1. Introduction
Reciprocal interactions between stress and pain are well documented. Physical and psychosocial stressors, including childhood adversity, predict onset and exacerbation of clinical pain [17]. Conversely, chronic pain is a persistent endogenous stressor that can contribute to a cascade of psychosocial stressors including decreased activity level, reduced social support, and unemployment [5]. Despite growing appreciation for the importance of interactions between stress and pain, the mechanisms whereby the environment influences pain-related biological processes remain unclear. Further, the biological consequences of exposure to pain as a chronic stressor have received limited empirical attention.
Converging work in telomere and epigenetics research indicates that adverse environmental experiences have a biological interface. The long-term effect of chronic stress appears to accelerate telomere shortening [13]. Epigenetic responses to the environment bridge stressful experiences with biological outcomes emphasizing the potential for epigenetic processes to influence individual pain experiences. Epigenome changes, occurring separate from and yet orchestrating the expression of the genome across the lifespan, may shape vulnerability and resilience factors implicated in chronic pain conditions [21].
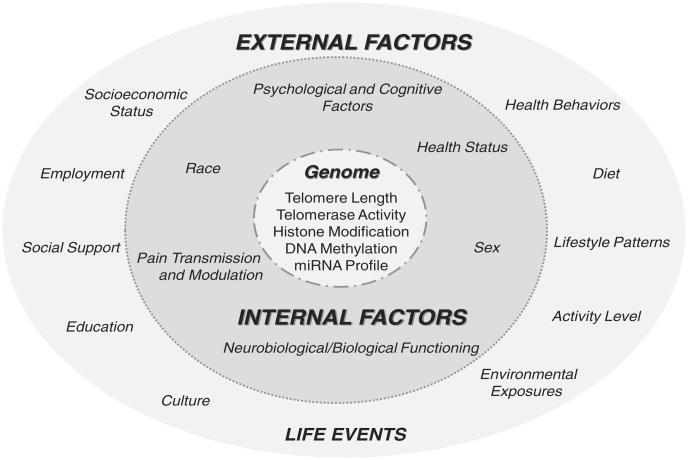
Fig. 1 provides a heuristic model that conceptualizes the interactions between environmental influences and genome functioning. The model illustrates how stress and pain might influence molecular processes central to genome function. Specifically, events arising from both external and internal environments can (1) contribute to accelerated cellular aging, resulting in alterations in telomere length and/or (2) propel epigenetic modifications that ultimately modulate genomic regulation of peripheral and central pain processes. Advances in telomere and epigenetic research hold substantial promise for revealing how adverse environmental experiences are transduced to the genome to influence pain processing. In an effort to highlight the potential application of the molecular consequences of environmental stress to pain research, we discuss below evidence supporting the relevance of telomeres and epigenetic processes to chronic pain.
Fig. 1.
A heuristic model for conceptualizing the interactions between environmental influences and genome functioning. External factors are “outside” the individual, exogenous in nature, and represented by the outer light gray circle. Internal factors are “within” the individual, endogenous in nature, and represented by the inner dark gray circle. Genome functioning is represented by the innermost white circle. The 3 circles are connected by dotted/spaced lines illustrating the reciprocal interaction between environmental influences (internal and external factors) and genome functioning. Of note, categorical assignment is not mutually exclusive, internal factors (eg, race and sex) interface with external factors and vice versa. Conceptually, stress or pain experiences can drive molecular changes that could result in accelerated cellular aging, reduced telomere length, or epigenetic changes influencing gene expression. Additionally, environmental influences may modulate the effects of stress or pain on the genome by buffering or reducing the impact (resilience factors) or exacerbating or increasing the impact (vulnerability factors) of negative environmental influences.
2. Telomeres
Pain elicits a stress response activating the autonomic, immune, and neuroendocrine systems [5]. While prior research has primarily addressed the biological profile of the acute stress response associated with clinical and experimental pain, the molecular consequences of chronic pain are not well understood. Identifying biological markers predicting the onset of chronic pain and reflecting the long-term impact of persistent pain would offer significant research and clinical utility. One such potential measure, leukocyte telomere length (TL), a marker of cellular aging, may benefit pain research given its association with the intensity and persistence of stress [10,38].
Telomeres are complexes comprising DNA and protein that cap and protect the ends of eukaryotic chromosomes, providing stability for replication and preventing fusions [4]. Telomeres are characterized by repetitive, single-strand DNA sequences that decrease in length with each cell division. As telomeres shorten to a critical length, cells typically become senescent; however, during this period, the risk of maladaptive cellular changes also increases [4,13]. Importantly, some cells are buffered by telomerase, a complex enzyme comprised of telomerase RNA, a reverse transcriptase, and accessory proteins. Telomerase adds telomeric DNA sequence repeats to the ends of chromosomes, providing protection, repair, and even potential lengthening of telomeres [4].
Although TL can be measured in various cells, a significant proportion of research has focused on peripheral blood mononuclear cells, leukocyte TL. Factors influencing mitosis in this cell population include oxidative stress, stress hormones, and inflammation [11–13,38]. In general, leukocyte TL shortens with chronological age, but the correlations are modest (r = −0.23 to −0.40) [10,28]. Some limitations identified in telomere research include differing measurement techniques, poor standardization across measures, and variability in TL across cell types within an individual [2].
Although conflicting findings exist, shorter leukocyte TL has been associated with age-related diseases, chronic mental and physical health conditions, and mortality [3,13,38], whereas longer TL has been associated with years of healthy life [27]. Growing evidence also indicates that TL is negatively associated with persistent psychosocial stress [13]; examples include caregiving stress [7,10] and a history of childhood adversity [18]. Importantly, in a group of adults providing extended care (average of 5 years) for a family member with Alzheimer's disease, TL was not only shorter compared to control subjects, impaired immune functioning was also demonstrated [7].
The effects of stress on TL may be modulated by external and internal factors (Fig. 1). For example, evidence demonstrates that shorter TL is related to high levels of trait pessimism in women [29], while moderate exercise is associated with longer TL [20]. Telomerase, which can buffer leukocyte telomere shortening, is theorized to be a dynamic measure with demonstrated responsiveness to acute stressors [11], and has been indicated as an outcome measure in short-term clinical interventions [39].
Consistent with the concept of allostatic overload, chronic pain may be the result of, or contribute to, dysregulated stress response systems [23]. Inversely correlated with the duration of stress [10,38], TL appears to function as a barometer of the organism's cumulative response to long-term endogenous and exogenous stressors. As such, TL may serve as a measure of system burden, the consequence of chronic pain and associated stressors on an individual system. Recent findings indicate that older adults reporting chronic pain and high stress had significantly shorter leukocyte TL than individuals reporting no chronic pain and low stress [33]. Further research is needed to determine if telomere measures are markers of pain-related system burden, sensitive to individual variability in the onset and persistence of pain-related conditions.
3. Epigenetics
Epigenetics refers to diverse and dynamic processes that modulate gene expression independent of DNA sequence and serve to translate environmental signals to the fixed genome. An epigenetic signature provides an additional layer of information that determines the level of gene expression. This encompasses heritable effects on gene expression, as well as stable modifications of a cell's transcriptional capability, which is not necessarily heritable [21]. Given the scope and diversity of epigenetics and associated research, a brief summary of key epigenetic processes is provided with references cited for additional information.
DNA methylation represents the most stable epigenetic modification of the genome in that it is part of the chemical structure of DNA [21]. DNA methylation occurs when a methyl group is attached to the backbone of DNA at position 5 of the cytosine found at CpG dinucleotides, which are associated with approximately 40% of human genes [31]. DNA methylation is catalyzed by a group of enzymes known as DNA methyltransferases and in general, promoter hypermethylation of DNA typically results in gene silencing. DNA methylation is dynamic and responsive to environmental exposure (eg, diet, physical or psychosocial stress, trauma), especially when occurring during critical periods of development or with aging [21].
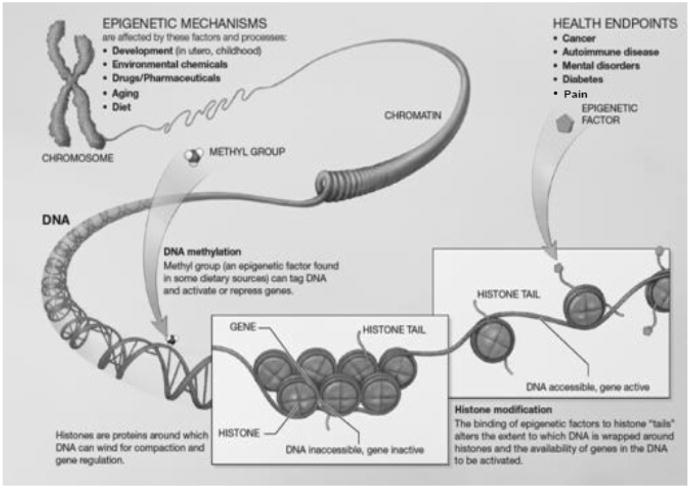
Another form of epigenetic regulation is histone modification. Within a cell, DNA is wrapped around histone proteins, which together compose chromatin. Chromatin remodeling occurs through chemical modifications of the amino terminal tails of histone proteins. These modifications alter how tightly or loosely chromatin is packaged and thus controls gene accessibility and expression. His tone modifications occur primarily through acetylation, phosphorylation, and/or methylation, which are mediated by enzymes that either add or remove these modifications, for example, histone acetyltransferases and deacetylases [30]. For example, deacetylation results in chromatin compaction, which blocks transcription (Fig. 2). It is important to note that DNA methylation and histone modification are mutually interactive and work in concert to affect epigenetic regulation of gene expression [19]. A third form of epigenetic regulation is through small noncoding RNA molecules (microRNAs) that exert epigenetic effects by regulating gene expression posttranscriptionally [32].
Fig. 2.
Epigenetic mechanisms include DNA methylation and histone modification. The addition of a methyl group to DNA results in repression of gene expression. Histones are proteins around which DNA is wrapped. The tightness of this wrapping determines the accessibility of DNA for transcription of genes. Histone modification occurs as a result of binding of epigenetic factors to histone “tails”. Such modification alters the extent to which DNA is wrapped around histones. If tightly wrapped, DNA is not accessible for transcription. http://www.nihroadmap.nih.gov/epigenomics/index.asp. Permission is granted to copy, distribute and/or modify this document under the terms of the GNU Free Documentation License, Version 1.2 or any later version published by the Free Software Foundation. This work is in the public domain in the United States because it is a work of the United States Federal Government under the terms of Title 17, Chapter 1, Section 105 of the US Code. The website for this figure is as follows: http://www.commons.wikimedia.org/wiki/File:Epigenetic_mechanisms.jpg.
Individual variability in the pain experience results from an interplay among a person's genetic makeup, past experience, and the environment; all of which interact to shape an individual's pain phenotype [15]. For example, children born preterm and who experience pain or surgery during early postnatal life exhibit alterations in sensory processing compared to term infants [36]. Pain-related developmental vulnerabilities are further supported by preclinical findings [1]. Evidence linking onset and persistence of pain, as well as analgesic responses to epigenetic regulation, is emerging [8,25,37]. For example, epigenetic modification has been posited to underlie variations in and susceptibility to pain syndromes such as: primary headaches, migraine and cluster headaches [24], chronic bladder pain syndrome [9], and endometriosis [14].
Epigenetic processes are also linked to nociceptive responses in rodents, including demethylating agents [16] and pan-histone acetylators [6]. Each of these agents has known epigenetic effects, but the effects are nonspecific and can affect molecules other than histones, DNA, or chromatin structure. More recent findings have linked persistent inflammation and neuropathic pain in that epigenetic changes decrease GABAergic synaptic functioning in areas of the brainstem associated with pain processing [40]. A review of current findings regarding the role of microRNAs in pain has been published [25].
A recent translational study associated increased DNA methylation of the gene promoter for the extracellular matrix protein SPARC (secreted protein, acidic, rich in cysteine) with chronic low back pain, in humans and in a mouse model. Findings suggest that epigenetic silencing of a gene, protective against accelerated disk degeneration, is linked to age-associated disk degeneration and chronic back pain. It is likely that epigenetic modulation of additional pain-relevant genes in both peripheral tissues and the central nervous system will be associated with the development and maintenance of chronic pain [34].
Other convincing evidence exists for epigenetic regulation of the opioid receptor genes, which exhibit various epigenetic modifications resulting in differential receptor expression [37]. Although the 3gene promoters are similar, they exploit different forms of epigenetic regulation and exhibit different patterns of expression. DNA methylation regulates both the μ and d receptor genes, and extensive chromatin remodeling and altered epigenetic marks have been demonstrated for μ and κ. κ undergoes reversible epigenetic modifications, and such “plasticity” suggests the potential for epigenetic modifications that couldbeexploitedtoimprove clinical conditions. For example, the μ promoter has been shown to be heavily methylated in former heroin addicts, and its expression may be subsequently downregulated [26]. In experimental animals, down-regulation of μ results in a loss of opiate-induced analgesia and increased sensitivity to pain [22]. Therapeutic potential has also been recognized for epigenetic manipulations of type-2 metabotropic glutamate receptors (mGlu2), as epigenetic modifications that increase mGlu2 expression in the dorsal root ganglion and spinal dorsal horn represent a potentially valuable therapeutic strategy [6]. It is tempting to speculate that epigenetic manipulation of receptor expression (ie, directed demethylation of the μ promoter) could, in the future, have profound implications for the relief of pain.
4. Conclusion
The above discussion suggests the potential relevance of epigenetics and telomere measures as molecular indicators of the biological consequences of stress and chronic pain. Specifically, epigenetic modification induced by environmental factors could influence the development of chronic pain by modulating genomic expression of one or more biological systems associated with pain or analgesia. Moreover, system burden (ie, the burden of chronic arousal of an individual's stress-related systems resulting from persistent pain and associated psychosocial stressors) may be reflected at the cellular level by the shortening of telomeres. Associations between stress and telomeres, and telomeres and morbidity/mortality have been reported; chronic pain has been related not only to stress but also to poor health and increased mortality [35]. Epigenetic and telomere changes may not only indicate individual differences in pain-related responses, but offer an array of targets that can be exploited for prevention and treatment interventions.
Additional research is warranted to ascertain the true relevance of these molecular responses to chronic pain and to explore possible clinical implications. Prospective studies should evaluate whether epigenetic modifications mediate the association between environmental stressors and future development of chronic pain. Also, the association of TL with chronic pain onset and the consequences of pain persistence await exploration. Additionally, evaluations of epigenetic, telomere, and telomerase changes with pain treatments are needed. Finally, identification of vulnerability and resilience factors that modulate the molecular influences of stress on pain and vice versa can enlighten understanding of individual differences in pain experiences. As a field, chronic pain research is well positioned to exploit recent advances in the molecular effects of stress. Such an approach could provide novel and important information leading to more effective pain management.
Acknowledgments
This work was supported by funding to include UF CTSI UL1TR000064 and KL2TR000065, the UF Claude D. Pepper OAIC, American Pain Society Future Leaders in Pain Research grant (K.T.S.); and OppNet AG0333906 07S1 (K.T.S. and R.B.F.); NIH/NIA grant R01 AG033906 (R.B.F.), and UF CTSI grant UL1 RR029890; and NIH/CA134736 (L.J. and H.L.M.). Special thanks to Taimour Langaee, Ph.D. for reviewing the telomere and telomerase sections of the manuscript.
Footnotes
Conflict of interest statement: Potential conflict of interest: R.B.F. is a stockholder in Algynomics.
References
- 1.Anand KJS, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–37. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aviv A. The epidemiology of human telomeres: faults and promises. J Gerontol A Biol Sci Med Sci. 2008;63:979–83. doi: 10.1093/gerona/63.9.979. [DOI] [PubMed] [Google Scholar]
- 3.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 4.Chan SR, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci. 2004;359:109–21. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain. 2008;9:122–45. doi: 10.1016/j.jpain.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiechio S, Zammataro M, Morales ME, Busceti CL, Drago F, Gereau RW, 4th, Copani A, Nicoletti F. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol Pharmacol. 2009;75:1014–20. doi: 10.1124/mol.108.054346. [DOI] [PubMed] [Google Scholar]
- 7.Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng N. Accelerated telomere erosion is asociated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol. 2007;179:4249–54. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denk F, McMahon SB. Chronic pain: emerging evidence for the involvement of epigenetics. Neuron. 2012;73:435–44. doi: 10.1016/j.neuron.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elgavish A. Epigenetic reprogramming: a possible etiological factor in bladder pain syndrome/interstitial cystitis? J Urol. 2009;181:980–4. doi: 10.1016/j.juro.2008.10.145. [DOI] [PubMed] [Google Scholar]
- 10.Epel E, Blackburn E, Lin J, Dhabhar F, Adler N, Morrow J, Cawthon R. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epel E, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, Blackburn EH. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav Immun. 2010;24:531–9. doi: 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epel E, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Dolbier C, Mendes W, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–87. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Epel ES. Telomeres in a life-span perspective: a new “psychobiomarker”? Curr Dir Psychol Sci. 2009;18:6–10. [Google Scholar]
- 14.Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15:587–607. doi: 10.1093/molehr/gap064. [DOI] [PubMed] [Google Scholar]
- 15.Hunt SP. Genes and the dynamics of pain control. Funct Neurol. 2009;24:9–15. [PubMed] [Google Scholar]
- 16.Hwang CK, Song KY, Kim CS, Choi HS, Guo XH, Law PY, Wei LN, Loh HH. Epigenetic programming of mu-opioid receptor gene in mouse brain is regulated by MeCP2 and Brg1 chromatin remodelling factor. J Cell Mol Med. 2008;13:3591–615. doi: 10.1111/j.1582-4934.2008.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones GT, Power C, Macfarlane GJ. Adverse events in childhood and chronic widespread pain in adult life: results from the 1958 British Birth Cohort Study. PAIN®. 2009;143:92–6. doi: 10.1016/j.pain.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Kananen L, Ssurakka I, Pirkola S, Suvisaari J, Lönnqvist J, Peltonen L, Ripatti S, Hovatta I. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between physical activity level, telomere length, and telomerase activity. Med Sci Sports Exerc. 2008;40:1764–71. doi: 10.1249/MSS.0b013e31817c92aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathews HL, Janusek L. Epigenetics and psychoneuroimmunology: mechanisms and models. Brain Behav Immun. 2011;25:25–39. doi: 10.1016/j.bbi.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kiefer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–23. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 23.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 24.Montagna P. The primary headaches: genetics, epigenetics and a behavioral genetic model. J Headache Pain. 2008;9:57–69. doi: 10.1007/s10194-008-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niederberger E, Kynast K, Lötsch J, Geisslinger G. MicroRNAs as new players in the pain game. PAIN®. 2012;152:1455–8. doi: 10.1016/j.pain.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen DA, Hamon S, Yuferov V, Jackson C, Ho A, Ott J, Kreek MJ. Ethnic diversity of DNA methylation in the OPRM1 promoter region in lymphocytes of heroin addicts. Hum Genet. 2010;127:639–49. doi: 10.1007/s00439-010-0807-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Njajou O, Hsueh WC, Blackburn EH, Newman AB, Wu SH, Li R, Simonsick EM, Harris TM, Cummings SR, Cawthon RM Healthy ABC study. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64:860–4. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Njajou OT, Cawthon RM, Damcott CM, Wu SH, Ott S, Garant MJ, Blackburn EH, Mitchell BD, Shuldiner AR, Hsueh WC. Telomere length is paternally inherited and is associated with paternal lifespan. Proc Natl Acad Sci USA. 2007;104:12135–9. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Donovan A, Lin J, Dhabhar FS, Wolkowitz O, Tillie JM, Blackburn E, Epel E. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2009;23:446–9. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:R546–51. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO J. 1998;17:4905–8. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278:1598–609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- 33.Sibille KT, Langaee T, Burkley B, Gong Y, Glover TG, King C, Riley JL, III, Leeuwenburgh C, Staud R, Bradley LA, Fillingim RB. Chronic pain, perceived stress, and cellular aging: an exploratory study. Mol Pain. 2012;8:12. doi: 10.1186/1744-8069-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tajerian M, Alvarado S, Millecamps M, Dashwood T, Anderson KM, Haglund L, Ouellet J, Szyf M, Stone LS. DNA methylation of SPARC and chronic low back pain. Mol Pain. 2011;7:65. doi: 10.1186/1744-8069-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torrance N, Elliott A, Lee A, Smith B. Severe chronic pain is associated with increased 10 year mortality. A cohort record linkage study. Eur J Pain. 2010;14:380–6. doi: 10.1016/j.ejpain.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. PAIN®. 2009;141:79–87. doi: 10.1016/j.pain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Wei LN, Loh HH. Transcriptional and epigenetic regulation of opioid receptor genes: present and future. Pharmacogenetics. 2011;51:75–97. doi: 10.1146/annurev-pharmtox-010510-100605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolkowitz OM, Mellon SH, Epel E, Lin J, Dhabhar FS, Su Y, Reus VI, Rosser R, Burke HM, Kupferman E, Compagnone M, Nelson JC, Blackburn EH. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress – preliminary findings. PLoS One. 2011;6:1–10. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R, Burke H, Compagnone M, Nelson J, Dhabhar FS, Blackburn EH. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatry. 2011:1–9. doi: 10.1038/mp.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Cai Y, Zou F, Bie B, Pan ZZ. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med. 2011;17:1448–56. doi: 10.1038/nm.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]