Abstract
Purpose
Wilms’ tumor protein (WT1) is overexpressed in most leukemias and many solid tumors, and is a promising target for tumor immunotherapy. WT1 peptide-based cancer vaccines have been reported but have limited application due to HLA-restriction of the peptides. We sought to vaccinate using adenoviral (Ad) vectors encoding tumor associated antigens (TAAs) such as WT-1 that can stimulate TAA-specific immunity across a broad array of HLA types and multiple Class I and Class II epitopes.
Experimental Design
We developed a novel Ad vector encoding a truncated version of WT1 (Ad-tWT1) lacking the highly-conserved C-terminus zinc finger domains and tested its ability to stimulate WT1-specific immune responses and antitumor immunity in two murine models of WT1-expressing tumors.
Results
Despite encoding a transcription factor, we found that Ad-tWT1 transduced murine and human dendritic cells showed cytoplasmic expression of the truncated WT1 protein. In addition, vaccination of C57BL/6 mice with Ad-tWT1 generated WT1-specific cell-mediated and humoral immune responses and conferred protection against challenge with the leukemia cell line, mWT1-C1498. Moreover, in a tumor therapy model, Ad-tWT1 vaccination of TRAMP-C2 tumor-bearing mice significantly suppressed tumor growth.
Conclusions
This is the first report of a WT1-encoding Ad vector that is capable of inducing effective immunity against WT1-expressing malignancies. Based upon these findings, Ad-tWT1 warrants investigation in human clinical trials to evaluate its applications as a vaccine for patients with WT1-expressing cancers.
Keywords: adenoviral vectors, WT-1, immunotherapy, tumor associated antigens, cancer vaccine
Introduction
The modest activity of systemic chemotherapies against the common malignancies has led to a search for more targeted approaches including immunotherapies such as cancer vaccines. The target of a specific vaccine approach is an antigen either uniquely expressed or overexpressed by a significant percentage of the malignant cells. Expression of a specific tumor antigen across a wide variety of malignancies is an attractive attribute for cancer vaccine based approaches. The Wilms’ tumor gene (WT1), a zinc-finger transcription factor, represents one such antigenic target. WT1 binds to the early growth response-1 (EGR-1) consensus sequence1 and plays a role in cellular proliferation and differentiation,2-5 apoptosis,6,7 and organogenesis.8,9WT1 was originally proposed as a tumor suppressor gene involved in the constellation of Wilms’ tumor, aniridia, genitourinary malformation, and mental retardation known as the WAGR syndrome.10 However, more recent evidence has demonstrated the overexpression of WT1 messenger RNA (mRNA) and protein in many tumor types (lung, breast, colon, ovary, thyroid, kidney) and in most cases of acute myelocytic leukemia (AML), acute lymphocytic leukemia, chronic myelocytic leukemia, and myelodysplastic syndrome (MDS).11-16 In contrast, normal tissue expression of WT1 in adults is limited to the podocyte layer of the glomerulus, Sertoli cells of the testis, granulosa cells of the ovary, decidual cells of the uterus, mesothelial cells, mammary duct and lobule, and CD34+ progenitor cells (reviewed in 17,18). Therefore, WT1 has been regarded as a specific target for cancer immunotherapy.19
That WT1 may serve as a tumor antigen is demonstrated by several lines of evidence. WT1-specific cytolytic T cells (CTL) have been detected following hematopoietic stem cell transplantation in patients with leukemias, MDS, and lymphomas.20 Furthermore, human WT1-specific CTL lyse WT1-expressing tumor cells in vitro, but do not cause damage to normal cell expressing physiologic levels of WT1.21,22 Humoral immune responses against WT1 have also been demonstrated in patients with leukemia.23 Immunization of mice with either MHC class I-restricted WT1 peptide or WT1 cDNAs both induced WT1-specific CTL, and immunized mice rejected WT1-expressing tumor cells, without damage to normal tissues.24 Human clinical trials with WT1 vaccines have now been reported using peptide vaccines.25-29 The immunizations have been well tolerated and evidence of decreased leukemic blasts in AML patients have been reported.29 However, these peptide based vaccine approaches are limited by MHC-restriction, and/or decreased immunogenicity, especially with regard to induction of potent cell mediated immune responses.
Adenoviruses have been studied extensively as platforms for gene delivery, and because in vivo Ad-mediated gene transfer often induces a transgene-specific immune response,30,31 they are promising agents for the vaccination against tumor associated antigens (TAA). Indeed, vaccination with adenoviral vectors encoding TAA has demonstrated the ability to break existing immune tolerance and stimulate TAA-specific immunity.32,33 Furthermore, recent human clinical trials with Ad vectors encoding viral antigens confirms their potency in inducing cell mediated immune responses when other peptide approaches have failed.34,35 We therefore designed an Ad vector encoding WT1. We chose to use a truncated version of WT1 (Ad-tWT1) for the following reasons: (1) in order to avoid any possibility of delivering a molecule with tumor-promoting capacity, zinc finger domains that are important for DNA and RNA binding are deleted,36 (2) because previous reports of a truncated version of human WT1, lacking the highly-conserved C-terminus zinc finger domain, having greater immunogenicity compared with its full-length counterpart,37 and (3) because WT1-specific antibodies isolated from the sera of patients with AML were shown to be directed against the N-terminus portion of the protein.38 These data indicate that truncated WT1 retains the high-affinity MHC binding domains,38 and therefore may be suitable for immunotherapeutic applications targeting WT1. We demonstrate that immunization with Ad-tWT1 induces WT1-specific cellular and humoral immunity sufficient to protect mice from challenge with a WT1-expressing leukemia cell line, mWT1-C1498, and also to suppress tumor growth in the WT-1 expressing TRAMP-C2 tumor-bearing mice. These are the first data supporting an adenoviral-based vaccine targeting WT1 that will be applicable to human malignancies.
Materials and Methods
WT1 isotype D cloning and creation of truncated WT1 pShuttle-CMV plasmid
Ten million K562 cells (American Type Culture Collection (ATCC), Manassas, VA) were grown in RPMI-1640 media (Invitrogen Corporation, Carlsbad, CA) with 10% fetal bovine serum (FBS) for several divisions and snap frozen in liquid nitrogen. Total cellular RNA was then extracted using the RNEasy kit (Qiagen Inc., Germantown, MD) according the manufacturer's instructions. cDNA was produced using 10 μL of the RNA mixture with the SuperScript-II kit (Invitrogen). A polymerase chain reaction (PCR) product containing the full-length open reading frame (ORF) of WT1 isotype D mRNA was amplified using nested PCR primers. The first PCR reaction was carried out using the primers Wt1ForOuter: 5′-TCTGAGCCTCAGCAAATG-3′ and WT1RevOuter: 5 -GAGGGAGACCCCTCAAAC-3′ (Invitrogen). 2 μL of cDNA substrate was used and PCR was carried out at 94°C for 60s, 55°C for 1 minute followed by 72°C for 1 minute for 30 cycles followed by 6 minutes at 72°C using Ex-Taq polymerase (Takara Bio, Inc., Otsu, Japan) in a 10% dimethyl sulfoxide (DMSO, Sigma-Aldrich Co., St. Louis, MO) solution. A second PCR reaction was performed using 5 μL of this product with the primers WT1ForInner: 5′-CAGCAAATGGGCTCCGAC-3′ and WT1RevInner 5′-CTCAAAGCGCCAGCTGGAG-3′ with Ex-Taq polymerase and 10% DMSO. This yielded a roughly 1500 base pair (bp) product that was subsequently cloned into the pCR2.1 vector (Invitrogen), propagated in chemically competent Escherichia coli (Invitrogen) and purified on QIAPrep spin columns (Qiagen). Clones were sequenced to confirm that the correct product was obtained.
The truncated WT1 sequence was created using the pCR2.1-WT1 plasmid in a PCR reaction using primer WT1ForInner and WT1RevTrunc: 5′-TCACTGAATGCCTCTGAAG-3′ in a 50 μL PCR reaction containing 0.5 μg/2 μL of the plasmid mix obtained after column purification. This product was then cloned into the pCR2.1 and sequenced to assure fidelity of the cloning reactions. Following this, the truncated WT1 product was subcloned into pShuttle-CMV (Stratagene, La Jolla, CA, USA) using NotI and HindIII digestion and ligation. Confirmation of correct orientation of the truncated WT1 construct (tWT1) within pShuttle-CMV was confirmed by PCR using the CMV promoter primer CMVFor: 5’-CGATGTACGGGCCAGATATACG-3’ with WT1RevTrunc and the same PCR conditions as described above. The pShuttle-CMV-tWT1 plasmid was then linearized using digestion with PmeI, recombined into a linearized [E1-E3-] serotype 5 pAdEasy construct, and propagated in chemically competent Escherichia coli (Invitrogen).
Adenoviral vector preparation
Construction and preparation of [E1-E3-] adenoviruses encoding LacZ, or tWT1 transgenes under control of the cytomegalovirus (CMV) immediate-early promoter/enhancer were undertaken as previously described.39,40 Briefly, the CMV-driven tWT1 transgene was subcloned into a shuttle plasmid, and used to generate a [E1-E3-] Ad vector using a previously delineated, BJ5183 bacterial recombination-based system.39 Complementing 293 cells (containing the Ad E1 gene) were used to produce high titers of these replication-deficient vectors, and cesium chloride double banding was performed to purify the vectors, essentially as previously detailed.40 All Ad vector stocks were evaluated for replication-competent adenovirus (RCA) via a polymerase chain reaction (PCR)-based RCA assay, as described previously.41,42 To ensure samples were not lipopolysaccharide (LPS)-contaminated, purified vectors were tested using an E-Toxate kit (Sigma-Aldrich) per the manufacturer's recommendations, with typical preparations containing < 0.025 endotoxin units (EU) per viral injection dose.
Peptides
A mixture of WT1 peptides containing 15 nmol of each of 15mer peptides spanning WT1 protein, each overlapping the next by 11 amino acids, was purchased from JPT Peptide Technologies (Berlin, Germany), and was used for immune monitoring, including the IFN-γ ELISPOT assay and anti-WT1 ELISA. An HIV peptide mix representing HIV gag protein was purchased from BD Biosciences (San Jose, CA) and was used as a negative control.
Animals, cell lines and human blood products
Female and male wild-type C57BL/6 (H-2Db) mice (Jackson Laboratory, Bar Harbor, ME, USA) were bred and maintained in the Duke University Medical Center pathogen-free Animal Research Facility, and used at 6 to 8 weeks of age. All animal studies described were approved by the Duke University Medical Center Institutional Animal Care & Use Committee, in accordance with guidelines published by the Commission on Life Sciences of the National Research Council.
The mWT1-C1498 murine leukemia cell line syngeneic to C57BL/6 mice was kindly provided by Dr. H. Sugiyama (Osaka University Graduate School of Medicine, Osaka, Japan). This line was established via transfection of C1498 murine leukemia cells with murine WT1 cDNA (Dr. D. Housman, Massachusetts Institute of Technology, Cambridge, MA, USA).43 WT1-expressing TRAMP-C2 prostate cancer cell line, syngenic to C57BL/6 mice, was purchased from ATCC.
All human blood products were obtained following signed informed consent from human donors according to an institutional review board-approved protocol.
Western Blotting
Monocytes were isolated from human PBMCs based on flask adherence, and cultured in AIM-V medium (Invitrogen) supplemented with GM-CSF (800 U/ml; Berlex, Richmond, CA) and IL-4 (25 ng/ml, R&D Systems, Minneapolis, MN) for 7 days to generate dendritic cells (DC). DCs were harvested from the flasks, infected with Ad-tWT1 at multiplicity of infection (moi) 5,000 to 40,000, further incubated for 2 days, and lysed for protein extraction. 30 μg of protein was applied for each lane, run on 12% Tris-HCl acrylamide gel, and transferred to Polyvinylidene Fluoride (PVDF) membranes. Membranes were incubated with anti-WT1 antibody (1:50 dilution, Dako, Carpinteria, CA) or anti-actin antibody (1:1000 dilution, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 1 h, followed by incubation with horseradish peroxidase-conjugated goat anti-mouse IgG antibody (1:2000 dilution, Bio-Rad, Hercules, CA). The chemiluminescent substrate kit (Thermo Scientific, Rockford, IL) was used for the development. Mouse DC were cultured from bone marrow cells of C57BL/6 mouse as previously reported44 and were infected with Ad-tWT1 at moi 20,000, incubated for 2 days, and protein was extracted. Western blotting was performed as described above.
Immunocytochemistry
Seven day-cultured human DCs were infected with Ad-tWT1 at moi 40,000, and incubated for 2 days on chamber slides (Lab-Tek, Hatfield, PA). Cells were fixed with 10% formalin, permeabilized with BD Permeabilizing Solution (BD Biosciences) for 20 min. Cells were incubated with rabbit monoclonal anti-WT1 mAb (1:50 dilution, Abcam, Cambridge, MA) for 1 h, washed three times with PBS, incubated with biotin-conjugated anti-rabbit IgG (1:2000 dilution, Bio-Rad), followed by Vectastain Elite ABC kit (Vector Lab, Burlingame, CA). Isotype matched mouse IgG was used as a negative control. Color was developed with diaminobenzidine (Sigma-Aldrich).
Flow cytometry of Ad-tWT1infected DC
Seven day-cultured human DCs were infected with Ad-tWT1 at moi 10,000 or 20,000, incubated for 2 days, and harvested for the analysis of maturation status. DCs were stained with Phycoerythrin (PE)-labeled anti-CD40, CD54, CD80, CD83, CD86, or CCR7 antibodies (BD Biosciences) for 30 min at 4°C. PE-labeled IgG was used as negative control. Cells were washed twice with PBS and analyzed on a FACSCaliber using CellQuest software (BD Biosciences).
IFN-γ enzyme-linked immunosorbent spot (ELISpot) assay
C57BL/6 mice were immunized via footpad injection with 2.6 × 1010 particles of the Ad-tWT1 vector in 40 μL of saline, or 40 μL of saline alone. Mice were euthanized weekly and splenocytes harvested.
Multiscreen-HA 96-well plates (Millipore Corporation, Bedford, MA, USA) were coated overnight at 4°C with 100 μL/well of 15 μg/mL goat anti-mouse IFN-γ capture antibody (Miltenyi Biotec, Bergisch Gladbach, Germany) in Dulbecco's PBS (DPBS; Invitrogen). Plates were rinsed and blocked with 100 μL/well of RPMI 1640 (Invitrogen), supplemented with 10% FBS, for 2 hours at 37°C in 5% CO2. Splenocytes were plated at 500,000 cells per well in the presence of medium, phorbol 12-myristate 13-acetate (0.125 μg/mL; PMA; Sigma-Aldrich) + ionomycin (1.0 μg/mL; Sigma-Aldrich), an HIV peptide mix (2.6 μg/mL), or a WT1 peptide mix (2.6 μg/mL; JPT Peptide Technologies) in a total volume of 200 μL. Replicates of six were performed for each sample. Plates were incubated for 18 hours at 37°C, 5% CO2, then washed 5 times with DPBS. 1 μg/mL biotinylated goat anti-mouse IFN-γ detection antibody was added to the plates (100 μL/well), which were subsequently incubated at room temperature for two hours. Following five washings with DPBS, 100 μL of streptavidin-horseradish peroxidase (prepared according to manufacturer instructions; Vector Laboratories) was added per well for 1 hour at room temperature. The plates were washed a final five times with DPBS. To develop color, 100 μL of the 3-amino-9-ethyl-carbazole (AEC; Sigma-Aldrich) solution was added per well and allowed to incubate for 4 minutes at room temperature in the dark. The color development process was terminated by running plates under distilled water. Membranes were read with a high-resolution automated ELISpot reader system (Carl Zeiss, Inc., Thornwood, NY, USA) using the KS ELISpot version 4.2 software.
Cytotoxicity Assay
C57BL/6 mice were immunized with 2.6 × 1010 particles of the Ad-tWT1 vector in 40 μL of saline via footpad injection on days 0 and 14. On day 28, mice were sacrificed and splenocytes were harvested and used as effector cells. WT1-expressing mWT1-C1498 cells were used as target cells and WT1-non-expressing C1498 cells as negative control for the assay. Target cells were labeled with 51Chromium. Five thousand target cells were placed into 96-well U-bottomed plates and splenocytes from Ad-tWT1 immunized mice were added to the wells to make 4:1 and 20:1 effector : target ratios. Plates were incubated for 5 h at 37 °C and chromium released into the supernatant was analyzed by MicroBeta Plus Scintillation counter (Wallac). Cytotoxicity was calculated as follows: percentage of target cell lysis = 100 × (cpm of experimental release – cpm of spontaneous release) / (cpm of maximum release – cpm of spontaneous release).
Antibody ELISA assay
Round bottom 96-well microplates (Corning, Inc., Acton, MA, USA) were coated with 50 μL/well of an N-terminal domain WT1 protein fragment (sc-4093; Santa Cruz) at 2 μg/mL in 1% BSA/DPBS. Plates were incubated for 3 hours at room temperature, washed twice with 200 μL DPBS per well with the Tecan 96PW plate washer, and blocked with 200 μL/well 1% BSA/PBS for 1 hour at room temperature. Serum was isolated from mice euthanized 14 days following immunization with 40 μL saline or 2.6 × 1010 particles of Ad-tWT1 or Ad-GFP, and serial dilutions were prepared (1:40, 1:200, 1:1000). 50 μL of the antisera dilutions was added per well in duplicate, and incubated for 3 hours at room temperature. The plates were subsequently washed with 200 μL/well DPBS, and 50 μL/well of alkaline phosphatase-labeled goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), diluted 1:1000 in 1% BSA/PBS was added and allowed to incubate for 3 hours at 37°C. The plates were again washed, and 50 μL reconstituted p-nitrophenyl phosphate (pNPP, prepared according to manufacturer instructions; Sigma-Aldrich) was added per well. Once the developed yellow color was visible to the naked eye, the reaction was terminated with 50 μL/well sodium hydroxide, and absorbance of each well measured at 405 nm.
Prophylactic anti-tumor model in C57Bl/6 mouse
C57BL/6 mice were immunized via footpad injection on days 0 and 14 with 2.6 × 1010 particles of the Ad-tWT1 vector or 2.6 × 1010 particles of an Ad-LacZ control in 40 μL of saline. On day 18, mice were inoculated with 5 × 105 mWT1-C1498 cells in 100 μL saline subcutaneously into the flank. Tumor dimensions were measured serially, and tumor volumes calculated using the following formula: long axis × (short axis)2 × 0.52.
Therapeutic anti-tumor model in C57Bl/6 mouse
Male C57BL/6 mice were inoculated with 1.0 × 106 TRAMP-C2 prostate cancer cells in 100 μL PBS subcutaneously into the flank on day 0. On day 7, mice were randomized and divided into 3 groups, immunized via footpad injection with 2.6 × 1010 particles of the Ad-tWT1 vector, 2.6 × 1010 particles of an Ad-LacZ control, or treated with saline only. Vaccination was repeated on days 7, 14, and 21. Tumor dimensions were measured serially, and tumor volumes calculated as described above. On day 35, two mice from each group were sacrificed for immune monitoring assays described above, and the remaining mice were sacrificed on day 56.
Statistical analysis
For the ELISpot and ELISA assays, differences in IFN-γ production and antibody binding, respectively, were analyzed using the Student's t test. Differences at p < 0.05 were considered statistically significant. In tumor prevention model and tumor therapeutic model in C57BL/6 mice, the differences of tumor volumes were assessed at the last time point of tumor volume measurements using Student's t test.
Results
Expression of truncated WT1 protein by Ad-tWT1 transduced human and murine dendritic cells
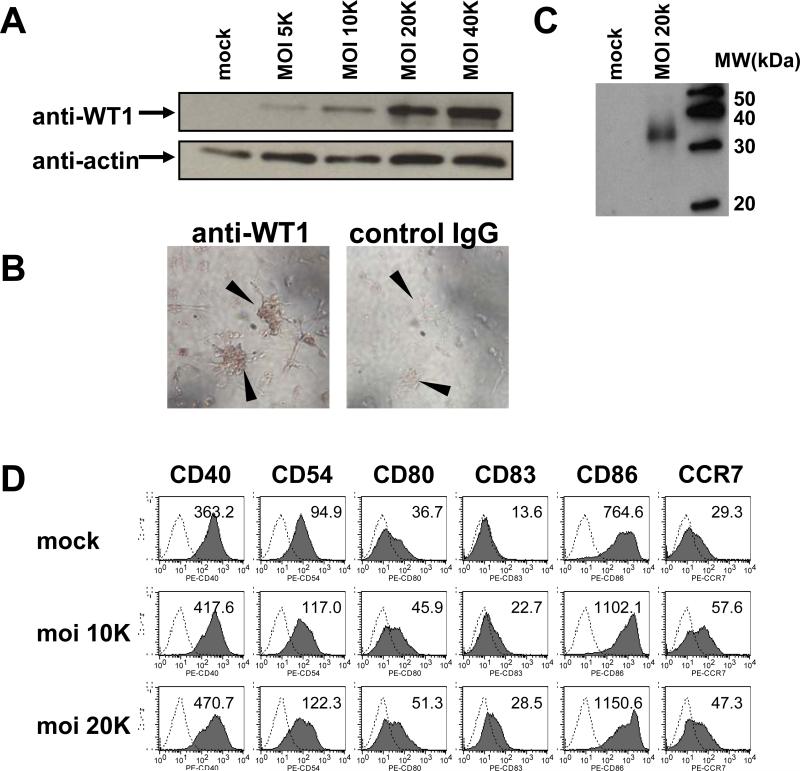
The Ad-tWT1 must be able to deliver the antigen (tWT1) to antigen presenting cells such as DC for subsequent processing and presentation to T cells. To evaluate the ability of Ad-tWT1 vector to transduce WT1 expression to antigen-presenting cells, cultured human and mouse dendritic cells (DC) were infected with Ad-tWT1 at multiplicity of infection (moi) 5,000 to 40,000 and after a two day-incubation, cells were lysed for Western Blotting. As shown in Figure 1A and 1C, transduced human and mouse DC efficiently expressed high levels of truncated WT1 protein, demonstrating that the Ad-tWT1 vaccine conferred antigen expression. Immunocytochemistry with anti-WT1 antibody (Figure 1B left) showed mainly cytoplasmic staining in human DC and the lack of accumulation in the nucleus. These data demonstrate that Ad-tWT1 successfully delivers the tWT1 transgene to murine and human DC, a pre-requisite for use as a cancer vaccine.
Figure 1. WT1 protein expression by Ad-tWT1 transduced human and mouse dendritic cells.
A) Seven (7) day-cultured human DC were infected with Ad-tWT1 at the indicated moi (5,000-40,000). After infection, cells were incubated for 2 days, and lysed for protein extraction. 30 μg of protein was applied for each lane, run on 12% Tris-HCl acrylamide gel, and transferred to Polyvinylidene Fluoride (PVDF) membranes. Membranes were incubated with anti-WT1 antibody or anti-actin antibody, followed by incubation with HRP-conjugated goat anti-mouse IgG antibody. An ECL kit was used for development. B) Human DC, cultured on chamber slides for 7 days, were infected with Ad-tWT1 at moi 40,000 and further incubated for 2 days. Then cells were fixed with 10% formalin and permeabilized with BD Permeabilizing Solution. Cells were stained with anti-WT1 mAb (left side of panel) or control IgG (right side of panel), followed by biotinylated anti-mouse IgG antibody. Color was developed with a Vectastain kit and DAB. Arrowheads indicate colonies of DC. C) Seven day-cultured mouse DCs were infected with Ad-tWT1 at moi 20,000, incubated for 2 days, and lysed for protein extraction. Immunoblotting with anti-WT1 mAb was performed as described in (A). D) Human DC were infected with Ad-tWT1 at the indicated moi. After infection, cells were incubated for 2 days, and stained with the indicated antibodies for analysis by flow cytometry. The mean fluorescence intensity of expression of each surface protein is indicated within each histogram.
An important consideration for the function of DC is their maturational status. We accessed whether Ad-tWT1 infected human DC underwent maturation as indicated by upregulation of maturational markers. As shown in Figure 1D, Ad-tWT1 resulted in increased expression of CD40, CD54, CD80, CD83, CD86, and CCR7 indicating maturation of the DC.
Vaccination with Ad-tWT1 stimulates WT1-specific IFN-γ production by T cells
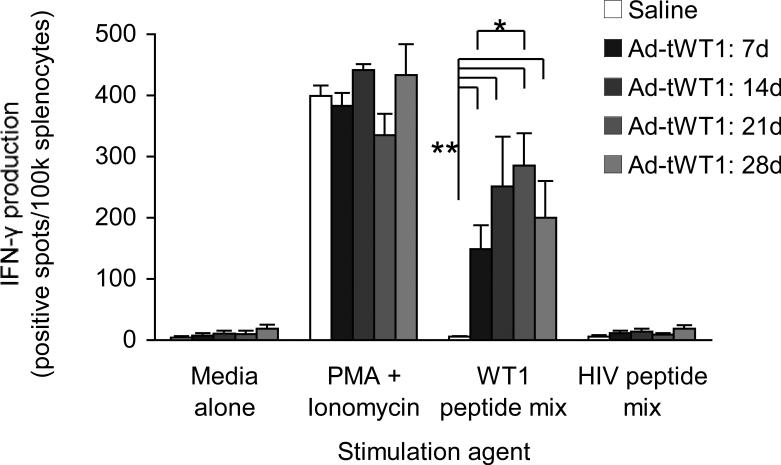
To evaluate the ability of Ad-tWT1 immunization to evoke a WT1-specific cellular immune response, C57BL/6 mice were vaccinated via footpad injection with 2.6 × 1010 particles of the Ad-tWT1 vector (n = 16) or 40 μL saline (n = 4) as a negative control. Four mice were euthanized at weekly intervals following vaccination. Antigen-specific IFN-γ secretion was assessed by stimulating splenocytes with a WT1 peptide mix or media and an HIV peptide mix as negative controls, and PMA + ionomycin as a positive control. As depicted in Figure 2, splenocytes from mice immunized with Ad-tWT1 demonstrated a significantly greater number of IFN-γ producing cells in response to WT1 peptide mix stimulation compared with those from the saline group (p < 0.005), indicating that Ad-tWT1 vaccination stimulated the expansion of WT1-specific T cells. This was true of all time points measured, with the peak response occurring 21 days post-immunization.
Figure 2. Induction of WT1-specific IFN-γ production by T cells following Ad-tWT1 vaccination.
C57BL/6 mice were vaccinated via footpad injection with either 40 μl of saline or 2.6 × 1010 particles of the Ad-tWT1 vector. Mice (n=4/group) were sacrificed weekly and the splenocytes were harvested. T cells producing IFN-γ in response to stimulation with WT1 peptide mix, an HIV peptide mix (negative control), media alone or PMA + ionomycin (positive control) were measured in an ELISpot assay. The mean (+/- s.d.) number of spots (T cells secreting cytokine) is depicted at each time point. * p < 0.005 for the comparison of saline control versus each time point of Ad-tWT1 vaccination. ** p < 0.01 for the comparison of the mean number of spots at day 21 versus day 7.
Vaccination with Ad-tWT1 elicits WT1-specific cytotoxic T cells
Splenocytes from Ad-tWT1 immunized mice were used as effector cells and co-incubated with 51Chromium labeled WT1-expressing mWT1-C1498 cells or WT1-negative C1498 cells. Supplementary Figure 1 shows the level of cytotoxicity after 5 hour incubation. Splenocytes from Ad-tWT1 immunized mice showed greater cytotoxicity against mWT1-C1498 cells compared to the cytotoxicity against WT1-negative C1498 cells, indicating WT1-specific killing of tumor cells. Thus, Ad-tWT1 can induce anti-WT1 cytotoxic T lymphocytes with anti-tumor activity.
Vaccination with Ad-tWT1 stimulates the generation of WT1-specific antibodies
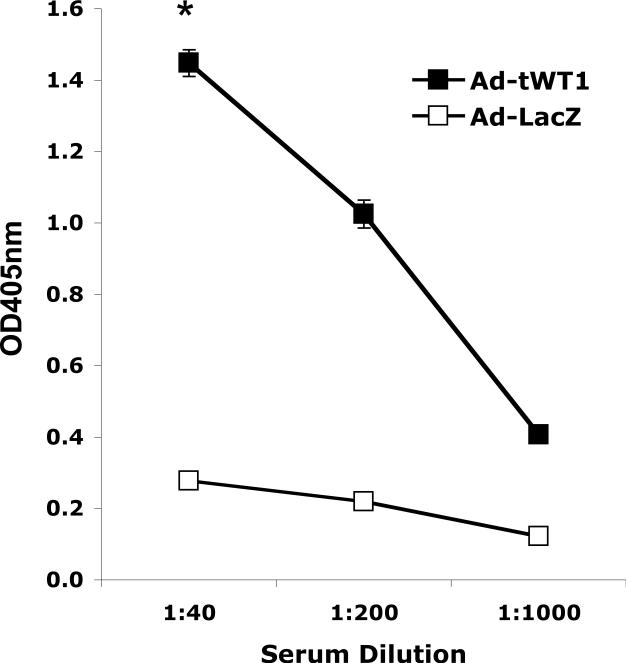
To evaluate the ability of Ad-tWT1 immunization to generate a WT1-specific humoral immune response, C57BL/6 mice were vaccinated via footpad injection with 2.6 × 1010 particles of the Ad-tWT1 vector or 2.6 × 1010 particles of an Ad-LacZ control vector. Mice were euthanized 14 days post-vaccination, and serum samples, pooled from two mice in each group, were assessed for the presence of WT1-specific antibodies via ELISA. As illustrated in Figure 3, anti-WT1 IgG antibodies at a titer of at least 1:1000 were detected in the serum of mice immunized with Ad-tWT1 but not mice immunized with Ad-LacZ, indicating that Ad-tWT1 vaccination induced anti-WT1 antibodies. These data indirectly indicate that Ad-tWT1 can also induce CD4 helper T cell responses that are necessary for IgG class switching of the antibody.
Figure 3. Detection of anti-WT1 antibodies in mice immunized with Ad-tWT1.
C57BL/6 mice (n= 5/group) were vaccinated via footpad injection with 2.6 × 1010 particles of Ad-LacZ, or 2.6 × 1010 particles of the Ad-tWT1 vector. Mice were sacrificed 14 days post-immunization, serum samples were obtained, pooled, and the quantity of anti-WT1 antibody was measured by ELISA. *p < 0.01 for the comparison of the anti-WT1 titer for mice immunized with Ad-tWT1 compared with Ad-LacZ..
Prophylactic Ad-tWT1 vaccination confers anti-tumor protection
The ability of Ad-tWT1 immunization to elicit immunity sufficient to prevent the growth of mWT1-C1498, a WT1-expressing murine leukemia line, was subsequently investigated. Mice were vaccinated via footpad injection on days 0 and 14 with 2.6 × 1010 particles of the Ad-tWT1 vector (n = 5), or 2.6 × 1010 particles of Ad-LacZ (n = 5), which served as the negative control. On day 18, mice were inoculated with 5 × 105 mWT1-C1498 cells in 100 μl saline subcutaneously. As shown in Figure 4, growth of tumors was significantly retarded in mice immunized with Ad-tWT1 vaccine throughout the time course compared to mice treated with Ad-LacZ (p < 0.05). Taken with the prior data indicating T cell responses against WT1, these results suggest that Ad-tWT1 vaccination was successful in eliciting the expansion of WT1-specific immune responses capable of reducing progression of mWT1-C1498 tumor cells.
Figure 4. Prevention of mWT1-C1498 tumor growth by Ad-tWT1 vaccination.
C57BL/6 mice (n= 5/group) were vaccinated via footpad injection with on days 0 and 14 with 2.6 × 1010 particles of Ad-LacZ, or 2.6 × 1010 particles of the Ad-tWT1 vector. On day 18, 5 × 105 mWT1-C1498 (WT1-expressing murine leukemia line) cells were inoculated subcutaneously. Mice were euthanized 25 days after tumor cell inoculation. The mean +/-s.d. of the tumor volume is presented at each time point and a t test was performed on the day 25 data. * p < 0.05 for the comparison of the tumor size .
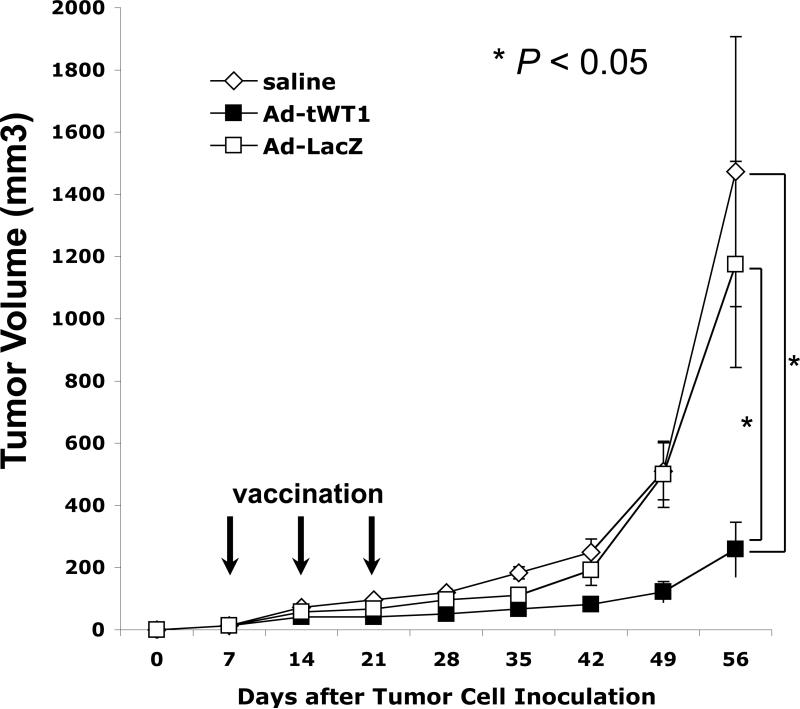
Ad-tWT1 vaccination confers tumor growth retardation in a therapeutic model
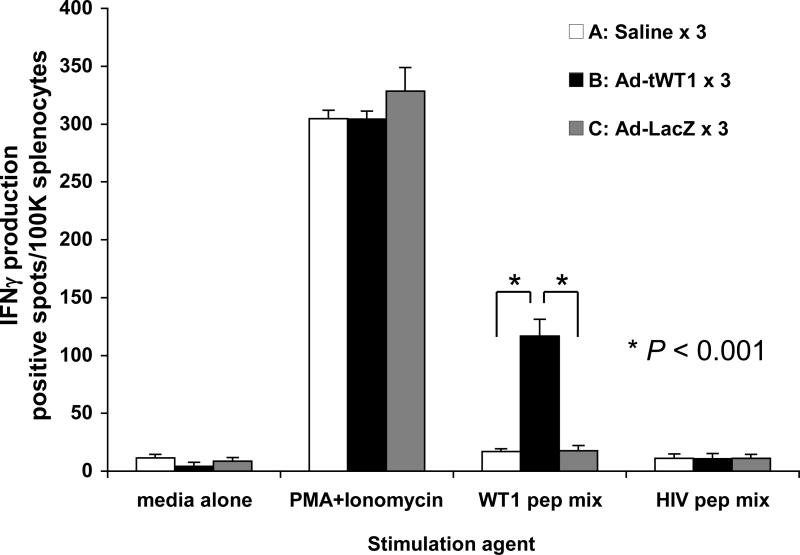
The ability of Ad-tWT1 vaccine to enhance therapeutic anti-tumor immunity in tumor-bearing mice was investigated using TRAMP-C2 cells (WT1-positive mouse prostate cancer cells) in C57BL/6 mice. 1 × 106 TRAMP-C2 cells were injected into the flank of mice on day 0, and immunization with Ad-tWT1 was administered on days 7, 14, and 21. As a negative control, saline only or Ad-LacZ was inoculated three times. Figure 5 shows the IFN-γ ELISPOT data of vaccinated mice on day 35, two weeks after the final vaccination. The Ad-tWT1 vaccine induced high levels of WT1-specific T cell response in TRAMP-C2 tumor-bearing mice. Tumor size was measured every week from day 0, and since subcutaneous tumors tended to ulcerate, all mice were sacrificed on day 56. As shown in Figure 6, Ad-tWT1 significantly inhibited the TRAMP-C2 tumor growth compared to control Ad-LacZ group or saline group (p < 0.05). This data demonstrates that the Ad-tWT1 vaccine has potent anti-tumor activity even in the setting of an established tumor.
Figure 5. Induction of WT1-specific T cell response following Ad-tWT1 vaccinations in TRAMP-C2 tumor-bearing mice.
C57BL/6 mice (n= 8/group) were inoculated with 1.0 × 106 TRAMP-C2 cells subcutaneously into the flank on day 0. On days 7, 14, and 21, mice were vaccinated via footpad injection with either 40 μl of saline, 2.6 × 1010 particles of the Ad-tWT1 vector, or 2.6 × 1010 particles of the Ad-LacZ vector. Mice were sacrificed on day 35 and the splenocytes were harvested. T cells producing IFN-γ in response to stimulation with WT1 peptide mix, an HIV peptide mix (negative control), media alone or PMA + ionomycin (positive control) were measured in an ELISpot assay. The mean (+/- s.d.) number of spots (T cells secreting cytokine) is depicted for each vaccine condition. * p < 0.001 for the comparison of the mean number of spots following vaccination with Ad-tWT1 than with saline or Ad-LacZ.
Figure 6. Inhibition of tumor growth following Ad-tWT1 vaccinations in TRAMP-C2 tumor-bearing mice.
C57BL/6 mice (n= 8/group) were inoculated with 1.0 × 106 TRAMP-C2 cells subcutaneously into the flank on day 0. On days 7, 14, and 21, mice were vaccinated via footpad injection with either 40 μl of saline, 2.6 × 1010 particles of the Ad-tWT1 vector, or 2.6 × 1010 particles of the Ad-LacZ vector. Tumor size was serially measured and on day 56 mice were sacrificed. The mean +/-s.d. of the tumor volume is presented at each time point and a t test was performed on the day 56 data. * p < 0.05 for the comparison of the tumor volume between the Ad-tWT1 vaccinated mice and those vaccinated with Ad-LacZ or saline.
Discussion
The goal of tumor immunotherapy is to break preexisting immune tolerance to tumor associated antigens (TAAs).45 On the basis of its overexpression in acute leukemia and solid tumors, limited physiologic expression, and potential oncogenic function, WT1 is an attractive TAA. As a result, induction of immune responses to WT1 has been attempted via peptide and DNA-based immunotherapy approaches. These approaches however are limited in their ability to induce potent immune responses, relative to virus based vaccine approaches. In this report, we describe the first example of successful WT1-specific immunotherapy utilizing a novel Ad vector encoding a truncated WT1 gene. Ad-tWT1 immunization resulted in the induction of both WT1-specific cellular and humoral immune responses and induced cytolytic T cells capable of killing WT1-expressing tumors in vitro. Subsequent tumor challenge studies demonstrated that this WT1-specific immunity was capable of protecting vaccinated mice against a murine WT1-expressing leukemia line. More importantly, in a cancer therapeutic model, Ad-tWT1 vaccination significantly suppressed the growth of a pre-existing and actively growing WT1-expressing prostate cancer (TRAMP-C2) in vivo. Although we used a human WT1 gene to generate our construct, the homology with murine WT1 is sufficiently high that all previous reports have used murine models to test the immunogeneticity of human WT1 peptide epitopes.38,43,46
There are several advantages to virus based vaccine approaches over prior peptide and DNA based strategies. The construction of a vector encoding a truncated version of WT1 allows for the delivery of a highly immunogenic protein that retains consensus amino acid motifs with high affinity for MHC class I and II molecules.38 The potential of inducing anti-tumor immunity with recombinant vectors, and/or recombinant vectors modifying dendritic cells (DCs) appears greater than with peptide based or cDNA based vaccines.
Although we have generated a recombinant vector, the truncation of the WT1 transgene enhances the safety profile of this vaccine, as any potential oncogenic function associated with WT1 transcriptional regulation is abrogated by the elimination of the protein's functional zinc finger domains. In addition, the risk of autoimmunity associated with the targeting of other zinc finger proteins, which are ubiquitous and have a high degree of homology in their zinc finger domains, is minimized. Interestingly, it was reported that a truncated construct of the WT1 protein, in which the highly-conserved C-terminus zinc finger domain is excluded, is more immunogenic than its full-length counterpart.37 In addition, WT1-specific antibodies isolated from the sera of patients with AML were shown to be directed against the N-terminus portion of the protein.38 We speculate that this increased immunogenicity might be a consequence of removal of tolerogenic epitopes in the conserved zinc finger domains or enhanced availability of the protein to the cytoplasmic processing and presentation apparatus of DCs because the lack of zinc fingers I and II results in a polypeptide incapable of nuclear concentration.47 Indeed, our study reported mainly cytoplasmic staining of WT1 in human DC, suggesting the lack of accumulation in the nucleus (Figure 1B).
Our choice of an adenoviral vector over other viral vectors was based upon the large clinical experience using this platform for vaccine studies, since our ultimate goal is to test this vaccine in cancer patients. Viral vectors are well-suited for immunotherapeutic applications. The infection of DCs – the most potent antigen presenting cells – by viral infection allows for the delivery of TAAs to DC. Ad vectors in particular are appealing due to their ability to transduce DC efficiently,48 as well their ability to induce multiple arms of the innate immune response. Viral infection provides Toll-like receptor signals to DC necessary for the reversal of regulatory T cell-mediated tolerance.49 Ad infection also initiates the maturation of DC,50 which is critical given that immature DC induce a tolerogenic immune response.51 Indeed, we observed enhanced expression of the maturation markers CD80, CD83, CD86 and CCR7 in DC infected with the Ad-tWT1 (Figure 1D).
This strategy is attractive in that the approach also leverages the cell's physiologic ability to process endogenous proteins via the transporter associated with antigen processing (TAP) pathway into peptides that are subsequently presented to CD8+ T cells in the context of MHC class I molecules. Proteosome degradation can generate a multitude of peptides from a single protein, thus facilitating the expansion of multiple CD8+ T cell clonal populations via delivery of a single transgene. Also, while tumor immunotherapy has traditionally focused on TAA-specific CD8+ CTLs, evidence has indicated that effective cancer vaccination requires the stimulation of both CD4+ and CD8+ T cells.52 CD4+ T cells support CD8+ T cell survival via their secretion of pro-inflammatory cytokines and orchestrate the simultaneous induction of TAA-specific T helper (Th)1 and Th2 responses, thus maximizing antitumor immunity. Indeed, the demonstration of WT1-specific IFN-γ production and detection of anti-WT1 IgG antibodies following Ad-tWT1 vaccination in our study indicate that this vector is effective in its capacity to prime both helper T functions. Since the WT1 protein expressed by Ad-tWT1 transduced cells contains many epitopes for MHC class I and II,53 clinical use of this vector would not be limited by HLA type. The primary disadvantage to Ad-mediated immunotherapy is the vector's inherent immunogenicity, which can result in the generation of serotype specific and cross-neutralizing anti-Ad antibodies that may restrict the ability to re-administer immunizations involving the same Ad serotype.54 In addition, given its ubiquitous nature of human infection by Ad serotype 5, the high prevalence of preexisting immunity in humans to this vector will likely attenuate its immunogenicity in the clinical setting. Both of these limitations may potentially be overcome by heterologous prime boosting strategies, in which rare Ad serotypes or different viral species such as avipoxvirus and alphavirus are utilized in subsequent immunizations.55 We are also developing a novel Ad vector deleted for the E2b gene that evades the effects of pre-existing anti-Ad immunity (Osada et al., submitted).
In conclusion, the demonstration that immunization with a novel Ad vector encoding a truncated version of WT1 can successfully generate WT1-specific anti-tumor immunity in mice supports further evaluation regarding the potential clinical efficacy of this vaccine. Given the high expression levels of WT1 in acute leukemias, and evidence indicating that a lymphopenic environment may enhance the efficacy of tumor-specific T cells,56 vaccination following immune ablation and bone marrow transplantation may be a promising avenue to pursue. Moreover, application of Ad-tWT1 vaccine for WT1-expressing solid tumors, including cancers of the brain, head and neck, lung, breast and colon, needs to be investigated in the future studies.
Supplementary Material
Supplementary Figure 1. Induction of WT1-specific cytotoxicity following Ad-tWT1 vaccination.
C57BL/6 mice were immunized with 2.6 × 1010 particles of the Ad-tWT1 vector via footpad injection on days 0 and 14. On day 28, splenocytes were harvested and used as effector cells. WT1-expressing mWT1-C1498 cells and WT1-non-expressing C1498 cells, were labeled with 51Chromium, and used as target cells. Five thousand target cells were placed into 96-well U-bottomed plates and splenocytes were added to the wells to make 4:1 and 20:1 effector : target ratios. Plates were incubated for 5 hours at 37°C and chromium released into the supernatant was analyzed by a scintillation counter. Percentage killing of WT1-positive mWT1-C1498 cells (filled symbols) and WT1-negative C1498 cells (open symbols), +/- s.d. is reported for two mice (square and diamond symbols).
Statement of Translational Relevance.
WT1 is a target for cancer immunotherapy because it is overexpressed in many tumor types (lung, breast, colon, ovary, thyroid, kidney) and in many leukemias, and has only minimal expression in normal tissues of the body. Human clinical trials with WT1 peptide vaccines have now been reported. The immunizations have been well tolerated and evidence of decreased leukemic blasts in AML patients has been reported. However, these peptide based vaccine approaches are limited by MHC-restriction, and/or decreased immunogenicity, especially with regard to induction of potent cell mediated immune responses. We chose as a platform, adenovirus encoding WT1 to enhance immunogenicity. This project demonstrates the immunogenicity and induction of antitumor activity of an adenovirus encoding WT1. This preliminary data supports the development of the ad-WT1 vaccine for human clinical trials.
Acknowledgments
This study was supported by research funding from NIH (NIH/NCI P01-CA047741) to N.C. C.Y.W. was funded by the Eugene A. Stead, Jr. Research Scholarship for Medical Students. A.A. was supported by the National Institutes of Health grants RO1DK-069884, P01 CA078673, the MSU Foundation as well the Osteopathic Heritage Foundation.
References
- 1.Rauscher FJ, III, Morris JF, Tournay OE, Cook DM, Curran T. Binding of the Wilms’ tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990;250:1259–62. doi: 10.1126/science.2244209. [DOI] [PubMed] [Google Scholar]
- 2.Drummond IA, Madden SL, Rohwer-Nutter P, Bell GI, Sukhatme VP, Rauscher FJ., III Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992;257:674–8. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- 3.Werner H, Re GG, Drummond IA, et al. Increased expression of the insulin-like growth factor I receptor gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R promoter activity by the WT1 Wilms tumor gene product. Proc. Natl. Acad. Sci. U. S. A. 1993;90:5828–32. doi: 10.1073/pnas.90.12.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hewitt SM, Hamada S, McDonnell TJ, Rauscher FJ, 3rd, Saunders GF. Regulation of the proto-oncogenes bcl-2 and c-myc by the Wilms’ tumor suppressor gene WT1. Cancer Res. 1995;55:5386–9. [PubMed] [Google Scholar]
- 5.Guan LS, Rauchman M, Wang ZY. Induction of Rb-associated protein (RbAp46) by Wilms’ tumor suppressor WT1 mediates growth inhibition. J Biol Chem. 1998;273:27047–50. doi: 10.1074/jbc.273.42.27047. [DOI] [PubMed] [Google Scholar]
- 6.Englert C, Hou X, Maheswaran S, et al. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J. 1995;14:4662–75. doi: 10.1002/j.1460-2075.1995.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayo MW, Wang CY, Drouin SS, et al. WT1 modulates apoptosis by transcriptionally upregulating the bcl-2 proto-oncogene. EMBO J. 1999;18:3990–4003. doi: 10.1093/emboj/18.14.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pritchard-Jones K, Fleming S, Davidson D, et al. The candidate Wilms’ tumour gene is involved in genitourinary development. Nature. 1990;346:194–7. doi: 10.1038/346194a0. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Prawitt D, Bardeesy N, et al. The Wilms’ tumor suppressor gene (wt1) product regulates Dax-1 gene expression during gonadal differentiation. Mol Cell Biol. 1999;19:2289–99. doi: 10.1128/mcb.19.3.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francke U, Holmes LB, Atkins L, Riccardi VM. Aniridia-Wilms’ tumor association: evidence for specific deletion of 11p13. Cytogenet Cell Genet. 1979;24:185–92. doi: 10.1159/000131375. [DOI] [PubMed] [Google Scholar]
- 11.Miwa H, Beran M, Saunders GF. Expression of the Wilms’ tumor gene (WT1) in human leukemias. Leukemia. 1992;6:405–9. [PubMed] [Google Scholar]
- 12.Rodeck U, Bossler A, Kari C, et al. Expression of the wt1 Wilms’ tumor gene by normal and malignant human melanocytes. Int J Cancer. 1994;59:78–82. doi: 10.1002/ijc.2910590116. [DOI] [PubMed] [Google Scholar]
- 13.Oji Y, Ogawa H, Tamaki H, et al. Expression of the Wilms’ tumor gene WT1 in solid tumors and its involvement in tumor cell growth. Jpn J Cancer Res. 1999;90:194–204. doi: 10.1111/j.1349-7006.1999.tb00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loeb DM, Evron E, Patel CB, et al. Wilms’ tumor suppressor gene (WT1) is expressed in primary breast tumors despite tumor-specific promoter methylation. Cancer Res. 2001;61:921–5. [PubMed] [Google Scholar]
- 15.Inoue K, Ogawa H, Sonoda Y, et al. Aberrant overexpression of the Wilms tumor gene (WT1) in human leukemia. Blood. 1997;89:1405–12. [PubMed] [Google Scholar]
- 16.Miyoshi Y, Ando A, Egawa C, et al. High expression of Wilms’ tumor suppressor gene predicts poor prognosis in breast cancer patients. Clin Cancer Res. 2002;8:1167–71. [PubMed] [Google Scholar]
- 17.Rauscher FJ., 3rd The WT1 Wilms tumor gene product: a developmentally regulated transcription factor in the kidney that functions as a tumor suppressor. FASEB J. 1993;7:896–903. [PubMed] [Google Scholar]
- 18.Baird PN, Simmons PJ. Expression of the Wilms’ tumor gene (WT1) in normal hemopoiesis. Exp Hematol. 1997;25:312–20. [PubMed] [Google Scholar]
- 19.Rosenfeld C, Cheever MA, Gaiger A. WT1 in acute leukemia, chronic myelogenous leukemia and myelodysplastic syndrome: therapeutic potential of WT1 targeted therapies. Leukemia. 2003;17:1301–12. doi: 10.1038/sj.leu.2402988. [DOI] [PubMed] [Google Scholar]
- 20.Morita Y, Heike Y, Kawakami M, et al. Monitoring of WT1-specific cytotoxic T lymphocytes after allogeneic hematopoietic stem cell transplantation. Int J Cancer. 2006;119:1360–7. doi: 10.1002/ijc.21960. [DOI] [PubMed] [Google Scholar]
- 21.Ohminami H, Yasukawa M, Fujita S. HLA class I-restricted lysis of leukemia cells by a CD8(+) cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood. 2000;95:286–93. [PubMed] [Google Scholar]
- 22.Gao L, Bellantuono I, Elsässer A, et al. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000;95:2198–203. [PubMed] [Google Scholar]
- 23.Elisseeva OA, Oka Y, Tsuboi A, et al. Humoral immune responses against Wilms tumor gene WT1 product in patients with hematopoietic malignancies. Blood. 2002;99:3272–9. doi: 10.1182/blood.v99.9.3272. [DOI] [PubMed] [Google Scholar]
- 24.Tsuboi A, Oka Y, Ogawa H, et al. Cytotoxic T-lymphocyte responses elicited to Wilms’ tumor gene WT1 product by DNA vaccination. J Clin Immunol. 2000;20:195–202. doi: 10.1023/a:1006637529995. [DOI] [PubMed] [Google Scholar]
- 25.Oka Y, Tsuboi A, Murakami M, et al. Wilms tumor gene peptide-based immunotherapy for patients with overt leukemia from myelodysplastic syndrome (MDS) or MDS with myelofibrosis. Int J Hematol. 2003;78:56–61. doi: 10.1007/BF02983241. [DOI] [PubMed] [Google Scholar]
- 26.Izumoto S, Tsuboi A, Oka Y, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108:963–71. doi: 10.3171/JNS/2008/108/5/0963. [DOI] [PubMed] [Google Scholar]
- 27.Iiyama T, Udaka K, Takeda S, et al. WT1 (Wilms’ tumor 1) peptide immunotherapy for renal cell carcinoma. Microbiol Immunol. 2007;51:519–30. doi: 10.1111/j.1348-0421.2007.tb03940.x. [DOI] [PubMed] [Google Scholar]
- 28.Morita S, Oka Y, Tsuboi A, et al. A phase I/II trial of a WT1 (Wilms’ tumor gene) peptide vaccine in patients with solid malignancy: safety assessment based on the phase I data. Jpn J Clin Oncol. 2006;36:231–6. doi: 10.1093/jjco/hyl005. [DOI] [PubMed] [Google Scholar]
- 29.Oka Y, Tsuboi A, Taguchi T, et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA. 2004;101:13885–90. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juillard V, Villefroy P, Godfrin D, Pavirani A, Venet A, Guillet JG. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur J Immunol. 1995;25:3467–73. doi: 10.1002/eji.1830251239. [DOI] [PubMed] [Google Scholar]
- 31.Tripathy SK, Black HB, Goldwasser E, Leiden JM. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–50. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 32.Hirschowitz EA, Leonard S, Song W, et al. Adenovirus-mediated expression of melanoma antigen gp75 as immunotherapy for metastatic melanoma. Gene Ther. 1998;5:975–83. doi: 10.1038/sj.gt.3300668. [DOI] [PubMed] [Google Scholar]
- 33.Salucci V, Mennuni C, Calvaruso F, et al. CD8+ T-cell tolerance can be broken by an adenoviral vaccine while CD4+ T-cell tolerance is broken by additional co-administration of a Toll-like receptor ligand. Scand J Immunol. 2006;63:35–41. doi: 10.1111/j.1365-3083.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 34.Catanzaro AT, Koup RA, Roederer M, et al. Phase I safety and immunogenicity evaluation of a multiclade HIV-a candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–49. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox KS, Clair JH, Prokop MT, et al. DNA gag/Adenovirus type 5 (Ad5) gag and Ad5 gag/Ad5 gag vaccines induce distinct T-cell response profiles. J Virol. 2008;82:8161–71. doi: 10.1128/JVI.00620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bardeesy N, Pelletier J. Overlapping RNA and DNA binding domains of the wt1 tumor suppressor gene product. Nucl Acids Res. 1998;26:1784–92. doi: 10.1093/nar/26.7.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kast WM, Levitsky H, Marincola FM. Synopsis of the 6th Walker's Cay Colloquium on Cancer Vaccines and Immunotherapy. J Transl Med. 2004;2:20. doi: 10.1186/1479-5876-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaiger A, Reese V, Disis ML, Cheever MA. Immunity to WT1 in the animal model and in patients with acute myeloid leukemia. Blood. 2000;96:1480–9. [PubMed] [Google Scholar]
- 39.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodges BL, Serra D, Hu H, Begy CA, Chamberlain JS, Amalfitano A. Multiply deleted [E1, polymerase-, and pTP-] adenovirus vector persists despite deletion of the preterminal protein. J Gene Med. 2000;2:250–9. doi: 10.1002/1521-2254(200007/08)2:4<250::AID-JGM113>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Amalfitano A, Hauser MA, Hu H, Serra D, Begy CR, Chamberlain JS. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. 1998;72:926–33. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lochmuller H, Jani A, Huard J, et al. Emergence of early region 1-containing replication-competent adenovirus in stocks of replication-defective adenovirus recombinants (delta E1 + delta E3) during multiple passages in 293 cells. Hum Gene Ther. 1994;5:1485–91. doi: 10.1089/hum.1994.5.12-1485. [DOI] [PubMed] [Google Scholar]
- 43.Oka Y, Udaka K, Tsuboi A, et al. Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. J Immunol. 2000;164:1873–80. doi: 10.4049/jimmunol.164.4.1873. [DOI] [PubMed] [Google Scholar]
- 44.Boczkowski D, Nair SK, Nam JH, Lyerly HK, Gilboa E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 2000;60:1028–34. [PubMed] [Google Scholar]
- 45.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–39. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima H, Kawasaki K, Oka Y, et al. WT1 peptide vaccination combined with BCG-CWS is more efficient for tumor eradication than WT1 peptide vaccination alone. Cancer Immunol Immunother. 2004;53:617–24. doi: 10.1007/s00262-003-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruening W, Moffett P, Chia S, Heinrich G, Pelletier J. Identification of nuclear localization signals within the zinc fingers of the WT1 tumor suppressor gene product. FEBS Lett. 1996;393:41–7. doi: 10.1016/0014-5793(96)00853-8. [DOI] [PubMed] [Google Scholar]
- 48.Dietz AB, Vuk-Pavlovic S. High efficiency adenovirus-mediated gene transfer to human dendritic cells. Blood. 1998;91:392–8. [PubMed] [Google Scholar]
- 49.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–15. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 50.Vujanovic L, Whiteside TL, Potter DM, Chu J, Ferrone S, Butterfield LH. Regulation of antigen presentation machinery in human dendritic cells by recombinant adenovirus. Cancer Immunol Immunother. 2009;58:121–33. doi: 10.1007/s00262-008-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174–7. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 52.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobayashi H, Nagato T, Aoki N, et al. Defining MHC class II T helper epitopes for WT1 tumor antigen. Cancer Immunol Immunother. 2006;55:850–60. doi: 10.1007/s00262-005-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–15. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hodge JW, McLaughlin JP, Kantor JA, Schlom J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15:759–68. doi: 10.1016/s0264-410x(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 56.Gattinoni L, Powell DJ, Jr., Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Induction of WT1-specific cytotoxicity following Ad-tWT1 vaccination.
C57BL/6 mice were immunized with 2.6 × 1010 particles of the Ad-tWT1 vector via footpad injection on days 0 and 14. On day 28, splenocytes were harvested and used as effector cells. WT1-expressing mWT1-C1498 cells and WT1-non-expressing C1498 cells, were labeled with 51Chromium, and used as target cells. Five thousand target cells were placed into 96-well U-bottomed plates and splenocytes were added to the wells to make 4:1 and 20:1 effector : target ratios. Plates were incubated for 5 hours at 37°C and chromium released into the supernatant was analyzed by a scintillation counter. Percentage killing of WT1-positive mWT1-C1498 cells (filled symbols) and WT1-negative C1498 cells (open symbols), +/- s.d. is reported for two mice (square and diamond symbols).