Abstract
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) has demonstrated clinical benefit in metastatic breast cancer (MBC) in a randomized phase III trial versus paclitaxel (CA012; N = 454) and in a randomized phase II trial versus docetaxel (CA024; N = 300). This retrospective analysis examines whether patients with poor prognostic factors demonstrate similar outcomes to the intent-to-treat (ITT) populations in these trials. This retrospective analysis evaluated the efficacy and safety of previously untreated patients with MBC with the following poor prognostic factors: visceral dominant metastases and short disease-free interval (DFI; ≤2 years). In CA012 (n = 186 first-line patients), nab-paclitaxel demonstrated a significantly higher overall response rate (ORR) versus paclitaxel in patients with visceral dominant metastases (42 vs. 23 %; P = 0.022), whereas the higher ORR for nab-paclitaxel in patients with a short DFI (43 vs. 33 %; P = NS) was not statistically significant. In CA024, a significantly higher ORR for nab-paclitaxel 150 mg/m2 versus docetaxel was observed in patients with visceral dominant metastases (76 vs. 37 %; P < 0.001). No significant differences in ORR were observed in patients with a short DFI. Although progression-free survival (PFS) and overall survival showed trends similar to ORR, statistical significance was only achieved for comparisons of PFS in patients with visceral dominant metastases in CA024 (13.1 months for nab-paclitaxel 150 mg/m2 vs. 7.8 months for docetaxel [P = 0.019] and 7.5 months for nab-paclitaxel 100 mg/m2 [P = 0.010]). Safety results were similar to previous reports of the ITT populations. nab-Paclitaxel demonstrated similar efficacy in patients with poor prognostic factors as in the ITT populations of these two trials. In each trial, ORR was significantly higher for nab-paclitaxel versus the comparator taxane among patients with visceral dominant metastases.
Electronic supplementary material
The online version of this article (doi:10.1007/s10549-013-2447-8) contains supplementary material, which is available to authorized users.
Keywords: nab-Paclitaxel, Visceral disease, Metastatic breast cancer, Taxanes
Introduction
Breast cancer has the highest incidence and second-highest mortality rate of any cancer in women worldwide [1]. Only lung cancer kills more women each year [1]. Although breast cancer mortality has declined over the last two decades [1, 2], approximately 30 % of women initially diagnosed with an earlier stage of breast cancer will develop metastatic disease, which remains essentially incurable [3]. Therefore, treatments that provide clinical benefit among these patients will continue to be sought. Although the 5-year survival rate for patients diagnosed with metastatic breast cancer (MBC) remains less than 25 % [2], a number of factors predict poor survival, including previous adjuvant chemotherapy, estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) status, short disease-free interval (DFI), the number of metastatic lesions, site(s) of recurrence, and visceral involvement [4–8].
Taxanes have proven to be one of the most active classes of antitumor agents for MBC [3]. The evolution of taxane approval for the treatment of MBC began with paclitaxel in 1994, continued with docetaxel (Taxotere) in 1996, and most recently included nanoparticle albumin-bound (nab-) paclitaxel (Abraxane) in 2005 [9]. The activity of taxane monotherapy in patients with MBC who have one or more poor prognostic factors has been well documented, both in the first-line setting and in the context of progressive/resistant disease [10–12].
nab-Paclitaxel was developed to take advantage of the antitumor activity of paclitaxel while decreasing or eliminating the toxicities typically associated with the solvent (Cremophor® EL) used to administer the most common formulation of paclitaxel [13–15]. Results from the phase III trial (N = 454) that led to the approval of nab-paclitaxel demonstrated that nab-paclitaxel at 260 mg/m2 every 3 weeks (q3w) achieved a higher overall response rate (ORR; 33 vs. 19 %; P = 0.001) and a longer time to tumor progression (23.0 vs. 16.9 weeks; HR 0.75; P = 0.006) compared with paclitaxel at 175 mg/m2 q3w in patients with MBC who were treated in the ≥first-line setting [14]. Among patients who received ≥second-line treatment for MBC, the median overall survival (OS) for nab-paclitaxel was significantly greater than that of paclitaxel (56.4 vs. 46.7 weeks; P = 0.024). Grade 4 neutropenia was less frequent among patients who received nab-paclitaxel (9 vs. 22 %; P < 0.001), but grade 3 sensory neuropathy occurred at a higher rate (10 vs. 2 %; P < 0.001).
In order to evaluate the activity of nab-paclitaxel in a first-line MBC population and explore whether a weekly schedule might offer favorable clinical outcomes relative to the approved q3w schedule, a randomized phase II trial (N = 300) was conducted [16]. Patients received one of the following treatments: nab-paclitaxel 300 mg/m2 q3w, 100 mg/m2 the first 3 of 4 weeks (qw 3/4), or 150 mg/m2 qw 3/4 or docetaxel at 100 mg/m2 q3w. This study demonstrated superior efficacy and safety of weekly nab-paclitaxel compared with docetaxel. nab-Paclitaxel 150 mg/m2 qw 3/4 had the highest investigator-assessed ORR (74 vs. 46 % for nab-paclitaxel 300 mg/m2 q3w [P = 0.002], 63 % for nab-paclitaxel 100 mg/m2 qw 3/4 [P not statistically significant, NS], and 39 % for docetaxel [P < 0.001]). nab-Paclitaxel 150 mg/m2 qw 3/4 also demonstrated the longest investigator-assessed progression-free survival (PFS; 14.6 vs. 10.9 months for nab-paclitaxel 300 mg/m2 q3w [P = NS], 7.5 months for nab-paclitaxel 100 mg/m2 qw 3/4 [P = 0.001], and 7.8 months for docetaxel [P = 0.012]). Patients who received nab-paclitaxel at 150 mg/m2 qw 3/4 also had the longest median OS (overall P = 0.047): 33.8 months vs. 27.7, 22.2, and 26.6 months in patients who received nab-paclitaxel 300 mg/m2 q3w, 100 mg/m2 qw 3/4, and docetaxel, respectively [17]. The 100 mg/m2 nab-paclitaxel arm exhibited the best safety profile with the lowest rates of grade ≥3 neutropenia (25 vs. 43–45 % in the other nab-paclitaxel arms and 92 % in the docetaxel arm), sensory neuropathy (8 vs. 14–17 % in the other nab-paclitaxel arms and 12 % in the docetaxel arm), and fatigue (0 vs. 3–5 % in the other nab-paclitaxel arms and 19 % in the docetaxel arm).
The objective of this analysis was to examine the efficacy and safety of nab-paclitaxel versus paclitaxel and docetaxel in patients with poor prognostic factors. As such, we performed a post hoc analysis of patients who received first-line treatment in the two randomized trials described above to determine whether the efficacy and safety of nab-paclitaxel were maintained across patient subgroups defined by DFI or visceral dominant metastases.
Methods
Patients
The patient populations for the two trials were analyzed separately due to differences in treatment and patient eligibility. In both trials, patients ≥18 years old with pathologically confirmed MBC, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and adequate hematologic, hepatic, and renal function were included. For the phase III CA012 trial, patients who had received docetaxel or paclitaxel in the adjuvant setting were permitted if ≥1 year had passed since completion of that therapy [14]. Patients in the phase II CA024 study had not received prior chemotherapy for metastatic disease, but patients who had received chemotherapy in the neoadjuvant or adjuvant setting were permitted if ≥1 year had passed since completion of that therapy [16]. Patients with preexisting sensory neuropathy of grade ≥1 were excluded from CA012, whereas patients in CA024 were permitted to enroll with grade ≤1 sensory neuropathy.
Study design and assessments
This is a retrospective analysis of patients from the randomized, multicenter phase III trial (CA012) and a randomized, multicenter phase II trial (CA024). For detailed information on the trial designs, see Gradishar et al. [14, 16], respectively. Although patients enrolled in CA012 could have received chemotherapy for metastatic disease if the treatment did not contain solvent-based paclitaxel or docetaxel, this analysis only includes those patients who had not received prior chemotherapy for metastatic disease. Trial CA024 included only patients who received first-line treatment. Within each of the six treatment arms among the two studies, patients were subdivided into the following subgroups for this analysis: patients with visceral dominant metastases and patients with a short DFI (≤2 years).
The primary efficacy endpoint for both studies was ORR, defined as complete response + partial response. Responses were assessed by the trial investigators using Response Evaluation Criteria in Solid Tumors [18]. Other efficacy endpoints in this analysis included median OS and PFS. The safety endpoints included in this analysis were treatment-related adverse events, including sensory neuropathy, neutropenia, and fatigue. Hematologic toxicity was reported on the basis of central laboratory data.
In the CA012 trial, patients received either nab-paclitaxel at 260 mg/m2 q3w or paclitaxel at 175 mg/m2 q3w. In the CA024 trial, patients received docetaxel at 100 mg/m2 q3w or nab-paclitaxel at 300 mg/m2 q3w, 100 mg/m2 qw 3/4, or 150 mg/m2 qw 3/4.
Statistical methods
The statistical analyses for the two trials were carried out separately as it was not appropriate to combine patient populations. Descriptive statistics were used to calculate the ORRs and 95 % binomial confidence intervals. Median OS and PFS values were calculated using Kaplan–Meier methods. For CA012, the median PFS represented the time from first dose to disease progression or death, and the median OS represented the time from first dose to death. For CA024, the median PFS represented the time from patient randomization to disease progression or death, and the median OS represented the time from patient randomization to death. Tumor shrinkage was calculated as the percent change in size from baseline of the target lesion to the smallest post-baseline measurement.
Results
Patients
The CA012 trial included 97 and 89 patients who received first-line therapy in the nab-paclitaxel and paclitaxel treatment arms, respectively. The numbers of patients per treatment arm in the intent-to-treat (ITT) population of CA024 ranged from 74 to 76. Baseline characteristics within each trial for the given patient subgroups were well balanced (Table 1).
Table 1.
Baseline patient characteristics
| Trial/treatment | n | Age (years), mean | White race, n (%) | Body weight (kg), mean | ECOG PS ≤1, n (%) | ECOG PS 2, n (%) | Post-menopausal, n (%) |
|---|---|---|---|---|---|---|---|
| Visceral dominant metastasis | |||||||
| CA012 | |||||||
| nab-P 260 mg/m2 q3w | 74 | 52.2 | 70 (95) | 72.4 | 68 (92) | 6 (8) | 52 (70) |
| P 175 mg/m2 q3w | 64 | 53.0 | 60 (94) | 71.5 | 63 (98) | 1 (2) | 45 (70) |
| CA024 | |||||||
| nab-P 300 mg/m2 q3w | 61 | 51.6 | 59 (97) | 73.2 | 55 (90) | 6 (10) | 39 (64) |
| nab-P 100 mg/m2 qw 3/4 | 60 | 55.6 | 59 (98) | 71.8 | 56 (93) | 4 (7) | 50 (83) |
| nab-P 150 mg/m2 qw 3/4 | 59 | 53.8 | 59 (100) | 76.1 | 55 (93) | 4 (7) | 45 (76) |
| Doc 100 mg/m2 q3w | 67 | 56.2 | 67 (100) | 75.9 | 65 (97) | 2 (3) | 57 (85) |
| Short DFI | |||||||
| CA012 | |||||||
| nab-P 260 mg/m2 q3w | 42 | 50.8 | 42 (100) | 72.4 | 39 (93) | 3 (7) | 31 (74) |
| P 175 mg/m2 q3w | 30 | 52.3 | 30 (100) | 70.0 | 30 (100) | 0 | 21 (70) |
| CA024 | |||||||
| nab-P 300 mg/m2 q3w | 20 | 49.5 | 20 (100) | 73.2 | 18 (90) | 2 (10) | 13 (65) |
| nab-P 100 mg/m2 qw 3/4 | 21 | 51.4 | 21 (100) | 75.7 | 21 (100) | 0 | 17 (81) |
| nab-P 150 mg/m2 qw 3/4 | 14 | 52.2 | 14 (100) | 80.1 | 12 (86) | 2 (14) | 9 (64) |
| Doc 100 mg/m2 q3w | 19 | 51.5 | 19 (100) | 84.5 | 19 (100) | 0 | 14 (74) |
DFI disease-free interval, nab-P nab-paclitaxel, Doc docetaxel, ECOG PS Eastern Cooperative Oncology Group performance status, P paclitaxel, q3w every 3 weeks, qw 3/4 the first 3 of 4 weeks
Efficacy
Overall response rate
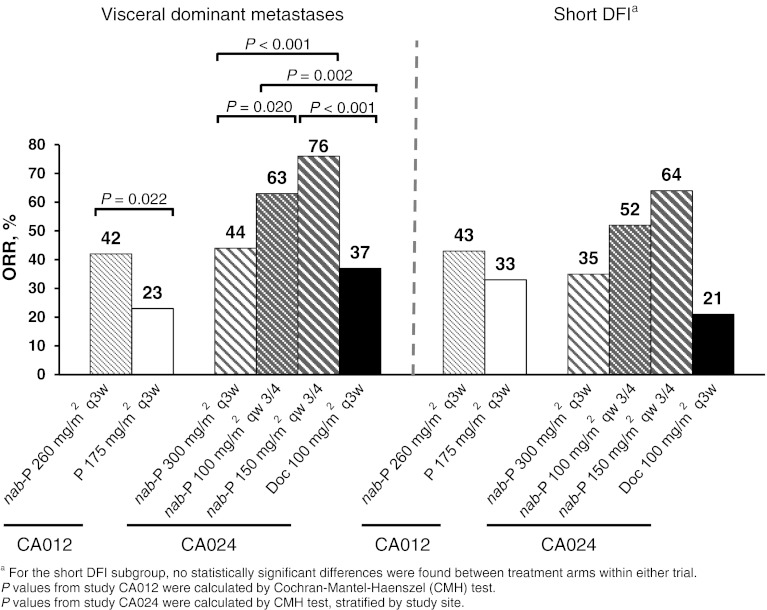
In the CA012 trial, ORR results for each poor prognostic factor subset were similar to the results from the general ITT population (Fig. 1). The ORR was higher with nab-paclitaxel versus paclitaxel in patients with visceral dominant disease (42 vs. 23 %; P = 0.022) and short DFI (43 vs. 33 %; P = 0.417). Similarly, ORR values among patients with poor prognostic factors in trial CA024 also corresponded to the trends in the ITT population. In both subsets, patients who received nab-paclitaxel on a qw 3/4 schedule exhibited higher ORRs compared with patients who received docetaxel (Fig. 1). Although comparisons within the short DFI subgroups failed to reach statistical significance, comparisons among patients with visceral dominant lesions did demonstrate significant differences for ORR.
Fig. 1.
Overall response rate. DFI disease-free interval, Doc docetaxel, nab-P nab-paclitaxel, ORR overall response rate, P paclitaxel, q3w every 3 weeks, qw 3/4 the first 3 of 4 weeks
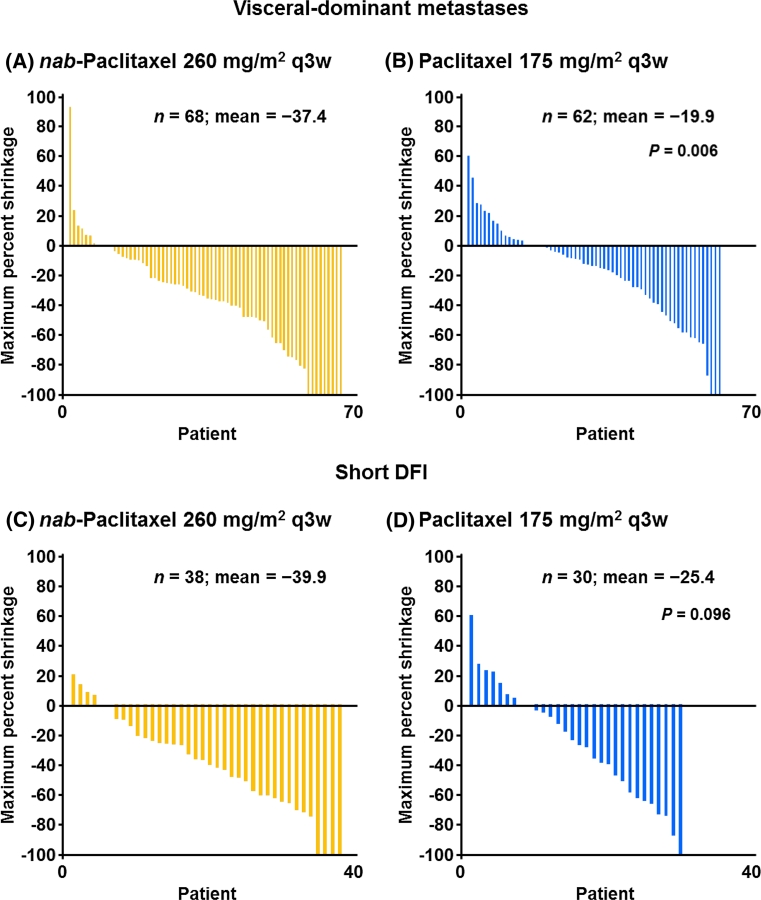
The mean maximum percent tumor shrinkage was also calculated for all patients in this analysis. Waterfall plots of tumor shrinkage for study CA012 are shown in Fig. 2 as a patient-by-patient overlay of treatment groups from the least tumor shrinkage to the most tumor shrinkage. Among patients with visceral dominant metastases (Fig. 2A), the mean maximum percent tumor shrinkage was 37.4 % in the nab-paclitaxel arm versus 19.9 % in the paclitaxel arm (P = 0.006). The difference in mean percent tumor shrinkage among patients with a short DFI was not statistically significant (Fig. 2B; 39.9 % for nab-paclitaxel vs. 25.4 % for paclitaxel; P = 0.096). The tumor shrinkage data for study CA024 are shown in Supplemental Figs. 1 (visceral dominant metastases) and 2 (short DFI). For patients with visceral dominant metastases, patients who received nab-paclitaxel 300 mg/m2 q3w, 100 mg/m2 qw 3/4, and 150 mg/m2 qw 3/4 had mean maximum percent tumor shrinkages of 35.5 %, 47.5 %, and 57.1 %, respectively, versus 37.0 % for the docetaxel group. For patients with a short DFI, patients who received nab-paclitaxel 300 mg/m2 q3w, 100 mg/m2 qw 3/4, and 150 mg/m2 qw 3/4 had mean maximum percent tumor shrinkages of 36.2 %, 48.1 %, and 56.1 %, respectively, versus 32.6 % for the docetaxel group. None of the differences for study CA024 were statistically significant.
Fig. 2.
Mean maximum percent tumor shrinkage in study CA012. DFI disease-free interval, q3w every 3 weeks
Progression-free survival
In trial CA012, PFS values in both poor prognostic factor subsets favored the nab-paclitaxel arm, although the differences were not statistically significant (Table 2). Trial CA024 also showed PFS trends among the patient subsets that reflected those of the ITT population. Comparisons of PFS in patients with visceral dominant metastases revealed statistically significant differences between nab-paclitaxel 150 mg/m2 qw 3/4 and docetaxel and between both qw 3/4 nab-paclitaxel treatment groups. In both prognostic factor subsets, the nab-paclitaxel 150 mg/m2 qw 3/4 treatment arm showed the longest PFS values (13.1 and 14.1 months in the visceral dominant metastases and short DFI groups, respectively).
Table 2.
Investigator-assessed PFS
| Trial/treatment | PFS (months), median | |||
|---|---|---|---|---|
| Visceral dominant metastasis | Short DFI | |||
| n | PFS (95 % CI) | n | PFS (95 % CI) | |
| Study CA012 | ||||
| nab-P 260 mg/m2 q3w | 74 | 5.6 (4.3–7.2) | 42 | 5.0 (3.6–6.6) |
| P 175 mg/m2 q3w | 64 | 3.8 (3.5–5.1) | 30 | 3.5 (2.7–5.1) |
| HR (95 % CI) | 0.717 (0.483–1.063) | 0.729 (0.437–1.215) | ||
| P valuesa | 0.094 | 0.220 | ||
| Study CA024 | ||||
| nab-P 300 mg/m2 q3w (A) | 61 | 10.9 (7.6–13.8) | 20 | 7.4 (2.4–10.3) |
| nab-P 100 mg/m2 qw 3/4 (B) | 60 | 7.5 (7.2–9.3) | 21 | 7.3 (7.2–9.3) |
| nab-P 150 mg/m2 qw 3/4 (C) | 59 | 13.1 (9.8–17.7) | 14 | 14.1 (6.2–18.4) |
| Doc 100 mg/m2 q3w (D) | 67 | 7.8 (5.8–10.3) | 19 | 5.5 (3.1–10.3) |
| HRb | C versus D: 0.600 | All NS | ||
| B versus C: 1.731 | ||||
| P valuesa,b | Overall: 0.049 | All NS | ||
| C versus D: 0.019 | ||||
| B versus C: 0.010 | ||||
CI confidence interval, DFI disease-free interval, Doc docetaxel, HR hazard ratio, nab-P nab-paclitaxel, NS not statistically significant, P paclitaxel, PFS progression-free survival, q3w every 3 weeks, qw 3/4 the first 3 of 4 weeks
a P values from log-rank test
bFor study CA024, only significant results shown
Overall survival
In this subset analysis, no comparisons for median OS reached statistical significance. In CA012, median OS was numerically higher with nab-paclitaxel versus paclitaxel in each of the subgroups (Table 3). Trial CA024 once again demonstrated similar trends in each patient subgroup compared with the ITT population. In patients with visceral dominant lesions, OS was numerically highest in patients who received nab-paclitaxel 150 mg/m2 qw 3/4, while in patients with a short DFI, the OS was numerically highest with nab-paclitaxel 100 mg/m2 qw 3/4.
Table 3.
Overall survival
| Trial/treatment | OS (months), median | |||
|---|---|---|---|---|
| Visceral dominant metastasis | Short DFI | |||
| n | OS (95 % CI) | n | OS (95 % CI) | |
| Study CA012 | ||||
| nab-P 260 mg/m2 q3w | 74 | 15.1 (11.5–19.0) | 42 | 14.6 (10.7–18.1) |
| P 175 mg/m2 q3w | 64 | 14.2 (11.8–22.5) | 30 | 11.7 (8.8–18.3) |
| HR (95 % CI) | 1.251 (0.841–1.859) | 0.942 (0.567–1.565) | ||
| P valuesa | 0.268 | 0.819 | ||
| Study CA024 | ||||
| nab-P 300 mg/m2 q3w | 61 | 27.7 (20.5–38.9) | 20 | 16.6 (12.1–22.0) |
| nab-P 100 mg/m2 qw 3/4 | 60 | 19.6 (14.5–26.0) | 21 | 19.1 (13.2–28.1) |
| nab-P 150 mg/m2 qw 3/4 | 59 | 32.1 (23.9–40.6) | 14 | 18.6 (10.6–>37.5) |
| Doc 100 mg/m2 q3w | 67 | 21.4 (18.0–31.3) | 19 | 14.4 (11.4–18.0) |
| P values | All NS | All NS | ||
CI confidence interval, DFI disease-free interval, Doc docetaxel, HR hazard ratio, nab-P nab-paclitaxel, NS not statistically significant, OS overall survival, P paclitaxel, q3w every 3 weeks, qw 3/4 the first 3 of 4 weeks
aValues from log-rank test
Treatment exposure
As the administered dose of nab-paclitaxel in the CA012 trial was higher than that of paclitaxel, patients in that treatment arm received a higher median cumulative dose (1,560 mg/m2 for patients in both subgroups who received nab-paclitaxel vs. 962.5 mg/m2 for patients in both subgroups who received paclitaxel) and a higher dose intensity (86.6 mg/m2/week for patients with visceral dominant metastases who received nab-paclitaxel and 85.3 mg/m2/week for patients with a short DFI who received nab-paclitaxel vs. 58.3 mg/m2/week for patients in both subgroups who received paclitaxel). Treatment delays and dose reductions occurred at similar frequencies between the two treatment arms across both prognostic subgroups. Patients in the nab-paclitaxel arm received a median of 6 cycles of treatment in both groups versus 5.5 for patients in the paclitaxel arm in both groups. The average dose intensities among the two patient subgroups for the nab-paclitaxel arms in trial CA024 ranged from 99.5 to 100 mg/m2/week in the 300 mg/m2 arm, 73.7–75 mg/m2/week in the 100 mg/m2 arm, and 98.7–103.1 mg/m2/week in the 150 mg/m2 arm. The median number of cycles received was highest for the nab-paclitaxel 150 mg/m2 qw 3/4 arm among patients with visceral dominant metastases (9 vs. 8 in the other arms), but among patients with a short DFI, patients in the nab-paclitaxel 100 mg/m2 qw 3/4 arm received the highest number of cycles (8 vs. 6–7 in the other arms). Among the poor prognostic factor subgroups in trial CA024, the highest rate of dose reductions took place in the nab-paclitaxel 150 mg/m2 qw 3/4 arm (44 and 50 % among patients with visceral dominant metastases and a short DFI, respectively), followed by docetaxel (30 and 26 %, respectively), and the two other nab-paclitaxel arms, which showed similar rates of dose reductions among the patient subgroups (14–18 %).
Safety
Tables 4 and 5 show the all-grade adverse events and the grade ≥3 adverse events, respectively, for the patients with poor prognostic factors in both trials. Safety results across the patient subgroups for trial CA012 were similar to the ITT population: grade 3/4 neutropenia was more frequent for paclitaxel in the poor prognostic factor subgroups, whereas sensory neuropathy and fatigue were both more frequent for nab-paclitaxel (Table 5). In trial CA024, rates of grade 3/4 adverse events in the patient subgroups very closely matched those of the ITT populations (Table 5). Accordingly, patients in each treatment arm had similar frequencies of specific adverse events for each poor prognostic factor subgroup. In the two subgroups, the rates of grade 3/4 neutropenia and fatigue were highest for the docetaxel group and lowest in the nab-paclitaxel 100 mg/m2 qw 3/4 group (no cases of grade 3/4 fatigue were reported in either qw 3/4 nab-paclitaxel arm). The nab-paclitaxel 100 mg/m2 qw 3/4 dose also produced the lowest rate of grade ≥3 sensory neuropathy in each prognostic factor subgroup.
Table 4.
All-grade toxicity
| Adverse events, n (%) | n | Neutropenia | Sensory neuropathy | Fatigue |
|---|---|---|---|---|
| Visceral dominant metastasis | ||||
| CA012 | ||||
| nab-P 260 mg/m2 q3w | 74 | 56 (76) | 51 (69) | 37 (50) |
| P 175 mg/m2 q3w | 64 | 51 (80) | 37 (58) | 31 (48) |
| CA024 | ||||
| nab-P 300 mg/m2 q3w | 61a | 55 (92) | 49 (80) | 23 (38) |
| nab-P 100 mg/m2 qw 3/4 | 60 | 48 (80) | 38 (63) | 20 (33) |
| nab-P 150 mg/m2 qw 3/4 | 59 | 53 (90) | 49 (83) | 28 (47) |
| Doc 100 mg/m2 q3w | 67b | 65 (100) | 44 (66) | 39 (58) |
| Short DFI | ||||
| CA012 | ||||
| nab-P 260 mg/m2 q3w | 42 | 30 (71) | 30 (71) | 20 (48) |
| P 175 mg/m2 q3w | 30 | 28 (93) | 16 (53) | 15 (50) |
| CA024 | ||||
| nab-P 300 mg/m2 q3w | 20c | 17 (89) | 14 (70) | 9 (45) |
| nab-P 100 mg/m2 qw 3/4 | 21 | 17 (81) | 15 (71) | 6 (29) |
| nab-P 150 mg/m2 qw 3/4 | 14 | 14 (100) | 11 (79) | 4 (29) |
| Doc 100 mg/m2 q3w | 19 | 19 (100) | 15 (79) | 9 (47) |
DFI disease-free interval, Doc docetaxel, nab-P nab-paclitaxel, P paclitaxel, q3w every 3 weeks, qw 3/4 the first 3 of 4 weeks
a60 Patients evaluable for neutropenia
b65 Patients evaluable for neutropenia
c19 Patients evaluable for neutropenia
Table 5.
Grade ≥3 toxicity
| Adverse events, n (%) | n | Neutropenia | Sensory neuropathy | Fatigue |
|---|---|---|---|---|
| Visceral dominant metastasis | ||||
| CA012 | ||||
| nab-P 260 mg/m2 q3w | 74 | 29 (39) | 9 (12) | 11 (15) |
| P 175 mg/m2 q3w | 64 | 37 (58) | 3 (5) | 1 (2) |
| CA024 | ||||
| nab-P 300 mg/m2 q3w | 61a | 27 (45) | 11 (18) | 3 (5) |
| nab-P 100 mg/m2 qw 3/4 | 60 | 15 (25) | 5 (8) | 0 |
| nab-P 150 mg/m2 qw 3/4 | 59 | 25 (42) | 13 (22) | 3 (5) |
| Doc 100 mg/m2 q3w | 67b | 61 (94) | 8 (12) | 13 (19) |
| Short DFI | ||||
| CA012 | ||||
| nab-P 260 mg/m2 q3w | 42 | 10 (24) | 4 (10) | 5 (12) |
| P 175 mg/m2 q3w | 30 | 20 (67) | 0 | 1 (3) |
| CA024 | ||||
| nab-P 300 mg/m2 q3w | 20c | 8 (42) | 4 (20) | 1 (5) |
| nab-P 100 mg/m2 qw 3/4 | 21 | 3 (14) | 1 (5) | 0 |
| nab-P 150 mg/m2 qw 3/4 | 14 | 6 (43) | 3 (21) | 0 |
| Doc 100 mg/m2 q3w | 19 | 19 (100) | 2 (11) | 4 (21) |
DFI disease-free interval, Doc docetaxel, nab-P nab-paclitaxel, P paclitaxel, q3w every 3 weeks, qw 3/4 the first 3 of 4 weeks
a60 Patients evaluable for neutropenia
b65 Patients evaluable for neutropenia
c19 Patients evaluable for neutropenia
Discussion
The results of this analysis indicate that the treatment benefits observed for nab-paclitaxel versus paclitaxel in the ITT populations of a randomized phase III trial and versus docetaxel in a randomized phase II trial also apply to patients with poor prognostic factors. In general, ORR, OS, PFS, safety, and treatment exposure were similar for each treatment arm among patients with visceral dominant metastases and patients with a short DFI. For trial CA012, the difference in ORR reached statistical significance in the subset of patients with visceral dominant metastases. In CA024, both qw 3/4 nab-paclitaxel arms exhibited higher ORRs versus docetaxel in the visceral dominant metastasis subgroup. The differences in ORR and the trends in median PFS and OS suggest that nab-paclitaxel provides clinical benefit to patients with virulent MBC who received first-line treatment.
This retrospective analysis was limited by two key factors. First, the patient subsets examined in this retrospective study were not large enough to allow statistical significance for some of the differences in efficacy outcomes, despite similar magnitudes of differences in absolute terms relative to the ITT populations of the two clinical trials. In addition, certain patient data, such as ER status, progesterone receptor status, and HER2 status were not collected in these trials.
A separate retrospective analysis examined patients ≥65 years old in these two studies [19]. It should be noted that for CA024, the numbers of patients ≥65 years old among the different treatment arms ranged from only 9 to 19. Nevertheless, that report reflected a common theme with the results of this study: the efficacy benefits of nab-paclitaxel relative to the other taxanes in these two trials were consistent among patient subgroups. Indeed, the ORR values for patients ≥65 years old in trial CA012 were 27 % for nab-paclitaxel versus 19 % for paclitaxel, and median PFS values were 5.6 and 3.5 months, respectively. Among patients ≥65 years old in CA024, ORR values were 22 % in the nab-paclitaxel 300 mg/m2 q3w group and 60–64 % in the qw 3/4 treatment groups versus 32 % in the docetaxel group. PFS values for all three nab-paclitaxel arms were longer than those for the docetaxel arm (9.2–18.9 vs. 8.5 months).
A recent presentation at the 2012 annual meeting of the American Society of Clinical Oncology gave results of a large phase III trial conducted by the Cancer and Leukemia Group B (CALGB) comparing three regimens for the first-line treatment of MBC: nab-paclitaxel ± bevacizumab (n = 271), paclitaxel ± bevacizumab (n = 283), and ixabepilone ± bevacizumab (n = 245) [20]. Although the trial protocol was amended to make bevacizumab use optional, 98 % of patients received it. The dose selected for nab-paclitaxel in this trial was 150 mg/m2 qw 3/4, marking the first time that a dose this high has been used in a phase III trial. Preliminary results from the trial suggest that the survival outcomes were similar with nab-paclitaxel and paclitaxel when given with bevacizumab; however, higher rates of dose reduction and discontinuation in the nab-paclitaxel arm suggest suboptimal delivery of the 150 mg/m2 dose of nab-paclitaxel when given in combination with bevacizumab. The preliminary findings of the CALGB study do not address a comparison of nab-paclitaxel versus paclitaxel outside of the combination with bevacizumab in patients with virulent MBC. Thus, further research may be required to determine the optimal weekly dose of nab-paclitaxel.
The results of this analysis of patients with visceral dominant metastases and a short DFI suggest that nab-paclitaxel exhibits substantial clinical activity in patients with virulent MBC. The efficacy of nab-paclitaxel versus paclitaxel and docetaxel in these two trials suggests that a phase III trial prospectively examining the effect of nab-paclitaxel in patients with poor prognostic factors may be warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The phase II and the phase III study were sponsored by Abraxis BioScience, a wholly owned subsidiary of Celgene, Summit, NJ, USA. Medical writing assistance was provided by John McGuire, PhD, MediTech Media, funded by Celgene Corporation. The authors are fully responsible for content and editorial decisions for this manuscript.
Conflict of interest
Joyce O’Shaughnessy—Consultant for Celgene Corporation, Paul Bhar—Former employee and stockholder of Celgene Corporation, Jose Iglesias—Former employee and stockholder of Celgene Corporation. William Gradishar had no conflicts of interest to disclose.
References
- 1.American Cancer Society (2012) Cancer facts and figures 2012. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf. Accessed 27 July 2012
- 2.National Cancer Institute (2012) Surveillance, epidemiology and end results. SEER stat fact sheets: breast. http://seer.cancer.gov/statfacts/html/breast.html. Accessed 27 July 2012
- 3.O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(Suppl 3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 4.Eichbaum MH, Kaltwasser M, Bruckner T, de Rossi TM, Schneeweiss A, Sohn C. Prognostic factors for patients with liver metastases from breast cancer. Breast Cancer Res Treat. 2006;96:53–62. doi: 10.1007/s10549-005-9039-1. [DOI] [PubMed] [Google Scholar]
- 5.Insa A, Lluch A, Prosper F, Marugan I, Martinez-Aquillo A, Garcia-Conde J. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67–78. doi: 10.1023/A:1006285726561. [DOI] [PubMed] [Google Scholar]
- 6.Alba E, Ribelles N, Sevilla I, Rueda A, Alonso L, Marquez A, Ruiz I, Miramón J. Adjuvant anthracycline therapy as a prognostic factor in metastatic breast cancer. Breast Cancer Res Treat. 2001;66:33–39. doi: 10.1023/A:1010616532332. [DOI] [PubMed] [Google Scholar]
- 7.Koenders PG, Beex LV, Kloppenborg PW, Smals AG, Benraad TJ. Human breast cancer: survival from first metastasis. Breast Cancer Study Group. Breast Cancer Res Treat. 1992;21:173–180. doi: 10.1007/BF01975000. [DOI] [PubMed] [Google Scholar]
- 8.Imkampe A, Bendall S, Bates T. The significance of the site of recurrence to subsequent breast cancer survival. Eur J Surg Oncol. 2007;33:420–423. doi: 10.1016/j.ejso.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Cortazar P, Justice R, Johnson J, Sridhara R, Keegan P, Pazdur R. US Food and Drug Administration approval overview in metastatic breast cancer. J Clin Oncol. 2012;30:1705–1711. doi: 10.1200/JCO.2011.39.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seidman AD, Tiersten A, Hudis C, Gollub M, Barrett S, Yao TJ, Lepore J, Gilewski T, Currie V, Crown J, et al. Phase II trial of paclitaxel by 3-hour infusion as initial and salvage chemotherapy for metastatic breast cancer. J Clin Oncol. 1995;13:2575–2581. doi: 10.1200/JCO.1995.13.10.2575. [DOI] [PubMed] [Google Scholar]
- 11.Nabholtz JM, Gelmon K, Bontenbal M, Spielmann M, Catimel G, Conte P, Klaassen U, Namer M, Bonneterre J, Fumoleau P, Winograd B. Multicenter, randomized comparative study of two doses of paclitaxel in patients with metastatic breast cancer. J Clin Oncol. 1996;14:1858–1867. doi: 10.1200/JCO.1996.14.6.1858. [DOI] [PubMed] [Google Scholar]
- 12.Fountzilas G, Athanassiades A, Giannakakis T, Bafaloukos D, Karakousis K, Dombros N, Kosmidis P, Skarlos D. A phase II study of paclitaxel in advanced breast cancer resistant to anthracyclines. Eur J Cancer. 1996;32A:47–51. doi: 10.1016/0959-8049(95)00398-3. [DOI] [PubMed] [Google Scholar]
- 13.Taxol [package insert] (2011) Bristol-Myers Squibb Company, Princeton. http://packageinserts.bms.com/pi/pi_taxol.pdf. Accessed 27 July 2012
- 14.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 15.ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–685. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]
- 16.Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, Bhar P. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27:3611–3619. doi: 10.1200/JCO.2008.18.5397. [DOI] [PubMed] [Google Scholar]
- 17.Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, Bhar P, McGuire JR, Iglesias J (2012) Phase II trial of nab-paclitaxel compared with docetaxel as first-line chemotherapy in patients with metastatic breast cancer: final analysis of overall survival. Clin Breast Cancer 12:313–321 [DOI] [PubMed]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verwell J, Van Glabbake M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Aapro M, Tjulandin S, Bhar P, Gradishar WJ. Weekly nab-paclitaxel is safe and effective in ≥65 years old patients with metastatic breast cancer: a post hoc analysis. Breast. 2011;20:468–474. doi: 10.1016/j.breast.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, Mayer EL, Naughton M, Layman RM, Carey LA, Somer RA, Perez EA, Hudis C, Winder EP (2012) CALGB 40502/NCCTG N063H: randomized phase III trial of weekly paclitaxel (P) compared to weekly nanoparticle albumin bound nab-paclitaxel (NP) or ixabepilone (Ix) with or without bevacizumab (B) as first-line therapy for locally recurrent or metastatic breast cancer (MBC). J Clin Oncol 30(Suppl):Abstract CRA 1002
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.