Abstract
Purpose
The purpose of this study was to evaluate the impact of tobacco abuse in the consolidation of fractures.
Methods
We retrospectively identified all patients with a diaphyseal fracture (femur, tibia, or humerus), between January 1999 and December 2010, in our orthopaedic trauma registry (Erasme hospital, Brussels, Belgium). Thirty-eight diaphyseal nonunions (ten femurs, 16 tibias and 12 humerus) were identified. Each nonunion was paired (on age, sex and location) with two control-healed fractures (76 control patients). The chi-squared test and a binary logistic regression were used for statistical analysis.
Results
In multivariate analysis, smoking (tobacco use) was significantly associated with nonunion, whether the fracture was open or closed (p < 0.01). In univariate analysis, open fracture was associated with a higher risk of nonunion (p < 0.05), while external fixation was associated with better bone healing (p < 0.05).
Conclusion
Tobacco is confirmed as a deleterious factor for diaphyseal bone healing.
Introduction
Experimental studies have shown that tobacco has negative effects on fracture healing [1]. Nicotine seems to affect the early revascularization of the fractured bone, probably through down-regulated gene transcription of fibroblast growth factor, vascular endothelial growth factor, and bone morphogenetic protein cytokines known to be important to angiogenesis and osteoblast function [1]. In experimental animals, exposure to nicotine decreases union rate and increases complications [2]. The influence of nicotine on bone healing in animals remains controversial; nicotine exposure enhances angiogenesis but cannot compensate for the adverse effect of vasoconstriction [3]. Clinically, the consequences of smoking on bone healing are less clear [4–10]. Several non-randomized and uncontrolled studies have suggested a deleterious effect of tobacco, resulting in delayed healing and increased rates of nonunion [11–13]. As nonunions are not so frequent and as various other factors can influence bone healing, prospective clinical studies are difficult to conduct. Indeed, the rate of nonunion in closed tibial fractures is low with modern methods of treatment. Open diaphyseal tibial fractures have been demonstrated to be associated with higher rates of nonunion and of re-operation to achieve union, and smoking has been demonstrated in these complex lesions to impair bone healing [4, 6, 11, 12]. Tobacco is not the only predictive factor in tibial fractures; other suggested barriers to fracture healing include chronic illness, malnutrition, prior radiation, bone loss, fracture comminution with bone and soft tissue devascularization, instability and infection [14]. In other diaphyseal fractures, smoking is believed to affect bone healing as well, but to our knowledge this has not been formally demonstrated yet, except for the negative effect of tobacco in healing of scaphoid nonunions [8] and of lumbar arthrodeses [15]. The hypothesis of this retrospective study was that tobacco impairs diaphyseal bone healing, whichever bone is fractured, and whether the fracture is open or closed.
Materials and methods
By using the orthopaedic trauma registry of our department, we were able to retrospectively identify all patients over 16 years of age treated from January 1999 to December 2010 at our institution for an unifocal diaphyseal fracture of the femur, the tibia, or the humerus. Three hundred thirty-two cases were identified. Among these cases, 38 were later re-operated on for a nonunion and constituted the studied group of nonunions. These cases were compared to similar fractures which underwent healing, without secondary surgery or primary bone grafting. A precise case–control study with two-to-one matching was conducted. This type of study was designed in an attempt to minimize the impact of small sample size, because of the relatively rare occurrence of the nonunions. A computer generated randomized list was created to select the control cases. All controls (n = 76) were skeletally mature adults operated on for a diaphyseal fracture of either the femur, the tibia or the humerus. Matching was based on age, gender and site of the fractured bone. The study therefore enrolled 114 patients who were followed for a diaphyseal fracture of either tibia, femur or humerus between 1999 and 2010. The following variables were studied: diabetes mellitus, tobacco abuse, multiple trauma/polytrauma, associated skin injury (open fracture), presence or absence of head injury, and type of osteosynthesis.
Study definitions
Clinical and radiographic criteria were used to define bone union or nonunion. A nonunion was defined as a fracture that had failed to show continuity of three of four cortices, six or more months from the time of the fracture-related injury, or had failed to demonstrate any radiographic change (improvement) for three consecutive months, and was associated with clinical findings consistent with a nonunion (inability to bear weight on the affected extremity, pain on palpation, or motion at the fracture site for three to six months or more following the incident traumatic event). Only cases operated on for a nonunion with peroperative confirmation of bone fragments mobility were included in the study. Clinical criteria of fracture healing included no pain on weight bearing, palpation, or attempted manual bending of the fracture site and no movement of the fracture fragments at the fracture site. Imaging assessment included antero-posterior and lateral radiographs made at the time of the initial presentation and at final follow-up. Re-establishment of cortical continuity of a minimum of three of four cortices and the absence of surgery at 12 months defined fracture healing.
Statistical methods
Primary analyses of patients included in the study were performed according to their original allocation (to the nonunion group or control group), regardless of whether or not they reported smoking. A multivariate analysis was conducted by binary logistic regression. The results were regarded as significant if p was <0.05 (two-tailed). We also used the chi-square test to compare the nonunion and control groups with regard to nominal values. The odds ratio as a measure of effect size was used to study the strength of association or non-independence between two binary data values. An analysis process was used to explore the risks of nonunion when patients had several risk factors of nonunion. This analysis is presented as a diagram. Factors that did not show a statistically significant association with the outcome were rejected for the diagram analysis.
Results
The 114 patients (mean age, 47 years; range, 16–85 years) consisted of 87 men (mean age, 42 years; range, 16–85 years) and 27 women (mean age, 63 years; range, 32–84 years). The study population included 38 nonunions (ten femurs, 16 tibias and 12 humerus) in nine female and 29 male patients. The control population consisted of 76 unions (20 femurs, 32 tibias and 24 humerus) in 18 female and 58 male patients. Among the whole group of 114 patients, 35 % were smokers (tobacco), with more smokers among men than women (Table 1)—40 % of the men smoked and 19 % of the women (p < 0.05). Note that the expected prevalence of smokers in Belgium [16] is 30 %. The distribution of open fractures among the three different anatomic sites is presented in Table 1. Among 114 patients, 29 % had an open fracture. Men had a higher percentage of open fractures than women (respectively, 32 % and 19 %) but this was not statistically significant.
Table 1.
Description of the population
| Distribution of smokers between male and female | |||
| Healing | Gender | smokers | non smokers |
| 76 Unions | 58 Men | 17 (29%) | 41 (71%) |
| 18 Women | 2 (11%) | 16 (89%) | |
| 38 Nonunions | 29 Men | 18 (62%) | 11 (38%) |
| 9 Women | 3 (33%) | 6 (67%) | |
| Distribution of open fracture | |||
| Healing | Site | Open fractures | Closed fractures |
| 76 Unions | 32 Tibias | 9 (28%) | 23 (72%) |
| 20 Femurs | 5 (25%) | 15 (75%) | |
| 24 Humerus | 3 (13%) | 21 (87%) | |
| 38 Nonunions | 16 Tibias | 13 (81%) | 3 (19%) |
| 10 Femurs | 3 (33%) | 7 (67%) | |
| 12 Humerus | 0 (0%) | 12 (100%) | |
Among the patients with tibial, femoral and humeral fractures the percentage of open fractures was respectively 46 %, 27 % and 8 %. Fifty-seven patients (50 %) had been treated by external fixation (Table 2), either for an open fracture, or for a closed fracture of the humerus or tibia. Indeed, following the pioneer work of F. Burny, the Brussels School of Orthopaedics and Traumatology continues to use external fixation as the primary treatment of closed diaphyseal fractures of the humerus and tibia, as this type of bone fixation is believed to better respect the biological healing of the fracture [17]. For closed diaphyseal fractures of the femur, nailing is the primary treatment option.
Table 2.
Distribution of treatment
| Healing | Site | External fixation | Flexible nail | Nailing | Open reduction | Non-operative treatment |
|---|---|---|---|---|---|---|
| 76 Unions | 32 Tibias | 26 (82%) | 0 (0%) | 2 (6%) | 2 (6%) | 2 (6%) |
| 20 Femurs | 5 (25%) | 0 (0%) | 13(65%) | 2 (10%) | 0 (0%) | |
| 24 Humerus | 12 (50%) | 0 (0%) | 10 (42%) | 1 (4%) | 1 (4%) | |
| 38 Nonunions | 16 Tibias | 10 (62.5%) | 0 (0%) | 1 (6%) | 3 (19%) | 2 (12.5%) |
| 10 Femurs | 2 (20%) | 0 (0%) | 6 (60%) | 2 (20%) | 0 (0%) | |
| 12 Humerus | 2 (17%) | 3 (25%) | 6 (50%) | 0 (0%) | 1 (8%) | |
| Total | 57 | 3 | 38 | 10 | 6 |
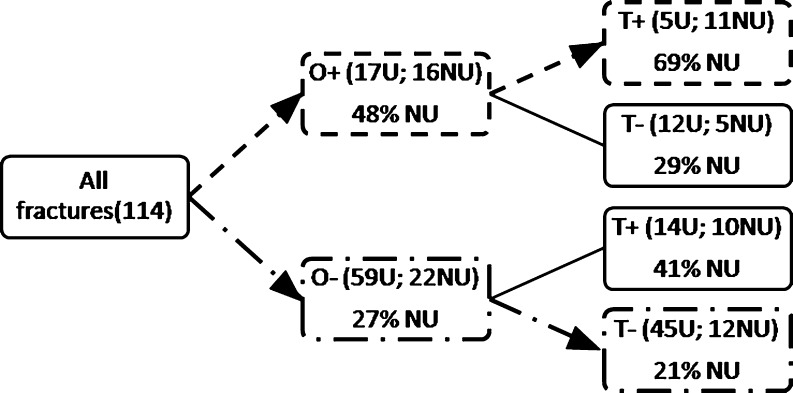
There were 55.3 % (21 patients) smokers in the nonunion group versus 25 % (19 patients) in the control group. There were 44.7 % (17 patients) of initially open fractures in the nonunion group, versus 22.4 % (17 patients) closed fractures in the control group. In multivariate analysis (binary logistic regression), tobacco was the only significant negative predictor of bone healing (Table 3, p < 0.01). In univariate analysis, two significant negative predictors were found—tobacco (p < 0.01) and opening of the fracture at the time of the trauma (p < 0.05). We found also that the risk of nonunion was decreased when external fixation had been chosen for the osteosynthesis (p < 0.05; Table 3). When the analysis process was used (Fig. 1), smoking associated with an open fracture was a predictive factor of nonunion for all anatomical sites (69 % of nonunions) as compared to closed fracture without tobacco (21 % of nonunions). This was statistically significant (p < 0.001) and the odds ratio was 8.25 (95% CI 2.4–28.34).
Table 3.
Results
| Risk factors | Univariate analysis: chi2 tests and OR | Multivariate analysis: binary logistic regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| General risk factors | ||||||
| Smoking | 3.71 | 1.6–8.45 | 0.0014 | 4.14 | 1.56–11 | 0.0046 |
| Open fractures | 2.52 | 1.09–5.84 | 0.0285 | 2.76 | 0.85–8.9 | 0.08 |
| Diabetes | 1.16 | 0.32–4.24 | 0.8 | 1.93 | 0.44–8.42 | 0.3 |
| Multiple trauma | 1.95 | 0.88–4.34 | 0.09 | 1.94 | 0.58–6.46 | 0.2 |
| No head injury | 1.42 | 0.51–3.98 | 0.5 | 2.2 | 0.6–8.25 | 0.2 |
| Treatments | ||||||
| External fixation | 0.45 | 0.2–1 | 0.04 | 0.4 | 0.04–5 | 0.5 |
| Nailing | − | − | − | 0.9 | 0.08–10 | 0.9 |
| Flexible nail | − | − | 0.01 | − | − | 0.9 |
| Plate fixation | 2.66 | 0.76–9.3 | 0.1 | 2.5 | 0.2–31 | 0.4 |
| Non-operative treatment | 4.17 | 0.37–47 | 0.2 | 8 | 0.3–244 | 0.2 |
OR odds ratio, 95% CI 95% confidence interval
Fig. 1.
Diagram analysis of the 114 fractures. Among the patients that presented open fractures associated with tobacco, 69 % had a nonunion versus 21 % for patients presenting closed fractures without tobacco used. This was a statistically significant difference with p < 0.001 and OR = 8.25, 95% CI (2.4–28.34). O+ open fracture, O− closed fracture, T+ smoking, T− no smoking, U union, NU nonunion
Discussion
This study demonstrates that smokers have a higher risk of developing a nonunion after a diaphyseal fracture of the humerus, femur or tibia, whether open or closed. The deleterious effects of smoking on bone healing were already known in tibial fractures [6, 11, 12, 18]. Adams compared complications rates in 140 smoking and 133 non-smoking patients with open tibial fractures [4]. Both groups were evenly matched demographically and in terms of primary fracture treatment. Bone grafting to stimulate union was required in 36 (26 %) smoking patients, compared with 24 (18 %) non-smoking patients. Kyro et al. [13] studied 135 patients with tibial fractures treated non-operatively; 86 % were closed injuries. They found the mean time to union was significantly longer in smokers as compared with non-smokers (166 vs. 134 days). Further surgery to achieve bone union was necessary in 25 % of smokers and 17 % of non-smokers. Schmitz et al. [19] reported on a series of 123 closed and grade I open tibial fractures. The mean time to union was significantly longer in smokers (276 days in smokers as compared with 146 days in non-smokers).
To a lesser extent than smoking, our study suggests also a higher risk of nonunion when the initial fracture was open. The prevalence of open fractures in our group of patients was especially high, due to the selection of patients with nonunions and also probably because the patients were treated in our University Hospital which receives many complex referred emergencies. It is not surprising that bone healing is impaired when the fracture is open, contaminated, usually with associated extensive lesions of the soft tissues, bone comminution and periosteal laceration.
An unexpected finding of this study was that the risk of nonunion was found to be decreased, when external fixation was used for the osteosynthesis. In this particular series, external fixation had been used for some closed fractures and for all open fractures. One would therefore expect an increased risk of nonunion in the group of patients treated by external fixation, but indeed the reverse was observed, possibly because external fixation is the osteosynthesis technique interfering the least with the biology of callus formation, while permitting micromovements stimulating early callus formation. This interesting observation might justify a future randomized prospective study, comparing external fixation with nailing for the treatment of closed diaphyseal fractures.
We recognize the limitations of this study. This retrospective analysis has a particular design, i.e. because nonunions occur infrequently, our study pooled data from three different anatomical locations in an attempt to identify general risk factors for nonunion. Matching of cases and controls was based on age, gender, and site of fracture. However, it would probably have been better to match also the patients by fracture type (closed vs open, type of fracture line) and type of treatment, which was unfortunately not possible. Also because of the particular matching of the patients, we could not investigate if age, gender or fracture location were significant predictive factors. We could also not investigate if the deleterious effects of tobacco on bone healing are dose-dependant or not. As the patients were noted in the charts as “smokers” or “non-smokers” at the time of the fracture, as the dose was not quantified, and as the post traumatic evolution of the tobacco habits were not recorded, we were unable to search for a possible relationship between bone healing and the number of daily cigarettes, nor if cessation of smoking could possibly have reduced the rate of nonunion (the patients received general information against tobacco addiction, but not about a possible increased risk of nonunion). In other published reports [6, 11, 12, 18], patients were considered as smokers according to the quantity of cigarettes smoked per day (for example, over 11 cigarettes). Probably only a prospective study could provide more precise answers. Another limitation of this study concerns the definition of nonunion and bone healing. As there is no standardization of clinical signs for defining delayed healing or nonunion, variability in judgment among surgeons may have occurred. Although variability in diagnosis cannot be quantified, a recent study [20] showed that the recording of tibial fracture healing by general impression was relatively reliable (kappa = 0.67). Nevertheless, to avoid this bias we selected cases with a minimum 12 months follow-up and strict criteria for healed fractures and patients with secondary surgery for nonunions.
We identified tobacco use, and, to a lesser extent, the initial opening of the fracture as significant predictors of nonunion after shaft fractures of the humerus, femur and tibia. Although the presence of associated open traumatic wounds cannot be modified (but early flap coverage could play a role in bone healing), nonunions and the need for secondary procedures to achieve union can be discussed with the patients at the time of the trauma. Although it remains unknown if modifying tobacco use [10] has an impact on fracture healing, it appears logical to advocate tobacco discontinuation in smokers, at least during the healing time of the bone.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Jacques Hernigou, Phone: +32-336-61169481, Email: jacques.hernigou@ulb.ac.be.
Frédéric Schuind, Email: frederic.schuind@erasme.ulb.ac.be.
References
- 1.Sloan A, Hussain I, Maqsood M, Eremin O, El-Sheemy M. The effects of smoking on fracture healing. Surgeon. 2010;8(2):111–116. doi: 10.1016/j.surge.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Raikin SM, Landsman JC, Alexander VA, Froimson MI, Plaxton NA (1998) Effect of nicotine on the rate and strength of long bone fracture healing. Clin Orthop Relat Res (353):231–237 [DOI] [PubMed]
- 3.Zheng LW, Ma L, Cheung LK. Changes in blood perfusion and bone healing induced by nicotine during distraction osteogenesis. Bone Aug. 2008;43(2):355–361. doi: 10.1016/j.bone.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Adams CI, Keating JF, Court-Brown CM. Cigarette smoking and open tibial fractures. Injury. 2001;32(1):61–65. doi: 10.1016/S0020-1383(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 5.Brown CW, Orme TJ, Richardson HD. The rate of nonunion (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine. 1986;11(9):942–943. doi: 10.1097/00007632-198611000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Castillo RC, Bosse MJ, MacKenzie EJ, Patterson BM. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19(3):151–157. doi: 10.1097/00005131-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Chen F, Osterman AL, Mahony K. Smoking and bony union after ulna shortening osteotomy. Am J Orthop. 2005;30(6):486–489. [PubMed] [Google Scholar]
- 8.Little CP, Burston BJ, Hopkinson-Woolley J, Burge P. Failure of surgery for scaphoid nonunion is associated with smoking. J Hand Surg Br. 2006;31(3):252–255. doi: 10.1016/j.jhsb.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Porter SE, Hanley EN., Jr The musculoskeletal effects of smoking. J Am Acad Orthop Surg. 2001;9(1):9–17. doi: 10.5435/00124635-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238(1):1–5. doi: 10.1097/01.SLA.0000074980.39700.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey EJ, Agel J, Selznick HS, Chapman JR, Henley MB. Deleterious effect of smoking on healing of open tibia-shaft fractures. Am J Orthop. 2002;31(9):518–521. [PubMed] [Google Scholar]
- 12.Hoogendoorn JM, van der Werken C. The adverse effects of smoking on healing of open tibial fractures. Ned Tijdschr Geneeskd. 2002;146(35):1640–1644. [PubMed] [Google Scholar]
- 13.Kyrö A, Usenius JP, Aarnio M, Kunnamo I, Avikainen V. Are smokers a risk group for delayed healing of tibial shaft fractures? Ann Chir Gynaecol. 1993;82(4):254–262. [PubMed] [Google Scholar]
- 14.Gaston P, Will E, Elton RA, McQueen MM, Court-Brown CM. Fractures of the tibia: can their outcome be predicted? J Bone Joint Surg. 1999;81(1):71–76. doi: 10.1302/0301-620X.81B1.8958. [DOI] [PubMed] [Google Scholar]
- 15.Glassman SD, Anagnost SC, Parker A, Burke D, Johnson JR, Dimar JR. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine. 2000;25(20):2608–2615. doi: 10.1097/00007632-200010150-00011. [DOI] [PubMed] [Google Scholar]
- 16.Institut Scientifique de la Sante Publique (2004) Enquête de santé par interview Belgique 2004. http://www.wiv-isp.be/epidemio/epifr/crospfr/hisfr/his04fr/his35fr.pdf. Accessed 28 January 2013.
- 17.Schuind F (2012) Technique de pose d’un fixateur externe unilatéral des membres. EMC. doi:10.1016/S0246-0467(12)58651-4
- 18.Gualdrini GD, Zati A, Degli Esposti S. The effects of cigarette smoke on the progression of septic nonunion of the tibia treated by Ilizarov external fixator. Chir Organi Mov. 1996;81(4):395–400. [PubMed] [Google Scholar]
- 19.Schmitz MA, Finnegan M, Natarajan R, Champine J (1999) Effect of smoking on tibial shaft fracture healing. Clin Orthop (365):184–200 [DOI] [PubMed]
- 20.Whelan DB, Bhandari M, McKee MD, Guyatt GH, Kreder HJ, Stephen D, Schemitsch EH. Interobserver and intraobserver variation in the assessment of the healing of tibial fractures after intramedullary fixation. J Bone Joint Surg. 2002;84(1):15–18. doi: 10.1302/0301-620X.84B1.11347. [DOI] [PubMed] [Google Scholar]