Abstract
Purpose
The haematoma at a fracture site plays an important role in fracture healing. Previously, we demonstrated that a fracture haematoma contains multilineage mesenchymal progenitor cells. We postulated that the haematoma provided a source of chondrogenic cells for endochondral ossification during fracture healing and preservation of the cells contributed to biological fracture healing. In this study, we investigated whether haematoma-derived cells (HCs) could differentiate into hypertrophic chondrocytes and finally induce calcification of the extracellular matrix in vitro.
Methods
Fracture haematomas were obtained from four patients. HCs were cultured for five weeks under conditions that induce chondrogenic differentiation, followed by two weeks of hypertrophic induction using a pellet culture system. The pellets were analysed histologically and immunohistochemically. The gene expression levels of chondrogenic, hypertrophic, osteogenic, and angiogenic markers were measured by real-time PCR.
Results
The histological and immunohistochemical analyses revealed that HCs differentiated into chondrocytes and hypertrophic chondrocytes, followed by calcification of the extracellular matrix. This sequential differentiation was also reflected in the gene expression profiles. After chondrogenic induction, expression of osteogenic and angiogenic markers was not significantly upregulated. However, the expression of these markers was significantly upregulated following hypertrophic induction. These in vitro observations mimicked the process of endochondral ossification during fracture healing.
Conclusions
Our results suggest that the fracture haematoma may offer a source of cells with chondrogenic potential that play key roles in endochondral ossification during fracture healing. These findings support the opinion that the haematoma should be preserved for biological fracture healing.
Introduction
Fracture haematomas play a critical role in fracture healing. Mizuno et al. [1] reported that the fracture haematoma has an inherent osteogenic potential that contributes significantly to fracture healing. During fracture healing, a bone-forming complex develops within the haematoma, composed of cells, inflammatory factors (interleukin-1, interleukin-6, cyclooxygenase-2, etc.), transforming growth factor (TGF)-β, insulin-like growth factor, fibroblast growth factor, platelet derived growth factor, vascular endothelial growth factor (VEGF), and macrophage-colony stimulating factor [2–6]. Recent studies have confirmed that these proteins are central regulators of cellular proliferation, differentiation and matrix synthesis during fracture healing.
Previously, we reported that the cells derived from fracture site haematomas (haematoma-derived cells: HCs) possessed osteogenic, chondrogenic, and adipogenic potential in vitro [7]. The cell-surface antigen profile obtained by flow cytometry revealed that HCs were strongly positive for the mesenchymal stem cell (MSC)-related markers CD29, CD44, CD105, and CD166, but negative for the haematopoietic cell markers CD14, CD34, CD45, and CD133, similar to bone marrow stromal cells [7]. These observations suggest that fracture haematomas contain multilineage mesenchymal progenitor cells that can serve as a reservoir of osteogenic and chondrogenic progenitors during fracture healing.
A haematoma forms as the first step of the healing process following a fracture. As healing progresses, bone is formed via two coordinated mechanisms: intramembranous and endochondral ossifications [2, 3, 8]. During intramembranous ossification, bone matrix is directly deposited by osteoblasts or committed osteoprogenitor cells residing in the periosteum [3, 8]. On the other hand, during endochondral ossification, proliferating chondrocytes differentiate into hypertrophic chondrocytes followed by calcification of the extracellular matrix (ECM) that is later replaced by bone. Previous reports have suggested that the periosteum, bone marrow, and surrounding soft tissues gave rise to the chondrocytes involved in endochondral ossification after a fracture [2, 3, 8–10]. We postulated that haematomas provide a source of chondrogenic cells for endochondral ossification during fracture healing. Furthermore, we hypothesised that preserving a fracture haematoma during fracture surgery using a modern osteosynthesis technique, such as minimally invasive osteosynthesis (MIO), contributes to biological fracture healing via preservation of the cell source for endochondral ossification. To date, several studies have used a pellet culture system in vitro to examine chondrogenic hypertrophic differentiation of human MSCs [11–13]. In this study, we investigated whether HCs could differentiate into hypertrophic chondrocytes and finally induce calcification of the ECM in vitro.
Materials and methods
Patients
Fracture haematomas were obtained from four patients with a mean age of 41 years (23–55) during osteosynthesis, at a mean of 4.8 days after the fracture incident. The fracture sites involved were the tibia (one patient), fibula (two patients) and clavicle (one patient). Patients taking anticoagulants, steroids, or nonsteroidal anti-inflammatory drugs in the three months prior to injury were excluded. The Institutional Review Board of Kobe University Hospital approved this study and informed consent was obtained from all patients.
Isolation and culture of HCs
Human fracture HCs were isolated and cultured as previously described [7, 14]. Following exposure of the fracture site, haematoma containing organised fibrin clots was removed manually before any manipulation or irrigation, and placed in sterile polypropylene containers. Special care was taken not to contain debris of muscle, periosteum, portions of bone marrow or adipose tissue. The samples were minced into small pieces in growth medium—α-modified Minimum Essential Medium (Sigma, St. Louis, MO) containing 10 % heat-inactivated foetal bovine serum (Sigma), 2 mM L-glutamine (Gibco BRL, Grand Island, NY) and antibiotics. The samples were cultured at 37 °C under 5 % humidified CO2. At five to seven days after initiating the culture, the culture medium was replaced with fresh medium. Thereafter, the culture medium was exchanged twice weekly. Approximately two weeks later, the adherent cells were harvested with 0.05 % trypsin/0.02 % ethylenediamine tetra-acetic acid (EDTA) (Wako, Osaka, Japan) and passaged for further expansion.
Chondrogenic and hypertrophic induction
For chondrogenic differentiation, a pellet culture was performed as a three-dimensional culture [11–13]. Approximately 2.5 × 105 cells were centrifuged at 2,000 rpm for four minutes in 15-ml polypropylene tubes. The pellets were cultured in medium for chondrogenic induction for five weeks, followed by culture in medium for hypertrophic induction for two weeks. The chondrogenic medium comprised high-glucose Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA), 100 nM dexamethasone (Sigma), 50 μg/ml L-ascorbic acid-2-phosphate (Sigma), 0.4 mM proline (Sigma), 1 % ITS+1 (Sigma), 10 ng/ml recombinant human TGF-β3 (R&D Systems, Minneapolis, MN) and 500 ng/ml recombinant human bone morphogenetic protein BMP-6 (R&D Systems). To promote hypertrophy, 50 ng/ml thyroxine and 20 mM β-glycerophosphate were added to the chondrogenic medium, while dexamethasone was reduced to 1 nM, and TGF-β3 and BMP-6 were omitted [11, 13].
Histological analysis
Pellets were fixed in 4 % paraformaldehyde for six hours, decalcified in 0.25 mol/L EDTA in phosphate buffered saline (pH 7.5), dehydrated in graded alcohol solutions and embedded in paraffin wax. The sections (6-μm thick) were stained with Safranin-O and Alizarin red S (AR) for microscopy.
Immunohistochemical analysis
For immunohistochemical analyses, deparaffinised sections were digested with proteinase (Dako Cytomation, Inc., Carpinteria, CA) for ten minutes and treated with 3 % hydrogen peroxide to block endogenous peroxidase activity. The sections were incubated overnight at 4 °C with the following primary antibodies: mouse polyclonal antibodies against collagen-II (Daiichi Fine Chemical, Toyama, Japan), collagen-X (Quartett GmbH, Berlin, Germany), alkaline phosphatase (ALP) (Wako, Osaka, Japan), and VEGF (Santa Cruz Biotechnology, Santa Cruz, CA), and a rabbit polyclonal antibody against osteocalcin (OC) (Santa Cruz Biotechnology). The sections were then treated with peroxidise-labelled anti-mouse immunoglobulins (Histofine Simplestain max PO (M); Nichirei Bioscience, Tokyo, Japan) or anti-rabbit immunoglobulins (Histofine Simplestain max PO (R); Nichirei Bioscience) at room temperature for 30 minutes. The signals were developed as a brown reaction product using the peroxidase substrate 3-amino-9-ethylcarbazole (Histofine Simplestain AEC Solution; Nichirei Bioscience). The sections were counterstained with methyl green, and examined microscopically.
Real-time PCR
Total RNA was extracted from the cultures after five and seven weeks using an RNeasy Mini Kit (Qiagen, Valencia, CA) with on-membrane DNase I (Qiagen) digestion to avoid genomic DNA contamination. Total RNA was extracted from HCs before chondrogenic induction as a day-0 control. Total RNA was reverse transcribed into single-stranded cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed using an ABI PRISM 7700 Sequence Detection System and SYBR Green reagents (Applied Biosystems) following the recommended protocols. The expression levels of all genes were normalised by the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels and expressed relative to the day-0 control culture levels (∆∆CT methods) [15]. All primer sequences are shown in Table 1.
Table 1.
Specific primers for real-time RT-PCR amplifications
| Gene name | Primer sequences (5’–3’) | GenBank accession number | |
|---|---|---|---|
| Forward | Reverse | ||
| Col II | GGCAATAGCAGGTTCACGTACA | CGATAACAGTCTTGCCCCACTTA | NM_001844 |
| Col X | GTCTGCTTTTACTGTTATTCTCTCCAAA | TGCTGTTGCCTGTTATACAAAATTTT | NM_000493 |
| MMP-13 | AAGGAGCATGGCGACTTCT | TGGCCCAGGAGGAAAAGC | NM_002427 |
| VEGF | CTCGTTGACACCTGGAAGAGCTTCAAACCG | GGTCCGTCACGTTGTTCCTGTTCAGC | NM_001025370 |
| ALP | CTCGTTGACACCTGGAAGAGCTTCAAACCG | GGTCCGTCACGTTGTTCCTGTTCAGC | NM_000478.4 |
| Runx 2 | ATGCTTCATTCGCCTCACAAAC | CCAAAAGAAGTTTTGCTGACATGG | NM_001024630.1 |
| OSX | CGGGACTCAACAACTCT | CCATAGGGGTGTGTCAT | NM_00117346.1 |
| OC | CATGAGAGCCCTCACA | AGAGCGACACCCTAGAC | NM_199173.2 |
| GAPDH | CGTCTTCACCACCATGGAGA | CGGCCATCACGCCACAGTTT | NM_002046.3 |
Col II type II collagen, Col X type X collagen, MMP-13 matrix metalloproteinase-13, VEGF vascular endothelial growth factor, ALP alkaline phosphatase, Runx 2 runt-related transcription factor 2, OSX osterix, OC osteocalcin, GADPH glyceraldehyde-3-phosphate-dehydrogenase
Statistical analysis
Data are presented as the mean and standard error. The Mann–Whitney U test was used to assess differences in the means among the day-0 control (before chondrogenic induction), as well as 5-week (chondrogenic induction group) and 7-week (hypertrophic induction group) time points. Values of p < 0.05 were considered statistically significant.
Results
Histological and immunohistochemical analyses
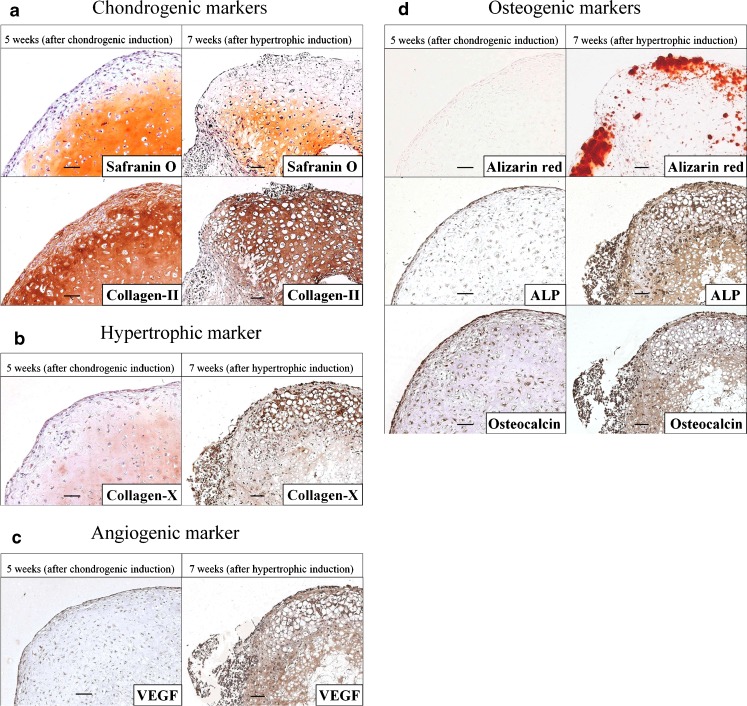
As shown in Fig. 1, the results indicated that HCs differentiated into hypertrophic chondrocytes via chondrocytes after five weeks of chondrogenic induction followed by two weeks of hypertrophic induction. After the five-week chondrogenic induction, HCs were successfully differentiated into chondrogenic cells. The pellets displayed clear chondrocytic features, including positive Safranin-O staining for glycosaminoglycans, and intense staining for collagen-II (Fig. 1a). In contrast, collagen-X deposition was only faintly observed (Fig. 1b). After the two-week hypertrophic induction, the pellets contained regions of hypertrophy (Fig. 1b), with the cells exhibiting a typical hypertrophic morphology, with large lacunae. The ECM surrounding the hypertrophic cells showed intense staining for collagen-II and collagen-X (Fig. 1a, b).
Fig. 1.
Histological and immunohistochemical analyses of the cell pellets at five weeks (after chondrogenic induction) and seven weeks (after hypertrophic induction). a Safranin-O and collagen-II. b Collagen-X. c VEGF. d Alizarin red S, ALP, and OC. Bars = 50 μm
Next, we determined whether the pellets were positive for expression of VEGF, an angiogenic marker. VEGF expression was not detected in the cell pellets after the five weeks of chondrogenic induction, but was detected after the two weeks of hypertrophic induction (Fig. 1c).
Finally, we investigated whether the pellets showed mineralised matrix formation (AR staining), and whether they expressed the osteogenic proteins ALP and OC. The pellets exhibited negative staining for AR after the five weeks of chondrogenic induction, while their outer rims showed positive staining for AR after the two weeks of hypertrophic induction (Fig. 1d). Similarly, expression of ALP and OC was not detected after the five weeks of chondrogenic induction, but was detected after the two weeks of hypertrophic induction (Fig. 1d).
Real-time PCR
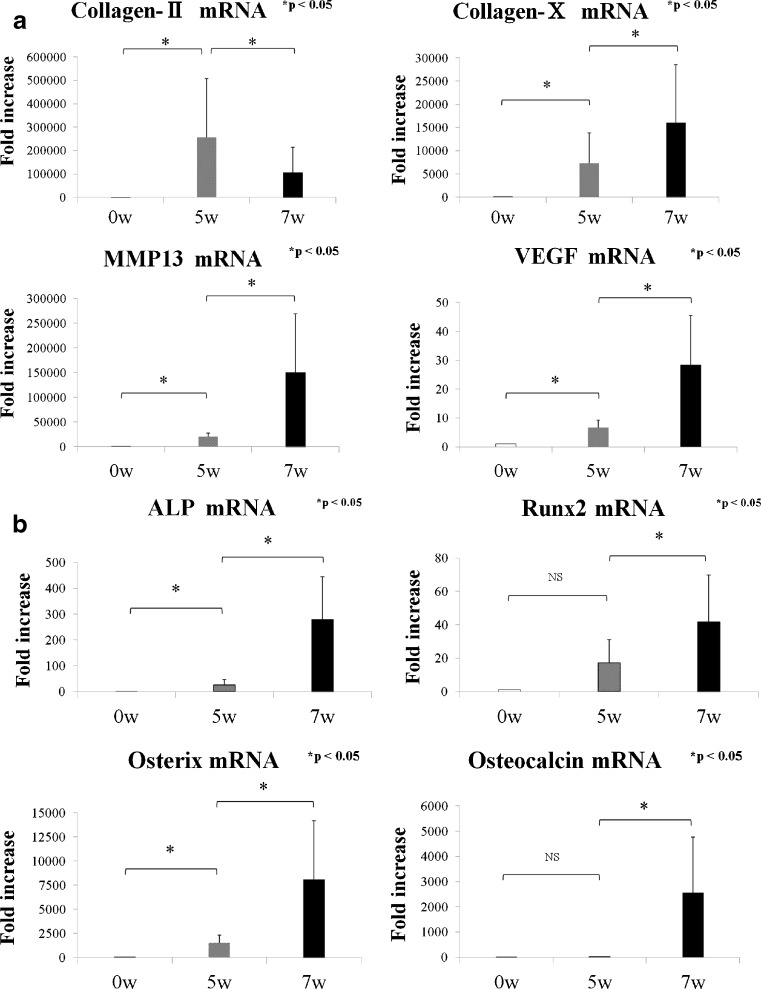
The expression of the chondrogenic marker collagen-II was robustly upregulated after the five-week chondrogenic induction, and then significantly downregulated after the 2-week hypertrophic induction (Fig. 2a). The expression levels of the hypertrophic markers collagen-X and MMP-13 were increased after the five weeks of chondrogenic induction, and then robustly increased after the two weeks of hypertrophic induction (Fig. 2a). Similar findings were observed for expression of the angiogenic marker VEGF at the five- and seven-week time points (Fig. 2a). For the osteogenic markers, the expression levels of ALP and osterix (OSX) were significantly upregulated at the five-week time point, and each of ALP, runt-related transcription factor 2 (Runx 2), OSX, and OC were significantly upregulated following the two weeks of hypertrophic induction, compared with the five-week time point (Fig. 2b).
Fig. 2.
Quantitative real-time PCR analyses at the 0-week (day 0, control), 5-week and 7-week time points. The mRNA levels are expressed relative to the value for the day-0 control (value set at 1 for each marker) and presented as fold increases. *p < 0.05 within the indicated group. a mRNA expression levels of collagen-II, collagen-X, MMP-13, and VEGF. b mRNA expression levels of ALP, Runx2, osterix, and osteocalcin
Discussion
Successful strategy for fracture repair consists of the intricate interplay between the three main constituents of the regenerative system: cells, environment and scaffolds [16]. Hematoma contains cells, factors which provides appropriate environment, and ECM as scaffold. Appropriate cell differentiation requires appropriate biological signalling including growth factors and cytokines [17, 18]. We demonstrated that the differentiation of HCs required appropriate biological signals.
In this study, we reproducibly demonstrated hypertrophic differentiation of HC-derived chondrocytes by switching the standard chondrogenic medium to a medium that favours hypertrophy. This is the first study to show that HCs can differentiate into hypertrophic chondrocytes and induce calcification of the ECM via chondrogenic differentiation in vitro.
During the endochondral ossification stage of fracture healing, MSCs are recruited to the fracture site, and induced to differentiate into chondrocytes that deposit a collagen-II-rich ECM. These cells further differentiate into hypertrophic chondrocytes and start to deposit an ECM composed of collagen-X. This ECM is then partially mineralised [3, 19]. MMP-13 is required for chondrocyte differentiation into a hypertrophic state and its expression occurs together with the upregulation of collagen-X expression [20–22]. Furthermore, VEGF plays a role in neo-angiogenesis found during the endochondral ossification. In this study, we showed significant upregulation of collagen-II upon chondrogenic induction, which was then significantly downregulated when hypertrophy was induced. Furthermore, the gene expressions of collagen-X, MMP-13, VEGF, ALP, Runx2, OSX, and OC were all significantly upregulated during the hypertrophic induction. The same trend was seen in the histological and immunohistochemical analyses. These findings suggest that HCs may differentiate into chondrocytes and hypertrophic chondrocytes, and finally induce calcification of the ECM given the right environmental cues over time. Our in vitro pellet culture system mimicked the process of endochondral ossification during fracture healing, and our findings suggest that the hematoma may offer a cell source during fracture-mediated endochondral ossification.
Several reports have described the hypertrophic differentiation of MSCs in chondrogenesis [11–13]. These reports demonstrated that MSCs differentiated into hypertrophic chondrocytes via chondrocytes in a pellet culture system, and that withdrawal of TGF-β3 and BMP-6, and addition of thyroxine and β-glycerophosphate were prerequisites for enhanced hypertrophy. In our pellet culture system, HCs were similarly able to differentiate into hypertrophic chondrocytes via the intermediate chondrocyte phase. Following a fracture, growth factors, hormones, and cytokines contained within the fracture haematoma may act as important elements for the initiation of HC chondrogenic and subsequent hypertrophic differentiation. Furthermore, it is possible that various cells surrounding the fracture site dynamically change the haematoma environment via the secretion of potent factors that promote chondrogenic and hypertrophic differentiation, and calcification of the ECM.
The goal in modern fracture fixation, using either an intramedullary nail or a plate, is to maintain the fracture hematoma and the blood supply of the bone, representing so-called biological osteosynthesis [23–25]. Biological osteosynthesis, such as MIO, involves minimally invasive surgical approaches. By preserving the osseous vascularity and fracture haematoma, biological osteosynthesis maintains a more biologically favourable environment for fracture repair, while reducing the risks of infection and nonunion [26–28]. Our previous study indicated that HCs were able to differentiate into osteogenic cells in vitro [7]. These findings indicated that HCs were able to differentiate into hypertrophic chondrocytes and induce calcification of the ECM via chondrogenic differentiation in vitro. Our previous and present studies suggest that HCs play key roles in the processes of intramembranous and endochondral ossifications during fracture healing. Our findings may help to shed light on how the preserved haematoma promotes fracture healing during biological osteosynthesis. We recommend preservation of the fracture haematoma where possible, unless it creates an obstacle in reducing the fracture or poses a risk of infection to the patient.
A limitation to our study is that it is an in vitro study. Under an in vivo environment, differentiation ability of HCs may differ to some degree. To confirm the differentiation capacity, further in vivo experiments are required.
In conclusion, our results suggest that the fracture haematoma may offer a source of cells that play key roles in endochondral ossification during fracture healing, and support the opinion that the haematoma should be preserved for biological fracture healing.
Acknowledgments
The authors wish to thank Ms. M. Yasuda, Ms. K. Tanaka, and Ms. M. Nagata (Department of Orthopaedic Surgery, Kobe University Graduate School of Medicine) for their excellent technical assistance.
References
- 1.Mizuno K, Mineo K, Tachibana T, Sumi M, Matsubara T, Hirohata K. The osteogenetic potential of fracture hematoma: subperiosteal and intramuscular transplantation of the hematoma. J Bone Joint Surg Br. 1990;72:822–829. doi: 10.1302/0301-620X.72B5.2211764. [DOI] [PubMed] [Google Scholar]
- 2.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Sarahrudi K, Mousavi M, Thomas A, Eipeldauer S, Vécsei V, Pietschmann P, Aharinejad S. Elevated levels of macrophage colony: stimulating factor in human fracture healing. J Orthop Res. 2010;28:671–676. doi: 10.1002/jor.21048. [DOI] [PubMed] [Google Scholar]
- 5.Kolar P, Gaber T, Perka C, Duda GN, Buttgereit F. Human early fracture hematoma is characterized by inflammation and hypoxia. Clin Orthop Relat Res. 2011;469:3118–3126. doi: 10.1007/s11999-011-1865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groothuis A, Duda GN, Wilson CJ, Thompson MS, Hunter MR, Simon P, Bail HJ, van Scherpenzeel KM, Kasper G. Mechanical stimulation of the pro-angiogenic capacity of human fracture haematoma: involvement of VEGF mechano-regulation. Bone. 2010;47:438–444. doi: 10.1016/j.bone.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Oe K, Miwa M, Sakai Y, Lee SY, Kuroda R, Kurosaka M. An in vitro study demonstrating that haematomas found at the site of human fractures contain progenitor cells with multilineage capacity. J Bone Joint Surg Br. 2007;89:133–138. doi: 10.1302/0301-620X.89B1.18286. [DOI] [PubMed] [Google Scholar]
- 8.Bielby R, Jones E, McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007;38:S26–S32. doi: 10.1016/j.injury.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Ueno M, Uchida K, Takaso M, Minehara H, Suto K, Takahira N, Steck R, Schuetz MA, Itoman M. Distribution of bone marrow-derived cells in the fracture callus during plate fixation in a green fluorescent protein-chimeric mouse model. Exp Anim. 2011;60:455–462. doi: 10.1538/expanim.60.455. [DOI] [PubMed] [Google Scholar]
- 10.Ozaki A, Tsunoda M, Kinoshita S, Saura R. Role of fracture hematoma and periosteum during fracture healing in rats: interaction of fracture hematoma and the periosteum in the initial step of the healing process. J Orthop Sci. 2000;5:64–70. doi: 10.1007/s007760050010. [DOI] [PubMed] [Google Scholar]
- 11.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 12.Mueller MB, Fischer M, Zellner J, Berner A, Dienstknecht T, Prantl L, Kujat R, Nerlich M, Tuan RS, Angele P. Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs. 2010;192:158–166. doi: 10.1159/000313399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, Lopez-Rios J, Zeller R, Barbero A, Martin I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA. 2010;107:7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasegawa T, Miwa M, Sakai Y, Niikura T, Kurosaka M, Komori T. Osteogenic activity of human fracture haematoma-derived progenitor cells is stimulated by low-intensity pulsed ultrasound in vitro. J Bone Joint Surg Br. 2009;91:264–270. doi: 10.1302/0301-620X.91B2.20827. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Ivkovic A, Marijanovic I, Hudetz D, Porter R, Pecina M, Evans C. Regenerative medicine and tissue engineering in orthopaedic surgery. Front Biosci (Elite Ed) 2011;3:923–944. doi: 10.2741/e299. [DOI] [PubMed] [Google Scholar]
- 17.Pecina M, Vukicevic S. Biological aspects of bone, cartilage and tendon regeneration. Int Orthop. 2007;31:719–720. doi: 10.1007/s00264-007-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borovecki F, Pecina-Slaus N, Vukicevic S. Biological mechanisms of bone and cartilage remodelling—genomic perspective. Int Orthop. 2007;31:799–805. doi: 10.1007/s00264-007-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behonick DJ, Xing ZQ, Lieu S, Buckley JM, Lotz JC, Marcucio RS, Werb Z, Miclau T, Colnot C. Role of matrix metalloproteinase 13 in both endochondral and Intramembranous ossification during skeletal regeneration. PLoS One. 2007;2:e1150. doi: 10.1371/journal.pone.0001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Angelo M, Yan Z, Nooreyazdan M, Pacifici M, Sarment D, Billings PC, Leboy PS. MMP-13 is induced during chondrocyte hypertrophy. J Cell Biochem. 2000;77:678–693. doi: 10.1002/(SICI)1097-4644(20000615)77:4<678::AID-JCB15>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 21.Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids—a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–429. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 22.Wu CW, Tchetina EV, Mwale F, Hasty K, Pidoux I, Reiner A, Chen J, Van Wart HE, Poole AR. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J Bone Miner Res. 2002;17:639–651. doi: 10.1359/jbmr.2002.17.4.639. [DOI] [PubMed] [Google Scholar]
- 23.Baumgaertel F, Buhl M, Rahn BA. Fracture healing in biological plate osteosynthesis. Injury. 1998;29:3–6. doi: 10.1016/S0020-1383(98)95002-1. [DOI] [PubMed] [Google Scholar]
- 24.Farouk O, Krettek C, Miclau T, Schandelmaier P, Guy P, Tscherne H. Minimally invasive plate osteosynthesis: does percutaneous plating disrupt femoral blood supply less than the traditional technique? J Orthop Trauma. 1999;13:401–406. doi: 10.1097/00005131-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Kesemenli C, Subasi M, Necmioglu S, Kapukaya A. Treatment of multifragmentary fractures of the femur by indirect reduction (biological) and plate fixation. Injury. 2002;33:691–699. doi: 10.1016/S0020-1383(02)00166-3. [DOI] [PubMed] [Google Scholar]
- 26.Collinge C, Kuper M, Larson K, Protzman R. Minimally invasive plating of high-energy metaphyseal distal tibia fractures. J Orthop Trauma. 2007;21:355–361. doi: 10.1097/BOT.0b013e3180ca83c7. [DOI] [PubMed] [Google Scholar]
- 27.Oh CW, Kyung HS, Park IH, Kim PT, Ihn JC. Distal tibia metaphyseal fractures treated by percutaneous plate osteosynthesis. Clin Orthop Relat Res. 2003;408:286–291. doi: 10.1097/00003086-200303000-00038. [DOI] [PubMed] [Google Scholar]
- 28.Redfern DJ, Syed SU, Davies SJM. Fractures of the distal tibia: minimally invasive plate osteosynthesis. Injury. 2004;35:615–620. doi: 10.1016/j.injury.2003.09.005. [DOI] [PubMed] [Google Scholar]