Abstract
Our previous work found the two yeast plasma membrane-localized casein kinases Yck1p and Yck2p to be palmitoylated on C-terminal Cys-Cys sequences by the palmitoyl transferase Akr1p. The present work examines a third casein kinase, Yck3p, which ends with the C-terminal sequence Cys-Cys-Cys-Cys-Phe-Cys-Cys-Cys. Yck3p is palmitoylated and localized to the vacuolar membrane. While the C-terminal cysteines are required for this palmitoylation, Akr1p is not. Palmitoylation requires the C-terminal Yck3p residues 463-524, whereas information for vacuolar sorting maps to the 409-462 interval. Vacuolar sorting is disrupted in cis through deletion of the 409-462 sequences and in trans through mutation of the AP-3 adaptin complex; both cis- and trans-mutations result in Yck3p missorting to the plasma membrane. This missorted Yck3p restores 37°C viability to yck1Δ yck2-ts cells. yck1Δ yck2-ts suppressor mutations isolated within the YCK3 gene identify the Yck3p vacuolar sorting signal—the tetrapeptide YDSI, a perfect fit to the YXXϕ adaptin-binding consensus. Although YXXϕ signals have a well-appreciated role in the adaptin-mediated sorting of mammalian cells, this is the first signal of this class to be identified in yeast.
INTRODUCTION
The four adaptin complexes AP-1, AP-2, AP-3, and AP-4 mediate membrane sorting at multiple points within the mammalian Golgi/endosomal system (Bonifacino and Traub, 2003). These protein complexes truly are adaptors, linking the cargo protein to the bud-forming coat proteins. This has been most clearly outlined in clathrin-mediated endocytosis for the AP-2 adaptin complex where the adaptin complex links through dual binding, the endocytic cargo and the heavy chain of the clathrin coat. Cargo and coat thus are recruited together, forming a nascent coated pit, which invaginates and is then released into the cytoplasm as a clathrin-coated vesicle.
Each adaptin complex is comprised of a conserved set of four subunits: two large subunits, a β (β1-4) and either a γ, α, δ, or ε, depending on the complex (i.e., AP-1, -2, -3, or -4); a medium subunit μ (μ1-4); and a small subunit σ (σ1-4). AP-2 functions at the plasma membrane to effect plasma membrane uptake, whereas AP-1, AP-3, and AP-4 function at the Golgi to mediate communication with endosomal compartments and the lysosome. Similar types of signals are recognized by the four complexes, with the signals conforming either to the tyrosine-based consensus YXXϕ (ϕ being a bulky, hydrophobic residue) or to the dileucine-based consensus [DE]XXXL[LI] (Bonifacino and Traub, 2003).
The yeast Saccharomyces cerevisiae has three adaptin complexes, AP-1, AP-2, and AP-3 (Boehm and Bonifacino, 2002). AP-1 has been suggested to function at the level of the Golgi, acting to maintain the integrity of this organelle through the AP-1/clathrin-mediated retrieval of Golgi proteins that escape to the early endosome (Stepp et al., 1995; Yeung et al., 1999; Valdivia et al., 2002). Despite the well-defined role for AP-2 in mammalian endocytosis, the function of the yeast AP-2 has proved elusive; deletion of the genes encoding the subunits of the yeast AP-2 complex is without discernible effect on endocytosis, or on any trafficking pathway yet examined (Huang et al., 1999; Yeung et al., 1999). Analysis of yeast AP-3 has proved particularly fruitful, leading to the definition of a new trafficking pathway that connects the Golgi to the vacuole (yeast lysosome) (Cowles et al., 1997a; Stepp et al., 1997). The AP-3-dependent pathway differs from the more well-studied VPS pathway, which also connects Golgi to vacuole, in that transport does not pass through the multivesicular body (MVB) (Cowles et al., 1997b; Piper et al., 1997). The MVB, also known as either the prevacuolar compartment or late endosome, is an intermediary compartment, located proximal to the vacuole, that functions to sort cargoes destined for the vacuolar lumen away from cargoes targeted to the limiting membrane of the vacuole (Odorizzi et al., 1998). Only two cargo proteins have been yet identified for the yeast AP-3 pathway: the vacuolar phosphatase ALP and the vacuolar t-SNARE Vam3p; both use classic dileucine-based signals to instigate their AP-3-mediated sorting to the limiting membrane of the vacuole (Darsow et al., 1998; Vowels and Payne, 1998). Rather than partnering with clathrin, the yeast AP-3 complex is thought instead to link to Vps41p, which has been suggested to provide the coat function (Darsow et al., 2001). AP-3 seems to fill a similar niche in mammalian cells, dileucine- and tyrosine-based signals are used to initiate the sorting from the Golgi to the lysosome (Boehm and Bonifacino, 2002; Bonifacino and Traub, 2003). The issue of whether clathrin participates in mammalian AP-3-dependent sorting remains to be settled (Newman et al., 1995; Simpson et al., 1997; Dell'Angelica et al., 1998).
Mutations in the yeast AP-3 subunit genes were first identified in a selection for suppressors of the yck1Δ yck2-ts mutations (Panek et al., 1997). Yck1p and Yck2p are homologous and functionally redundant type I casein kinases that localize to the yeast plasma membrane (Robinson et al., 1993; Vancura et al., 1994). Given that yck1Δ yck2Δ cells are inviable, a conditional yck1Δ yck2-ts strain was constructed and was found to be defective both in endocytosis and in cell growth and morphogenesis (Robinson et al., 1993; Panek et al., 1997). Suppressors conferring 37°C growth to the yck1Δ yck2-ts strain were found to map to four complementation groups, the four AP-3 subunit genes; suppression also could be achieved through total deletion of the subunit genes, indicating suppression depended on a loss of AP-3 function (Panek et al., 1997). At the time, nothing was known regarding AP-3 function and it was thought, given the Yck1p/Yck2p participation in endocytosis, that this new adaptin complex might also participate in endocytosis. However, although they suppressed the yck1Δ yck2-ts endocytosis defect, the AP-3 mutations were themselves not associated with any endocytosis defect. Even with the understanding of AP-3 function in Golgi-vacuole trafficking that has been since gleaned, the mechanism underlying the yck1 yck2 suppression has remained a puzzle.
The present study, which focuses on Yck3p, a third and related type I casein kinase, resolves this mystery. Having recently demonstrated that Yck2p is tethered to the plasma membrane through palmitoylation of a C-terminal Cys-Cys sequence (Roth et al., 2002), we were intrigued to find that the Yck3p C-terminal sequence is the striking cysteine-rich sequence CCCCFCCC. Yck3p, we find, also is palmitoylated. However, rather than localizing to the plasma membrane like Yck1p and Yck2p, Yck3p, instead localizes to the cytoplasmic surface of the vacuolar membrane. The trafficking of Yck3p to the vacuole is via the AP-3-dependent, ALP pathway: in apm3Δ or in aps3Δ cells, deleted for the AP-3 μ or σ subunit genes, respectively, Yck3p is missorted to the plasma membrane. This Yck3p missorting might explain the yck1Δ yck2-ts suppression by AP-3 mutations (Panek et al., 1997): cell surface casein kinase function, presumably deficient in yck1Δ yck2-ts cells at 37°C, might be replenished through the diversion of Yck3p to plasma membrane. To test this hypothesis, Yck3(Δ409-462)p, a mutant Yck3p deleted for its AP-3-dependent vacuolar sorting information, was expressed in yck1Δ yck2-ts cells: Yck3(Δ409-462)p is missorted to the plasma membrane and consequently, 37°C growth is restored to the yck1Δ yck2-ts cells. The yck1Δ yck2-ts suppression phenotype was exploited to isolate point mutations within the Yck3p sorting signal; these mutations identify a classic tyrosine-based YXXϕ consensus signal for adaptin-mediated sorting. Although such signals are known to participate in the AP-1-, AP-2-, and AP-3-mediated sorting of mammalian cells, this is the first YXXϕ signal to be identified in yeast.
MATERIALS AND METHODS
Strains
Two strain backgrounds were used in this work. Most of the work was done with the LRB759 (MATα ura3-52 leu2 his3) strain background (Panek et al., 1997). In addition to the wild-type LRB759 strain, the isogenic yck1-1::ura3 yck2-2ts strain LRB757 (Panek et al., 1997), and the akr1Δ version NDY1405 (Roth et al., 2002) were also used. Other strains (used for Figure 5) were from the Saccharomyces Deletion Consortium, including the wild-type MATa ura3Δ0 leu2Δ0 his3Δ1 met15Δ0 strain BY4741 and the aps3Δ, apm3Δ, vps2Δ, and vps4Δ deletion derivatives, in which the relevant open reading frame (ORF) is replaced with the G418R marker (Research Genetics, Huntsville, AL). Polymerase chain reaction (PCR) was used for confirming the presence of each deletion within the Consortium strains.
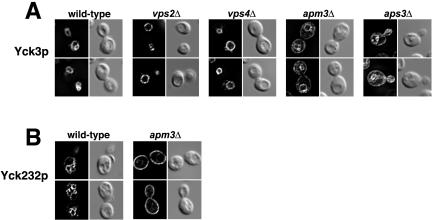
Figure 5.
Yck3p traffics to the vacuole via the AP-3 pathway. HA-tagged Yck3p (A) or HA-tagged Yck232 (B) expressed at normal endogenous levels under the respective control of YCK3 or YCK2 upstream sequences, in either the wild-type Saccharomyces Deletion Consortium strain or in the isogenic consortium strains individually deleted for the indicated genes. For each mutant strain, two typical cells are shown, with the fluorescent images of the cells on the left and the same cells visualized by DIC optics shown to the right.
Plasmids
All YCK2 and YCK3 constructs were introduced into yeast on the URA3/CEN/ARS vector plasmid pRS316 (Sikorski and Hieter, 1989). A YCK3P-4xHA/YCK3 construct was used for most of the indirect immunofluorescent experiments. This construct, which has four copies of the hemagglutinin (HA) epitope tandemly inserted immediately after the initiator codon, retains the natural YCK3 regulatory sequences, both upstream (the 438 base pairs immediately upstream of the initiator codon) and downstream (420 base pairs downstream of the terminator codon) of the YCK3 ORF. GAL1P-6xHis/FLAG/HA/YCK3, used both in the palmitate-labeling experiment and for some immunofluorescence, differs in having the GAL1 regulatory sequences in place of the YCK3 upstream sequences and in having an amino-terminal 6xHis/FLAG/HA tripartite tag instead of the 4xHA sequence. Two plasmids were used for expression of tagged YCK2: YCK2P-HA/YCK2 has Yck2p, N-terminally tagged with a single HA epitope controlled by the 623 base pairs upstream of the YCK2 ORF, whereas GAL1P-6xHis/FLAG/HA/YCK2, Yck2p is N-terminally tagged with the tripartite 6xHis/FLAG/HA and is controlled by the GAL1 promoter.
The ΔCys truncation was constructed through the insertion of a terminator codon just before the C-terminal CCCCFCCC encoding sequence of YCK3. The Δ409-462 mutation is an in-frame deletion of YCK3 codons 409 through 462, with Thr-Arg-encoding a Mlu I site in place of the missing codons. The YCK232 construct encodes a chimeric protein having the N-terminal portion of YCK2 (codons 1-396), followed by a portion of YCK3 (codons 409-462), and ending with the C-terminal portion of YCK2 (codons 496-546). This plasmid was constructed by in vivo recombination in yeast. A 264-base pair PCR fragment was generated from a YCK3 template by using two 76-nt primers having 51 nt of YCK2 sequence at their 5′ ends and 25 nt of YCK3 sequence at their 3′ ends to serve as the template-specific primers. The resulting PCR fragment has a core 162 base pairs of YCK3 sequences (codons 409-462) flanked by 51 base pairs of upstream and downstream YCK2 sequence (upstream YCK2 codons 382-396; downstream YCK2 codons 496-512) to direct the in vivo recombination. The PCR fragment was cotransformed into yeast along with YCK2P-HA/YCK2 plasmid linearized at its unique Mfe I site (located at YCK2 codons 435 and 436). Circularized plasmids repaired through homologous recombination with the PCR fragment were recovered from the Ura+ yeast colonies.
A GAL1P-HA/URA3 construct (single HA epitope tag at the Ura3 N terminus) on the LEU2/CEN/ARS plasmid pRS315 was the starting point for the HA/URA3/YCK3 fusions. XhoI restriction sites that had been introduced into YCK3 just before codon 1, codon 409, codon 462, and codon 517, were used in ligations to a SalI site, introduced just before the URA3 terminator codon.
YCK3 Suppressors of yck1Δ yck2-ts
The YCK3 C-terminal truncation mutation Δ409-524 was constructed in the YCK3P-4xHA/YCK3 plasmid context by using PCR-based methods; the 116 deleted codons in this mutation were replaced by a Mlu I restriction site. Yck3(Δ409-524)p lacks the C-terminal palmitoylation sequences and, like Yck3(ΔCys)p, is expected to be cytoplasmic. A 796-base pair fragment of YCK3 (corresponding to codons 336-524 plus 230 base pairs of downstream sequence), generated from a YCK3 plasmid template by mutagenic PCR (reactions included 10 mM MnCl2 in addition to the usual 50 mM MgCl2 and 80 μM dATP and 400 μM of the other dNTPs) was transformed into yck1Δ yck2-ts yeast cells together with the Mlu I-linearized YCK3P-4xHA/YCK3(Δ409-524) plasmid. Ura+ transformants that grew at 37°C were selected. Plasmid DNA was isolated from these cells and the C-terminal region of YCK3 was sequenced.
Palmitate Labeling
GAL1-driven YCK2 and YCK3 constructs, tagged with amino-terminal 6xHis/FLAG/HA sequences, were introduced both into the AKR1+ wild-type strain LRB759 and the isogenic akr1Δ strain NDY1405. The in vivo labeling with [(9,10)[3H]palmitic acid (60 Ci/mmol; DuPont, Wilmington, DE), along with the subsequent preparation of extracts and the anti-FLAG immunoprecipitation of the labeled Yck kinases were as described previously (Roth et al., 2002). Quantitation of the Yck protein recovery from the immunoprecipitation by anti-HA Western blotting was used to normalize the amount of sample applied to the SDS-polyacrylamide gel for assessing incorporation of the labeled palmitate.
Indirect Immunofluorescence Microscopy
For most experiments, cells that carried HA-tagged YCK URA3/CEN/ARS plasmid constructs were cultured in minimal yeast medium lacking uracil. For experiments that used GAL1-driven constructs, the Yck proteins were expressed from cultures grown in YP medium containing 2% raffinose with a 2-h addition of 2% galactose, followed by a 20-min “chase” period with 3% glucose (giving newly synthesized proteins the opportunity to attain their endpoint destinations). Cells were fixed, spheroplasted, and otherwise developed for immunofluorescent microscopy as described previously (Chen and Davis, 2002). The HA-tagged proteins were detected using a 1:1000 dilution of the HA.11 monoclonal antibody (Covance, Princeton, NJ) as the primary antibody, followed by a 1:500 dilution of the Cy3-conjugated goat anti-mouse IgG secondary antibody. Z-stacks of digital images with focal planes spaced at 0.2-μm increments were collected using the 100× objective on a Leica DMRA2 microscope and Hamamatsu Orca ER charge-coupled device camera. Images were deconvolved with 25 iterations of Autoquant Auto-Deblur software (Autoquant) and contrast-enhanced with Photoshop (Adobe Systems, Mountain View, CA).
RESULTS
Yck3p Palmitoylation
The Yck3p striking cysteine-rich C-terminal sequence Cys-Cys-Cys-Cys-Phe-Cys-Cys-Cys (CCCCFCCC), suggested the possibility of its palmitoylation. To test this possibility, cells were labeled with [3H]palmitic acid and Yck3p was immune precipitated via N-terminally attached epitope tags (Figure 1). Indeed, we found that palmitate label is incorporated into Yck3p. In contrast, Yck3(ΔCys)p, a deletion mutant missing just the cysteine-rich C-terminal eight Yck3p residues, was not labeled, indicating a likely role of some or all of the C-terminal cysteines as palmitoyl acceptors (Figure 1).
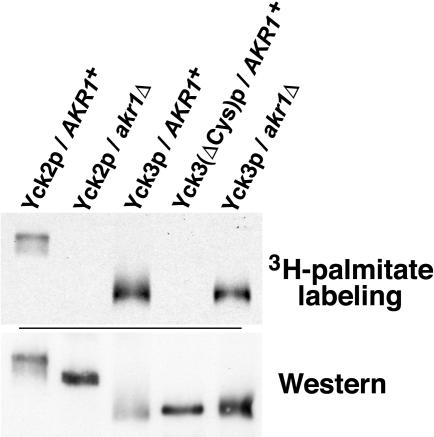
Figure 1.
Yck3p is subject to Akr1p-independent palmitoylation. Yck2 and Yck3 proteins, tagged at their amino termini with a 6xHis/FLAG/HA sequence, were expressed from the GAL1 promoter in either wild-type (AKR1+) cells or isogenic akr1Δ cells. The ΔCys mutation is a deletion of the last C-terminal eight Yck3p residues, CCCCFCCC. After labeling of the cells with [3H]palmitic acid, extracts were prepared, the tagged kinases were immune precipitated and subjected to SDS-PAGE and fluorography (top). Recovery of the immune-precipitated kinases was assessed by Western blotting by using an anti-HA monoclonal antibody (bottom).
Our recent work showed the polytopic membrane protein Akr1p to be the palmitoyl transferase responsible for the palmitoylation of Yck1p and Yck2p (Roth et al., 2002). Furthermore, our work indicated that cellular palmitoylation is likely carried out by multiple, distinct palmitoyl transferase activities. Indeed, a second yeast activity comprised by Erf2p and Shr5p has been identified as the devoted palmitoyl transferase for yeast Ras protein (Lobo et al., 2002). Here, we examined the dependence of Yck3p palmitoylation on Akr1p function (Figure 1). As we have shown previously (Roth et al., 2002), the palmitoyl labeling of Yck2p was abolished in akr1Δ cells (Figure 1). In contrast, Yck3p labeling seemed undiminished. Although Akr1p clearly is not required for Yck3p palmitoylation, we cannot rule out that Akr1p participates in some partial capacity, perhaps functioning redundantly with some other palmitoyl transferase.
Yck3p Localizes to the Vacuolar Membrane
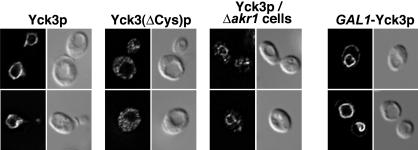
The palmitoylation of Yck1p and Yck2p serves to tether them to the plasma membrane (Roth et al., 2002). Here, Yck3p localization was examined using indirect immunofluorescent detection of N-terminal HA epitope tags (Figure 2). Like Yck1p and Yck2p, palmitoylated Yck3p also is membrane localized; however, rather than being localized to the plasma membrane, it localized instead to the limiting membrane of the yeast vacuole, the vacuole being easily visualized by differential interference contrast (DIC) optics as apparent surface depressions (a well-known artifact of this optical method). The Yck3(ΔCys)p mutant, which is not palmitoylated, was mislocalized; it showed a diffuse distribution throughout the cytoplasm and nucleus. We conclude that the palmitoylation of Yck3p serves to tether it to the cytoplasmic face of the vacuolar membrane.
Figure 2.
Yck3p localizes to the vacuolar membrane. Indirect immunofluorescent detection of Yck3 proteins is through amino-terminal HA epitope tags. The tagged constructs were expressed at normal endogenous levels under the control of YCK3 upstream sequences or in one instance, overexpressed from the GAL1 promoter (GAL1P-Yck3p). Two typical cells for each condition are shown, with the fluorescent images of the cells on the left and the same cells as visualized by DIC optics shown to the right.
In akr1Δ cells, consistent with its continued palmitoylation, Yck3p was seen to be largely localized to the vacuole membrane (Fig. 2). However, this vacuolar membrane localization was somewhat less complete than that seen in the wild-type AKR1+ context; in the akr1Δ cells, in addition to vacuolar membrane staining, some Yck3p localization to cytoplasmic puncta was also apparent (Figure 2). Thus, although no difference was discerned in the palmitate labeling of Yck3p in AKR1+ and akr1Δ cells, Akr1p does seem to play some partial role in the Yck3p localization process. A possibility discussed below (see DISCUSSION) is that Yck3p palmitoylation might involve Akr1p acting together with other palmitoyl transferases.
Subtle effects on Yck3p localization also were seen with overexpression from the GAL1 promoter (Figure 2). Although the bulk of the staining was again at the limiting membrane of the vacuole, some Yck3p clearly could be seen also localizing to the plasma membrane. Overexpression may partially overwhelm the normal Yck3p vacuolar sorting mechanism, resulting in some Yck3p escape to the plasma membrane.
Mapping the Vacuolar Sorting Signals of Yck3p
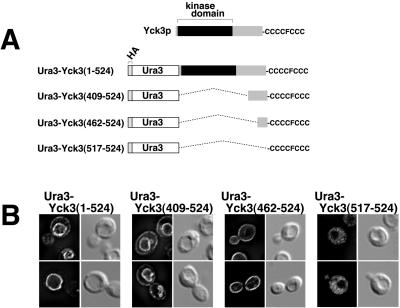
To delimit the Yck3p signals directing both palmitoylation and localization, we constructed a series of chimeric proteins which fuse various C-terminal portions of Yck3p to the C terminus of Ura3p, a cytoplasmic enzyme of the uracil biosynthesis pathway (Figure 3A). Fusion proteins were expressed from the GAL1 promoter and have the HA epitope tag attached to the N terminus of the Ura3 sequences to facilitate immunofluorescent detection. The starting Ura3p construct with no fused Yck3p sequences was found to be diffusely distributed through the cytoplasm (our unpublished data). When the entire Yck3 coding sequence was fused to Ura3, the Ura3-Yck3(1-524) fusion was found to localize primarily to the vacuolar membrane (Figure 3B). Consistent with results reported above for GAL1 promoter overexpression (Figure 2), the Ura3-Yck3 fusion, which was also expressed from the GAL1 promoter, also was partially mislocalized to the plasma membrane. The Ura3-Yck3(409-524) fusion protein localized like Ura3-Yck3(1-524) (Figure 3B); thus the Yck3 C-terminal 116 residues contain sufficient information for directing both palmitoylation and vacuolar sorting. In contrast, the Ura3-Yck3(462-524) fusion protein localized exclusively to the plasma membrane (Figure 3B). The failure of vacuolar targeting indicates a requirement for Yck3 409-462 sequences for proper vacuolar sorting - loss of this vacuolar sorting information, resulted in Ura3-Yck3(462-524)p being delivered, presumably by default, to the plasma membrane. The Ura3-Yck3(517-524) fusion protein, having just the C-terminal CCCCFCCC sequence as its Yck3 contribution, was found diffusely distributed throughout the cell cytoplasm (Figure 3B); this localization was identical to that seen both for the parental Ura3 construct with no fused Yck3 sequences (our unpublished data) and for the unpalmitoylated Yck3(ΔCys)p mutant (Figure 2). Thus, although the CCCCFCCC sequence is required for palmitoylation, it is not, by itself, a sufficient signal. Instead, palmitoylation apparently requires additional sequences mapping within the C-terminal 63 Yck3 residues (i.e., the sequences required for the membrane localization of the Ura3-Yck3(462-524) fusion protein). We conclude that the Yck3 409-462 sequences are required for vacuolar targeting and 462-524 sequences are required for palmitoylation.
Figure 3.
Deletional mapping of the Yck3 sequences that direct palmitoylation and vacuolar localization. (A) Schematic of Ura3-Yck3 fusion proteins. At the top is a schematic of Yck3p; the highly conserved (among the Yck proteins) N-terminal kinase domain is followed by the poorly conserved C-terminal domain, terminating in the indicated cysteine-rich sequence. The different Ura3-Yck3 fusion protein constructs with the indicated portions of Yck3 fused in-frame to the C terminus of the cytoplasmic enzyme Ura3p are shown below. The Ura3 amino terminus is tagged with a copy of the HA epitope to facilitate immune detection. (B) Indirect immunofluorescent localization of the Ura3-Yck3 constructs. Fusion proteins were expressed from the GAL1P. For each construct, two typical cells are shown, with the fluorescent images of the cells on the left and the same cells visualized by DIC optics shown to the right.
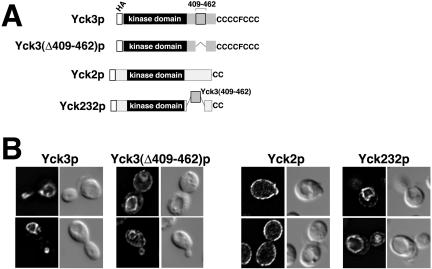
As a further test of the involvement of the 409-462 sequence in vacuolar targeting, a mutant Yck3p having an in-frame deletion of this interval was constructed, Yck3(Δ409-462)p (Figure 4A). The Δ409-462 mutation indeed did perturb Yck3p localization; however, the effect was only partial: whereas some Yck3(Δ409-462)p clearly was mislocalized to the plasma membrane, much still continued to be delivered to the vacuolar membrane (Figure 4B). Thus, although we can conclude that “efficient” sorting to the vacuole requires elements within the 409-462 interval, the continued delivery of substantial amounts of the Yck3(Δ409-462)p to the vacuole suggests that Yck3p may have additional sorting information, that maps outside of the 409-462 interval. Alternatively, the residual vacuolar transport could represent default transport to the vacuole (Roberts et al., 1992), with default transport being transport that occurs in the absence of sorting information (see DISCUSSION).
Figure 4.
The Yck3 409-462 sequence interval harbors a vacuolar sorting signal. (A) Schematic of the different HA-tagged Yck mutant constructs. (B) Indirect immunofluorescent localization of the different HA-tagged mutant Yck proteins. Expression of the Yck2 and Yck3 constructs was directed by the natural YCK2 and YCK3 upstream regulatory sequences. Two typical cells are shown for each construct with the fluorescent images of the cells on the left and the same cells visualized by DIC optics shown to the right.
To test the sufficiency of the sorting information within the 409-462 interval, we transplanted this sequence into the Yck2p context. The resulting Yck2/Yck3/Yck2 chimeric protein (Yck232p) has the Yck3 409-462 interval in place of Yck2 residues 396 through 496 (Figure 4A). The Yck2 396-496 interval is a glutamine-rich sequence, located C-terminal to the kinase domain, that is required neither for Yck2p palmitoylation, nor for its transport to the plasma membrane; the YCK2(Δ396-496) allele also fully complements a temperature-sensitive yck2 allele (Babu et al., 2002; Roth and Davis, unpublished data). Finally, the C-terminal 50 amino acids of Yck2p retained by the Yck232 chimera suffice to direct Yck2p palmitoylation (Babu et al., 2002; Roth and Davis, unpublished data).
The addition of the Yck3 sequences to Yck2p resulted in a striking change in localization: rather than being localized to the plasma membrane like Yck2p, Yck232p instead was found to localize predominately to the vacuolar membrane (Figure 4B). Thus, the Yck3 409-462 interval does contain a sufficient signal for vacuolar sorting. We note, however, that the redirection to the vacuole is not fully complete—some Yck232p also was seen to localize to the surface membrane. This partial localization to the plasma membrane might again be the result of overexpression; the Yck232 chimeric construct is expressed from upstream YCK2 promoter sequences, which may be substantially stronger than the YCK3 promoter: expressed from their native promoters, Yck2p accumulates to 20-fold higher levels within the cell than does Yck3p (our unpublished data).
AP-3-dependent Sorting
In yeast, two distinct trafficking mechanisms have been found to deliver membrane proteins to the vacuolar membrane. The carboxypeptidase Y (CPY) pathway, which transports soluble hydrolases such as CPY from the Golgi lumen to vacuolar lumen, also transports membrane proteins to the vacuolar membrane (examples being the protease DPAP-B and the vacuolar ATPase). The second pathway for Golgito-vacuole integral membrane protein traffic is the ALP or AP-3 pathway. We used mutants separately disabling these two pathways to see which might participate in Yck3p transport to the vacuole. Class E vps mutants were used to disrupt traffic along the CPY pathway. Acting at the level of the multivesicular body (MVB), these mutants accumulate CPY pathway cargoes within so-called class E bodies, aberrant compartments located just adjacent to the vacuole (Conibear and Stevens, 1998; Katzmann et al., 2002). Blockade of the ALP pathway was achieved through deletion of the genes encoding subunits of the AP-3 complex. Two class E mutants, vps2Δ and vps4Δ, were tested and neither showed discernible effects on Yck3p transport. In both mutant strains, Yck3p was found to localize exclusively to the vacuolar membrane; no staining of perivacuolar class E bodies, characteristic of CPY pathway cargoes, was discerned (Figure 5A). This lack of an effect of the class E mutations also excludes scenarios in which Yck3p might access the vacuole via an endocytic route (after first being delivered to the cell surface); endocytic traffic to the vacuole also goes through the MVB and endocytic cargoes consequently also accumulate within the class E bodies of class E vps mutants (Davis et al., 1993; Piper et al., 1995). To more directly rule out the participation of the endocytic pathway, we assessed Yck3p localization in two strains mutationally disabled for the plasma membrane internalization step of endocytosis, namely, end3Δ and sac6Δ cells (D'Hondt et al., 2000). No impairment in the vacuolar transport of Yck3p was seen in either of these end mutant strains (our unpublished data). For blockade of AP-3 pathway sorting, again two mutants were tested, aps3Δ and apm3Δ, deleted for the AP-3 σ and μ subunit genes, respectively. In both mutant backgrounds, a clear mislocalization of Yck3p to the plasma membrane was observed (Figure 5A). However, again, as previously observed with Yck3(Δ409-462)p, the missorting was not total: a substantial fraction of the Yck3p continued to be delivered to the vacuolar membrane (the issue of this residual vacuolar sorting is further explored in DISCUSSION). Nonetheless, we can conclude that Yck3p depends, at least partially, on the AP-3 pathway for its transport to the vacuole.
We have also examined Yck232p sorting in apm3Δ cells (Figure 5B). Although in wild-type cells, Yck232p localized mainly to the vacuolar membrane, in apm3Δ cells, its sorting to the vacuole was abolished as the chimeric protein localized instead, fully to the plasma membrane (Figure 5B). Thus, the vacuolar sorting signal(s) that map within the 409-462 interval are fully AP-3 dependent.
Yck3p Missorting to the Plasma Membrane Suppresses yck1Δ yck2-ts
As described in INTRODUCTION, disabling mutations within the AP-3 subunit genes were first identified as suppressors of the 37°C inviability of yck1Δ yck2-ts cells (Panek et al., 1997). Given the role described here for AP-3 in Yck3p sorting, we wondered whether the Yck3p missorting to the plasma membrane might account for yck1Δ yck2-ts suppression. Does the Yck3p that is diverted to the plasma membrane in AP-3 mutants help to compensate for the casein kinase deficit of the yck1Δ yck2-ts cells? Supporting such an explanation, yck1Δ yck2-ts suppression can also be achieved through Yck3p overexpression (Wang et al., 1996), a condition that also results in some Yck3p being diverted to the plasma membrane (Figure 2).
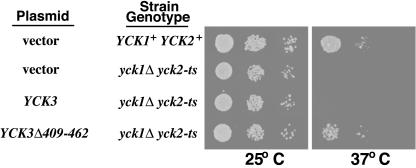
To test the role of Yck3p missorting in yck1Δ yck2-ts suppression, we have assessed the 37°C growth of a yck1Δ yck2-ts strain transformed with plasmids carrying either the wild-type YCK3 allele or the YCK3(Δ409-462) mutant allele. Although the addition of the wild-type YCK3 allele to the YCK3+ yck1Δ yck2-ts strain provided no growth benefit, introduction of the YCK3(Δ409-461) allele clearly conferred substantial 37°C growth to the yck1Δ yck2-ts strain (Figure 6). Thus, Yck3p missorting does provide a possible mechanism for yck1Δ yck2-ts suppression.
Figure 6.
Yck3p missorting confers 37°C viability to yck1Δ yck2-ts cells. Serial 10-fold dilutions of yck1Δ yck2-ts cells, transformed by the CEN/ARS vector pRS316 or by pRS316 carrying either the wild-type YCK3 or the mutant YCK3Δ409-462 allele, were plated at both permissive (25°C) and nonpermissive (37°C) temperatures. Growth of the yck1Δ yck2-ts transformants is compared with that of the isogenic YCK1+ YCK2+ parental strain at the two temperatures.
Point Mutants Disabling the Vacuole Sorting Signal Identify a Tyrosine-based Adaptin Recognition Sequence
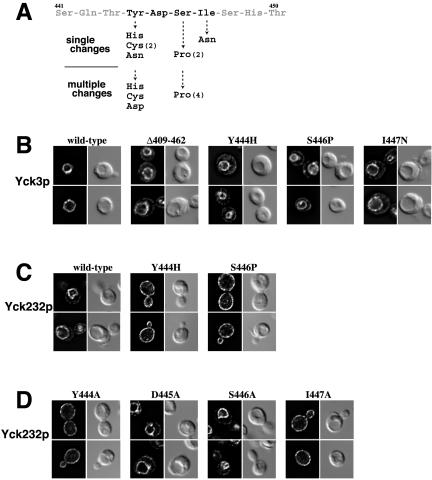
The yck1Δ yck2-ts suppression by Yck3p missorting suggested a scheme for isolating mutants within the Yck3p sorting signal. Like the Δ409-462 deletion, point mutants that inactivate the sorting signal also should confer suppression. Mutations were randomly directed into the C-terminal region of YCK3 (including the 409-462 interval) by mutagenic PCR, and the resulting library of plasmid-borne mutant YCK3 alleles was introduced into the yck1Δ yck2-ts strain, with growth at 37°C selected. From an estimated 40,000 transformants, 14 colonies surviving at 37°C were selected. Plasmid DNA was recovered from each and mutations were identified by sequencing. Although seven of the isolates had multiple changes within the 409-462 interval, the other seven showed changes just at single residues (Figure 7A). The seven single changes all clustered within the four-residue sequence YDSI from Tyr444 to Ile447. Suppression was conferred by changes of Tyr444 to His, Cys, and Asn, with a change of Ser446 to Pro, and by changing Ile447 to Asn. Of the seven mutants with multiple changes within the 409-462 interval, all had one of the mutations within the YDSI tetrapeptide sequence, with Tyr444 changed to His, Cys, or Asp, or with Ser446 again changed to Pro.
Figure 7.
Point mutations within the Yck3 YDSI tetrapeptide disrupts vacuolar sorting. (A) Mutations within the Yck3 YDSI tetrapeptide conferring yck1Δ yck2-ts suppression. The ten residue-long Yck3p sequence harboring the YDSI tetrapeptide is shown. Just below are the substitutions identified from the seven mutants having only a single substitution within the 409-462 interval. Below this are substitutions within YDSI peptide found among the seven mutants that had more than one amino acid changes within the 409-462 interval. For substitutions that were reisolated multiple times, the number of times the particular change was found is indicated (in parentheses). (B-D) Effects of YDSI mutations on localization of HA-tagged Yck3 and Yck232 proteins. Localization of wild-type and mutant versions of Yck3p and the chimeric Yck232p, expressed under the control of YCK3 and YCK2 promoter sequences, respectively, were detected by indirect immunofluorescent microscopy. For each mutant, two typical cells are shown, with the fluorescent images of the cells on the left and the same cells visualized by DIC optics shown to the right. (B) Effects of the indicated suppressor mutations on Yck3p localization. (C) Effect of suppressor mutations within the Yck232 chimeric protein context. (D) Effect of alanine substitutions at each position within the YDSI tetrapeptide tested on Yck232p localization.
Localization of three of these mutant Yck3 proteins was assessed by indirect immunofluorescence (Figure 7B). Each showed a missorting defect similar to that seen for Yck3(Δ409-462)p, giving a dual localization - some of the Yck3p sorted to the vacuolar membrane and some missorted to the plasma membrane. The effects of two of these mutations, namely, Y444H and S446P, were also assessed within the Yck232 chimeric protein context (Figure 7C). Here, the effects of the mutations were more dramatic. Rather than being localized to the vacuolar membrane like wild-type Yck232p, the two mutant Yck232 proteins were found to localize exclusively to the plasma membrane. Thus, introduction of either of these two point mutations fully destroys the AP-3-dependent sorting determinant contained within the Yck3 409-462 interval.
The YDSI tetrapeptide fits nicely to the YXXϕ consensus for tyrosine-based adaptin recognition motifs (Bonifacino and Traub, 2003). To further explore this connection with the adaptin consensus, alanine substitution mutants were constructed at each of the four residues within the YDSI tetrapeptide. Examining the effect of these mutations within the context of the Yck232 construct, we found that localization to the vacuole is abolished by both the Y444A and I447A substitutions, whereas the D445A and S446A changes were without effect (Figure 7D). Thus, the Yck3 YDSI fits well with the YXXϕ consensus motif for adaptin-mediated recognition: the initial Tyr as well as the ϕ residue at the Y + 3 position are critical, whereas residues at the Y + 1 and Y + 2 make little or no contribution.
DISCUSSION
The present work localizes Yck3p to the limiting membrane of the vacuole. This localization requires both palmitoylation for membrane association and the AP-3 pathway for sorting. Blocking the AP-3-mediated sorting either through mutation of the AP-3 complex itself or through mutation of the AP-3-dependent sorting signal resulted in Yck3p missorting to the plasma membrane. This AP-3 involvement in Yck3p sorting suggests an answer to an old puzzle: the suppression of yck1Δ yck2-ts by AP-3 disabling mutations (Panek et al., 1997). The answer, our results suggest, is that the Yck3p missorting that occurs in these AP-3 mutants serves to replenish active kinase to the kinase-deficient yck1Δ yck2-ts plasma membranes. Exploiting this suppression phenotype, we have isolated mutations within the Yck3p AP-3-dependent sorting signal that identify a classic YXXϕ adaptin-sorting signal.
The Yck3p vacuolar localization suggests the possibility of vacuolar function. Indeed, a recent genomic study in which the yeast deletion collection was screened for aberrant vacuolar morphology (vam) mutants found yck3Δ cells to have fragmented vacuoles (Seeley et al., 2002), i.e., having multiple small vacuoles rather than the one to three large vacuoles found in wild-type cells. vam mutants often show correlated defects in vacuolar homotypic fusion (Wickner, 2002). The same genomic analysis also found yck3Δ cells to have an associated Vps- phenotype, i.e., CPY is partially missorted to the extracellular milieu rather than to the vacuolar lumen (Seeley et al., 2002). Tethered to the external surface of the vacuole, Yck3p is well-positioned to modulate both transport into the vacuole, as well as the vacuolar fusion-fission reactions that control vacuolar number and size.
Yck3p Palmitoylation
We previously showed that Yck1p and Yck2p are palmitoylated and that Akr1p is the enzyme responsible for the palmitoyl modification (Roth et al., 2002). Akr1p is one of only two palmitoyl transferases (PTases) to be identified to date from any species. The other PTase, which was identified concurrently with Akr1p, is composed of two proteins, Erf2p and Shr5p, and is apparently devoted to yeast Ras protein palmitoylation (Lobo et al., 2002). The two transferases differ in their specificity for substrate, with Akr1p being devoted to the palmitoylation of Yck1p and Yck2p and Erf2p/Shr5p, to the palmitoylation of the yeast Ras proteins, Ras1p and Ras2p. Erf2p/Shr5p has been localized to the endoplasmic reticulum (ER) and Akr1p has been preliminarily localized to the yeast Golgi (Lobo et al., 2002; Roth et al., 2002). Akr1p and Erf2p both are polytopic integral membrane proteins and both have DHHC cysteine-rich domains (DHHC-CRD), i.e., a novel 58-residue-long, zinc finger-like domain. DHHC-CRD sequences have been identified in an evolutionarily widespread family of proteins found in all eukaryotic genomes sequenced to date; seven DHHC-CRD proteins have been identified in S. cerevisiae and 28, in human. These proteins all are predicted to be polytopic integral membrane proteins and all have the DHHC-CRD sequence similarly disposed within an intertransmembrane domain loop segment. The integrity of the DHHC-CRD is required for the activity of both the Akr1p and Erf2p/Shr5p PTases (Lobo et al., 2002; Roth et al., 2002). We have suggested that the DHHC-CRD sequence may be a signature palmitoyl transferase feature, with the DHHC-CRD protein family constituting a diverse family of palmitoyl transferase specificities, needed to accommodate the diverse range of substrates known to undergo palmitoylation (Lobo et al., 2002; Roth et al., 2002).
What PTase mediates Yck3p palmitoylation? As palmitoylation is required for Yck3p membrane attachment, we expect that palmitoylation temporally precedes the AP-3-dependent Golgi to vacuole sorting. Therefore, the cognate PTase for Yck3p should localize either to the Golgi or to a compartment with access to the Golgi (e.g., the ER). The unimpaired Yck3p palmitoylation seen in akr1Δ cells by [3H]palmitate labeling (Figure 1), indicates a participation for some PTase other than Akr1p. Nonetheless, we cannot rule out that Akr1p also participates, perhaps in some partial or redundant capacity. Indeed, consistent with such participation, a subtle impairment to Yck3p localization was noted in akr1Δ cells; in addition to the usual vacuolar membrane localization, some Yck3p was found to localize in the akr1Δ cells to cytoplasmic puncta (Figure 2). This mislocalization is accentuated with GAL1-driven Yck3p overexpression in akr1Δ cells, which results in Yck3p being predominantly localized to such extravacuolar membranous structures (our unpublished data). Given the seven potential palmitoyl acceptors in the C-terminal CCCCFCCC sequence, the involvement of multiple PTases seems reasonable; Akr1p could be the devoted PTase for some of the modifications, with the other cysteines modified by other palmitoyl transferases; an incompletely palmitoylated Yck3p might be poorly delivered to the vacuole. Alternatively, Akr1p effects on Yck3p localization could be indirect; perhaps the Akr1p-dependent palmitoylation of some other protein is required for the proper functioning of the Yck3p vacuolar trafficking mechanism.
Akr2p would seem to be the obvious candidate for a collaborating PTase with Akr1p. However, despite its striking and extensive homology to Akr1p, we have been unable to detect a role for Akr2p in the palmitoylation or function of any of the Yck proteins (our unpublished data). For Yck3p, no localization defect is seen in akr1Δ akr2Δ cells, beyond the subtle mislocalization previously noted in akr1Δ cells (Figure 2; Sun and Davis, unpublished data). Future experiments will examine Yck3p palmitoylation in strains having AKR1 deleted in combination with each of the other five yeast DHHC-CRD genes.
Deletion of the Yck3 C-terminal CCCCFCCC eliminates both palmitoylation and Yck3p membrane association, suggesting a likely role for these cysteines as the palmitoyl acceptors. We do not expect that this sequence also is prenylated. First, this sequence does not fit to the rigid CaaX prenylation consensus: the fourth residue from the C terminus, conspicuously, is not a cysteine. Furthermore, although C-terminal CC or CxC sequences can be geranylgeranylated, such prenylation is limited to members of the Rab GTPase family; this Rab protein prenylation requires, in addition to the geranylgeranyl transferase, a second essential participant, Rab escort protein (REP) (Mrs6p in S. cerevisiae), which specifically binds to the generic Rab structure and presents it to the transferase for modification (Jiang and Ferro-Novick, 1994; Zhang and Casey, 1996).
Although the Yck3 CCCCFCCC sequence is required for palmitoylation, it is not sufficient. Results from the Ura3-Yck3 fusion protein analysis indicated that additional sequences mapping within the Yck3 C-terminal 62 residues also are required. This is similar to what has been found for Yck2p, where the sequence requirements for palmitoylation have been more extensively analyzed: in addition to the C-terminal Cys-Cys sequence, palmitoylation also requires sequence elements distributed through the Yck2 C-terminal 50 residues (Babu et al., 2002; Roth et al., 2002) (Roth and Davis, unpublished data). Thus, it seems that relatively large, perhaps folded protein domains may serve as palmitoylation signals for the Yck kinases.
Residual AP-3-independent Yck3p Transport to the Vacuole
We demonstrated that the AP-3 pathway plays a major role in trafficking of Yck3p to the vacuole. However, it is not absolutely essential for this vacuolar transport. Substantial Yck3p traffic to the vacuole continues when the AP-3 complex is disabled or when the Yck3p AP-3-sorting signal is deleted. This is also true for the two other yeast AP-3 substrate proteins Vam3p and ALP. For both, substantial transport to the vacuole persists when their AP-3 sorting is blocked either through cis-mutation of their dileucine sorting signals or through trans-mutation of the subunits of the AP-3 complex (Stepp et al., 1997; Darsow et al., 1998; Vowels and Payne, 1998). For Vam3p and ALP, this default, AP-3-independent sorting to the vacuole seems to route predominantly through the CPY pathway (Darsow et al., 1998; Vowels and Payne, 1998). To test whether the residual, AP-3-independent, Yck3p sorting to the vacuole might also be via the CPY pathway, we have examined the localization of two AP-3-sorting-defective Yck3p mutants Yck3(Δ409-462)p and Yck3(Y444H)p, in three different class E vps mutants: in vps2Δ, in vps4Δ, or in vps27Δ cells. Although these same vps mutations showed no effect on the trafficking of wild-type Yck3p (Figure 5B), the two mutant Yck3 proteins both did show substantial localization to perivacuolar class E bodies in each of the three different vps mutant strains (our unpublished data). The block imposed by these class E vps mutations was not total: in addition to the class E body accumulation, substantial immunostaining of the vacuolar membrane still persisted (our unpublished data). Nonetheless, the class E body localization that was observed indicates that at least some of the residual, AP-3-independent traffic to the vacuole is through the MVB and perhaps also indicates the CPY pathway is being used for the default transport, as previously suggested for bothVam3p and ALP.
Rather than using the CPY pathway for this secondary trafficking from Golgi to vacuole, a second plausible scenario would involve endocytosis of Yck3p that is first missorted to the plasma membrane due to impaired AP-3 sorting. Like CPY pathway cargoes, endocytic cargoes destined for the vacuole also pass through the MVB and thus also get trapped within the aberrant prevacuolar, class E compartments of class E vps mutant cells (Davis et al., 1993; Piper et al., 1995). To assess the contribution made by the endocytic pathway to the AP-3-independent, residual sorting, Yck3(Δ409-462)p and Yck3(Y444H)p localization was examined in end3Δ and in sac6Δ cells - two end mutants disabled for the initial plasma membrane uptake step of endocytosis (D'Hondt et al., 2000). The two AP-3-sorting-deficient Yck3p mutants showed a localization in the two end mutants qualitatively very similar to that seen in the isogenic endocytosis-competent, wild-type cell, i.e., a dual localization to both vacuolar and plasma membranes (our unpublished data). Thus, blocking the endocytic pathway does not, by itself, block the residual transport of the mutant Yck3 proteins to the vacuole. Nonetheless, we cannot rule out that such routing of Yck3p to vacuole via the plasma membrane makes some partial contribution to this phenomenon of secondary or default Yck3p transport.
YXXϕ Sorting Signal
The Yck3p AP-3-dependent sorting signal was first mapped to the 409-462 sequence interval. This interval inserted into Yck2p, redirected sorting from the plasma membrane to the vacuole, demonstrating the sufficiency of this interval in signaling vacuolar sorting. Using the yck1Δ yck2-ts suppression strategy, we isolated point mutations within this interval that abolished function of this signal. These mutations identified the tetrapeptide sequence YDSI, a classic example of a tyrosine-based adaptin sorting signal.
In mammalian cells, the AP-1, AP-2, and AP-3 complexes recognize their cargoes through a remarkably similar set of tyrosine- or dileucine-based signals, with the tyrosine signals based on the YXXϕ consensus and with the dileucine-based signals generally conforming to a [DE]XXXL[LI] consensus. The two signals bind different sites within the adaptin complexes. Of the two, the binding site for YXXϕ signals, located on the surface of the μ subunit, has been more extensively characterized. Crystal structures of mammalian AP-2 μ2 complexed with YXXϕ-containing peptides have shown that the YXXϕ sequence binds in an extended conformation, with the critical Y and ϕ side chains contributing the major contacts, fitting into hydrophobic pockets on the μ surface (Owen and Evans, 1998). Consistent with the primacy of the Y and ϕ interactions, most of the changes within the Yck3 YDSI sequence were isolated in these two residues, i.e., the Tyr and the Ile at the Y + 3 position. Indeed, only one change was found that did not occur at either of these two residues: the Ser-to-Pro substitution at the Y + 2 position (Figure 7A). Although this Ser-to-Pro change abolished function of the signal, a Ser-to-Ala change at the same position had no effect (Figure 7D). Although the residues at the Y + 1 or Y + 2 positions are not predicted to contribute key binding interactions, one can easily see from the crystal structure how the rigidifying effect of a Pro substitution at either of these positions might disrupt interaction of the Y and ϕ side chains with their binding pockets. Given the fixed positions of these binding pockets on the μ surface, accommodation of the simultaneous binding of Y and ϕ depends on the rotational flexibility of the intervening peptide backbone at the Y + 1 and Y + 2 positions.
Yck3p is only the third cargo protein yet identified for AP-3-dependent sorting in yeast. The two other yeast AP-3 cargoes yet identified, i.e., ALP and Vam3p, both use classic dileucine signals (Darsow et al., 1998; Vowels and Payne, 1998). AP-3-dependent sorting in mammalian cells makes extensive use of both dileucine- and tyrosine-based sorting signals. This first identification of a yeast YXXϕ signal indicates that the adaptin-signal interaction is remarkably well-conserved through evolution. Additional examples of this classic sorting signal likely will be found as more is learned about adaptin-mediated sorting in yeast.
Acknowledgments
We are indebted to Alex Gow for sharing a microscope and expertise. We thank Lucy Robinson for strains and Cynthia Chang for contributions in the initial phase of this work. This work was supported by grants from the National Science Foundation (MCB 99-83688) and the National Institutes of Health (GM-65525).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-09-0682. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0682.
References
- Babu, P., Bryan, J.D., Panek, H.R., Jordan, S.L., Forbrich, B.M., Kelley, S.C., Colvin, R.T., and Robinson, L.C. (2002). Plasma membrane localization of the Yck2p yeast casein kinase 1 isoform requires the C-terminal extension and secretory pathway function. J. Cell Sci. 115, 4957-4968. [DOI] [PubMed] [Google Scholar]
- Boehm, M., and Bonifacino, J.S. (2002). Genetic analyses of adaptin function from yeast to mammals. Gene 286, 175-186. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J.S., and Traub, L.M. (2003). Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72, 395-447. [DOI] [PubMed] [Google Scholar]
- Chen, L., and Davis, N.G. (2002). Ubiquitin-independent entry into the yeast recycling pathway. Traffic 3, 110-123. [DOI] [PubMed] [Google Scholar]
- Conibear, E., and Stevens, T.H. (1998). Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim. Biophys. Acta 1404, 211-230. [DOI] [PubMed] [Google Scholar]
- Cowles, C.R., Odorizzi, G., Payne, G.S., and Emr, S.D. (1997a). The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell 91, 109-118. [DOI] [PubMed] [Google Scholar]
- Cowles, C.R., Snyder, W.B., Burd, C.G., and Emr, S.D. (1997b). Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO J. 16, 2769-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow, T., Burd, C.G., and Emr, S.D. (1998). Acidic di-leucine motif essential for AP-3-dependent sorting and restriction of the functional specificity of the Vam3p vacuolar t-SNARE. J. Cell Biol. 142, 913-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow, T., Katzmann, D.J., Cowles, C.R., and Emr, S.D. (2001). Vps41p function in the alkaline phosphatase pathway requires homo-oligomerization and interaction with AP-3 through two distinct domains. Mol. Biol. Cell 12, 37-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, N.G., Horecka, J.L., and Sprague, G.F., Jr. (1993). Cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J. Cell Biol. 122, 53-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica, E.C., Klumperman, J., Stoorvogel, W., and Bonifacino, J.S. (1998). Association of the AP-3 adaptor complex with clathrin. Science 280, 431-434. [DOI] [PubMed] [Google Scholar]
- D'Hondt, K., Heese-Peck, A., and Riezman, H. (2000). Protein and lipid requirements for endocytosis. Annu. Rev. Genet. 34, 255-295. [DOI] [PubMed] [Google Scholar]
- Huang, K.M., D'Hondt, K., Riezman, H., and Lemmon, S.K. (1999). Clathrin functions in the absence of heterotetrameric adaptors and AP180-related proteins in yeast. EMBO J. 18, 3897-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., and Ferro-Novick, S. (1994). Identification of yeast component A: reconstitution of the geranylgeranyltransferase that modifies Ypt1p and Sec4p. Proc. Natl. Acad. Sci. USA 91, 4377-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann, D.J., Odorizzi, G., and Emr, S.D. (2002). Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 3, 893-905. [DOI] [PubMed] [Google Scholar]
- Lobo, S., Greentree, W.K., Linder, M.E., and Deschenes, R.J. (2002). Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 277, 41268-41273. [DOI] [PubMed] [Google Scholar]
- Newman, L.S., McKeever, M.O., Okano, H.J., and Darnell, R.B. (1995). Beta-NAP, a cerebellar degeneration antigen, is a neuron-specific vesicle coat protein. Cell 82, 773-783. [DOI] [PubMed] [Google Scholar]
- Odorizzi, G., Babst, M., and Emr, S.D. (1998). Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95, 847-858. [DOI] [PubMed] [Google Scholar]
- Owen, D.J., and Evans, P.R. (1998). A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282, 1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek, H.R., Stepp, J.D., Engle, H.M., Marks, K.M., Tan, P.K., Lemmon, S.K., and Robinson, L.C. (1997). Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J. 16, 4194-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper, R.C., Bryant, N.J., and Stevens, T.H. (1997). The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J. Cell Biol. 138, 531-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper, R.C., Cooper, A.A., Yang, H., and Stevens, T.H. (1995). VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J. Cell Biol. 131, 603-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, C.J., Nothwehr, S.F., and Stevens, T.H. (1992). Membrane protein sorting in the yeast secretory pathway: evidence that the vacuole may be the default compartment. J. Cell Biol. 119, 69-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, L.C., Menold, M.M., Garrett, S., and Culbertson, M.R. (1993). Casein kinase I-like protein kinases encoded by YCK1 and YCK2 are required for yeast morphogenesis. Mol. Cell Biol. 13, 2870-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, A.F., Feng, Y., Chen, L., and Davis, N.G. (2002). The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 159, 23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, E.S., Kato, M., Margolis, N., Wickner, W., and Eitzen, G. (2002). Genomic analysis of homotypic vacuole fusion. Mol. Biol. Cell 13, 782-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R.S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, F., Peden, A.A., Christopoulou, L., and Robinson, M.S. (1997). Characterization of the adaptor-related protein complex, AP-3. J. Cell Biol. 137, 835-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp, J.D., Huang, K., and Lemmon, S.K. (1997). The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J. Cell Biol. 139, 1761-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp, J.D., Pellicena-Palle, A., Hamilton, S., Kirchhausen, T., and Lemmon, S.K. (1995). A late Golgi sorting function for Saccharomyces cerevisiae Apm1p, but not for Apm2p, a second yeast clathrin AP medium chain-related protein. Mol. Biol. Cell 6, 41-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia, R.H., Baggott, D., Chuang, J.S., and Schekman, R.W. (2002). The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell 2, 283-294. [DOI] [PubMed] [Google Scholar]
- Vancura, A., Sessler, A., Leichus, B., and Kuret, J. (1994). A prenylation motif is required for plasma membrane localization and biochemical function of casein kinase I in budding yeast. J. Biol. Chem. 269, 19271-19278. [PubMed] [Google Scholar]
- Vowels, J.J., and Payne, G.S. (1998). A dileucine-like sorting signal directs transport into an AP-3-dependent, clathrin-independent pathway to the yeast vacuole. EMBO J. 17, 2482-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Hoekstra, M.F., DeMaggio, A.J., Dhillon, N., Vancura, A., Kuret, J., Johnston, G.C., and Singer, R.A. (1996). Prenylated isoforms of yeast casein kinase I, including the novel Yck3p, suppress the gcs1 blockage of cell proliferation from stationary phase. Mol. Cell Biol. 16, 5375-5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner, W. (2002). Yeast vacuoles and membrane fusion pathways. EMBO J. 21, 1241-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, B.G., Phan, H.L., and Payne, G.S. (1999). Adaptor complex-independent clathrin function in yeast. Mol. Biol. Cell 10, 3643-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F.L., and Casey, P.J. (1996). Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65, 241-269. [DOI] [PubMed] [Google Scholar]