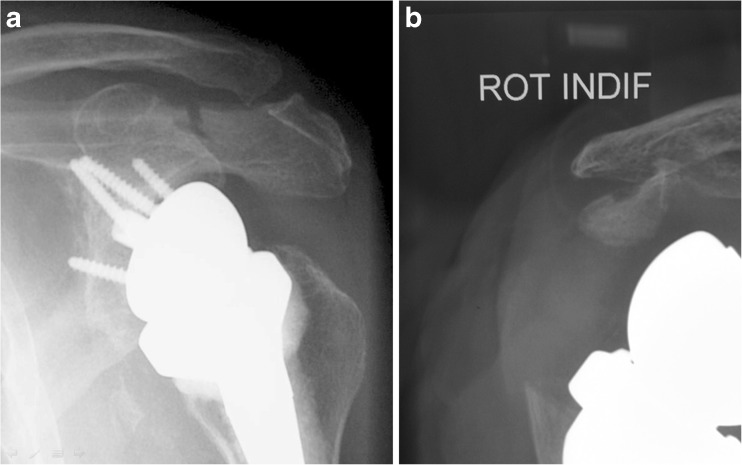
Acromial insufficiency with reverse arthroplasty was studied by Walch and colleagues in a multicentre study including 457 RTSA implantations in 430 patients [47]. A total of 283 prostheses were implanted in patients with no history of shoulder surgery, while 174 cases were performed for revision of a previously failed procedure. The acromial situation was evaluated before and after surgery. Lengthening of the arm and subsequent increased tension in the deltoid may be responsible for a fatigue fracture of the acromion; nine percent of the cases showed different previous acromial congenital or acquired modifications that had no or little influence on the result. Perioperative fracture of the scapular spine was seen in one case and resulted in a poor outcome [47]. In our personal experience we observed two cases of acromial fractures following RTSA; both fractures occurred between three and eight weeks after surgery and had no major effect on the outcome (Fig. 6a, b).
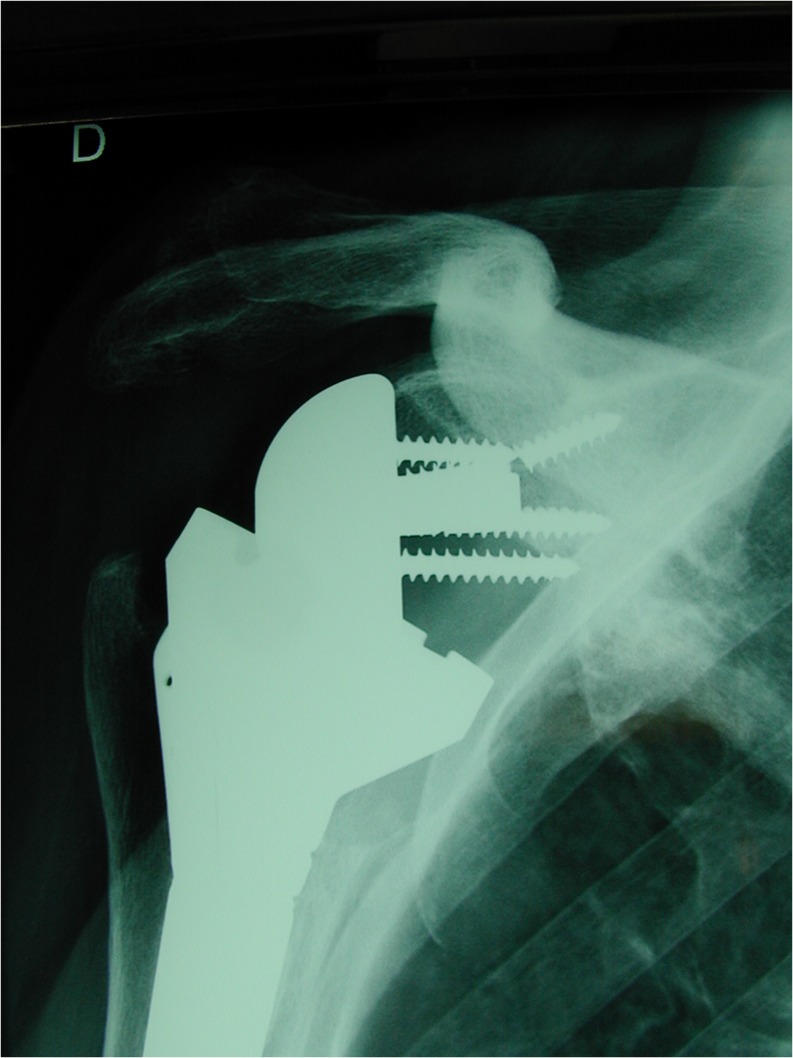
In a clinical series of 60 implantations of an RTSA model with a lateralised centre of rotation Frankle and colleagues observed a total of 13 complications in ten patients (17 %), including three acromial fractures and one scapular fracture as well as five failures of the glenoid baseplate fixation, including screw breakage and dismantling. The follow-up was performed at an average of 33 months [7]. An experimental study by Roche and colleagues demonstrates that severe notching may play a role in initial glenoid baseplate stability [48]. In our experience glenoid screw breakage can be observed in association with severe notching and obvious instability of the glenosphere (Fig. 7).
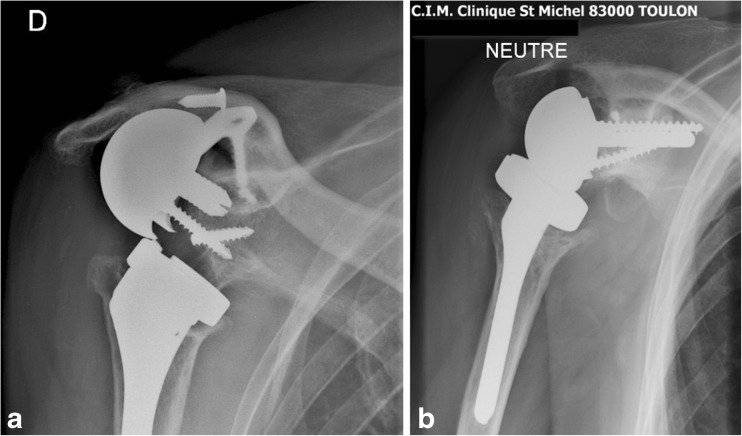
Glenoid loosening by glenoid snatching may be observed in cases of bone insufficiency or severe osteoporosis. In our experience two cases were resolved by using a 40-mm-long tip glenoid baseplate (Duocentric®, Aston Medical, Saint-Étienne, France) (Fig. 8a, b).
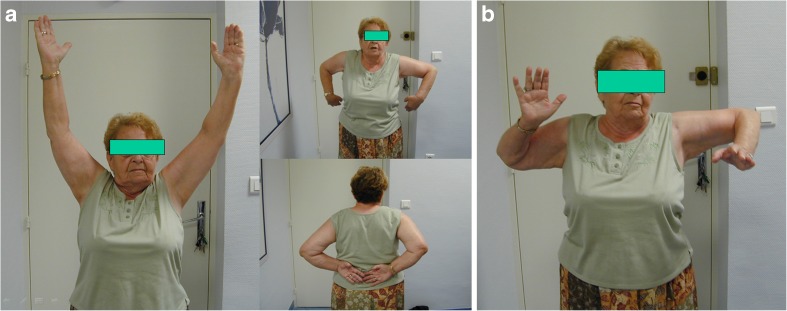
Limited external rotation may be related to regional stiffness or to a weakness of the remaining teres minor muscle. This situation is easily recognised clinically, but is quite well tolerated by the patients, when weak or no external rotation is diagnosed preoperatively (Fig. 9a, b).
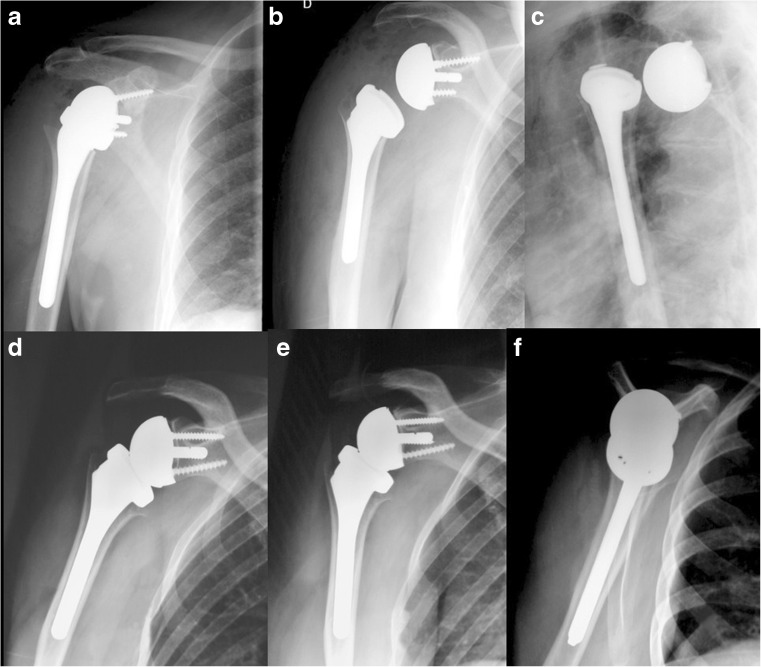
Shoulder instability: dislocation is a major complication of RTSA. Despite the constrained situation of this prosthesis some dislocations may occur, mainly in revision surgery or in patients with neurological deficit or associated conditions such as Parkinson’s disease or seizures [11]. A revision with a more constrained couple of friction may be useful as well as the initial use of a constrained humeral polyethylene in cases “at risk”. Instability may sometimes be difficult to demonstrate with anteroposterior (AP) views (Fig. 10a–b). The lateral transthoracic view is easy to perform and may bring essential information in case of instability. In a stable arthroplasty the centre of the humeral baseplate is in front of the glenosphere on all of the views (Fig. 10d–f).
Fig. 5.
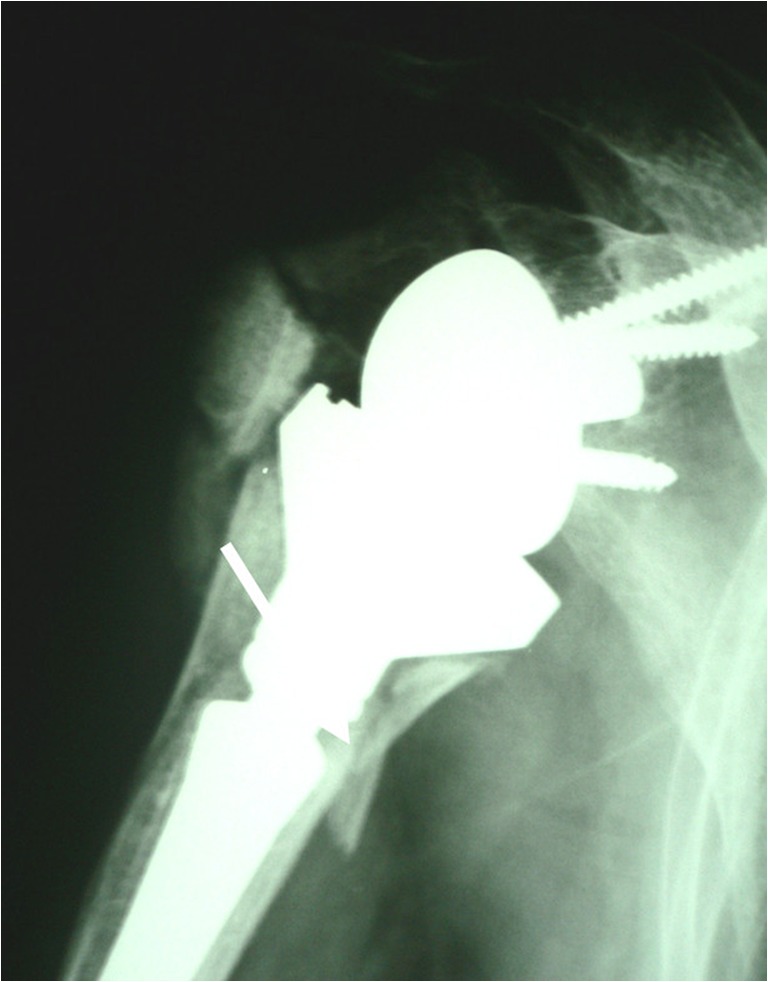
Dismantling of the proximal humerus in a Delta 2 reverse shoulder inserted for fracture. Note the fact that this type of incident is recorded only in arthroplasties for the right shoulder, the unscrewing coming about by internal rotation
Fig. 6.
a, b Acromial fractures following two implantations of a reverse shoulder arthroplasty for cuff tear arthropathy
Fig. 7.
Severe glenoid notching with lysis of the inferior pillar, insufficiency of fixation, screw breakage and instability
Fig. 8.
a, b Glenoid snatching revised by using a 40-mm-long tip glenoid baseplate
Fig. 9.
a, b Reduced external rotation with the Delta reverse arthroplasty is obvious in this case. However, the implant provides outstanding ranges of motion in a previous pseudoparalytic shoulder and high satisfaction of the patient
Fig. 10.
a–f Instability may sometimes be difficult to demonstrate with AP views (a, b). In this case the posterior dislocation of the RTSA implant is seen on the lateral transthoracic view (c). After reduction the centre of the humeral baseplate is in front of the glenosphere on all of the views (d–f)
General problems related to the outcome of an arthroplasty include:
Humeral loosening
Stiffness in a defined direction
Specific problems related to a brand
Tuberosity lysis related to a voluminous proximal humeral metaphysis
Polyethylene dissociation from the humeral component (different brands)
Mechanical breakage of prosthetic parts (different brands)
A comprehensive list of complications reported in the literature has been published by Wierks et al. [49]. They define early complications such as glenoid intraoperative fracture or unscrewing of the glenosphere.
A study by Molé and Favard from a multicentre European group included 527 patients undergoing RTSA, mainly with a Delta prosthesis. There were 3 % haematomas, 5 % infections, 3.4 % instability, 5 % complications on the glenoid side, 2 % complications on the humeral side, 3 % fractures of the acromion and 1 % neurological complications [35].
A study by Farshad and Gerber follows two series, a first one of 111 cases of Delta III (DePuy) and a second series of 230 cases of Anatomical Reverse® (Zimmer, Inc.) prostheses. Non-specific complications such as haematoma, infection and neurological impairment are reported, as well as 50 % cases of scapular notching in the case series of 230 patients followed up by Gerber at an average follow-up of 22.3 months. Glenoid component loosening was observed in 3 % of the cases [37].
Solutions and perspectives
About notching, glenoid morphology and new prosthetic designs:
Scapular notching is by far the most common situation encountered with RTSA. Its occurrence is dependent on several factors, but it seems to be related to the glenoid morphology because the same implant may result in notching in a short-neck glenoid and no notching in a long-neck glenoid as demonstrated by Pierre Trouilloud and co-workers from Dijon in anatomical experiments. Different solutions have been proposed to prevent notching: Frankle et al. suggested lateralising the centre of rotation outside the scapula, using an eccentric glenosphere that approaches two thirds of a sphere volume instead the one half used in the original Delta concept, creating more constraint and torque on the glenosphere and increasing the risk of glenoid loosening. Increasing the inclination (neck-shaft angle) of the humeral component will avoid inferior scapular notching [27], but then may create a superior conflict and enhance prosthetic loosening. Baulot and co-workers propose a new glenoid design manufactured by Aston Medical (Duocentric®) with extended inferior coverage that provoked no notching in a case series of 50 patients (unpublished data). A retrospective study compares clinical and radiological results of 47 Delta III reverse prostheses and 49 Arrow reverse prostheses at a minimum of 12 months follow-up. Scapular notching was noted in 32 patients with the Delta III prosthesis and in no instance with the Arrow prosthesis. The design features of this implant were found to be associated with improvement in range of motion and absence of scapular notching [50].
Limited or weak external rotation may arise from the limited lateral offset that acts by interior impingement with the scapula pillar as showed by Nyffeler in a presentation at the European Shoulder and Elbow Surgeons meeting in Madrid, 2009. Based on mechanical experience with anatomical specimens, the author suggests that the positioning of the humeral stem in RTSA should not be retroverted. He recommends the use of an implant that has a modular baseplate able to be implanted at various degrees of rotation in an eccentric position.
Acromial fractures are probably related to a very important lengthening of the arm. It is difficult to appreciate exactly how much tension to put in the deltoid with a modified anatomy such as the reverse arthroplasty. It is acknowledged that using a smaller size glenosphere reduces lengthening, but the instability is another concern that needs to be checked in this case.
Failure of fixation in four-part fractures may be related to the voluminous prosthetic metaphysis. A smaller metaphysis in shoulder reconstruction with a reverse implant after trauma may be useful for better fixation of tuberosities.
Poor outcomes in revision surgery are related to indications, bone quality and muscular balance. It is difficult to make a synthesis for these cases because revision after osteosynthesis for trauma is very different from revision after a previous arthroplasty failure or after failed rotator cuff repair. Failures of reverse arthroplasty in revision cases should be analysed separately for each type of indication.
References
- 1.Baulot E, Garron E, Grammont PM. Grammont prosthesis in humeral head osteonecrosis. Indications—results. Acta Orthop Belg. 1999;65(Suppl 1):109–115. [PubMed] [Google Scholar]
- 2.Rockwood CA., Jr The reverse total shoulder prosthesis. The new kid on the block. J Bone Joint Surg. 2007;89(2):233–235. doi: 10.2106/JBJS.F.01394. [DOI] [PubMed] [Google Scholar]
- 3.Sirveaux F, Favard L, Oudet D, et al. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388–395. doi: 10.1302/0301-620X.86B3.14024. [DOI] [PubMed] [Google Scholar]
- 4.Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy. Oper Orthop Traumatol. 2005;17(1):1–24. doi: 10.1007/s00064-005-1119-1. [DOI] [PubMed] [Google Scholar]
- 5.Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17–22. doi: 10.1067/mse.2001.110515. [DOI] [PubMed] [Google Scholar]
- 6.Laudicina L, D’Ambrosia R. Management of irreparable rotator cuff tears and glenohumeral arthritis. Orthopedics. 2005;28(4):382–388. doi: 10.3928/0147-7447-20050401-13. [DOI] [PubMed] [Google Scholar]
- 7.Frankle M, Siegal S, Pupello D, et al. The Reverse Shoulder Prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg. 2005;87(8):1697–1705. doi: 10.2106/JBJS.D.02813. [DOI] [PubMed] [Google Scholar]
- 8.Cuff D, Pupello D, Virani N, et al. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg. 2008;90(6):1244–1251. doi: 10.2106/JBJS.G.00775. [DOI] [PubMed] [Google Scholar]
- 9.Boileau P, Watkinson D, Hatzidakis AM, et al. Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Ekelund A, Nyberg R. Can reverse shoulder arthroplasty be used with few complications in rheumatoid arthritis? Clin Orthop Relat Res. 2011;469(9):2483–2488. doi: 10.1007/s11999-010-1654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drignei M, Scarlat MM. Treatment of chronic dislocations of the shoulder by reverse total shoulder arthroplasty: a clinical study of six cases. Eur J Orthop Surg Traumatol. 2009;19(8):541–546. doi: 10.1007/s00590-009-0472-4. [DOI] [Google Scholar]
- 12.De Wilde L, Boileau P, Van der Bracht H. Does reverse shoulder arthroplasty for tumors of the proximal humerus reduce impairment? Clin Orthop Relat Res. 2011;469(9):2489–2495. doi: 10.1007/s11999-010-1758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieckmann R, Liem D, Gosheger G et al (2013) Evaluation of a reconstruction reverse shoulder for tumour surgery and tribological comparison with an anatomical shoulder arthroplasty. Int Orthop. doi:10.1007/s00264-012-1771-7 [DOI] [PMC free article] [PubMed]
- 14.Austin L, Zmistowski B, Chang ES, et al. Is reverse shoulder arthroplasty a reasonable alternative for revision arthroplasty? Clin Orthop Relat Res. 2011;469(9):2531–2537. doi: 10.1007/s11999-010-1685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elhassan B, Ozbaydar M, Higgins LD, et al. Glenoid reconstruction in revision shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):599–607. doi: 10.1007/s11999-007-0108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flury MP, Frey P, Goldhahn J, et al. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure-midterm results. Int Orthop. 2011;35(1):53–60. doi: 10.1007/s00264-010-0990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gohlke F, Rolf O. Revision of failed fracture hemiarthroplasties to reverse total shoulder prosthesis through the transhumeral approach: method incorporating a pectoralis-major-pedicled bone window. Oper Orthop Traumatol. 2007;19(2):185–208. doi: 10.1007/s00064-007-1202-x. [DOI] [PubMed] [Google Scholar]
- 18.Levy J, Frankle M, Mighell M, et al. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg. 2007;89(2):292–300. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 19.Walker M, Willis MP, Brooks JP et al (2012) The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg 21(4):514–522. doi:10.1016/j.jse.2011.03.006 [DOI] [PubMed]
- 20.Cazeneuve JF, Cristofari DJ. Delta III reverse shoulder arthroplasty: radiological outcome for acute complex fractures of the proximal humerus in elderly patients. Orthop Traumatol Surg Res. 2009;95(5):325–329. doi: 10.1016/j.otsr.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Martin TG, Iannotti JP. Reverse total shoulder arthroplasty for acute fractures and failed management after proximal humeral fractures. Orthop Clin North Am. 2008;39(4):451–457. doi: 10.1016/j.ocl.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Young SW, Everts NM, Ball CM, et al. The SMR reverse shoulder prosthesis in the treatment of cuff-deficient shoulder conditions. J Shoulder Elbow Surg. 2009;18(4):622–626. doi: 10.1016/j.jse.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219–225. [PubMed] [Google Scholar]
- 24.Guery J, Favard L, Sirveaux F, et al. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg. 2006;88(8):1742–1747. doi: 10.2106/JBJS.E.00851. [DOI] [PubMed] [Google Scholar]
- 25.Grassi FA, Murena L, Valli F, et al. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong) 2009;17(2):151–156. doi: 10.1177/230949900901700205. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Sotelo J. Reverse total shoulder arthroplasty. Clin Anat. 2009;22(2):172–182. doi: 10.1002/ca.20736. [DOI] [PubMed] [Google Scholar]
- 27.Kasten P, Maier M, Rettig O, et al. Proprioception in total, hemi- and reverse shoulder arthroplasty in 3D motion analyses: a prospective study. Int Orthop. 2009;33(6):1641–1647. doi: 10.1007/s00264-008-0666-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy JC, Virani N, Pupello D, et al. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189–195. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 29.Ortmaier R, Resch H, Matis N, et al. Reverse shoulder arthroplasty in revision of failed shoulder arthroplasty-outcome and follow-up. Int Orthop. 2013;37(1):67–75. doi: 10.1007/s00264-012-1742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Kempton LB, Ankerson E, Wiater JM. A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res. 2011;469(9):2496–2504. doi: 10.1007/s11999-011-1811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zavala JA, Clark JC, Kissenberth MJ, et al. Management of deep infection after reverse total shoulder arthroplasty: a case series. J Shoulder Elbow Surg. 2012;21(10):1310–1315. doi: 10.1016/j.jse.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 33.Lädermann A, Lübbeke A, Mélis B, et al. Prevalence of neurologic lesions after total shoulder arthroplasty. J Bone Joint Surg. 2011;93(14):1288–1293. doi: 10.2106/JBJS.J.00369. [DOI] [PubMed] [Google Scholar]
- 34.Dines JS, Fealy S, Strauss EJ, et al. Outcomes analysis of revision total shoulder replacement. J Bone Joint Surg. 2006;88(7):1494–1500. doi: 10.2106/JBJS.D.02946. [DOI] [PubMed] [Google Scholar]
- 35.Molé D, Favard L. Excentered scapulohumeral osteoarthritis. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(Suppl 6):37–94. doi: 10.1016/S0035-1040(07)92708-7. [DOI] [PubMed] [Google Scholar]
- 36.Samuelson EM, Cordero GX, Fehringer EV. Fracture of a reverse total shoulder arthroplasty retentive liner. Orthopedics. 2009;32(3):211. doi: 10.3928/01477447-20090301-25. [DOI] [PubMed] [Google Scholar]
- 37.Farshad M, Gerber C. Reverse total shoulder arthroplasty-from the most to the least common complication. Int Orthop. 2010;34(8):1075–1082. doi: 10.1007/s00264-010-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Wilde L, Walch G. Humeral prosthetic failure of reversed total shoulder arthroplasty: a report of three cases. J Shoulder Elbow Surg. 2006;15(2):260–264. doi: 10.1016/j.jse.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36–41. doi: 10.1016/j.jse.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Cheung E, Willis M, Walker M, et al. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439–449. [PubMed] [Google Scholar]
- 41.Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157–168. [PubMed] [Google Scholar]
- 42.Gallo RA, Gamradt SC, Mattern CJ, et al. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20(4):584–590. doi: 10.1016/j.jse.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 43.Dedy NJ, Stangenberg M, Liem D, et al. Effect of posterior offset humeral components on range of motion in reverse shoulder arthroplasty. Int Orthop. 2011;35(4):549–554. doi: 10.1007/s00264-010-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizuno N, Denard PJ, Raiss P, et al. The clinical and radiographical results of reverse total shoulder arthroplasty with eccentric glenosphere. Int Orthop. 2012;36(8):1647–1653. doi: 10.1007/s00264-012-1539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middernacht B, De Wilde L, Molé D, et al. Glenosphere disengagement: a potentially serious default in reverse shoulder surgery. Clin Orthop Relat Res. 2008;466(4):892–898. doi: 10.1007/s11999-007-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res. 2011;469(9):2544–2549. doi: 10.1007/s11999-011-1881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walch G, Mottier F, Wall B, et al. Acromial insufficiency in reverse shoulder arthroplasties. J Shoulder Elbow Surg. 2009;18(3):495–502. doi: 10.1016/j.jse.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Roche CP, Stroud NJ, Martin BL et al (2013) The impact of scapular notching on reverse shoulder glenoid fixation. J Shoulder Elbow Surg. doi:10.1016/j.jse.2012.10.035 [DOI] [PubMed]
- 49.Wierks C, Skolasky RL, Ji JH, et al. Reverse total shoulder replacement: intraoperative and early postoperative complications. Clin Orthop Relat Res. 2009;467(1):225–234. doi: 10.1007/s11999-008-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalouche I, Sevivas N, Wahegaonker A, et al. Reverse shoulder arthroplasty: does reduced medialisation improve radiological and clinical results? Acta Orthop Belg. 2009;75(2):158–166. [PubMed] [Google Scholar]