Abstract
Background
Bowel resection may lead to short bowel syndrome (SBS), which often requires parenteral nutrition (PN) due to inadequate intestinal adaptation. The objective of this study was to determine the time course of adaptation and proglucagon system responses after bowel resection in a PN-dependent rat model of SBS.
Methods
Rats underwent jugular catheter placement and a 60% jejunoileal resection + cecectomy with jejunoileal anastomosis or transection control surgery. Rats were maintained exclusively with PN and killed at 4 hours to 12 days. A nonsurgical group served as baseline. Bowel growth and digestive capacity were assessed by mucosal mass, protein, DNA, histology, and sucrase activity. Plasma insulin-like growth factor I (IGF-I) and bioactive glucagon-like peptide 2 (GLP-2) were measured by radioimmunoassay.
Results
Jejunum cellularity changed significantly over time with resection but not transection, peaking at days 3–4 and declining by day 12. Jejunum sucrase-specific activity decreased significantly with time after resection and transection. Colon crypt depth increased over time with resection but not transection, peaking at days 7–12. Plasma bioactive GLP-2 and colon proglucagon levels peaked from days 4–7 after resection and then approached baseline. Plasma IGF-I increased with resection through day 12. Jejunum and colon GLP-2 receptor RNAs peaked by day 1 and then declined below baseline.
Conclusions
After bowel resection resulting in SBS in the rat, peak proglucagon, plasma GLP-2, and GLP-2 receptor levels are insufficient to promote jejunal adaptation. The colon adapts with resection, expresses proglucagon, and should be preserved when possible in massive intestinal resection.
Keywords: intestinal failure, intestinal adaptation, GI hormones, short bowel syndrome, bowel resection
It is estimated that approximately 30,000 people in the United States suffer from short bowel syndrome (SBS), a condition resulting from inadequate nutrient and water absorption and altered intestinal transit that can lead to debilitating and life-threatening malnutrition, dehydration, and vitamin deficiencies. Patients with SBS resulting in intestinal failure often require parenteral nutrition (PN) for survival, a therapy that is life saving but fraught with serious morbidity due to the need for long-term central venous access.1 Massive intestinal resection due to conditions such as trauma, mesenteric ischemia, and inflammatory bowel disease leads to reduced intestinal length and mucosal surface area, and if the residual intestine does not adapt by increasing digestive capacity, intestinal failure may result.
Glucagon-like peptide 2 (GLP-2) is a 33–amino acid hormone that is derived from tissue-specific posttranslational processing of the parent peptide proglucagon in the enteroendocrine L cells of the ileum and colon.2,3 Plasma concentrations of endogenous GLP-2 and ileum proglucagon gene expression increase after massive intestinal resection in rodent models. Exogenous GLP-2 has been shown to increase plasma GLP-2 concentration and GLP-2 receptor (GLP-2R) mRNA expression, as well as to augment adaptive growth and digestive capacity of the residual small intestine while maintaining endogenous proglucagon expression in a model of mid–small bowel resection in orally fed rats.4 However, resection of the ileum and proximal colon reduces the endogenous production of GLP-2, and in this setting, adaptation does not occur.5–7 Recent investigations suggest that replacement of GLP-2 in the setting of inadequate endogenous production augments adaptation after massive intestinal resection in animal SBS models using PN or PN plus supplemental enteral nutrients.8–10
Human studies using GLP-2 in SBS patients with reduced endogenous GLP-2 secretion due to massive bowel resection have shown increases in fluid absorption and nutrition parameters.11 These findings have been supported by clinical trials using teduglutide, a GLP-2 analog resistant to degradation by dipeptidyl peptidase IV (DPP-IV).12 Human studies of GLP-2 administration have been limited to patients with SBS who are at least 12 months from their most recent bowel resection.11,12 It is unknown if earlier administration of GLP-2 to coincide with massive resection might improve adaptation, as there are currently no human or animal studies that have examined the effect that timing of GLP-2 administration has on intestinal adaptation after massive intestinal resection. Therapy immediately after resection has primarily been supportive, and additional treatments, such as growth hormone and GLP-2 analogs, are usually delayed until the patient is stabilized. Given the controversy of whether morphologic adaptation occurs in humans with SBS,13,14 data characterizing the time course of the adaptive response in a rat model that mimics human SBS are needed to better understand how early intestinal adaptation might occur in humans and guide the design of future animal studies and GLP-2 clinical trials.
Materials and Methods
Animals and Experimental Design
We investigated the time course of the intestinotrophic response to massive small bowel resection in a rat model of human SBS, defined as resection of the gastrointestinal (GI) tract that does not permit complete intestinal adaptation and requires PN for survival.15 This model was originally developed by Kripke et al6 and adapted by our laboratory.5 The animal facilities and research protocols were approved by the University of Wisconsin–Madison Institutional Care and Use Committee. Male Sprague-Dawley rats (Harlan, Madison, WI) initially weighing 150–200 g were housed in individual stainless steel cages with unlimited water access. The animal facilities were maintained at 22°C on a 12:12-h light-dark cycle. All animals were acclimated to their new environment for 5–7 days while being fed semipurified elemental powdered diet ad libitum.16
Rats were randomly assigned to undergo 60% bowel resection (Figure 1) and to be killed at one of the following 10 time points postsurgery: 4 hours (n = 4), 12 hours (n = 6), 1 day (n = 4), 2 days (n = 6), 3 days (n = 5), 4 days (n = 3), 5 days (n = 4), 7 days (n = 10), 10 days (n = 5), and 12 days (n = 6). A baseline group that received the same presurgery care as surgery animals was also included. This group was killed without undergoing surgery and served as time point 0 for both transection and resection groups (n = 4). In addition, rats were randomly assigned to 3 transection control surgery groups and were killed at 1 day (n = 4), 3 days (n = 5), or 7 days (n = 3).
Figure 1.

Illustration of 60% jejunoileal resection plus cecectomy in the rat that mimics human short bowel syndrome. The resected area, designated by shading, consists of intestine from 40 cm distal to the ligament of Treitz to 1 cm distal to the cecum. Continuity of the remaining 40 cm of jejunum and colon is restored with an end-to-end jejunocolic anastomosis. Transection consists of 2 parallel cuts with restoration of bowel continuity.
Surgical Procedures and Animal Care
Rats were fed a low-residue, partially hydrolyzed complete liquid diet (Vital; donated by Ross Products Division, Abbott Laboratories, Columbus, OH) ad libitum for 3 days to prepare the bowel and were then fasted for 18 hours prior to surgery. On the day of surgery, rats were anesthetized by inhalation of isoflurane (Isoflo, Abbot Laboratories, North Chicago, IL) via an anesthetic machine. After anesthesia, rats underwent a 60% jejunoileal resection + cecectomy with end-to-end jejunocolic anastomosis or transection as described previously.5 Briefly, small intestine was resected from 40 cm distal to the ligament of Treitz to 1 cm distal to the cecum. The remaining 40 cm of jejunum was attached to the proximal colon primarily with an end-to-end anastomosis using 6-0 silk suture. Transected animals received transection with primary anastomosis at 40 cm distal to the ligament of Treitz and 1 cm proximal to the cecum. Animals were given 5 mL of intraperitoneal saline for fluid resuscitation. The peritoneum was closed with absorbable suture, and the abdominal skin incision was closed with wound clips. After abdominal closure, an intravenous catheter was placed in the superior vena cava via the external jugular vein as previously described.16 Rats received oxymorphone (0.18 mg/kg body weight) every 6 hours for 24 hours after surgery for analgesia, and ampicillin (200 mg/kg body weight) was administered every 12 hours for 48 hours postoperatively as perioperative prophylaxis.17
All resection and transection control rats were maintained exclusively with an isonitrogenous and isoenergetic PN regimen for the duration of the study, and provided water ad libitum. PN solution was prepared aseptically as a total nutrient admixture using commercial preparations of amino acids, dextrose, and lipid emulsions. PN was infused via a syringe infusion pump (Harvard apparatus, Inc, Holliston, MA) at rates of 1.0 mL/h on postsurgery day 0, 1.67 mL/h on day 1, and then a rate to provide 250 kcal/kg body weight per day. Electrolytes, vitamins, trace elements, and choline were added based on reported requirements of the intravenously fed rat.16 The rat PN solutions have a caloric density of ~1.0 kcal/mL and provide 1.5 g N/kg/d and 32% of nonprotein energy from Intralipid. At the prespecified postsurgery time points, the rats were anesthetized with isoflurane and killed by exsanguination, at which time the entire small and large intestine, liver, and kidneys were removed for analysis.
Intestinal Composition and Histology
After removal, the duodenum, jejunum, and colon were flushed with ice-cold saline and placed on a chilled glass plate for further sectioning. Intestine 1 cm on either side of the anastomosis was discarded. The first 2 cm of each section was used for measuring wet and dry mucosal mass. The third centimeter was fixed in 10% formalin for 24 hours, transferred to 70% ethanol, paraffin-embedded, cut into 5-μm sections, and stained with hematoxylin and eosin for histomorphology as previously described.5 The next 2 cm was used to determine mucosal protein (bicinchoninic acid protein assay; Pierce Chemicals, Rockford, IL) and DNA content, along with sucrase activity.18,19 The remaining sections from each segment of bowel were snap-frozen intact in liquid nitrogen and stored at −70°C for RNA extraction.
Plasma Hormone Determinations
Blood was collected in chilled tubes containing 1 mg EDTA, 0.1 mmol diprotin A/L (MP Biomedicals, Aurora, OH), and 0.01 mmol aprotinin/L (Calbiochem, La Jolla, CA). Plasma bioactive GLP-2 was quantified by radioimmunoassay (RIA) with the use of an antibody specific to the N-terminus of GLP-2.2 The concentration of free insulin-like growth factor I (IGF-I) in plasma was determined by RIA after IGF binding proteins were removed by high-performance liquid chromatography (HPLC) under acid conditions.5,20,21 Materials for the RIA include recombinant human IGF-I (rhIGF-I) as a standard, 125I-IGF-I, polyclonal antibodies to human IGF-I, goat antirabbit Ig-G, and normal rabbit serum. Plasma extracts were assayed in triplicate in a single assay.
RNA Extraction and Quantification of Proglucagon and GLP-2R mRNA
Total RNA was extracted from intact jejunum and colon using the TRIzol reagent (Gibco BRL Life Technologies, Grand Island, NY). All RNA solutions were quantitated by absorbance at 260 nm, and quantity and integrity were confirmed by electrophoresis through 1.25% agarose/2.2 M formaldehyde gels and staining with ethidium bromide to visualize ribosomal RNA bands.
Proglucagon and GLP-2R mRNA expression was measured in a 2-step real-time quantitative polymerase, chain reaction (RT-qPCR) using the SYBR Green detection method as previously described.22 Then, 10 μg of jejunum or colon total RNA was treated with DNase (TURBO DNA-free kit, Ambion, Austin, TX) to eliminate genomic DNA and reverse transcribed using random hexamers (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Control reactions without reverse transcriptase were performed to confirm the absence of genomic DNA. A 4-step hot-start real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) and the Applied Biosystems 7000 Real-Time PCR instrument (Applied Biosystems) with conditions as follows:
Step 1: 50°C for 2 minutes
Step 2: 95°C for 10 minutes
Step 3: 50 cycles of 95°C for 15 seconds followed by 55°C or 49.5°C for 1 minute
Step 4 (dissociation): 95°C for 15 seconds, 60°C for 20 seconds, and 95°C for 15 seconds
No reverse transcriptase or template control reactions were done with every assay to ensure the specificity of the reaction and absence of any contamination. Data were analyzed using 7000 system software (Applied Biosystems), and relative quantification was done using the ΔΔC(t) method with 18S (QuantumRNA 18S Internal Standards, Ambion) or beta-actin as the internal control and the orally fed group as the reference control.23 Sequences for primers were as follows (Integrated DNA Technologies, Inc, Coralville, IA):
Proglucagon (72-bp amplicon): forward 5′-GAA TTC ATT GCT TGG CTG GT-3′ and reverse 5′-TTC CTC AGC TAT GGC GAC TT-3′
GLP-2R (109-bp amplicon): forward 5′-CGA CGA CCA AGT TCA AGG AT-3′ and reverse 5′-TCC ATT GGC AAA GCC ATA CT-3′
Beta-actin (272-bp amplicon), forward 5′-CAC ACT GTG CCC ATC TAT GA-3′ and reverse 5′-CCG ATA GTG ATG ACC TGA CC-3′
Statistical Analyses
SAS Version 8.2 (SAS Institute, Cary, NC) and R (downloaded from www.r-project.org) were used for statistical analysis. The differences between treatment groups were examined by 1-way analysis of variance followed by the protected least significant differences technique. General linear models were used to analyze the main effects of resection, time, and their interactions. Changes in body weight were assessed by repeated-measures analysis. Among resected animals, simple linear regression was used to examine the correlations between different endpoints. All values are presented as means ± standard error; P < .05 was considered statistically significant.
Results
Body Weight
Resected animals demonstrated a significant gain in body weight over time (P < .0001), whereas the transected animals did not (P = .76; see Figure 2). Animals in the transection group weighed, on average, 6 g more than animals in the resection group at the time of surgery (206 ± 2 vs 200 ± 2 g). Resection animals lost ~5 g of tissue due to their resection and initially lost weight, reaching a nadir of 187 ± 2 g on day 3 after surgery. This weight loss was not recovered until day 12 with a final weight of 202 ± 2 g.
Figure 2.
Mean (± SE) daily body weight of rats maintained with parenteral nutrition after transection or resection surgery. n = 3–6 per surgery group per time point. Repeated-measures analysis was used to analyze the main effect of type of surgery on body weight. Resected rats had significant gains in body weight (P < .0001), whereas transected rats did not have a significant change in body weight.
Intestinal Adaptive Growth
In resected animals, there were significant changes over time in duodenal mucosal protein and DNA, jejunum mucosal dry mass, protein and DNA, and colon intact dry mass and crypt depth (see Figures 3 and 4). Duodenal mucosal protein and DNA decreased 1 day after resection; returned to baseline levels on days 3 and 4, respectively; and then stably decreased to levels significantly below baseline by day 7. In addition, transected animals showed significantly decreased duodenal mucosal protein (P = .006) and DNA (P = .02) over time and, like resected animals, reached values significantly below baseline by day 7.
Figure 3.
Mean (± SE) mucosal dry mass and concentrations of protein and DNA in the duodenum (left) and jejunum (right) in rats subjected to transection or resection surgery and parenteral nutrition. n = 3–10 per surgery group per time point. * denotes means that are significantly different from baseline (time 0), # denotes time points that are significantly different from baseline (time 0) for both resection and transection surgery, and † denotes time points with statistically different means for resection and transection surgery; P < .05. The differences between means were examined by 1-way analysis of variance (ANOVA). General linear models were used to analyze the main effect of time on resection and transection surgery. In the duodenum (resection and transection respectively) P values for dry mass are .40 and 27; protein, .002 and .006; and DNA, .003 and .02. In the jejunum (resection and transaction respectively) P values for dry mass are .003 and .58; protein, .0004 and .46; and DNA, .01 and .46.
Figure 4.
Mean (± SE) intact dry mass (A) and crypt depth (B) in the colon in rats subjected to transection or resection surgery and parenteral nutrition. n = 3–6 per surgery group per time point. *denotes means that are significantly different from baseline (time 0), # denotes time points that are significantly different from baseline (time 0) for both resection and transection surgery, and † denotes time points with statistically different means for resection and transection surgery; P < .05. There was a significant change in colon dry mass (P = .0009) and crypt depth (P < .0001) over time with resection but not transection. The differences between means were examined by 1-way analysis of variance (ANOVA). General linear models were used to analyze the main effect of time on resection and transection surgery.
The residual jejunum of resected animals did not adapt as increases in mucosal dry mass, protein, and DNA were not significantly above baseline values. Jejunum mucosal dry mass, protein, and DNA in resected animals reached maximal increases above baseline of 15% at day 3, 16% at day 3, and 13% at day 4, respectively, although none of these increases was statistically significant compared with baseline. By day 12, jejunum mucosal dry mass decreased by 19%, mucosal protein decreased by 43%, and mucosal DNA decreased by 31% from baseline, with the latter 2 reaching statistical significance. Transected animals had no significant changes over time with respect to jejunum dry mass, protein, or DNA, and at day 7, jejunal mucosal DNA was significantly higher in transected (6.3 ± 0.51) than in resected (4.5 ± 0.51) animals (P = .04). In addition, jejunum villus height and crypt depth were not increased above baseline with resection at any time points and were significantly decreased from baseline at day 12.
In contrast to the proximal intestine, residual colon showed increases in intact dry mass, DNA, and crypt depth over time in resected animals, reaching peak dry mass and DNA at day 10 and peak crypt depth at day 7. Colon crypt depth was significantly greater in the resected than in the transected animals over time and was >90% greater than baseline on days 7 and 12.
Sucrase Activity
Duodenum and jejunum sucrase activity in resected animals showed significant declines over time both when normalized to bowel length (segmental activity) and mucosal protein levels (specific activity). Duodenum sucrase-specific activity in resected rats decreased below baseline levels by day 3, reaching significance at day 4 and days 7–12 and a nadir at day 10 (24% of baseline activity). Jejunum sucrase-specific activity in resected rats decreased by day 1 and reached significantly decreased levels below baseline from days 2–12, reaching a nadir at day 12 (58% of baseline activity). In addition, jejunum sucrase-specific activity decreased over time in transected animals, which approached significance (P = .057), reaching a nadir at day 7 (62% of baseline levels). Furthermore, there were no significant differences in jejunum sucrase activity between resected or transected animals at any time points.
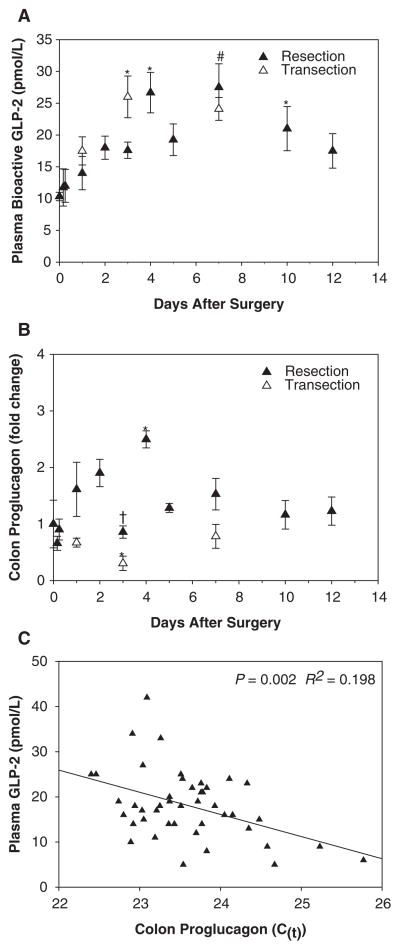
Plasma Bioactive GLP-2 and Colon Proglucagon mRNA Expression
Plasma bioactive GLP-2 showed significant changes over time with resection (P = .02) but not transection (Figure 5A). GLP-2 levels increased significantly over baseline levels on days 4 and 7 by 158% and 166%, respectively. Levels declined by day 10 but remained significantly increased from baseline. At day 12, GLP-2 was 69% greater than baseline levels, but this was no longer significantly different. There were no significant differences in plasma GLP-2 levels between resected animals and transected animals at any time points. Colon proglucagon mRNA levels increased in resected but not transected animals, reaching a significant peak level of 250% of baseline at day 4 (Figure 5B). After day 4, levels decreased near baseline from days 5–12. In resected animals, the colon proglucagon curve paralleled the curve for plasma GLP-2. Plasma GLP-2 levels were significantly correlated to colon proglucagon levels (R2 = 0.198, P = .002; see Figure 5C; lower C(t) signifies higher mRNA levels).
Figure 5.
A) Mean (± SE) plasma bioactive glucagon-like peptide 2 (GLP-2) and (B) colon proglucagon mRNA in rats subjected to transection or resection surgery and parenteral nutrition. (C) Correlation between colon proglucagon mRNA and concentration of plasma GTLP-2 in resected rats only (lower C(t) signifies higher mRNA levels). n = 2–10 per surgery group per time point. * denotes means that are significantly different from baseline (time 0), # denotes time points that are significantly different from baseline (time 0) for both resection and transection surgery, and † denotes time points with statistically different means for resection and transection surgery; P < .05. There was a significant change in plasma GLP-2 in resected rats (P = .02) over time. The differences between means were examined by 1-way analysis of variance (ANOVA). General linear models were used to analyze the main effect of time on resection and transection surgery and to obtain correlation coefficients. Colon proglucagon mRNA was analyzed using the ΔΔC(t) method with beta-actin as an internal control and was expressed relative to the baseline group (time 0).
Plasma Total IGF-I
Plasma total IGF-I levels decreased in both resected and transected animals on day 1 and then increased, reaching peak levels that were near baseline in the transected group at day 7, and significantly increased 22% above baseline in the resected group on day 12 (Figure 6A). There were significant changes in IGF-I levels over time in both the resected (P < .0001) and transected (P = .03) animals. There was a trend toward a correlation between plasma IGF-I levels in resected animals and colon crypt depth by linear regression analysis (R2 = 0.202, P = .053; see Figure 6B).
Figure 6.
(A) Mean (± SE) plasma total insulin growth factor I (IGF-I) in rats subjected to transection or resection surgery and parenteral nutrition. (B) Correlation between plasma IGF-I levels and colon crypt depth in resected rats only. n = 2–6 per surgery group per time point. * denotes means that are significantly different from baseline (time 0), and # denotes time points that are significantly different from baseline (time 0) for both resection and transection surgery; P < .05. There was a significant change in plasma IGF-I in both resected (P < .0001) and transected (P = .03) rats over time. The differences between means were examined by 1-way analysis of variance (ANOVA). General linear models were used to analyze the main effect of time on resection and transection surgery and to obtain correlation coefficients.
Jejunum and Colon GLP-2R mRNA Expression
Jejunum GLP-2R showed a significant change over time in resected animals (P = .005) but not transected animals. There was a sharp initial increase in resected animals at 4 hours after surgery that was significant from baseline and remained significantly increased compared to transected animals on day 1 (Figure 7A). Levels declined to significantly below baseline levels at day 3 but then showed a second increase with a peak at day 5 that was 378% higher than baseline, which was significant. Levels declined to that of transected animals by day 7 and remained below baseline through day 12. Colon GLP-2R levels showed a significant change over time in resected animals (P = .007) and transected animals (P = .03; Figure 7B). Resected animals had an early peak on day 1 that was significantly higher than baseline and significantly different from transected animals. There was no peak in the transected group, and by day 3, both groups had levels that were significantly lower than baseline, which persisted through the later time points.
Figure 7.
Mean (± SE) jejunum (A) and colon (B) glucagon-like peptide 2 receptor (GLP-2R) mRNA expression in rats subjected to transection or resection surgery and parenteral nutrition. n = 3–6 per surgery group per time point.* denotes means that are significantly different from baseline (time 0), # denotes time points that are significantly different from baseline (time 0) for both resection and transection surgery, and † denotes time points with statistically different means for resection and transection surgery; P < .05. There was a significant change in jejunum GLP-2R in resected rats (P = .005) and colon GLP-2R in both resected (P = .007) and transected (P = .03) rats over time. The differences between means were examined by 1-way analysis of variance (ANOVA). General linear models were used to analyze the main effect of time on resection and transection surgery. Jejunum and colon GLP-2R mRNA was analyzed using the ΔΔC(t) method with 18S (jejunum) and beta-actin (colon) as an internal control and was expressed relative to the baseline group (time 0).
Discussion
GLP-2 regulation of intestinal adaptation to massive bowel resection is a poorly understood process. It is unclear what, if any, adaptation occurs in patients with SBS and no residual ileum, and there are virtually no data regarding the early phase of human structural adaptation to intestinal resection.13,14,24,25 In addition to the lack of human data, there are no animal data characterizing the early phase of PN-dependent SBS. Previous studies demonstrating time-dependent changes in GLP-2 and intestinal adaptation used models of resection with residual ileum and cecum.26–28 These models are not clinically relevant as the residual intestine adapts, and animals do not require PN for survival. The model used in the present study reliably mimics human SBS and, to our knowledge, is the only one able to study the effects of growth factors on intestinal adaptation to resection using a “clinical” animal model for human SBS, which does not adapt and requires PN for survival. Our group and others have previously shown that in this “clinical” SBS model, mucosal growth is not stimulated by enteral feeding, and rats are unable to survive on enteral feeding alone.5,6,8,15 Thus, use of this SBS model to evaluate the time course of the endogenous intestinotrophic response to massive intestinal resection may provide valuable insight into the early phase of human SBS, in which patients are typically maintained on PN, enteral feedings are deferred, and recovery is prolonged.
We demonstrated in this study that, after a limited peak in duodenum and jejunum cellularity between days 3 and 4 after resection, proximal intestinal mucosal cellularity rapidly declines to levels that are significantly below baseline levels. This occurred despite significantly elevated plasma GLP-2 levels on days 4, 7, and 10. This finding can be explained by the fact that, despite being significantly elevated above baseline, peak plasma GLP-2 levels in the resected animals were too low to promote proximal intestinal adaptation. In an earlier publication from our group, Liu et al8 used this surgical model to show that plasma GLP-2 levels >50 pmol/L are required for significant adaptation to occur in the proximal intestine, and only animals that received exogenous GLP-2 after resection reached that level. The peak mean plasma GLP-2 level in resected animals in this study was 27.5 pmol/L, demonstrating that animals without an ileal source of proglucagon are incapable of generating adequate endogenous GLP-2 to promote structural adaptation of the proximal bowel.
We also found in this study that there was an absence of functional adaptation after resection despite increased plasma GLP-2 levels. Duodenum and jejunum sucrase-specific activity showed a rapid, steep decline after resection to levels that were significantly below baseline. A similar decline in sucrase activity was seen in the jejunum of transected animals, suggesting that the reduction in enzyme activity was related to the PN and lack of luminal nutrients and not to type of surgery. In fact, enteral feedings along with PN have been shown to increase jejunum sucrase activity after both transection and resection in a rat model of SBS, whereas the addition of GLP-2 alone to PN had no effect.8
Human studies have shown that patients with SBS and resection of their entire ileum and colon and a jejunostomy have an impaired GLP-2 response to a meal compared to control patients with a normal GI tract.29 However, patients with SBS and no ileum but with a preserved colon are able to generate a GLP-2 response to a meal that is larger than control patients.30 These data suggest that the colon is capable of producing supra-physiologic levels of GLP-2 in the absence of ileum that does not occur in the presence of functioning ileum. Our study confirms this human finding in the rat, as plasma bioactive GLP-2 was found to be positively correlated with colon proglucagon mRNA levels in resected animals. Transected animals, despite having significantly increased GLP-2 levels, had a decrease in colon proglucagon mRNA, suggesting that, in the presence of an intact ileum, colon proglucagon is not upregulated. Although colon proglucagon and GLP-2 were only transiently elevated due to resection in this study, this is not surprising as rats were maintained exclusively with PN, and enteral nutrients are the primary stimulus to GLP-2 secretion.27 Exogenous GLP-2 has been shown to increase both colon proglucagon and plasma GLP-2 in ileum-resected rats with SBS; the addition of luminal nutrients to GLP-2 treatment leads to a synergistic GLP-2 response, possibly through decreased plasma DPP-IV.31
Adaptation occurred in the colon in a GLP-2-independent fashion. Colon dry mass and crypt depth increased to peak levels by day 7 after resection and remained elevated despite declining levels of colon proglucagon and plasma GLP-2. Colon adaptation after resection mirrored plasma IGF-I levels, which showed an increase over time through day 12. The increase in colon structural adaptation in this model has been shown before.5,6 In addition, although colonic electrical parameters are unchanged after resection and PN in this model, the addition of gastric feeding increases electroneutral NaCl absorption in the colon, suggesting functional adaptation as well.5,32 Furthermore, a correlation between plasma IGF-I and colonic structural and functional adaptation is supported by a study using this model that showed increased colonic mucosal growth, water absorption, and IGF-I mRNA with resection and 7 days of IGF-I infusion.7 The increase in colonic adaptation in our study, coupled with the finding that colon proglucagon expression is upregulated after ileal resection, supports colon-preserving strategies in massive intestinal resections that have the potential to lead to intestinal failure. Indeed, human studies have identified colon preservation as a predictor of successful PN weaning in children33 and both weaning and survival in adults.34,35
The differential adaptation whereby the colon adapts but jejunum does not is further explained by the disparity in hormone receptors between tissues. Jejunum GLP-2R increased 8-fold after resection and remained significantly elevated above transection controls 1 day after surgery. In addition, a second peak occurred in the jejunum 7 days after resection and coincided with the peak plasma GLP-2 level. We have previously shown that mid–small bowel resection and exogenous GLP-2 increases ileum GLP-2R mRNA,4 and although jejunum GLP-2R mRNA was not increased in that study, levels of circulating GLP-2 were much higher due to the presence of functional ileum. It is possible that, due to lower GLP-2 levels after ileum resection in this study, jejunum GLP-2R mRNA expression is more sensitive to rises in plasma GLP-2 than when ileum is present. In contrast, colon GLP-2R mRNA increased 2-fold after resection and then rapidly declined to levels significantly below baseline levels. This is not surprising given the decreased overall GLP-2R density in the colon compared to the jejunum and the absence of colonic adaptation in response to GLP-2.31,36 Rather, colon tissue has been shown to have a greater density of IGF-I receptors than the jejunum, with a high degree of receptor expression in the crypt epithelium,37 findings that further support an IGF-I-mediated adaptation mechanism in the colon.
This study is the first to characterize the time course of intestinal adaptation and hormonal responses after resection in a PN-dependent rat model of human SBS. There is an absence of significant structural or functional adaptation of the proximal intestine, despite transient increases in the expression of both colon proglucagon and jejunal GLP-2R mRNAs, as well as plasma concentrations of bioactive GLP-2. Colon adaptation occurs in response to resection and may reflect increased levels of plasma IGF-I. These findings support the practice of colon preservation in massive intestinal resection. Given the lack of proximal intestinal adaptation in this model, further animal and human studies are needed to determine if early replacement of growth factors such as GLP-2 are of benefit during the postresection period in the absence of an ileal source of proglucagon.
Acknowledgments
We thank Michael J. Grahn and Patrick M. Solverson for their expert technical assistance and Ross Laboratories for donating the Vital diet.
Footnotes
Financial disclosure: This work was supported by NIH grants R01-DK-42835 and T32-DK-07665.
References
- 1.Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology. 2003;124:1111–1134. doi: 10.1016/s0016-5085(03)70064-x. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann B, Johnsen AH, Orskov C, Adelhorst K, Thim L, Holst JJ. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides. 2000;21:73–80. doi: 10.1016/s0196-9781(99)00176-x. [DOI] [PubMed] [Google Scholar]
- 3.Sinclair EM, Drucker DJ. Proglucagon-derived peptides: mechanisms of action and therapeutic potential. Physiology (Bethesda) 2005;20:357–365. doi: 10.1152/physiol.00030.2005. [DOI] [PubMed] [Google Scholar]
- 4.Koopmann MC, Nelson DW, Murali SG, et al. Exogenous glucagon-like peptide-2 (GLP-2) augments GLP-2 receptor mRNA and maintains proglucagon mRNA levels in resected rats. JPEN J Parenter Enteral Nutr. 2008;32:254–265. doi: 10.1177/0148607108316198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillingham MB, Dahly EM, Carey HV, Clark MD, Kritsch KR, Ney DM. Differential jejunal and colonic adaptation due to resection and IGF-I in parenterally fed rats. Am J Physiol Gastrointest Liver Physiol. 2000;278:G700–G709. doi: 10.1152/ajpgi.2000.278.5.G700. [DOI] [PubMed] [Google Scholar]
- 6.Kripke SA, De Paula JA, Berman JM, Fox AD, Rombeau JL, Settle RG. Experimental short-bowel syndrome: effect of an elemental diet supplemented with short-chain triglycerides. Am J Clin Nutr. 1991;53:954–962. doi: 10.1093/ajcn/53.4.954. [DOI] [PubMed] [Google Scholar]
- 7.Mantell MP, Ziegler TR, Adamson WT, et al. Resection-induced colonic adaptation is augmented by IGF-I and associated with upregulation of colonic IGF-I mRNA. Am J Physiol. 1995;269 (pt 1):G974–G980. doi: 10.1152/ajpgi.1995.269.6.G974. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Nelson DW, Holst JJ, Ney DM. Synergistic effect of supplemental enteral nutrients and exogenous glucagon-like peptide 2 on intestinal adaptation in a rat model of short bowel syndrome. Am J Clin Nutr. 2006;84:1142–1150. doi: 10.1093/ajcn/84.5.1142. [DOI] [PubMed] [Google Scholar]
- 9.Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2004;286:G964–G972. doi: 10.1152/ajpgi.00509.2003. [DOI] [PubMed] [Google Scholar]
- 10.Scott RB, Kirk D, MacNaughton WK, Meddings JB. GLP-2 augments the adaptive response to massive intestinal resection in rat. Am J Physiol. 1998;275(pt 1):G911–G921. doi: 10.1152/ajpgi.1998.275.5.G911. [DOI] [PubMed] [Google Scholar]
- 11.Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806–815. doi: 10.1053/gast.2001.22555. [DOI] [PubMed] [Google Scholar]
- 12.Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224–1231. doi: 10.1136/gut.2004.061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Keefe SJ, Haymond MW, Bennet WM, Oswald B, Nelson DK, Shorter RG. Long-acting somatostatin analogue therapy and protein metabolism in patients with jejunostomies. Gastroenterology. 1994;107:379–388. doi: 10.1016/0016-5085(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 14.Porus RL. Epithelial hyperplasia following massive small bowel resection in man. Gastroenterology. 1965;48:753–757. [PubMed] [Google Scholar]
- 15.Scarpello JH, Cary BA, Sladen GE. Effects of ileal and caecal resection on the colon of the rat. Clin Sci Mol Med. 1978;54:241–249. doi: 10.1042/cs0540241. [DOI] [PubMed] [Google Scholar]
- 16.Dahly EM, Guo Z, Ney DM. Alterations in enterocyte proliferation and apoptosis accompany TPN-induced mucosal hypoplasia and IGF-I-induced hyperplasia in rats. J Nutr. 2002;132:2010–2014. doi: 10.1093/jn/132.7.2010. [DOI] [PubMed] [Google Scholar]
- 17.Gillingham MB, Clark MD, Dahly EM, Krugner-Higby LA, Ney DM. A comparison of two opioid analgesics for relief of visceral pain induced by intestinal resection in rats. Contemp Top Lab Anim Sci. 2001;40:21–26. [PubMed] [Google Scholar]
- 18.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 19.Dahlqvist A. Method for assay of intestinal disaccharidases. Anal Biochem. 1964;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- 20.Gillingham MB, Dahly EM, Murali SG, Ney DM. IGF-I treatment facilitates transition from parenteral to enteral nutrition in rats with short bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2003;284:R363–R371. doi: 10.1152/ajpregu.00247.2002. [DOI] [PubMed] [Google Scholar]
- 21.Ney DM, Yang H, Smith SM, Unterman TG. High-calorie total parenteral nutrition reduces hepatic insulin-like growth factor-I mRNA and alters serum levels of insulin-like growth factor-binding protein-1, -3, -5, and -6 in the rat. Metabolism. 1995;44:152–160. doi: 10.1016/0026-0495(95)90258-9. [DOI] [PubMed] [Google Scholar]
- 22.Nelson DW, Murali SG, Liu X, Koopmann MC, Holst JJ, Ney DM. Insulin-like growth factor I and glucagon-like peptide-2 responses to fasting followed by controlled or ad libitum refeeding in rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1175–R1184. doi: 10.1152/ajpregu.00238.2007. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Bell MJ, Martin LW, Schubert WK, Partin J, Burke J. Massive small-bowel resection in an infant: long-term management and intestinal adaptation. J Pediatr Surg. 1973;8:197–204. doi: 10.1016/s0022-3468(73)80085-5. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein LD, Shoemaker CP, Hersh T, Wright HK. Enhanced intestinal absorption after small bowel resection in man. Arch Surg. 1969;99:560–562. doi: 10.1001/archsurg.1969.01340170012003. [DOI] [PubMed] [Google Scholar]
- 26.Ljungmann K, Hartmann B, Kissmeyer-Nielsen P, Flyvbjerg A, Holst JJ, Laurberg S. Time-dependent intestinal adaptation and GLP-2 alterations after small bowel resection in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G779–G785. doi: 10.1152/ajpgi.2001.281.3.G779. [DOI] [PubMed] [Google Scholar]
- 27.Dahly EM, Gillingham MB, Guo Z, et al. Role of luminal nutrients and endogenous GLP-2 in intestinal adaptation to mid-small bowel resection. Am J Physiol Gastrointest Liver Physiol. 2003;284:G670–G682. doi: 10.1152/ajpgi.00293.2002. [DOI] [PubMed] [Google Scholar]
- 28.Welters CF, Dejong CH, Deutz NE, Heineman E. Intestinal function and metabolism in the early adaptive phase after massive small bowel resection in the rat. J Pediatr Surg. 2001;36:1746–1751. doi: 10.1053/jpsu.2001.28813. [DOI] [PubMed] [Google Scholar]
- 29.Jeppesen PB, Hartmann B, Hansen BS, Thulesen J, Holst JJ, Mortensen PB. Impaired meal stimulated glucagon-like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut. 1999;45:559–563. doi: 10.1136/gut.45.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeppesen PB, Hartmann B, Thulesen J, et al. Elevated plasma glucagon-like peptide 1 and 2 concentrations in ileum resected short bowel patients with a preserved colon. Gut. 2000;47:370–376. doi: 10.1136/gut.47.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Murali SG, Holst JJ, Ney DM. Enteral nutrients potentiate the intestinotrophic action of glucagon-like peptide-2 in association with increased insulin-like growth factor-I responses in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1794–R1802. doi: 10.1152/ajpregu.90616.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fabritius J, Nell G, Loeschke K. Adaptation of electrolyte transport in rat large intestine after proximal resection: II. Colon after 50% jejunoilectomy combined with cecectomy. Pflugers Arch. 1986;406:328–332. doi: 10.1007/BF00640923. [DOI] [PubMed] [Google Scholar]
- 33.Spencer AU, Neaga A, West B, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005;242:403–409. doi: 10.1097/01.sla.0000179647.24046.03. discussion 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117:1043–1050. doi: 10.1016/s0016-5085(99)70388-4. [DOI] [PubMed] [Google Scholar]
- 35.Quiros-Tejeira RE, Ament ME, Reyen L, et al. Long-term parenteral nutritional support and intestinal adaptation in children with short bowel syndrome: a 25-year experience. J Pediatr. 2004;145:157–163. doi: 10.1016/j.jpeds.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Munroe DG, Gupta AK, Kooshesh F, et al. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA. 1999;96:1569–1573. doi: 10.1073/pnas.96.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinz-Erian P, Kessler U, Funk B, Gais P, Kiess W. Identification and in situ localization of the insulin-like growth factor-II/mannose-6-phosphate (IGF-II/M6P) receptor in the rat gastrointestinal tract: comparison with the IGF-I receptor. Endocrinology. 1991;129:1769–1778. doi: 10.1210/endo-129-4-1769. [DOI] [PubMed] [Google Scholar]