Abstract
Objective
To create a clinical prediction tool to differentiate women at risk for postoperative complications after benign gynecologic surgery.
Methods
We utilized the 2005 to 2009 American College of Surgeons National Surgical Quality Improvement Program participant use data files to perform a secondary dataset analysis of women over the age of 16 years who underwent benign gynecologic procedures. We then temporally divided women into two similar cohorts. Our derivation cohort included all women undergoing benign gynecologic procedures in the 2005 to 2008. Our validation cohort included all women undergoing benign gynecologic procedures in the 2009. The primary outcome, composite 30-day major postoperative complications, was analyzed as a dichotomous variable. A prediction tool was then constructed to predict the occurrence of postoperative complications built from the logistic regression model by rounding the value of each estimated β coefficient to the nearest integer. An individual’s risk score was then computed by summing the number of points based on her preoperative characteristics. This risk score was then used to categorize women into low, medium, and high-risk groups.
Results
A prediction tool for benign gynecologic procedures identified women at low (2.7% and 2.4%), medium (6.3% and 6.8%), and high (29.5% and 23.8%) risk of complications in the derivation and validation cohorts, respectively.
Conclusion
A prediction tool can differentiate women at risk for postoperative complications after benign gynecologic surgery.
Keywords: ACS NSQIP, gynecology, medical comorbidities, prediction tool, surgical outcomes
Introduction
Clinical prediction tools (also known as clinical decision tools or risk scores) are helpful tools that can increase the accuracy of clinical assessments, aid complex decision making, and identify patients at risk for poor outcomes. The creation of a clinical prediction tool involves the quantification of known variables, such as the patient’s medical history, physical exam, and diagnostic tests, in order to predict a diagnosis or prognosis.[1] These tools have been used to identify patients at risk for postoperative cardiac events and delirium.[2,3] In addition, prediction tools specific to individual surgical procedures have been created to predict postoperative morbidity and mortality.[4,5]
There is currently only one prediction model for perioperative morbidity after vaginal hysterectomy developed by Heisler et al.[6] This model includes both preoperative and postoperative variables, prohibiting the use of Heisler’s model as a preoperative medical risk assessment tool.[6] Our goal was to create a clinical prediction tool for use as a preoperative medical risk assessment tool to identify women at risk for postoperative complications after benign gynecologic surgery.
Materials and Methods
Study Design
We performed a secondary dataset analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) participant use data files from 2005 to 2009. Previously, we have reported on the occurrence of 30-day major postoperative complications from the ACS NSQIP dataset.[7]
Dataset
The ACS NSQIP is a national program for surgical quality assessment and improvement in academic and private hospitals.[8] Hospital participation is currently voluntary and has increased from 121 participating hospitals in 2005 to 237 hospitals in 2009. ACS NSQIP collects extensive information by a formal chart review process. Trained nurse abstractors collect data on the first 40 cases performed within consecutive 8-day cycle. Between 2005 and 2008 these cases included patients 16 years of age and older, while 2009 included patients 18 years and older. Over 200 HIPAA-compliant variables are collected for each patient, including: preoperative characteristics, procedures performed, and 30-day postoperative complications. The ACS NSQIP program requires 30-day postoperative follow-up even after discharge. Hospitals contact both patients and providers to obtain this follow-up. ACS NSQIP participating hospitals with less than 80% 30-day follow-up are excluded from the program and their data is not eligible for inclusion in the analyzed dataset. To ensure the quality of data within the dataset and reduce potential bias, inter-rater reliability audits are conducted by the ACS NSQIP with an overall disagreement rate of 1.8%. This dataset has been validated by previous studies.[8,9] Further information on the ACS NSQIP program and database is available at: http://acsnsqip.org. This study was exempt from review by the Institutional Review Board as it involves research of an existing dataset from a public source.
Target population
The target population for this study included women over the age of 16 years who underwent benign gynecologic surgery. Women were considered for potential inclusion in this analysis based on a variable in the ACS NSQIP signifying the specialty of the primary surgeon was gynecology. Women were excluded from analysis for the following reasons: 1) current pregnancy, 2) previous operation within 30-days of current procedure, 3) Physicians’ Current Procedural Terminology Coding System, 4th edition (CPT-4) code inconsistent with benign gynecologic procedure, and 4) missing preoperative hematocrit values. CPT-4 codes for gynecologic procedures can be found in Table 1.
Table 1.
Physicians’ Current Procedural Terminology Coding System, 4th edition (CPT-4) codes for gynecologic procedures
| Procedure category | CPT-4 codes |
|---|---|
| Benign hysterectomy | |
| TAH | 51925, 58150, 58152 |
| SCH | 58180 |
| TVH | 58260, 58262, 58263, 58267, 58270, 58275, 58280, 58290, 58291, 58292, 58293, 58294 |
| LAVH | 58550, 58552, 58553, 58554 |
| TLH | 58570, 58571, 58572, 58573 |
| LASH | 58541, 58542, 58543, 58544 |
|
| |
| Laparotomy | 44005, 49000, 49010, 49020, 49402, 51900, 57296, 57540, 58140, 58146, 58410, 58520, 58540, 58700, 58720, 58740, 58750, 58752, 58760, 58770, 58805, 58822, 58920, 58925, 58940 |
|
| |
| Procedures for prolapse and/or urinary incontinence | 45560, 51840, 51841, 51990, 51992, 57120, 57200, 57210, 57220, 57240, 57250, 57260, 57265, 57267, 57268, 57270, 57280, 57282, 57283, 57284, 57285, 57288, 57292, 57295, 57423, 57425, 57556, 58400 |
|
| |
| Vaginal procedures | 53230, 56405, 56420, 56440, 56441, 56501, 56515, 56620, 56625, 56740, 56800, 56810, 57000, 57010, 57065, 57130, 57135, 57300, 57308, 57310, 57320, 57330, 57456, 57505, 57510, 57522, 57530, 57720, 58120, 58145, 58356, 58565, 58579 |
|
| |
| Laparoscopy | 44180, 49321, 49322, 49329, 58350, 58578 |
TAH = total abdominal hysterectomy, SCH= supracervical hysterectomy, TVH = total vaginal hysterectomy, LAVH = laparoscopic assisted vaginal hysterectomy, TLH = total laparoscopic hysterectomy, LASH = laparoscopic supracervical hysterectomy
We chose to exclude women with missing preoperative hematocrit values due to the demonstrated value of preoperative anemia in predicting postoperative complications.[6,10] Additionally, women undergoing procedures for gynecologic cancer were excluded because they have more complications than women undergoing procedures for benign gynecologic conditions.[7] This exclusion allowed us to identify unique independent predictors of postoperative complications among women undergoing benign gynecologic procedures, rather than confirming that women undergoing procedures for malignancy are at the highest risk of postoperative complications.
We then temporally divided women into two similar cohorts, one to derive the prediction tool and the second to validate the prediction tool. Our derivation cohort included all women undergoing benign gynecologic procedures in the 2005 to 2008 ACS NSQIP participant use data files. Our validation cohort included all women undergoing benign gynecologic procedures in the 2009 ACS NSQIP participant use data files. Intervals of unequal lengths were used due to increasing hospital enrollments into the ACS NSQIP after 2004. The prevalence of preoperative predictors and postoperative complications were compared between the derivation cohort (ACS NSQIP 2005–2008) and the validation cohort (ACS NSQIP 2009) to determine whether these changed over time.
Analysis
Independent variables
We examined all preoperative risk factors available in the ACS NSQIP dataset. Age and BMI were explored as both continuous and dichotomous variables in relation to postoperative complications. We did not examine intraoperative risk factors that would fit better into a global model, such as route of anesthesia, operative time, intraoperative blood loss, or change in hematocrit after surgery, because we wanted to build a prediction tool that could be used in preoperative medical risk assessment as termed a counseling model.
Procedures were classified based on CPT-4 coding. Women could undergo multiple concomitant procedures, however they were classified based on their primary CPT-4 codes and only counted once in the dataset. Total work relative value units (RVUs), including primary procedure and all concomitant procedures were analyzed as a measure of procedural difficulty.
Dependent variable
The primary outcome, composite 30-daymajor postoperative complications, was analyzed as a dichotomous variable (Yes/No). Components included the following 30-day outcome measures: 1) mortality, 2) cardiac arrest requiring cardiopulmonary resuscitation, 3) acute transmural myocardial infarction, 4) stroke or cerebrovascular accident, 5) a non-medication induced coma for greater that 24 hours after surgery, 6) wound dehiscence, 7) wound seroma or deep wound surgical site infection of fascial or muscle layers, 8) organ space surgical site infection, 9) pulmonary embolism, 10) deep vein thrombosis, 11) prolonged mechanical ventilation > 48 hours after surgery, 12) unplanned reintubation, 13) pneumonia, 14) sepsis, 15) septic shock, 16) progressive renal insufficiency, 17) acute renal failure with need for dialysis, 18) return to the operating room within 30-days after surgery and 19) postoperative blood transfusion within the first 72 hours after surgery. Urinary tract infections and superficial surgical site infections are common postoperative morbidity easily treated with antibiotics; therefore urinary tract infections and superficial surgical site infections were not included in the composite outcome of 30-day major postoperative complications.
Prediction tools
A logistic regression analysis was conducted to estimate the association of preoperative predictors with 30-day postoperative morbidity in women undergoing benign gynecologic procedures. Preoperative variables were identified for inclusion in the final model based on bivariate analysis (p <.1). Variables were then identified for inclusion in the model using backwards stepwise selection (p<.05) to identify factors independently associated with postoperative morbidity. Goodness-of-fit of the final models was confirmed using Hosmer-Lemeshow testing.
A prediction tool was then constructed to predict the occurrence of postoperative complications. The prediction tool was built from the logistic regression model by assigning the value of 1 point each to each variable that had a β coefficient of > 1.5 and 2 points each for β coefficients ≥1.5. An individual’s risk score was then computed by summing the number of points based on her preoperative characteristics. This risk score was then used to categorize women into low, medium, and high-risk groups. Performances in the derivation and validation sets were quantified and compared using receiver operating characteristic analysis to determine the concordance-index or “c-index” of the models. The c-index was calculated to check the predictive ability of the model.[11] Possible values for a c-index range from 0 to 1.0. A c-index of 1.0 indicates a model that perfectly predicts the outcome of interest. A c-index of 0.5 would indicate that the predictive model is equivalent to random chance. Statistical analysis was performed using STATA 11.0 (StataCorp, College Station, TX) and SAS 9.2 (SAS Institute, Inc, Cary, NC).
Results
A total of 23,665 women were identified as undergoing a primary gynecologic procedure in the ACS NSQIP dataset. Women were excluded from analysis for the following reasons: 1) current pregnancy (n=416), 2) previous operation within 30-days of current procedure (n=185), 3) CPT-4 code inconsistent with benign gynecologic procedure (n= 2,117), and 4) missing preoperative hematocrit value (n =1,508). A total of 19,439 women who underwent benign gynecologic procedures were included in our final analysis. This was further broken down into the derivation and validation cohorts, containing 9,572 and 9,867 women respectively. (Figure 1)
Figure 1.
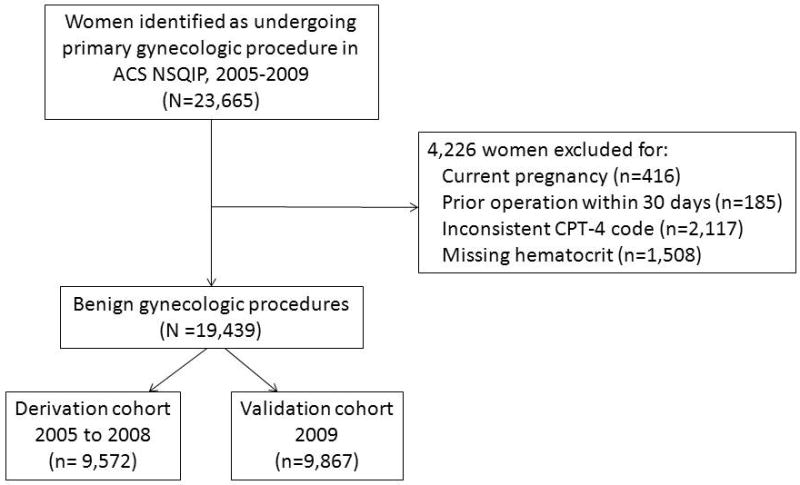
Flow diagram for women included in analysis of postoperative complications after benign gynecologic procedures and procedures for gynecologic cancer.
The most commonly performed benign gynecologic procedures included 12,492 hysterectomies (64.3%), 1,997 laparotomies (10.3%), 3,483 procedures for prolapse and/or urinary incontinence (17.9%), 996 vaginal procedures (5.1%), and 471 laparoscopies (2.4%). Major postoperative complications occurred in 3.3% (n= 636) of women undergoing benign gynecologic procedures (3.33% (319/9,572) vs. 3.21% (317/9,867) in the derivation and the validation cohort, respectively). Sixty-six women who experience three or more complications and these women had an occurrence of 30 day postoperative mortality of 21.2% (14/66). Women who were anemic were more likely to undergo postoperative blood transfuse than women with hematocrit >36% (0.32% (13/4,041) vs. 0.14% (21/15,364), p=.01), however the occurrence of postoperative blood transfusion was rare. Two-hundred forty women (1.23%) had preoperative infection (SIRS, sepsis, or septic shock). Women who had preoperative infection had a higher risk of having postoperative sepsis or septic shock compared with women without preoperative infection (6.25% (15/240) vs. 0.55% (106/19,199), p <.001).
No association was found between age and postoperative complications following benign gynecologic procedures. When body mass index (BMI) was analyzed as a continuous variable, no significant association with major postoperative complications was found. However, an association was seen between postoperative complications when BMI was analyzed as a dichotomous variable (BMI ≥ 40 kg/m2). This dichotomous classification is clinically significant as BMI ≥ 40 kg/m2 commonly represents the definition of morbid obesity by the National Institutes of Health.[12]
The prevalence of preoperative predictors in the derivation cohort (ACS NSQIP 2005–2008) was similar to the validation cohort (ACS NSQIP 2009). (Table 2) There was a statistically significant difference in the percentage of benign hysterectomies performed in the derivation and validation benign gynecology cohorts (62.3% vs. 66.2%, p=.001) although these percentages are clinically similar. There was no difference in the procedures for prolapse or urinary incontinence between the derivation and validation benign cohorts (17.9% vs. 17.9%, p =.99) Procedural difficulty measured by mean total work RVUs for primary and all concomitant procedures were statistically different between the derivation and validation cohorts (15.1 (±5.0) vs. 14.9 (±3.5), p= .04), but clinically similar. The postoperative outcomes of the derivation cohort were similar to the validation cohort. (Table 3)
Table 2.
Bivariate analysis of preoperative predictors of major postoperative complications for benign gynecologic surgery (N = 19,439)
| Derivation cohort 2005 to 2008 (n= 9,572) | Validation cohort 2009 (n= 9,867) | p-value | |
|---|---|---|---|
| Age category | .10 | ||
| < 80 years old | 9,368 (97.9) | 9,689 (98.2) | |
| ≥ 80 years old | 204 (2.1) | 178 (1.8) | |
| Race | |||
| White | 5,457 (57.0) | 6,276 (63.6) | 0 |
| Ethnicity | |||
| Hispanic | 1,998 (20.9) | 1316 (13.3) | 0 |
| Hypertension | 2,735 (28.6) | 2,893 (29.3) | .25 |
| Diabetes mellitus | 711 (7.4) | 712 (7.2) | .57 |
| History of CVA with neurologic deficit | 47 (0.49) | 43 (0.44) | .57 |
| Known bleeding disorder | 82 (0.86) | 100 (1.01) | .26 |
| Steroid use for chronic condition | 83 (0.87) | 106 (1.07) | .14 |
| Preoperative systemic infection | |||
| SIRS | 110 (1.15) | 78 (0.79) | .01 |
| Sepsis | 11 (0.11) | 36 (0.36) | 0 |
| Septic Shock | 1 (0.01) | 4 (0.04) | .19 |
| Preoperative blood transfusion > 4 units | 5 (0.05) | 11 (0.11) | .15 |
| Current smoker | 1,819 (19.0) | 1,825 (18.5) | .40 |
| Current pneumonia | 4 (0.04) | 2 (0.02) | .39 |
| Morbid obesity (BMI ≥ 40 kg/m2) | 910 (9.5) | 1014 (10.3) | .07 |
| Prior myocardial infarction within 6 months | 5 (0.05) | 0 | .02 |
| New or exacerbated congestive heart failure | 8 (0.08) | 9 (0.09) | .86 |
| Prior percutaneous coronary intervention | 111 (1.16) | 82 (0.83) | .02 |
| Prior cardiac surgery | 78 (0.81) | 53 (0.54) | .02 |
| Angina within 30 days of surgery | 21 (0.22) | 18 (0.18) | .57 |
| History of peripheral vascular disease (amputation) | 16 (0.17) | 20 (0.2) | .56 |
| Rest pain in lower extremities | 1 (0.01) | 1 (0.01) | .98 |
| New York Heart Class III or IV | 498 (5.2) | 478 (4.8) | .25 |
| Chronic obstructive pulmonary disease | 125 (1.3) | 145 (1.5) | .33 |
| Ventilator dependency | 4 (0.04) | 3 (0.03) | .68 |
| Acute or chronic dialysis | 6 (0.06) | 6 (0.06) | .96 |
| Acute or chronic renal failure | 4 (0.04) | 7 (0.07) | .39 |
| Ascites | 27 (0.28) | 32 (0.32) | .59 |
| Esophageal varicies | 2 (0.02) | 1 (0.01) | .55 |
| Unintentional weight loss of > 10% in last 6 months | 29 (0.3) | 31 (0.31) | .89 |
| Functional status(dependent for ADLs) | 68 (0.71) | 78 (0.79) | .52 |
| Preoperative anemia (hematocrit < 36%) | 1,971 (20.6) | 2,083 (21.1) | .37 |
| ASA score of III, IV, or V | 1,713 (17.9) | 1,819 (18.4) | .33 |
All values listed as n(%)
CVA = cerebrovascular accident; SIRS = systemic inflammatory response syndrome; ADLs = activities of daily living; ASA = American Society of Anesthesiologist
Table 3.
Major postoperative complications after benign gynecologic procedures (N =19,439)
| Complication | Derivative Cohort N =9,572 | Validation Cohort N = 9,867 | P-value |
|---|---|---|---|
| Death | 12 (0.13) | 10 (0.10) | .62 |
| Cardiac arrest | 8 (0.08) | 5 (0.05) | .38 |
| Acute myocardial infarction | 2 (0.02) | 6 (0.06) | .17 |
| Postoperative coma > 24 hours | 1 (0.01) | 1 (0.01) | .98 |
| CVA with neurologic deficit | 2 (0.02) | 1 (0.01) | .55 |
| Acute renal failure | 7 (0.07) | 5 (0,05) | .53 |
| Progressive renal insufficiency | 7 (0.07) | 6 (0.06) | .74 |
| Deep wound surgical site infection | 35 (0.37) | 22 (0.22) | .07 |
| Organ space surgical site infection | 62 (0.65) | 57 (0.58) | .53 |
| Wound dehiscence | 37 (0.39) | 25 (0.25) | .10 |
| Pulmonary embolism | 20 (0.21) | 27 (0.27) | .36 |
| Deep vein thrombosis | 10 (0.10) | 12 (0.12) | .83 |
| Prolonged mechanical ventilation | 20 (0.21) | 15 (0.15) | .35 |
| Unplanned reintubation | 25 (0.26) | 17 (0.17) | .18 |
| Pneumonia | 19 (0.20) | 30 (0.30) | .14 |
| Sepsis | 42 (0.44) | 50 (0.51) | .49 |
| Septic Shock | 21 (0.22) | 10 (0.10) | .04 |
| Blood transfusion | 22 (0.23) | 12 (0.12) | .07 |
| Return to the Operating Room | 150 (1.57) | 156 (1.58) | .94 |
| Composite Major Morbidity | 319 (3.33) | 317 (3.21) | .64 |
All values listed as n(%) unless otherwise specified
Blood transfusion includes the transfusion of packed red blood cells or whole blood cells within 72 hours of completion of surgery.
Return to the Operating Room includes the return to the surgical operating room for any reasons within 30 days of surgery.
The final multivariable logistic regression model for the occurrence of major postoperative complications after a benign gynecologic procedure is presented in Table 4. The following predictors of postoperative complications after benign gynecologic procedures were identified and assigned points for use in the preoperative predictive tool: preoperative sepsis (2 points), ascites (2 points), unintentional weight loss (> 10% weight loss in the six months prior to surgery) (1 point), preoperative systemic inflammatory response syndrome (1 point), history of cerebrovascular accident (1 point), morbid obesity (1 point), American Society of Anesthesiologists score III or greater (1 point), current smoker (1 point), and preoperative anemia (Hematocrit < 36.0%) (1 point). The c-index of the model for benign gynecology procedures in the derivation and validation cohort was 0.611 and 0.642, respectively. Women were assigned a preoperative risk category of low (0 to 1 point), medium (2 to 3 points) and high (≥ 4 points). Performance of the prediction tool for postoperative complications after benign gynecologic procedures is shown in Table 5. The prediction tool successfully differentiated women at risk for postoperative complications. Women in the derivation and validation cohorts undergoing benign gynecologic procedures had an occurrence of postoperative complications in the low-risk group of 2.7% and 2.4%, medium-risk group of 6.3% and 6.8%, and high-risk group of 29.5% and 23.8%.
Table 4.
Multivariable logistic regression model for postoperative complications after benign gynecologic procedures
| β | Odds Ratio (95% Confidence Interval) | Points | |
|---|---|---|---|
| Intercept | −3.77 | ||
| Preoperative sepsis | 2.07 | 7.93 (1.92, 32.81) | 2 |
| Ascites | 1.88 | 6.54 (2.62, 16.29) | 2 |
| Unintentional weight loss | 1.22 | 3.40 (1.12, 10.19) | 1 |
| Preoperative SIRS | 1.21 | 3.34 (1.84, 6.08) | 1 |
| History of CVA with neurologic deficit | 0.99 | 2.68 (1.03, 6.96) | 1 |
| Morbid obesity (BMI ≥ 40 kg/m2) | 0.54 | 1.72 (1.24, 2.39) | 1 |
| ASA score of III, IV, or V | 0.47 | 1.60 (1.22, 2.08) | 1 |
| Current smoker | 0.42 | 1.52 (1.17, 1.97) | 1 |
| Preoperative anemia (hematocrit < 36.0%) | 0.29 | 1.34 (1.04, 1.74) | 1 |
SIRS = systemic inflammatory response syndrome; CVA = cerebrovascular accident; BMI = body mass index; ASA = American Society of Anesthesiologist.
Preoperative risk scoring: 0–1 point is low risk; 2–3 points is medium risk; ≥ 4 points is high risk
Table 5.
Prediction model for preoperative risk stratification of major postoperative complications after benign gynecologic surgery (N = 19,439)
| Low risk (0 to 1 points) | Medium risk (2 to 3 points) | High risk (≥ 4 points) | |
|---|---|---|---|
| Derivation cohort (2005 to 2008) | |||
| Major complications absent (n = 9,253) | 7,909 (97.3) | 1,313 (93.7) | 31 (70.5) |
| Major complications present (n =319) | 217 (2.7) | 89 (6.3) | 13 (29.5) |
| Validation cohort (2009) | |||
| Major complications absent (n = 9,550) | 8,150 (97.6) | 1,352 (93.2) | 48 (76.2) |
| Major complications present (n = 317) | 204 (2.4) | 98 (6.8) | 15 (23.8) |
All values listed as n(%)
Discussion
We have developed a clinical prediction tool that can be used preoperatively to identify risk of postoperative complications in women undergoing benign gynecologic procedures. The preoperative predictors for postoperative complications after benign gynecologic procedures included preoperative sepsis, ascites, unintentional weight loss, preoperative systemic inflammatory response syndrome, history of cerebrovascular accident, morbid obesity, American Society of Anesthesiologists score of III or greater, current smoking status, and preoperative anemia. The prediction tool performed well in both the derivation and validation cohorts as indicated by the c-index and the ability of the tool to differentiate between women with low-, medium-, and high-risk of postoperative complications.
This study adds to our previous work on major postoperative complications after gynecologic surgery. The prediction tool we developed only includes preoperative variables and differs from our previous logistic regression model in two important ways.[7] First, we excluded women with missing preoperative hematocrit values who were included in the first logistic regression model. Second, the operative time was not included in our predictive tool as this was an intraoperative variable. Consistent with the findings of Wu et al[10] after general surgical procedures and Heisler et al[6] after vaginal hysterectomy, we also found anemia to be a predictor of postoperative complications. Unfortunately, the dataset does not include preoperative blood transfusion, but does include information on intraoperative and postoperative transfusion. However, the most recent hematocrit before the surgery was recorded as the preoperative hematocrit value and would include a post-transfusion hematocrit if performed. Not surprisingly, we found that the presence of preoperative systemic infection, either systemic inflammatory response syndrome or sepsis, predicted increases in the occurrence of major postoperative complications. We also found that increased American Society of Anesthesiologists score and current smoking status were predictive of increased postoperative complication.
Additionally, unintentional weight loss of greater than 10% in the six months prior to surgery and ascites were also identified as predictors of postoperative complications. Unintentional weight loss has been used as a marker of frailty.[13] Frailty has been independently linked to increased postoperative complications in addition to American Society of Anesthesiologists score, Lee’s revised cardiac index score, and Eagle scores.[3,14,15] Another explanation is that both unintentional weight loss and ascites could represent women with unrecognized malignancy. Both unintentional weight loss and ascites represented less than 0.3% of the women in the benign gynecologic cohort, but were independent predictors of postoperative complications.
Finally, we saw an increase in postoperative complications in women with morbid obesity (BMI ≥ 40 kg/m2). We dichotomized women into morbidly obese and non-morbidly obese categories due to a lack of association between BMI analyzed as a continuous variable and postoperative complications. We did not find an association if women were dichotomized into obese (BMI ≥ 30 kg/m2) and non-obese women. Similarly, Chen et al[16] did not find an association between obesity (BMI ≥ 30 kg/m2) and increased complications in women undergoing benign vaginal gynecologic procedures. Our findings demonstrated that increased postoperative complications after benign gynecologic procedures were associated with morbid obesity.
A limitation of a prediction tool based on a single institution’s data is that the prediction tool may not be generalizable to other hospitals. One strength of our study is that the ACS NSQIP dataset allowed for the development of this clinical prediction tool across multiple participating hospitals, instead of a single institution, increasing the generalizability of the prediction tools. A possible limitation of our prediction tool is the temporal division of the derivation and validation cohorts, however our analysis demonstrated that the two cohorts were similar in baseline data, preoperative characteristics, and procedures performed. Additionally, the 2005 to 2008 cohort included less total participating institutions than the 2009 cohort which is a difference in the two temporally divided cohorts. However both cohorts included over 100 and 200 institutions that were both tertiary care centers and community hospitals. Missing data is another potential limitation. According to Harrell et al[11], models may be inaccurate if there is a high frequency of missing data or improper imputation methods. Because of the quality of data in the ACS NSQIP participant use data file, very little missing data existed in the dataset. However, missing data from preoperative bloodwork is present in the ACS NSQIP. Preoperative bloodwork is not a requirement of every participating hospital before women undergo gynecologic procedures. Missing data on preoperative hematocrit occurred in 6.7% of women undergoing gynecologic procedures. We chose to exclude women with missing preoperative hematocrit values due to the association of preoperative anemia with postoperative complications demonstrated by Wu et al[10] in general surgery and the predicative value preoperative hemoglobin reported by Heisler et al[6] in gynecologic surgery. If women who had missing hematocrit values had less composite postoperative complications, we may have slightly overestimated the true occurrence of postoperative complications. Some postoperative complication data may be missing if patients were managed as outpatients or readmitted to another hospital. The ACS NSQIP dataset does not include information on the reasons for return to the operating room within 30 days of the index procedure. It is possible that the return to the operating room is unrelated to the original surgery. However, an additional strength of the ACS NSQIP is that 30-day follow-up is available even after a patient is discharged from the hospital. Participating hospitals are required to document 30-day follow-up on over 80% of their sampled case. If this is not maintained, the hospital is excluded from the participant use dataset. Finally, even though tests of internal validation of these prediction tools were performed, the ultimate test will be external validation of these prediction tools to predict postoperative outcomes.
In conclusion, we have developed and validated a preoperative prediction tool to differentiate women at high-risk for major postoperative complications after benign gynecologic procedures.
Acknowledgments
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Footnotes
No reprints available
Disclosures: Dr. Terri Fried is supported by K24 AG28443, National Institute on Aging. No funding was provided for this research or development of the manuscript. There are no conflicts of interest to disclose.
References
- 1.McGinn TG, Guyatt GH, Wyer PC, et al. Users’ guides to the medical literature. JAMA. 2000;284(1):79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction tool for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134–9. [PubMed] [Google Scholar]
- 3.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–9. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 4.Greenblatt D, Kelly K, Rajamanickam V, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18(8):2126–35. doi: 10.1245/s10434-011-1594-6. [DOI] [PubMed] [Google Scholar]
- 5.Gupta P, Franck C, Miller W, et al. Development and validation of a bariatric surgery morbidity risk calculator using the prospective, multicenter NSQIP dataset. J Am Coll Surg. 2011;212(3):301–9. doi: 10.1016/j.jamcollsurg.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Heisler CA, Aletti GD, Weaver AL, et al. Improving quality of care: development of a risk-adjusted perioperative morbidity model for vaginal hysterectomy. Am J Obstet Gynecol. 2010;202(2):137.e1–5. doi: 10.1016/j.ajog.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erekson EA, Yip SO, Ciarleglio MM, et al. Postoperative complications after gynecologic surgery. Obstet Gynecol. 2011;118(4):785–93. doi: 10.1097/AOG.0b013e31822dac5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson W, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: Why is it what it is? Am J Surg. 2009;198(5 Suppl):S19. doi: 10.1016/j.amjsurg.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Dhungel B, Diggs BS, Hunter JG, et al. Patient and peri-operative predictors of morbidity and mortality after esophagectomy: American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), 2005–2008. J Gastrointest Surg. 2010;14(10):1492–501. doi: 10.1007/s11605-010-1328-2. [DOI] [PubMed] [Google Scholar]
- 10.Wu W, Schifftner T, Henderson W, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297(22):2481. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 11.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--the evidence report. National Institutes of Health. Obes Res. 1998;6 (Suppl 2):51–209S. [PubMed] [Google Scholar]
- 13.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 14.Makary M, Segev D, Pronovost P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Eagle K, Berger P, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery--executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2002;39(3):542–53. doi: 10.1016/s0735-1097(01)01788-0. [DOI] [PubMed] [Google Scholar]
- 16.Chen CCG, Collins S, Rodgers A, et al. Perioperative complications in obese women vs normal-weight women who undergo vaginal surgery. Am J Obstet Gynecol. 2007;197(1):98.e1. doi: 10.1016/j.ajog.2007.03.055. [DOI] [PubMed] [Google Scholar]


