Abstract
Purpose
Although serum hyaluronan (HA) levels increase in patients with osteoarthritis (OA), the association between OA severity and elevation of serum HA levels is not clear. Our purpose was to investigate the relationship between serum HA levels and OA in various anatomical sites and to detect which joints are strongly correlated with elevated serum HA levels.
Methods
Seven hundred and ten individuals from the general population who participated in the Iwaki Health Promotion Project in 2008 were involved. Kellgren–Lawrence grade 2 or higher in the knee, hip, lumbar spine, finger and wrist was defined as OA. Serum HA levels were determined on the same day. Spearman’s correlation coefficients between serum HA levels and total number of joints affected by OA were calculated. Linear regression was analysed with serum HA levels as the independent variable; age, gender, presence of OA and intake of supplements were used as dependent variables.
Results
Prevalence of knee OA was 30.7 %, hip 16.8 %, lumbar spine 65.1 %, wrist 9.0 % and finger 22.0 %. Serum HA levels had a positive correlation with the number of involved joints, and the correlation coefficient was 0.410 (p < 0.001). Serum HA was significantly affected by age (β = 0.382), knee OA (β = 0.163) and finger OA (β = 0.164).
Conclusion
Although this biomarker reflects a systemic condition, higher serum HA levels were associated with total number of OA joints. Knee and finger OA were key joints related to increased serum HA levels. These results are valuable in understanding characteristics of serum HA levels as a biomarker for osteoarthritis.
Introduction
Osteoarthritis (OA) is one of the most common disorders to affect elderly populations and is a major cause of pain and disability [1–3], resulting in enormous financial costs for therapy, culminating in surgical treatment. Therefore, OA patients should be detected at an early stage of disease to ensure a precise therapeutic response, such as strength training and alignment correction of the lower extremity. However, diagnosis and detection of OA progression at an early stage by radiographs alone is considered to be inadequate because of the low sensitivity of radiographs to detect minor changes in detail [4].
Biomarkers are attracting attention as new diagnostic tools. Advantages of biomarkers include less invasiveness, low cost, easy collection, short examination time and no requirement for extra devices. There are many biomarkers for cartilage, bone and synovitis [5]. Among them, serum hyaluronan (HA) level is considered a good biomarker for reflecting cartilage damage and synovitis in OA patients [6, 7]. Previous studies show its association with the presence [7–9], duration [10] and severity of radiographic OA [11]; degree of pain [5, 11, 12]; and OA progression [13]. However, systemic biomarkers have negative aspects, as they are affected by many factors related to aging [14] and physical activity [15]. Furthermore, these previous studies were performed only in disease-specific joints despite the fact that most or many OA patients have disease in several joints. The number of OA joints increases with age regardless of gender [3]. OA is generally recognised as a part of the aging process, and people with osteoarthritic painful joints in multiple sites experience more disabilities and have relatively lower health status than people with knee or hip OA only [16, 17]. Therefore, it is necessary to consider the presence of comorbid OA in the elderly. However, the relationship between the systemic OA activity and serum HA levels is unclear.
The purpose of this study was to investigate the relationship between serum HA levels and total number of comorbid OA and to detect which joints are related to elevated serum HA levels. In order to understand serum HA level as a biomarker, it is necessary to elucidate its relationship with systemic OA activity. We postulated that serum HA levels increase with the presence of systemic OA and that the number of involved joints increases in proportion to elevations in serum HA levels.
Materials and methods
Patients were voluntary participants from the Iwaki Health Promotion Project of 2008, which is a community-based programme to prevent lifestyle diseases and improve average life expectancy by performing general health checkups and prophylactic interventions [11]. It is an annual programme that has been performed in the general population living in the Iwaki area of Hirosaki City located in western Aomori prefecture, Japan, since 2005. This cohort study allows evaluation of many kinds of diseases and disorders from various aspects and research into the risk factors of locomotive disability. All participants provided written informed consent, and the study was conducted with the approval of the ethics committee of the Hirosaki University School of Medicine.
Participants
A total of 866 volunteers from approximately 12,000 residents participated in this project in 2008. They were recruited by calls from public health nurses and an advertisement in the mass media: 710 of 866 individuals were selected as appropriate for analysis. Exclusion criteria included those who had undergone surgery for joint diseases, renal failure, liver failure, rheumatoid arthritis, malignant tumours and incomplete questionnaires. Those who did not undergo radiographic examination were also excluded. A total of 269 men and 441 women were assessed. Their mean age was 58.3 ± 11.6 (21–85) years. All participants completed a questionnaire regarding age, gender and intake of supplements (glucosamine or HA orally) for joints. Height and body weight were measured, and body mass index (BMI) was calculated.
Serum HA level
Blood samples were taken from all participants in the early morning for biochemical examination. Blood sampling was performed before breakfast because circulating HA levels increase following a meal [18]. Serum HA levels were determined using the Hyaluronan Assay Kit (Seikagaku Corporation, Tokyo, Japan).
Radiographic GOA
A total of four radiographs were taken to evaluate five joint groups: anterior–posterior view of weight-bearing bilateral knees, weight-bearing bilateral hips, bilateral hands for wrists and fingers and lateral lumbar spine. The following regions were evaluated from each joint group. Finger OA consisted of 30 regions: proximal interphalangeal, distal interphalangeal, metacarpophalangeal and carpometacarpal joints of both hands. Wrist OA evaluated eight regions: intercarpal, radiocarpal, ulnocarpal and distal radioulnar joints of both hands. Knee and hip joints were evaluated individually for OA. Lumbar OA consisted of five regions: degeneration of intervertebral space between L1/2 to L5/S1. Each joint was graded according to Kellgren–Lawrence (KL) grade [19]. The presence of OA was defined as KL grade 2 or more in the severe region in each joint. All joints were graded by two orthopaedic surgeons (YI and RI). If their findings differed, they came to a conclusion after mutual consultation.
Statistical analysis
Data input and calculations were performed with SPSS ver. 12.0 J (SPSS Inc., Chicago, IL, USA). Differences in mean values of age, height, weight, BMI and serum HA levels between men and women were compared using Mann–Whitney U test. Rates of supplement use between men and women were compared by chi-square test. Percentages of involved joints amongst age groups (<50, 50s, 60s, >70) in men and women were compared by analysis of variance (ANOVA) and Tukey method. In each joint, Spearman’s correlation coefficient was calculated between serum HA levels and total number of involved regions, and serum HA levels of those with or without OA were compared using Mann–Whitney U test. Serum HA levels by the number of involved joints were compared using analysis of covariance (ANCOVA). Serum HA levels were adjusted by age, gender, BMI and joint supplements. For comparison among involved joints as a post hoc analysis, the Bonferroni method for multiple comparisons was applied regardless of the significant differences of ANCOVA. Linear regression analysis was performed with serum HA levels as a dependent variable and age, gender, intake of joint supplements and presence of OA in knee, hip, lumbar region, wrist and fingers were used as dependent variables. Standard partial regression coefficient (β) was calculated to indicate the strength of correlation to serum HA levels. A p value <0.05 was considered to be statistically significant.
Results
There was no significant difference of mean age between the 269 men and 441 women (p = 0.156). Body height (p = 0.011) and BMI (p < 0.001) in men were significantly higher than in women. The number of supplement users was 91, and use was significantly higher in women than men (p = 0.002) (Table 1). The average serum HA level of participants was 68.3 ± 44.9 ng/ml. There was no significant difference of serum HA level between men and women (p = 0.111) (Table. 1).
Table 1.
Summary of physical features among gender groups
| Men (n = 269) | Women (n = 441) | Total (n = 710) | |
|---|---|---|---|
| Age (years) | 57.4 ± 12.6 | 58.8 ± 11.0 | 58.3 ± 11.7 |
| Height (cm) | 159.5 ± 8.9 | 157.8 ± 9.1* | 158.4 ± 9.0 |
| Weight (kg) | 58.5 ± 10.9 | 57.8 ± 10.1 | 58.1 ± 10.4 |
| BMI (kg/m2) | 23.5 ± 2.8 | 22.8 ± 3.2* | 23.1 ± 3.1 |
| Serum HA levels (ng/ml) | 65.2 ± 42.5 | 70.2 ± 46.2 | 68.3 ± 44.9 |
| Supplement (%) | 7.8 | 15.9* | 12.8 |
Values are means ± standard deviation of age, height, weight, BMI and serum HA levels and percentage of joint supplement intake. Differences between men and women were compared using Mann–Whitney U test
BMI body mass index, HA hyaluronan
*P value <0.05 significant
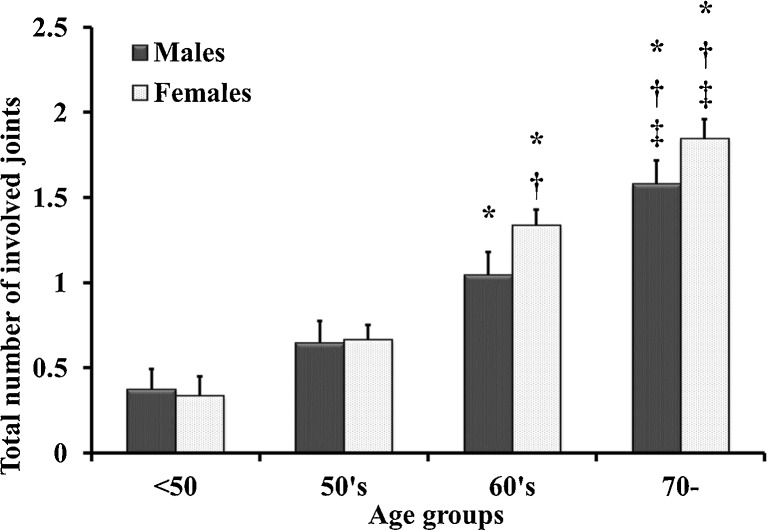
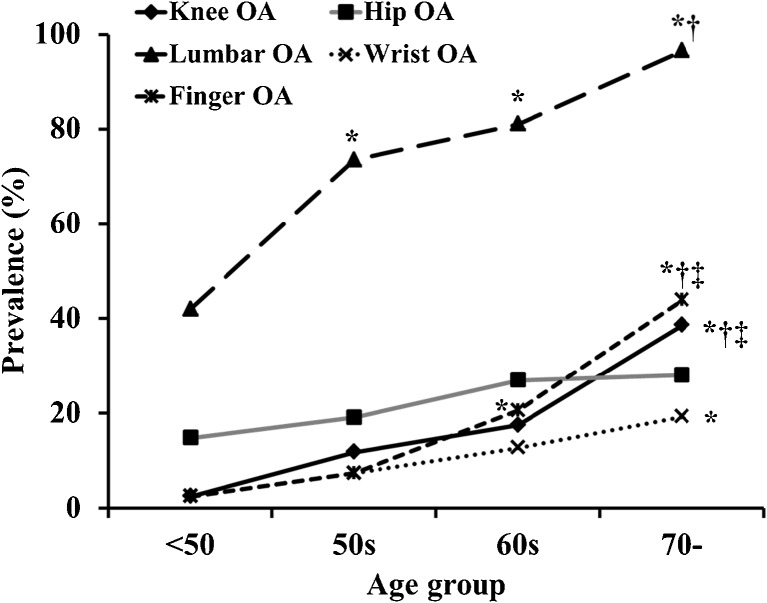
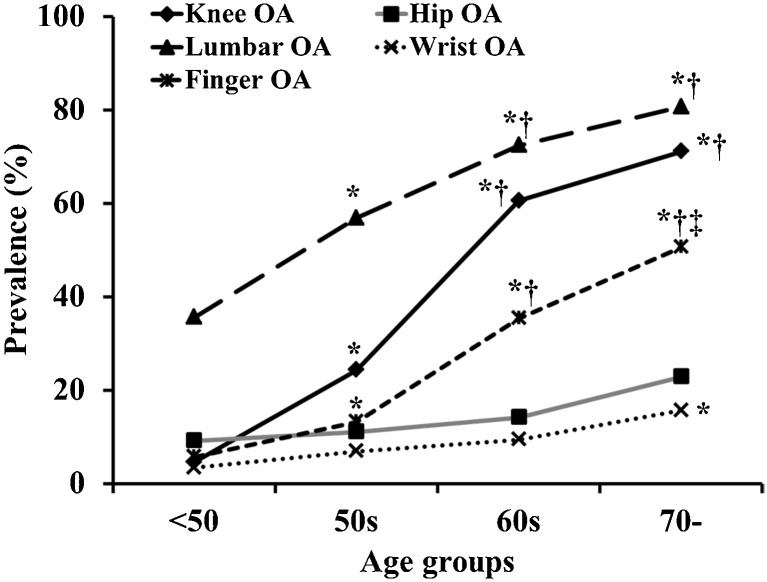
Overall prevalence of OA in the knee was 30.7 %, hip 16.8 %, lumber spine 65.1 %, wrist 9.0 % and finger 22.0 %. Total number of involved joint groups increased with age, especially in men and women over 60 years (Fig. 1). The number of patients with OA in five joints was three (0.4 %), four joints 31 (4.4 %), three joints 40 (5.6 %), two joints 127 (17.9 %) and any one joint 163 (23.0 %); there was no OA in 346 (48.7 %) study participants. The prevalence of lumbar OA in men and women was high, and knee OA showed a high prevalence in women (Figs. 2 and 3).
Fig. 1.
Total number of involved joint groups among age groups in men and women. Total number of involved joints, adjusted for intake of supplements and body mass index (BMI), was compared using analysis of covariance. For post hoc analysis, the Bonferroni method for multiple comparisons was applied. * p < 0.05 vs <40 age group, † p < 0.05 vs 50s age group, ‡ p < 0.05 vs 60s age group
Fig. 2.
Process of prevalence in knee, hip, lumbar spine, finger and wrist in men. Prevalences among age groups were compared using analysis of variance. Post hoc analysis used the Tukey method for multiple comparisons. * p < 0.05 vs <50s age group, † p < 0.05 vs 50s age group, ‡ p < 0.05 vs 60s age group
Fig. 3.
Process of prevalence in knee, hip, lumbar spine, finger and wrist in women. Prevalence among age groups were compared using analysis of variance. Post hoc analysis used the Tukey method for multiple comparisons. * p < 0.05 vs <50 age group, † p < 0.05 vs 50s age group, ‡ p < 0.05 vs 60s age group
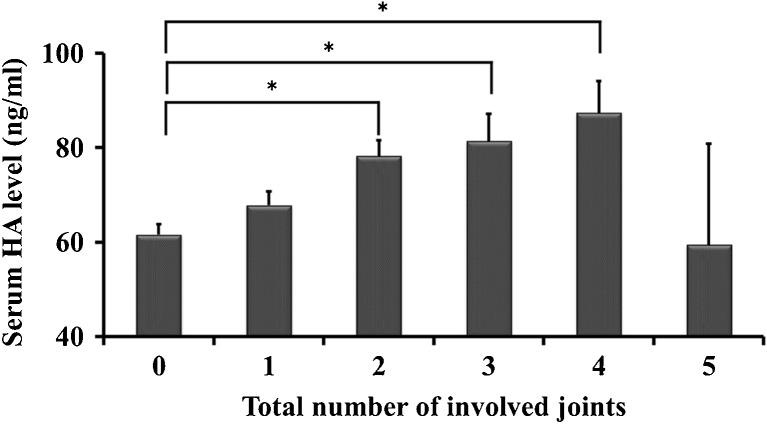
Presence of OA was associated with higher serum HA levels: knee (p < 0.001), hip (p = 0.019), lumbar (p < 0.001), wrist (p = 0.002) and finger (p < 0.001). Total number of involved joints was correlated with the increase in serum HA levels, and the correlation coefficient was 0.410 (p < 0.001) (Table 2). Furthermore, those with OA in two, three or four joints had significantly higher serum HA levels than those without OA, adjusted for age (Fig. 4). Also, serum HA levels had significant correlations with the number of OA regions. Correlation coefficient with knee OA was the highest among joint groups (Table 2).
Table 2.
Correlation coefficient between serum hyaluronan (HA) levels and the number of regions with osteoarthritis (OA) change in each joint group
| Total regions (min–max) | Correlation coefficient | P value |
|---|---|---|
| Knee 0–2 | 0.432 | <0.001 |
| Hip 0–2 | 0.105 | 0.005 |
| Lumbar spine 0–5 | 0.392 | <0.001 |
| Wrist 0–8 | 0.141 | <0.001 |
| Finger 0–25 | 0.363 | <0.001 |
Correlation coefficients were estimated between the number of regions and serum HA levels
P values <0.05 significant
Fig. 4.
Serum hyaluronan (HA) level among number of involved joints adjusted by age, gender, body mass index (BMI) and intake of joint supplements (value ± standard error). P values <0.05 significant (*) in multiple comparisons using the Bonferroni method
The multiple correlation coefficient was 0.593 and a squared multiple correlation coefficient adjusted for degrees of freedom was 0.344 (P < 0.001); a regression model adequate to evaluate factors related to serum HA levels was used. This analysis showed that knee and finger OA and age were significantly correlated with serum HA levels. The influence of knee and finger OA on serum HA levels was equal, with a β value of 0.163 and 0.164, respectively (Table 3).
Table 3.
Evaluation of joints affecting serum hyaluronan (HA) levels
| Standard partial regression coefficient (β) | P value | 95 % confidence interval (CI) | |
|---|---|---|---|
| Gender | −0.034 | 0.302 | −9.00–2.80 |
| Age | 0.382 | <0.001 | 1.20–1.78 |
| Knee OA | 0.163 | <0.001 | 8.87–22.68 |
| Hip OA | −0.006 | 0.846 | −8.06–6.60 |
| Lumbar OA | 0.032 | 0.340 | −3.20–9.26 |
| Wrist OA | 0.039 | 0.217 | −3.57–15.67 |
| Finger OA | 0.164 | <0.001 | 10.49–24.86 |
| Joint supplement intake | 0.049 | 0.125 | −1.82–14.87 |
Linear regression analysis was performed with the serum HA levels as a dependent variable and with gender; age; presence of knee, hip, lumbar, finger and hand OA; and joint supplement intake as dependent variables. P value <0.05 significant
Discussion
Our results show that serum HA levels increased with the total number of involved joints. Also, serum HA levels were higher in those with knee, hip, lumbar spine, wrist, and finger OA than those without OA in these joints. Furthermore, knee and finger OA were strongly related to increases in serum HA levels. To date, this relationship has been unclear, although there are many reports on the relationship between systemic biomarkers and a specific OA joint. Our results therefore suggest that it is important to consider serum HA levels as a biomarker for OA diagnostic purposes. Our study suggests that serum HA values are affected by the presence of involved OA joints in multiple sites in individuals without other serum-HA-related factors. This biomarker increases in the presence of articular synovitis and cartilage damage [6, 7]. Inflamed synovium produces more HA locally and secretes it into the synovial fluid [20]. Increasing intra-articular HA is then forced into blood and lymph due to increasing intra-articular pressure [21]. Furthermore, HA is a component of cartilage, and radiographic joint-space narrowing by cartilage damage is correlated with higher serum HA levels [13]. On the other hand, it is considered that serum biomarkers are affected by other systemic factors, such as medical problems associated with aging, circadian rhythm, food intake and vigorous activities [15] because data are obtained from blood samples. In this study, to analyse the relationship between a serum biomarker and OA, major diseases were excluded, such as liver failure, renal failure, rheumatoid arthritis and presence of malignant tumours [22–24]. Also, the timing of blood sampling was standardised to the early morning to diminish the effect of physical activity, food intake or circadian rhythms. This study was designed to reveal the relationship between serum HA levels and OA joints.
The presence of OA increased serum HA levels, and among these joints, knee and finger OA were key factors for this biomarker. Previous reports have suggested that serum HA levels are associated with several joints [5, 7–13]. In the literature, there is a major focus on the relationship between knee OA and serum HA levels [5, 7, 10–13]. The presence of knee OA indicates higher serum HA levels, even at an early stage, in OA patients [12]. Inoue et al. reported that radiographic severity of knee OA and knee pain were correlated with serum HA levels, which suggests that serum HA levels reflect OA activity [11]. Furthermore, it is expected that this biomarker predicts OA progression [13]. Also, the presence of erosive hand OA in interphalangeal and wrist joints increases serum HA levels [8]. These reports support the results of our study. There are also some reports investigating the association with lumbar OA [9, 25]. Researchers report that mild degenerative lumbar scoliosis or lumbar OA did not significantly correlate with increasing serum HA levels [9], and similar results were shown in our study.
Although knee and finger OA were significantly related to serum HA levels, the reasons for this finding have not been investigated. It is considered that the degree of synovitis and cartilage damage may be associated with these correlations. The knee is the largest among weight-bearing joints and has a large volume of cartilage and synovium. Although the individual size of interphalangeal joints is very small, their number is large, resulting in large cartilage and synovium volume. Kraus et al. reported similar results showing that joint-space narrowing in the carpometacarpal, metacarpophalangeal, knee and hip joints were significantly related to serum HA levels in an analysis that focused on familial hand OA patients [25]. A more detailed evaluation of cartilage and inflamed synovium volume by MRI would further help elucidate the significance of serum HA levels.
There are several limitations to this study. First, presence or possibility of familial OA was not examined. Second, radiographic ankle, elbow and shoulder OA were not evaluated, as OA is more prevalent in the hands, knees, hips and spine and less prevalent in the wrist, elbow, shoulder and ankle [1, 19]. Third, painful joints, intensity and presence of joint effusion were not investigated, although serum HA levels reflect the degree of synovitis which is related to pain and effusion. Fourth, this was a cross-sectional study.
Despite these limitations, this study shows the relationship between serum HA levels and total number of involved joints. In particular, knee and finger OA had correlations with serum HA levels. These results indicate the importance of understanding the characteristics of serum HA level as a diagnostic biomarker for OA or as a research and observational tool because serum biomarkers reflect not only systemic medical conditions but also systemic OA activity. A longitudinal study would further enhance knowledge about serum biomarkers.
Conclusions
Although this biomarker reflects the systemic condition, higher serum HA levels are associated with total number of comorbid OA joints. Knee and finger OA are key joints related to increased serum HA levels. These results are valuable in elucidating the characteristics of serum HA levels as a biomarker for OA.
Acknowledgements
This study was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No.18200044), Japanese Society for the Promotion of Science (No.21500676) and JOA-Subsidized Science Project Research from the Japanese Orthopaedic Association.
Conflict of interest
None.
References
- 1.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Kellgren JH, Lawrence JS. Osteo-arthrosis and disk degeneration in an urban population. Ann Rheum Dis. 1958;17:388–397. doi: 10.1136/ard.17.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;8:646–656. [PMC free article] [PubMed] [Google Scholar]
- 4.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishijima M, Watari T, Naito K, Kaneko H, Futami I, Yoshimura-Ishida K, Tomonaga A, Yamaguchi H, Yamamoto T, Nagaoka I, Kurosawa H, Poole RA, Kaneko K. Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Res Ther. 2011;13:R22. doi: 10.1186/ar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poole AR, Witter J, Roberts N, Piccolo F, Brandt R, Paquin J, Baron M. Inflammation and cartilage metabolism in rheumatoid arthritis. Studies of the blood markers hyaluronic acid, orosomucoid, and keratan sulfate. Arthritis Rheum. 1990;33:790–799. doi: 10.1002/art.1780330605. [DOI] [PubMed] [Google Scholar]
- 7.Garnero P, Piperno M, Gineyts E, Christgau S, Delmas PD, Vignon E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis. 2001;60:619–626. doi: 10.1136/ard.60.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filková M, Senolt L, Braun M, Hulejová H, Pavelková A, Sléglová O, Kupka K, Gatterová J, Pavelka K. Serum hyaluronic acid as a potential marker with a predictive value for further radiographic progression of hand osteoarthritis. Osteoarthr Cartil. 2009;17:1615–1619. doi: 10.1016/j.joca.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Hosogane N, Watanabe K, Tsuji T, Miyamoto T, Ishii K, Niki Y, Nakamura M, Toyama Y, Chiba K, Matsumoto M (2012) Serum cartilage metabolites as biomarkers of degenerative lumbar scoliosis. J Orthop Res 13. doi:10.1002/jor.22067 [DOI] [PubMed]
- 10.Turan Y, Bal S, Gurgan A, Topac H, Koseoglu M. Serum hyaluronan levels in patients with knee osteoarthritis. Clin Rheumatol. 2007;26:1293–1298. doi: 10.1007/s10067-006-0499-4. [DOI] [PubMed] [Google Scholar]
- 11.Inoue R, Ishibashi Y, Tsuda E, Yamamoto Y, Matsuzaka M, Takahashi I, Danjo K, Umeda T, Nakaji S, Toh S. Knee osteoarthritis, knee joint pain and aging in relation to increasing serum hyaluronan level in the Japanese population. Osteoarthr Cartil. 2011;19:51–57. doi: 10.1016/j.joca.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Golightly YM, Marshall SW, Kraus VB, Renner JB, Villaveces A, Casteel C, Jordan JM. Biomarkers of incident radiographic knee osteoarthritis: do they vary by chronic knee symptoms? Arthritis Rheum. 2011;63:2276–2283. doi: 10.1002/art.30412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavelka K, Forejtová S, Olejárová M, Gatterová J, Senolt L, Spacek P, Braun M, Hulejová M, Stovícková J, Pavelková A. Hyaluronic acid levels may have predictive value for the progression of knee osteoarthritis. Osteoarthr Cartil. 2004;12:277–283. doi: 10.1016/j.joca.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Engström-Laurent A, Laurent UB, Lilja K, Laurent TC. Concentration of sodium hyaluronate in serum. Scand J Clin Lab Invest. 1985;45:497–504. doi: 10.3109/00365518509155249. [DOI] [PubMed] [Google Scholar]
- 15.Rössler A, László Z, Kvas E, Hinghofer-Szalkay HG. Plasma hyaluronan concentration: no circadian rhythm but large effect of food intake in humans. Eur J Appl Physiol Occup Physiol. 1998;78:573–577. doi: 10.1007/s004210050463. [DOI] [PubMed] [Google Scholar]
- 16.Hopman-Rock M, Odding E, Hofman A, Kraaimaat FW, Bijlsma JW. Differences in health status of older adults with pain in the hip or knee only and with additional mobility restricting conditions. J Rheumatol. 1997;24:2416–2423. [PubMed] [Google Scholar]
- 17.Buchman AS, Shah RC, Leurgans SE, Boyle PA, Bennett DA. Musculoskeletal pain and incident disability in community-dwelling older adults. Arthritis Care Res. 2010;62:1287–1293. doi: 10.1002/acr.20200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engström-Laurent A. Changes in hyaluronan concentration in tissues and body fluids in disease states. CIBA Found Symp. 1989;143:233–240. doi: 10.1002/9780470513774.ch14. [DOI] [PubMed] [Google Scholar]
- 19.Kellgren JH, Lawrence RC. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells AF, Klareskog L, Lindblad S, Laurent TC. Correlation between increased hyaluronan localized in arthritic synovium and the presence of proliferating cells. A role for macrophage-derived factors. Arthritis Rheum. 1992;35:391–396. doi: 10.1002/art.1780350405. [DOI] [PubMed] [Google Scholar]
- 21.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 22.Hällgren R, Engström-Laurent A, Nisbeth U, Circulating hyaluronate A potential marker of altered metabolism of the connective tissue in uremia. Nephron. 1987;46:150–154. doi: 10.1159/000184331. [DOI] [PubMed] [Google Scholar]
- 23.Valva P, Casciato P, Diaz Carrasco JM, Gadano A, Galdame O, Galoppo MC, Mullen E, De Matteo E, Preciado MV. The role of serum biomarkers in predicting fibrosis progression in pediatric and adult hepatitis C virus chronic infection. PLoS One. 2011;6:e23218. doi: 10.1371/journal.pone.0023218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emlen W, Niebur J, Flanders G, Rutledge J. Measurement of serum hyaluronic acid in patients with rheumatoid arthritis: correlation with disease activity. J Rheumatol. 1996;23:974–978. [PubMed] [Google Scholar]
- 25.Kraus VB, Kepler TB, Stabler T, Renner J, Jordan J. First qualification study of serum biomarkers as indicators of total body burden of osteoarthritis. PLoS One. 2010;5:e9739. doi: 10.1371/journal.pone.0009739. [DOI] [PMC free article] [PubMed] [Google Scholar]