Abstract
The sialomucin endolyn is a transmembrane protein with a unique trafficking pattern in polarized Madin-Darby canine kidney cells. Despite the presence of a cytoplasmic tyrosine motif that, in isolation, is sufficient to mediate basolateral sorting of a reporter protein, endolyn predominantly traverses the apical surface en route to lysosomes. Apical delivery of endolyn is disrupted in tunicamycin-treated cells, implicating a role for N-glycosylation in apical sorting. Site-directed mutagenesis of endolyn's eight N-glycosylation sites was used to identify two N-glycans that seem to be the major determinants for efficient apical sorting of the protein. In addition, apical delivery of endolyn was disrupted when terminal processing of N-glycans was blocked using glycosidase inhibitors. Missorting of endolyn occurred independently of the presence or absence of the basolateral sorting signal, because apical delivery was also inhibited by tunicamycin when the cytoplasmic tyrosine motif was mutated. However, we found that apical secretion of a soluble mutant of endolyn was N-glycan independent, as was delivery of glycosylphosphatidylinositol-anchored endolyn. Thus, specific N-glycans are only essential for the apical sorting of transmembrane endolyn, suggesting fundamental differences in the mechanisms by which soluble, glycosylphosphatidylinositol-anchored, and transmembrane proteins are sorted.
INTRODUCTION
Proper functioning of polarized epithelial cells necessitates the maintenance of differentiated apical and basolateral plasma membranes, which requires appropriate sorting of newly synthesized proteins to these distinct domains. In the biosynthetic pathway, newly synthesized apical and basolateral proteins are sorted upon reaching the trans-Golgi network (TGN). Basolateral sorting signals, including some tyrosine-containing tetrapeptide motifs and di-leucine motifs, are generally localized to the cytoplasmically exposed portions of these proteins (Nelson and Yeaman, 2001). These signals are thought to bind directly to adaptor protein complexes, which mediate sorting of the respective transmembrane proteins into basolaterally directed transport vesicles, analogous to the sorting of plasma membrane proteins into endocytic vesicles by AP-2 (Folsch et al., 2001; Simmen et al., 2002; Bonifacino and Traub, 2003). By contrast, apical sorting signals are less well defined and reside frequently within the membrane- or lumenally oriented regions of these molecules (Nelson and Yeaman, 2001). Membrane-embedded signals include glycosylphosphatidylinositol (GPI)-anchors attached to the carboxy terminus of some proteins and amino acid sequences within the transmembrane domains of other apical proteins. It has been postulated that association with glycolipid-enriched lipid microdomains may play a role in the apical delivery of these proteins (Ikonen and Simons, 1998; Paladino et al., 2002). In addition, both N- and O-linked glycosylation have been demonstrated to be necessary for the correct apical delivery of several proteins (Scheiffele et al., 1995; Wagner et al., 1995; Yeaman et al., 1997; Naim et al., 1999; Scheiffele and Fullekrug, 2000).
We have been examining the trafficking of the sialomucin endolyn, a type I transmembrane protein that contains both lysosomal and apical targeting information (Ihrke et al., 1998, 2001). The lumenal domain of endolyn consists of two highly O-glycosylated mucin subdomains connected by a nonmucin region with a suggested compact structure defined by two disulfide bridges (Ihrke et al., 2000). Each of these regions possesses two to four N-glycosylation sites. Endolyn is often most highly concentrated in lysosomes at steady state, but it is found to varying degrees in other endocytic compartments and at the plasma membrane, depending on the cell type and state of differentiation (Croze et al., 1989; Ihrke et al., 1998; Chan et al., 2001). Lysosomal delivery of endolyn requires a carboxy-terminal YXXϕ motif (Ihrke et al., 2000). Although the function of endolyn is poorly understood, studies of the human and mouse proteins (also called CD164) in hematopoietic cells and myoblasts, respectively, indicate a role in cell adhesion and cell differentiation highlighting the importance of the plasma membrane pool of the protein (Zannettino et al., 1998; Lee et al., 2001).
We previously demonstrated that a sizable fraction of newly synthesized endolyn, unlike other lysosomal membrane proteins that contain similar tyrosine sorting motifs, traverses the apical plasma membrane en route to lysosomes in polarized Madin-Darby canine kidney (MDCK) cells (Ihrke et al., 2001). This unusual route is not due to lack of basolateral sorting information in the cytoplasmic tail of the protein, because analysis of chimeras demonstrated that endolyn's tyrosine motif can serve as an efficient basolateral targeting signal. Rather, the lumenal domain of endolyn confers dominant apical targeting information that overrides the lysosomal and basolateral sorting information in the cytoplasmic tail in the biosynthetic pathway. Apical sorting of endolyn occurs by a mechanism that does not involve glycolipid rafts, but, intriguingly, requires N-glycosylation of the protein. Endolyn thus represents the first example of a transmembrane protein in which N-glycosylation seems to generate an apical sorting signal that is dominant over basolateral tyrosine motif-dependent sorting information.
Here, we have examined in detail the structural requirements for glycan-dependent apical sorting of endolyn. Whereas all of endolyn's eight N-glycosylation consensus sequences seem to be glycosylated, only a subset of these glycans was found to be both necessary and sufficient for efficient apical sorting of endolyn. In addition, terminal oligosaccharide processing rather than simply core glycosylation was required for interpretation of the apical sorting information, suggesting that specific receptor-ligand interactions are involved in the process. We also addressed the question whether N-glycans were a general requirement for apical sorting of differentially tethered forms of the same protein. Interestingly, manufactured soluble and GPI-anchored forms of endolyn were targeted apically independently of N-glycosylation. This indicates that at least two distinct mechanisms exist to sort proteins to the apical surface of MDCK cells. Sorting of transmembrane proteins such as endolyn seem to be restricted to one (N-glycan-dependent) mechanism, whereas secretory and GPI-linked proteins can be sorted in entirely different way(s), possibly in addition to the mechanism used by transmembrane endolyn.
MATERIALS AND METHODS
Antibodies, Reagents, and Drug Treatments
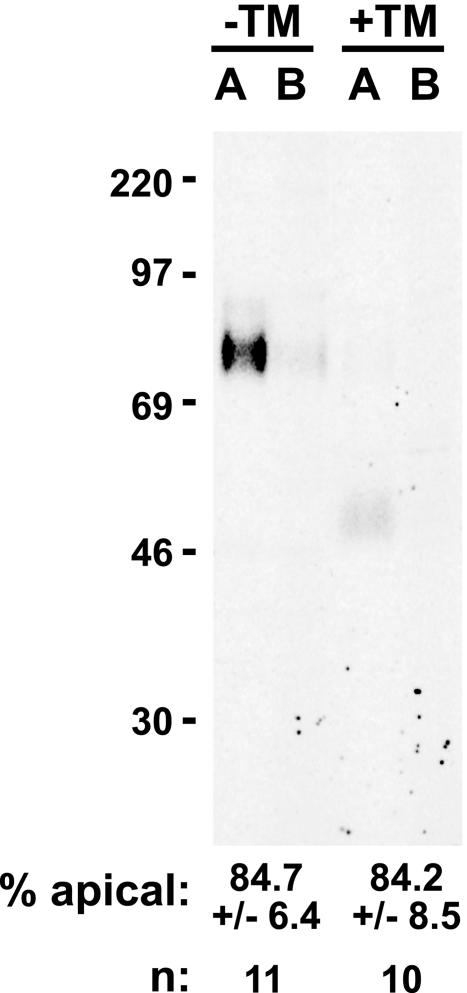
Rabbit antiserum no. 6431 and mouse monoclonal antibodies (mAbs) 501 and 502 to rat endolyn have been described previously (Ihrke et al., 1998, 2001); the latter were used interchangeably with similar results. Secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). The glycosylation perturbants were obtained from Sigma-Aldrich (St. Louis, MO) and used at the following concentrations: tunicamycin (TM, 3 μg/ml), kifunensine (KIF, 21.5 μM), deoxymannojirimycin (DMJ, 1 mM), deoxynojirimycin (DNJ, 1 mM), and benzyl 2-acetamido-2-deoxy-α-d-galactopyranoside (BGN, 4 mM). Drugs were added 30 min-2 h before the radiolabeling of cells and were included in the starvation and pulse steps but omitted thereafter. Short pretreatment with tunicamycin (30 min) resulted in synthesis of both fully N-glycosylated and non-N-glycosylated forms of proteins, allowing direct comparison of their polarized distribution in the same gel lanes (Figures 5, 6, 7). On longer pretreatment (2 h), only the non-N-glycosylated forms were observed. The polarized distribution of non-N-glycosylated forms was the same regardless of the pretreatment protocol used.
Figure 5.
Apical secretion of a soluble form of endolyn does not require N-glycans. Filter-grown Ensol-expressing cells were starved and radiolabeled for3hinthe presence or absence of TM. The apical and basolateral media were collected and secreted Ensol immunoprecipitated and analyzed by SDS-PAGE. A representative gel is shown, and the polarity of secreted Ensol (mean ± SD determined from 10-11 experiments) is noted below each pair of lanes.
Figure 6.
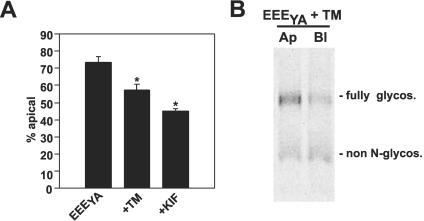
Apical delivery of EEEYA is N-glycosylation dependent. (A) EEEYA-expressing cells were starved and radiolabeled for 2 h in the presence of TM, BGN, or KIF and then chased for 15 min before domain-selective biotinylation and quantitation of cell surface polarity. The apical fraction of total biotinylated EEEYA is plotted (mean ± SEM; control, n = 11; TM, n = 6; BGN, n = 9; KIF, n = 2). *p < 0.01 versus endolyn by t test. (B) A typical gel showing apically and basolaterally biotinylated EEEYA recovered from TM-treated cells, with the migration of fully glycosylated and non-N-glycosylated protein indicated.
Figure 7.
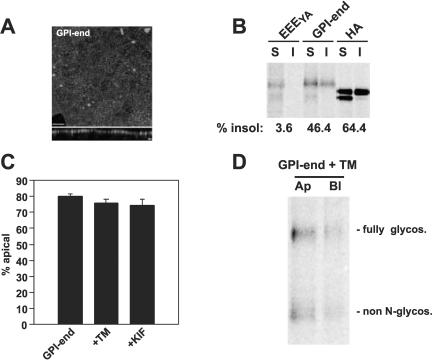
Apical delivery of GPI-anchored endolyn does not require N-glycosylation. (A) Surface distribution of GPI-endolyn. Filter-grown GPI-endolyn expressing cells were surface labeled on ice with anti-endolyn mAb, fixed and processed for indirect immunofluorescence, and analyzed by confocal microscopy. An xy projection of multiple sections comprising the entire cell volume and a corresponding xz section are shown. The position of the filter in the xz section is marked with an arrowhead. Bar, 8 μm. (B) Detergent solubility of GPI-endolyn. MDCK cells expressing influenza hemagglutinin, EEEYA, or GPI-endolyn were radiolabeled for 1 h and then chased for 1 h before solubilization on ice and isolation of detergent-soluble (S) and -insoluble (I) fractions. Immunoprecipitated fractions were analyzed by SDS-PAGE. The fraction of total protein that was insoluble in cold detergent is given beneath each pair of lanes. (C) Effect of glycosylation perturbants on surface distribution of GPI-endolyn. GPI-endolyn-expressing cells were starved and radiolabeled for 2 h in the presence of TM or KIF and then chased for 15 min before domain-selective biotinylation. The apical fraction of biotinylated GPI-endolyn is plotted (mean ± SEM; control, n = 13; TM, n = 6; KIF, n = 6). (D) Representative gel showing apically and basolaterally biotinylated GPI-endolyn recovered from TM-treated cells.
cDNA Constructs
The cDNA of wild-type rat endolyn and construction of the EEEYA and Ensol mutants have been described previously (Ihrke et al., 1998, 2001). To create ECEYA, the Y-to-A mutation was first introduced into the CD8-endolyn chimera CCE by polymerase chain reaction (PCR) and the CC-portion of this construct was then replaced by the EC-portion of ECCΔ by using the AflII sites at the transmembrane/cytoplasmic boundaries of both constructs (Ihrke et al., 2001). EECΔ was constructed by replacing the EC-portion of ECCΔ with endolyn's luminal and transmembrane domains, into which an AflII site had been introduced at the 3′ end by PCR. Individual and double glycosylation mutants were generated by site-directed mutagenesis by using primers to cDNA sequences in the region of interest, both containing the desired mutation. Generation of the N-null construct was done by sequentially introducing the N68A/N74A, N86A, N26A/N32A, N97A, and N144A mutations. Constructs with one or two intact glycosylation sites were generated by reintroducing the required codons into the N-null construct. To make the GPI-linked version of endolyn, we first generated an intermediate construct in pBSII SK- coding for the lumenal domain of endolyn and ending with a PstI site introduced by PCR. The fragment of rat 5′ nucleotidase cDNA coding for the C-terminal signal sequence necessary for GPI-anchor attachment to the protein was retrieved from the full-length cDNA by digest with PstI/BamH I and then cloned into the PstI site of the intermediate. The resulting expressed amino acid sequence at the domain border was KSTFSAAS, with the first four amino acids from endolyn and the last four from 5′ nucleotidase. All mutations were initially introduced into cDNAs inserted in pBSII SK-, which were then subcloned behind the butyrate-inducible cytomegalovirus promoter of the pCB6 vector (Brewer and Roth, 1991) and verified by DNA sequencing. Primer sequences will be provided on request.
Immunofluorescence Microscopy
Both surfaces of filter-grown cells were exposed to anti-endolyn mAb (25 μg/ml) for 1 h on ice. Filters were washed extensively before fixation and incubation with Cy3-conjugated goat anti-mouse antibody. Confocal images were collected using a Leica TCS SP system equipped with a 100× HCX PL-APO objective (numerical aperture 1.4) at a resolution of 1024 × 1024 pixels and a zoom of 2.0-3.0. Adobe Photoshop software (Adobe Systems, Mountain View, CA) was used for image processing.
Generation and Polarity of Endolyn Mutants
Generation of MDCK II stable transfectants expressing endolyn mutants was performed as described previously (Weisz et al., 1993; Ihrke et al., 2001). For most constructs, the polarity of at least two individual clones of drug-resistant cells was assessed. Domain selective biotinylation was performed as described previously (Weisz et al., 1993; Ihrke et al., 2001). Briefly, duplicate samples of filter-grown cells were starved in cys-free medium for 30 min, radiolabeled with [35Cys] for 2 h, and then chased for 15 min before biotinylation of the apical or basolateral cell surface on ice. The cells were solubilized and endolyn immunoprecipitated. Antibody-antigen complexes were eluted, and one-fifth was reserved to determine the total radiolabeled endolyn in each sample. The remaining four-fifths was incubated with streptavidin-agarose beads to recover biotinylated (surface) protein. After electrophoresis of the surface and total protein samples, the fraction of cell surface endolyn recovered from each apically or basolaterally biotinylated filter was determined by normalizing to the total endolyn recovered from that filter. The ratio of apical surface endolyn to total surface endolyn (apical plus basolateral) in each filter pair was then calculated.
Antibody uptake to assess vectorial delivery of newly synthesized endolyn and endolyn mutants to the cell surface, and determination of GPI-endolyn detergent insolubility was performed as described previously (Ihrke et al., 2001). Data were analyzed using Sigma Stat (SPSS Science, Chicago, IL); outlying data points were identified using Chauvenet's criterion.
RESULTS
Only a Subset of Endolyn's N-Glycans Is Required for Efficient Apical Sorting
Previously, we demonstrated that TM treatment dramatically disrupted endolyn polarity in MDCK cells (Ihrke et al., 2001). To examine the potential role of individual N-glycans in endolyn sorting, we generated MDCK cell lines stably expressing endolyn mutants in which one or two of the eight N-glycosylation consensus sequences were disrupted by alanine substitution for the respective asparagines(s) (Figure 1). To assess the loss of N-glycans, cells were radiolabeled for 3 h before solubilization and immunoprecipitation by using anti-endolyn mAb. To minimize oligosaccharide heterogeneity and facilitate the electrophoretic analysis, the O-glycosylation inhibitor BGN was included during the radiolabeling period. All of the mutants migrated distinctly from wild-type endolyn on SDS-PAGE, with the double mutations resulting in a larger shift than the single mutations, suggesting all eight possible glycosylation sites are normally used (Figure 1B).
Figure 1.
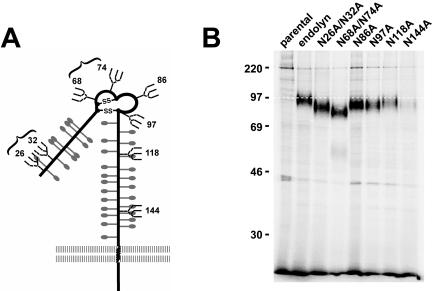
Characterization of endolyn N-glycosylation mutants. (A) Schematic of rat endolyn with predicted sites for N- and O-glycosylation indicated according to Ihrke et al. (2000). Numbers indicate the positions of asparagines in N-glycosylation consensus sequences; these sites were mutated individually or in pairs (as indicated by brackets) by substituting alanines for asparagines. (B) Analysis of endolyn glycosylation mutants by SDS-PAGE. MDCK cells expressing the indicated endolyn glycosylation mutants were radiolabeled for 3 h in the presence of BGN (to reduce oligosaccharide heterogeneity and thus provide sharper resolution of endolyn upon SDS-PAGE) and then solubilized and endolyn immunoprecipitated from lysates by using anti-endolyn mAb. The faster migration on SDS-PAGE of all mutants compared with wild-type endolyn (white dotted line) suggests that all eight consensus sequences are normally N-glycosylated.
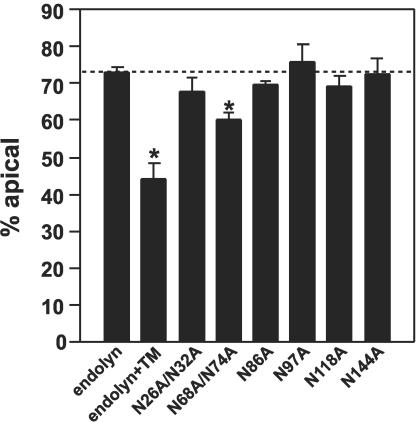
To determine the polarized distribution of these endolyn glycosylation mutants the corresponding cell lines were subjected to domain selective biotinylation after metabolic labeling for 2 h and a 15-min chase (Figure 2). Importantly, the fraction of each mutant protein biotinylated at the cell surface (apical and basolateral membranes combined) was similar to wild-type endolyn (with or without tunicamycin treatment), and generally ranged between 10 and 20% of the total with no statistical difference between any of the cell lines. This suggests that the mutations did not disrupt endolyn folding or cell surface delivery. Only one mutant (N68A/N74A) displayed a cell surface distribution distinct from that of wild-type endolyn (60 versus 73% apical for wild-type endolyn). Although the effect of this double mutation did not approach the effect of TM treatment on endolyn polarity (44% apical), the decreased polarity of this mutant was highly reproducible and statistically significant (p < 0.001). To further dissect the importance of the N-glycans at positions 68 and 74, we generated endolyn mutants in which these sites were altered individually (N68A and N74A, respectively). Whereas the polarity of N68A was slightly reduced relative to wild-type endolyn (64 versus 71% apical; p < 0.02, n = 21), the polarity of N74A was indistinguishable from wild type (71% apical, n = 19). Thus, within the context of otherwise fully glycosylated endolyn, the glycan at position 68 seems to be most critical for glycan-dependent apical sorting of the protein.
Figure 2.
Surface polarity of endolyn glycosylation mutants. Duplicate filters of polarized MDCK cells stably expressing wild-type endolyn or the indicated mutant proteins were radiolabeled for 2 h and chased for 15 min before domain-selective biotinylation. TM was included 30 min before and during the radiolabeling period in the indicated samples for cells expressing wild-type endolyn. The apical fraction of biotinylated endolyn in each apical/basolateral pair of filters is shown (mean % apical ± SEM; endolyn, n = 37; endolyn + TM, n = 11; N26A/N32A, n = 11; N68A/N74A, n = 30; N86A, n = 11; N97A, n = 8; N118A, n = 11; N144A, n = 4). *p < 0.001 versus endolyn by t test.
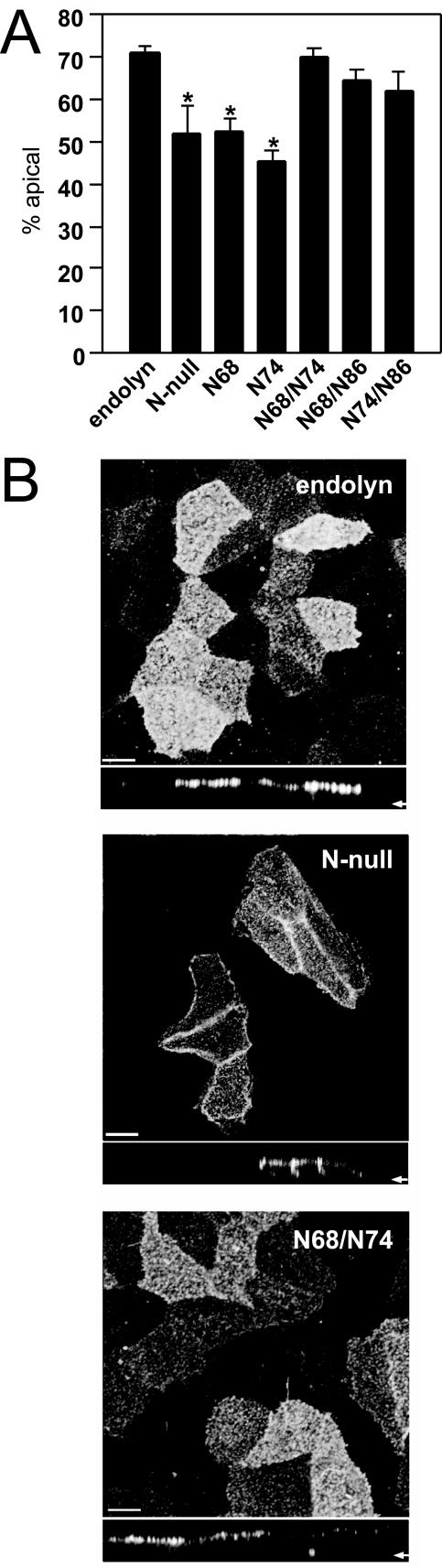
To further investigate the role of glycans at positions 68 and 74 in endolyn sorting, we generated an additional panel of endolyn mutants. The parent for these mutants was a construct we call N-null, in which all eight glycosylation sites were mutagenized. Importantly, the distribution of cell surface N-null was nonpolarized (Figure 3), similar to that of non-N-glycosylated endolyn in TM-treated cells (Figure 2). Thus, missorting of endolyn in tunicamycin-treated cells cannot be explained by a global effect on the apical sorting machinery. This is an important control, because a previous study demonstrated that tunicamycin treatment altered the polarity of chromogranin A, a proteoglycan that lacks N-glycans, in MDCK cells (Kuhn et al., 2000). Reconstruction of the N-glycosylation consensus sequences at either position 68 or 74 (N68 and N74, respectively) did not restore polarized sorting of endolyn. Strikingly, however, when both N-glycosylation consensus sequences were reintroduced (N68/N74), the resultant construct was sorted with polarity similar to that of wild-type endolyn (70 versus 71%), demonstrating that both glycans contribute to the sorting determinant (Figure 3A). Comparison of the N-null mutant with N68/N74 by indirect immunofluorescence after simultaneous binding of anti-endolyn mAb to both surface domains confirms their different distributions: whereas N68/N74 showed preferential apical binding of the antibody like wild-type endolyn, anti-endolyn mAb bound efficiently to both apical and basolateral membranes of cells expressing N-null (Figure 3B). We also tested, by surface biotinylation, whether different pairs of glycosylation sites in the nonmucin “knob” domain of endolyn, namely, N68/N86 or N74/N86, led to restored apical sorting (Figure 3A). Both combinations could partially substitute for N68/N74 with apical polarities of the corresponding mutants of 65 and 62%, respectively.
Figure 3.
Addition of two glycans to endolyn N-null mutant restores apical transport. (A) Filter-grown MDCK cells stably expressing wild-type endolyn, endolyn lacking all N-glycosylation consensus sequences (N-null), or containing one or two restored sites (N68, N74, and N68/N74) were subjected to domain-selective biotinylation as described in the legend to Figure 2. The surface polarity of endolyn is plotted (mean % apical ± SEM; endolyn, n = 22; N-null, n = 4; N68, n = 8; N74, n = 8; N68/N74, n = 12; N68/N86, n = 14; NN74/N86, n = 13). *p < 0.001 versus endolyn by t test. (B) Filter-grown endolyn, N-null, and 68/74-expressing cells were surface-labeled on ice with anti-endolyn mAb, fixed, and processed for indirect immunofluorescence and analyzed by confocal microscopy. An xy projection of multiple sections comprising the entire cell volume and a corresponding xz section are shown. The arrow marks the location of the filter in each xz section. Bar, 8 μm.
Role of Oligosaccharide Processing in Endolyn Sorting
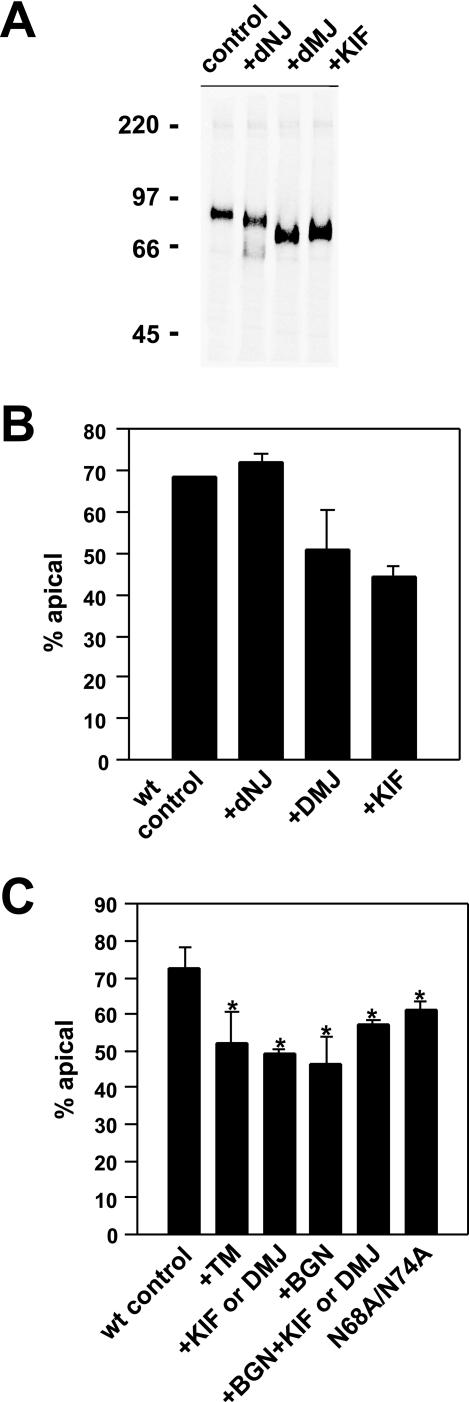
Because glycosylated proteins travel through the early biosynthetic pathway, the N-glycan precursors added en bloc to asparagine residues are trimmed by glucosidases and mannosidases. Glycan-dependent apical sorting of gp80 (a.k.a. clusterin), the major endogenous protein secreted by MDCK cells, has been shown to require core N-glycosylation but not subsequent processing of the N-glycans (Parczyk and Koch-Brandt, 1991; Wagner et al., 1995). Thus, we assessed the effects of various inhibitors of glycan processing on apical delivery of endolyn. DNJ inhibits glucose trimming of N-glycan core structures, whereas DMJ and KIF inhibit mannose trimming and interfere with further processing of the glycan on all branches, i.e., addition of N-acetylglucosamine, galactose, and sialic acid. Treatment with these drugs altered the mobility of endolyn on SDS-PAGE (Figure 4A), suggesting that N-glycan processing was disrupted in each case, although the effect of DNJ on endolyn mobility was minor and thus more difficult to observe than that of DMJ and KIF.
Figure 4.
Terminal sugars on N-glycans participate in apical sorting of endolyn. (A) Mobility of endolyn from DNJ-, DMJ-, or KIF-treated cells on SDS-PAGE. Endolyn-expressing cells were starved and radiolabeled for 2 h in the presence of DNJ, DMJ, or KIF, and chased for 15 min. Endolyn was immunoprecipitated from the cells after solubilization and analyzed on a 7.5% SDS-PAGE gel. (B) Effect of glycosylation perturbants on surface distribution of endolyn. Endolyn-expressing cells were starved and radiolabeled for 2 h in the presence of the indicated drugs and chased for 15 min before domain-selective biotinylation. The apical fraction of total biotinylated endolyn is plotted (average ± range of two experiments). (C) Effect of glycosylation perturbants on vectorial delivery of endolyn. Cells expressing wild-type endolyn or the N68A/N74A mutant were starved and radiolabeled for 20 min in the presence or absence of the indicated drugs and chased for 60 min at 37°C with anti-endolyn mAb added to either the apical or basolateral media of a pair of filters. After extensive washing, cells were solubilized and antibody-antigen complexes collected. The remaining endolyn was then immunoprecipitated from the samples, and the fraction of newly synthesized endolyn that reached the apical or basolateral membranes in each pair of filters was quantitated after SDS-PAGE. The fraction of endolyn accessible to apically added antibody (relative to total endolyn reaching the surface) is plotted (mean ± SEM; endolyn control, n = 9; + TM, n = 4; + either KIF or DMJ, n = 3; + BGN, n = 8; + BGN and either KIF or DMJ, n = 2 [average ± range]; N68A/N74A, n = 3). Because very similar effects were observed using KIF and dMJ, statistical analysis was performed on the combined data from cells treated with either of these drugs. *p < 0.005 versus endolyn by t test.
To determine the effects of these drugs on the apical polarity of endolyn, domain selective biotinylation experiments were performed (Figure 4B). Although these drugs affect endoplasmic reticulum (ER) export of some proteins, we observed no effect on the overall efficiency of transport of endolyn to the cell surface, consistent with our observations by using TM. Strikingly, both DMJ and KIF dramatically altered endolyn polarity; by contrast, DNJ treatment had no effect on endolyn polarity. The effects of DMJ and KIF were comparable with that observed in TM-treated cells or in the N-null mutant, suggesting that mannose trimming of endolyn's N-glycans (allowing subsequent processing of complex glycans) was critical for generating the apical sorting determinant.
To confirm that these drugs altered the vectorial delivery of endolyn to the apical plasma membrane, we used an antibody internalization assay designed to assess direct transport of newly synthesized endolyn to either cell surface domain in the biosynthetic pathway (Ihrke et al., 2001). Here, cells were radiolabeled over a brief period (20 min) and chased for 60 min in the presence of anti-endolyn mAb in either the apical or basolateral medium. As expected, treatment with TM, DMJ, or KIF disrupted apical sorting of newly synthesized endolyn (Figure 4C). We also confirmed that substitution of alanines for asparagines 68 and 74 resulted in significantly lower direct apical transport (62%), consistent with the compromised polarity of the corresponding mutant protein in our biotinylation experiments. In addition, we assessed the effect of BGN in this assay: this drug inhibits O-glycan processing but may also block galactose addition to N-glycans. BGN had a similar effect on endolyn polarity as DMJ, KIF, or TM. Importantly, the effect of BGN on endolyn polarity was not additive to KIF or DMJ, suggesting that BGN′s effect was not due to perturbation of O-glycan processing, but N-glycan processing. These results are consistent with the notion that terminal processing, rather than simply inhibition of mannose trimming, is important for formation of endolyn's N-glycan-dependent apical sorting determinant.
Polarized Sorting of Soluble and GPI-anchored Endolyn Is N-Glycan Independent
We previously demonstrated that Ensol, a truncated, soluble mutant of endolyn lacking a transmembrane domain, is efficiently secreted into the apical medium of polarized MDCK cells (Ihrke et al., 2001). Surprisingly, although Ensol mobility on SDS-PAGE was altered by TM treatment of stably expressing cell lines as expected, the polarity of Ensol secretion was unaffected by this drug (Figure 5). Thus, N-glycosylation-independent apical sorting of Ensol might reflect the presence of additional apical sorting information in the lumenal domain of endolyn. Treatment with BGN also had no effect on the polarity of Ensol secretion (our unpublished data).
The difference between apical sorting of transmembrane and secreted forms of endolyn in respect to their dependence on glycans could be explained in two ways: The tyrosine-containing motif in the cytoplasmic tail of endolyn, which can function as a basolateral sorting signal, might supercede recognition of redundant apical info and redirect non-N-glycosylated molecules basolaterally. Alternatively, the soluble form of endolyn might be sorted via a mechanism entirely distinct from that of wild-type endolyn. To distinguish between these possibilities, we examined whether polarity of a transmembrane form of endolyn lacking a functional basolateral sorting signal (EEEYA, a mutant in which the critical tyrosine is substituted by alanine; Ihrke et al., 2001) was compromised by perturbation of N-glycans (Figure 6). Interestingly, EEEYA polarity was sensitive to TM treatment, as well as to perturbation of terminal N-glycan processing by KIF treatment (Figure 6). The discrepant polarity between fully N-glycosylated and non-N-glycosylated forms of EEEYA was striking under conditions where both forms were simultaneously expressed, i.e., when cells were radiolabeled after relatively short (30 min) pretreatment with TM (Figure 6B). These results suggest that the nonpolarized distribution of non-N-glycosylated endolyn and EEEYA does not depend on a functional basolateral sorting signal, and by inference, that Ensol is sorted apically via a distinct, glycan-independent mechanism unavailable to transmembrane forms of endolyn.
We next examined whether GPI-anchored endolyn requires N-glycosylation for apical sorting. A mutant expressing GPI-endolyn was constructed by fusing the C-terminal signal sequence of 5′-nucleotidase providing a GPI-anchor attachment site to the luminal domain of endolyn (see MATERIALS AND METHODS). Indirect immunofluorescence of surface-labeled polarized MDCK cells stably expressing GPI-endolyn demonstrated a predominant apical distribution of the cell surface protein (Figure 7A). Domain-selective biotinylation of radiolabeled GPI-endolyn (Figure 7C) and antibody internalization experiments after brief metabolic labeling (our unpublished data) confirmed that the protein was delivered primarily to the apical cell surface. Unlike wild-type endolyn (Ihrke et al., 2001) and EEEYA, which are almost completely extracted from cells by Triton X-100, GPI-endolyn was partly insoluble in cold Triton X-100, similar to virally introduced influenza hemagglutinin (included as an positive control as this protein is known to have this characteristic; Figure 7B). This resistance to extraction by cold Triton X-100 is a hallmark of GPI-anchored proteins that reflects their association with glycolipid-enriched microdomains (Brown and Rose, 1992). To determine the role of N-glycans in GPI-endolyn sorting, stably expressing polarized MDCK cells were pretreated with glycosylation perturbants, and the distribution of radiolabeled GPI-endolyn analyzed by using domain-selective biotinylation (Figure 7, C and D). None of the perturbants tested, including TM (Figure 7, C and D), KIF (Figure 7C), or BGN (our unpublished data) disrupted the polarity of GPI-endolyn delivery. Thus, similar to the soluble form, apical sorting of GPI-linked endolyn seems to occur independently of the presence of N-glycans.
Finally, we tested whether the mode of anchoring, i.e., a transmembrane anchor in general, or the presence of endolyn-specific transmembrane or cytoplasmic sequences, were additional requirements for glycan-dependent apical sorting. To address this, we examined the targeting and sensitivity to tunicamycin treatment of two chimeric proteins in stably expressing polarized MDCK cells. In one of these chimeras, ECEYA, the transmembrane domain of EEEYA was replaced with that of CD8α, an O-glycosylated plasma membrane protein. In the other chimera, EECΔ, the cytoplasmic domain of endolyn was replaced with a “neutral” cytoplasmic tail consisting of the first 10 amino acids of the CD8α tail. Both of these proteins were targeted apically with a polarity similar to that of wild-type endolyn (70.3% apical for EECΔ; 73.7% for ECEYA; n = 6), and treatment with TM disrupted their apical delivery (54.7 and 49.8% apical, respectively; n = 5; p < 0.05 versus untreated controls.) Treatment with KIF also disrupted the polarized sorting of these mutants (our unpublished data). Thus, N-glycans in endolyn's luminal domain are required apical sorting determinants uniquely in the context of a transmembrane protein, but the underlying sorting mechanism does not depend on specific sequences in the transmembrane or cytoplasmic domain of endolyn.
DISCUSSION
We previously found that the sialomucin endolyn is uniquely trafficked to lysosomes via the apical surface of polarized MDCK cells. A dominant N-glycan-dependent apical sorting signal was localized to the lumenal domain of endolyn. Here, we have further characterized the N-glycosylation requirement for apical sorting of endolyn, revealing specificity both in the position of glycans involved and in glycan structure. The amino acid sequence of endolyn contains eight potential sites for N-glycosylation, all of which seem to be glycosylated in vivo. Of these eight, we have identified two specific sites located within a putative disulfide-bonded loop domain that are necessary and sufficient for efficient apical delivery. Proper sorting of newly synthesized endolyn requires terminal processing of endolyn's N-glycans rather than simply the presence of core N-glycans, suggesting that the terminal sugars of these glycans are an integral part of the apical targeting signal. Surprisingly, however, apical sorting of both soluble and GPI-anchored forms of endolyn was found to be N-glycan independent. These results suggest that closely related apically destined proteins can be efficiently sorted via distinct, nonredundant mechanisms.
Specific N-Glycosylation Sites on Endolyn Are Required for Apical Sorting of Endolyn along the Biosynthetic Pathway
When we examined the polarity of mutant endolyn proteins with one or two missing N-glycosylation sites, only one mutant, N68A/N74A, was significantly less polarized than wild-type endolyn (∼60 versus ∼72% apical), and another mutant with the single substitution N68A exhibited intermediate polarity (∼64% apical). This effect on polarity was observed both by domain selective biotinylation and in antibody uptake experiments in cells that were metabolically labeled over shorter periods, demonstrating that the missing N-glycans disrupt direct delivery of newly synthesized endolyn to the apical membrane as opposed to subsequent trafficking of the protein. A mutant that contained intact glycosylation sites only at positions 68 and 74 was correctly sorted to the apical domain. This suggests that N-glycans in these positions are sufficient to mediate apical targeting and thus are probably the major sorting determinants in the fully glycosylated protein in vivo. Neither N68 nor N74 alone was sufficient to confer apical sorting, indicating that both N-glycans are necessary. However, the effect of the N68A/N74A double mutation on polarity was less dramatic than that observed for endolyn in tunicamycin-treated cells or for the N-null mutant in which all asparagines of putative glycosylation sites were substituted by alanines. This partial effect may reflect the ability of the remaining N-glycans to compensate for the loss of N-glycans at positions 68 and 74. In fact, in the context of the N-null background, reconstruction of pairs of N-glycosylation consensus sequences at positions 68 and 86 or 74 and 86 resulted in preferential apical sorting of the corresponding proteins, although the fidelity of sorting seemed to be somewhat lower compared with the N68/N74 mutant.
In contrast to some previously described apical proteins whose exit from the TGN was blocked by inhibition of N-glycosylation (Gut et al., 1998), endolyn mutants lacking N-glycans were not retained in the TGN. We interpret our results to indicate that apical targeting of endolyn requires N-glycans not as a general structural support to allow a “transport-permissive” conformation of the protein, but as a sorting determinant that enables specific interactions with the sorting machinery.
Terminal Processing of N-Glycans Is Important for Apical Delivery of Endolyn
We observed that only drugs that block mannose trimming and further processing of N-glycans (KIF and DMJ), but not a drug that inhibits glucose trimming (DNJ), resulted in nonpolarized cell surface delivery of endolyn. N-glycan processing to form complex oligosaccharides can occur in the absence of endogenous glucosidase activity or in DNJ-treated cells (Matter et al., 1989; Fujimoto and Kornfeld, 1991). This suggests that the terminal glycans on endolyn expressed under these conditions can form the necessary sorting determinant, whereas this is not possible when terminal processing is completely abrogated. Our results using glycosylation inhibitors are in contrast to those published for the primary secreted protein in MDCK cells, gp80 (a.k.a. clusterin), whose proper apical delivery requires the addition of core oligosaccharides but not further processing of N-glycans (Parczyk and Koch-Brandt, 1991; Wagner et al., 1995). This discrepancy points toward differences in the N-glycan-dependent sorting of individual proteins or secreted and transmembrane proteins in general.
We also observed that treatment with BGN, a drug that scavenges UDP-galactose and thus prevents its addition to oligosaccharide chains, significantly disrupted polarized delivery of newly synthesized endolyn. This overrides our earlier results showing that acute (2-h) treatment with BGN does not affect the polarized distribution of endolyn (Ihrke et al., 2001). The discrepancy between these results is likely due to the mixing of preexisting (fully glycosylated) recycling endolyn pools with newly synthesized molecules at the apical plasma membrane (Bruns and Weisz, unpublished data), such that short-term drug treatment is insufficient to alter the glycosylation profile of the majority of cell surface endolyn. The effects of BGN treatment on the surface distribution of sucrase-isomaltase were previously interpreted to reflect a requirement for O-glycan processing in the apical sorting of this protein (Alfalah et al., 1999). However, this drug has also been shown to block terminal processing of N-glycans in some cell lines (Gouyer et al., 2001). Treatment with both BGN and DMJ or KIF disrupted endolyn sorting to the same extent as either drug alone, consistent with a common inhibitory mechanism. We conclude that the effect of BGN on endolyn polarity is most likely due to its effects on N-glycosylation rather than to an additional role for O-glycosylation in endolyn sorting, although the latter cannot be ruled out completely.
Differential Sorting Requirements for Soluble and GPI-anchored Endolyn
N-glycans were initially implicated as sorting signals for gp80 and glycosylated human growth hormone, both of which are soluble proteins (Parczyk and Koch-Brandt, 1991; Scheiffele et al., 1995). Thus, we were surprised to find that apical delivery of a soluble form of endolyn, Ensol, was completely insensitive to treatment with TM. The N-glycan-independent delivery of Ensol could be due to the unmasking of a recessive apical sorting signal upon removal of endolyn's cytoplasmic tail, which contains a tyrosine-dependent basolateral sorting motif (Ihrke et al., 2001). In this scenario, the nonpolarized sorting we observed for TM-treated endolyn or the N-null mutant would reflect the competition between cytoplasmic basolateral sorting information and residual lumenal apical targeting information in these proteins. However, the polarity of EEEYA, a full-length construct in which this critical tyrosine residue was mutated to alanine, was disrupted by cell treatment with TM or KIF to an extent equal to that observed for wild-type endolyn. This suggests that the apical sorting determinant responsible for glycan-independent secretion of Ensol is absent or inaccessible in EEEYA and wild-type endolyn. Indeed, other studies suggest that soluble and transmembrane proteins can be sorted via distinct mechanisms; for example, trafficking of soluble and membrane proteins is differentially sensitive to perturbation of microtubule structure and pH (Caplan et al., 1987; Boll et al., 1991).
We also found that apical delivery of a GPI-anchored form of endolyn is N-glycan independent. The ability of GPI-anchors to function as apical sorting signals is currently controversial (Lisanti et al., 1989; Benting et al., 1999; Lipardi et al., 2000; Paladino et al., 2002). Early studies demonstrated that addition of a GPI-attachment signal to herpes simplex glycoprotein D or to human growth hormone resulted in apical delivery (Lisanti et al., 1989). It has been hypothesized that the GPI-lipid anchor facilitates the inclusion of proteins into detergent-insoluble glycosphingolipid-enriched membrane domains that are selectively trafficked to the apical surface. However, a recent study, which showed that GPI-anchored proteins lacking N-glycans are not always apically targeted, has challenged the role of GPI-anchors as autonomous apical targeting signals (Benting et al., 1999). In the case of endolyn, the addition of a GPI-anchor undoubtedly facilitates apical sorting in the absence of N-glycans; however, it is possible that other additional apical targeting information is also needed. This determinant could be identical to the information that directs soluble endolyn to the apical surface. At this stage the nature of such “recessive” apical information is unclear; it could consist of residual O-glycans, a weak peptide motif, and/or depend on a receptor-independent mechanism, such as aggregation of cargo mediated by the lumenal domain of endolyn.
Mechanisms for Glycan-dependent Sorting
The mechanism by which N-glycans mediate apical sorting is not known. In one of the two general models that have been proposed, N-glycans are required to stabilize transport permissive conformations of proteins (Rodriguez-Boulan and Gonzalez, 1999). Our results are more compatible with the second proposed mechanism, i.e., that N-glycans are required for protein binding to a cargo receptor in the TGN that concentrates the protein into apically destined vesicles. There is in fact precedence for lectins that function as cargo receptors in the biosynthetic pathway (Hauri et al., 2000; Hara-Kuge et al., 2002). One such lectin, VIP36, has been suggested to act as a receptor for soluble and membrane proteins with high-mannose-type N-glycans, including gp80 (Hara-Kuge et al., 2002) and is therefore an unlikely candidate to bind endolyn or other proteins that rely on complex N-glycans for sorting. Thus, rather than a single receptor recognizing all proteins that require N-glycans for apical sorting, there may be several such binding proteins.
Our observation that two of endolyn's eight N-glycans are critical for efficient apical sorting suggests that there are specific structural requirements for recognition of the apical sorting determinant. Similarly, it was previously demonstrated that only one of the three N-glycans on erythropoietin, a secreted apical protein whose distribution is perturbed by tunicamycin, is critical for its apical targeting (Kitagawa et al., 1994). The key N-glycans identified for endolyn and erythropoietin sorting may be critical because they possess oligosaccharide structures that are selectively recognized by a lectin receptor or they may stabilize a proteinaceous motif that binds to a sorting receptor. The N-glycans at positions 68 and 74 of endolyn are located on a predicted disulfide-bonded loop that carries no or few O-glycans. This part of the protein is likely to have a well-defined secondary structure, different from the mucin-like regions that constitute much of the remaining lumenal domain. Moreover, these N-glycans and several other amino acids in this region are well conserved among species and could therefore serve as a distinct structural sorting determinant. It is important to keep in mind that complex N-glycans are bulky carbohydrate clusters that can reach more than 3 nm from the protein surface and that their outer parts can therefore act as independent domains of glycoproteins (Helenius and Aebi, 2001). Thus, terminal sugars could be part of an epitope recognized by a sorting receptor, or they alone could create the crucial epitope.
Glycans and Endolyn Function
Glycans have previously been implicated in the function of endolyn/CD164. A mAb recognizing an N- and O-glycan-dependent epitope on the N-terminal mucin domain of human CD164 reduced the adhesion of hematopoietic progenitor cells to bone marrow stroma and negatively regulated cell proliferation in vitro (Zannettino et al., 1998; Doyonnas et al., 2000). This observation is consistent with the paradigm that specific sugar modifications are essential for the interaction of sialomucins with their extracellular binding partners (Varki, 1997). Assuming that similar principles operate in human and rat, the N-glycans involved in apical targeting and those possibly involved in extracellular protein-protein interactions do not seem to be the same. The amino-terminal epitopes of human CD164 are differentially expressed in various tissues (Watt et al., 2000). We do not yet know whether the N-glycans (or their specific modifications) important for apical sorting in polarized MDCK cells are ubiquitously expressed. Because the structure of N- and O-glycans is developmentally regulated (Trugnan et al., 1987; Youakim et al., 1989), it is possible that the terminal carbohydrates on these N-glycans are not always processed equivalently. Thus, glycan processing may be one of the modifications by which the trafficking of endolyn/CD164 is regulated to adjust its distribution between cell surface and endosomal/lysosomal compartments according to the functional requirements for this protein at different stages during tissue development.
Acknowledgments
We thank J. Paul Luzio for support and for critically reading the manuscript. This project was funded by National Institutes of Health grant DK-54407 to O.A.W. K.M.W. was supported by National Institutes of Health grant T32-DK61296. G.I. was supported by Wellcome Trust project grants 057263 and 067206 to J. Paul Luzio and G.I., respectively. The Laboratory of Epithelial Cell Biology is supported in part by Dialysis Clinic Inc. The Cambridge Institute for Medical Research is in receipt of a strategic award from the Wellcome Trust.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-08-0550. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-08-0550.
Abbreviations used: BGN, benzyl 2-acetamido-2-deoxy-α-d-galactopyranoside; DMJ, deoxymannojirimycin; DNJ, deoxynojirimycin; GPI, glycosylphosphatidylinositol; KIF, kifunensine; mAb, monoclonal antibody; MDCK, Madin-Darby canine kidney; TGN, trans-Golgi network; TM, tunicamycin.
References
- Alfalah, M., Jacob, R., Preuss, U., Zimmer, K.P., Naim, H., and Naim, H.Y. (1999). O-Linked glycans mediate apical sorting of human intestinal sucrase-isomaltase through association with lipid rafts. Curr. Biol. 9, 593-596. [DOI] [PubMed] [Google Scholar]
- Benting, J.H., Rietveld, A.G., and Simons, K. (1999). N-Glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells. J. Cell Biol. 146, 313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll, W., Partin, J.S., Katz, A.I., Caplan, M.J., and Jamieson, J.D. (1991). Distinct pathways for basolateral targeting of membrane and secretory proteins in polarized epithelial cells. Proc. Natl. Acad. Sci. USA 88, 8592-8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino, J.S., and Traub, L.M. (2003). Signals for Sorting of Transmembrane Proteins to Endosomes and Lysosomes. Annu. Rev. Biochem. 72, 395-447. [DOI] [PubMed] [Google Scholar]
- Brewer, C.B., and Roth, M.G. (1991). A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J. Cell Biol. 114, 413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D.A., and Rose, J.K. (1992). Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68, 533-544. [DOI] [PubMed] [Google Scholar]
- Caplan, M.J., Stow, J.L., Newman, A.P., Madri, J., Anderson, H.C., Farquhar, M.G., Palade, G.E., and Jamieson, J.D. (1987). Dependence on pH of polarized sorting of secreted proteins. Nature 329, 632-635. [DOI] [PubMed] [Google Scholar]
- Chan, J.Y., Lee-Prudhoe, J.E., Jorgensen, B., Ihrke, G., Doyonnas, R., Zannettino, A.C., Buckle, V.J., Ward, C.J., Simmons, P.J., and Watt, S.M. (2001). Relationship between novel isoforms, functionally important domains, and subcellular distribution of CD164/endolyn. J. Biol. Chem. 276, 2139-2152. [DOI] [PubMed] [Google Scholar]
- Croze, E., Ivanov, I.E., Kreibich, G., Adesnik, M., Sabatini, D.D., and Rosenfeld, M.G. (1989). Endolyn-78, a membrane glycoprotein present in morphologically diverse components of the endosomal and lysosomal compartments: implications for lysosome biogenesis. J. Cell Biol. 108, 1597-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyonnas, R., Yi-Hsin Chan, J., Butler, L.H., Rappold, I., Lee-Prudhoe, J.E., Zannettino, A.C., Simmons, P.J., Buhring, H.J., Levesque, J.P., and Watt, S.M. (2000). CD164 monoclonal antibodies that block hemopoietic progenitor cell adhesion and proliferation interact with the first mucin domain of the CD164 receptor. J. Immunol. 165, 840-851. [DOI] [PubMed] [Google Scholar]
- Folsch, H., Pypaert, M., Schu, P., and Mellman, I. (2001). Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol. 152, 595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto, K., and Kornfeld, R. (1991). alpha-Glucosidase II-deficient cells use endo alpha-mannosidase as a bypass route for N-linked oligosaccharide processing. J. Biol. Chem. 266, 3571-3578. [PubMed] [Google Scholar]
- Gouyer, V., Leteurtre, E., Zanetta, J.P., Lesuffleur, T., Delannoy, P., and Huet, G. (2001). Inhibition of the glycosylation and alteration in the intracellular trafficking of mucins and other glycoproteins by GalNAcalpha-O-bn in mucosal cell lines: an effect mediated through the intracellular synthesis of complex GalN.Acalpha-O-bn oligosaccharides. Front. Biosci. 6, D1235-D1244. [DOI] [PubMed] [Google Scholar]
- Gut, A., Kappeler, F., Hyka, N., Balda, M.S., Hauri, H.P., and Matter, K. (1998). Carbohydrate-mediated Golgi to cell surface transport and apical targeting of membrane proteins. EMBO J. 17, 1919-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Kuge, S., Ohkura, T., Ideo, H., Shimada, O., Atsumi, S., and Yamashita, K. (2002). Involvement of VIP36 in intracellular transport and secretion of glycoproteins in polarized Madin-Darby canine kidney (MDCK) cells. J. Biol. Chem. 277, 16332-16339. [DOI] [PubMed] [Google Scholar]
- Hauri, H., Appenzeller, C., Kuhn, F., and Nufer, O. (2000). Lectins and traffic in the secretory pathway. FEBS Lett. 476, 32-37. [DOI] [PubMed] [Google Scholar]
- Helenius, A., and Aebi, M. (2001). Intracellular functions of N-linked glycans. Science 291, 2364-2369. [DOI] [PubMed] [Google Scholar]
- Ihrke, G., Bruns, J.R., Luzio, J.P., and Weisz, O.A. (2001). Competing sorting signals guide endolyn along a novel route to lysosomes in MDCK cells. EMBO J. 20, 6256-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrke, G., Gray, S.R., and Luzio, J.P. (2000). Endolyn is a mucin-like type I membrane protein targeted to lysosomes by its cytoplasmic tail. Biochem. J. 345, 287-296. [PMC free article] [PubMed] [Google Scholar]
- Ihrke, G., Martin, G.V., Shanks, M.R., Schrader, M., Schroer, T.A., and Hubbard, A.L. (1998). Apical plasma membrane proteins and endolyn-78 travel through a subapical compartment in polarized WIF-B hepatocytes. J. Cell Biol. 141, 115-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen, E., and Simons, K. (1998). Protein and lipid sorting from the trans-Golgi network to the plasma membrane in polarized cells. Semin. Cell Dev. Biol. 9, 503-509. [DOI] [PubMed] [Google Scholar]
- Kitagawa, Y., Sano, Y., Ueda, M., Higashio, K., Narita, H., Okano, M., Matsumoto, S., and Sasaki, R. (1994). N-glycosylation of erythropoietin is critical for apical secretion by Madin-Darby canine kidney cells. Exp. Cell Res. 213, 449-457. [DOI] [PubMed] [Google Scholar]
- Kuhn, U., Cohn, D.V., and Gorr, S.U. (2000). Polarized secretion of the regulated secretory protein chromogranin A. Biochem. Biophys. Res. Commun. 270, 631-636. [DOI] [PubMed] [Google Scholar]
- Lee, Y.N., Kang, J.S., and Krauss, R.S. (2001). Identification of a role for the sialomucin CD164 in myogenic differentiation by signal sequence trapping in yeast. Mol. Cell. Biol. 21, 7696-7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipardi, C., Nitsch, L., and Zurzolo, C. (2000). Detergent-insoluble GPI-anchored proteins are apically sorted in Fischer rat thyroid cells, but interference with cholesterol or sphingolipids differentially affects detergent insolubility and apical sorting. Mol. Biol. Cell 11, 531-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti, M.P., Caras, I.W., Davitz, M.A., and Rodriguez-Boulan, E. (1989). A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J. Cell Biol. 109, 2145-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter, K., McDowell, W., Schwartz, R.T., and Hauri, H.P. (1989). Asynchronous transport to the cell surface of intestinal brush border hydrolases is not due to differential trimming of N-linked oligosaccharides. J. Biol. Chem. 264, 13131-13139. [PubMed] [Google Scholar]
- Naim, H.Y., Joberty, G., Alfalah, M., and Jacob, R. (1999). Temporal association of the N- and O-linked glycosylation events and their implication in the polarized sorting of intestinal brush border sucrase-isomaltase, aminopeptidase N, and dipeptidyl peptidase IV. J. Biol. Chem. 274, 17961-17967. [DOI] [PubMed] [Google Scholar]
- Nelson, W.J., and Yeaman, C. (2001). Protein trafficking in the exocytic pathway of polarized epithelial cells. Trends Cell Biol. 11, 483-486. [DOI] [PubMed] [Google Scholar]
- Paladino, S., Sarnataro, D., and Zurzolo, C. (2002). Detergent-resistant membrane microdomains and apical sorting of GPI-anchored proteins in polarized epithelial cells. Int. J. Med. Microbiol. 291, 439-445. [DOI] [PubMed] [Google Scholar]
- Parczyk, K., and Koch-Brandt, C. (1991). The role of carbohydrates in vectorial exocytosis. The secretion of the gp 80 glycoprotein complex in a ricin-resistant mutant of MDCK cells. FEBS Lett. 278, 267-270. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan, E., and Gonzalez, A. (1999). Glycans in post-Golgi apical targeting: sorting signals or structural props? Trends Cell Biol. 9, 291-294. [DOI] [PubMed] [Google Scholar]
- Scheiffele, P., and Fullekrug, J. (2000). Glycosylation and protein transport. Essays Biochem. 36, 27-35. [DOI] [PubMed] [Google Scholar]
- Scheiffele, P., Peranen, J., and Simons, K. (1995). N-glycans as apical sorting signals in epithelial cells. Nature 378, 96-98. [DOI] [PubMed] [Google Scholar]
- Simmen, T., Honing, S., Icking, A., Tikkanen, R., and Hunziker, W. (2002). AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat. Cell Biol. 4, 154-159. [DOI] [PubMed] [Google Scholar]
- Trugnan, G., Rousset, M., Chantret, I., Barbat, A., and Zweibaum, A. (1987). The posttranslational processing of sucrase-isomaltase in HT-29 cells is a function of their state of enterocytic differentiation. J. Cell Biol. 104, 1199-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki, A. (1997). Selectin ligands: will the real ones please stand up? J. Clin. Investig. 99, 158-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, M., Morgans, C., and Koch-Brandt, C. (1995). The oligosaccharides have an essential but indirect role in sorting gp80 (clusterin, TRPM-2) to the apical surface of MDCK cells. Eur. J. Cell Biol. 67, 84-88. [DOI] [PubMed] [Google Scholar]
- Watt, S.M., et al. (2000). Functionally defined CD164 epitopes are expressed on CD34(+) cells throughout ontogeny but display distinct distribution patterns in adult hematopoietic and nonhematopoietic tissues. Blood 95, 3113-3124. [PubMed] [Google Scholar]
- Weisz, O.A., Swift, A.M., and Machamer, C.E. (1993). Oligomerization of a membrane protein correlates with its retention in the Golgi complex. J. Cell Biol. 122, 1185-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman, C., Le Gall, A.H., Baldwin, A.N., Monlauzeur, L., Le Bivic, A., and Rodriguez-Boulan, E. (1997). The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J. Cell Biol. 139, 929-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youakim, A., Romero, P.A., Yee, K., Carlsson, S.R., Fukuda, M., and Herscovics, A. (1989). Decrease in polylactosaminoglycans associated with lysosomal membrane glycoproteins during differentiation of CaCo-2 human colonic adenocarcinoma cells. Cancer Res. 49, 6889-6895. [PubMed] [Google Scholar]
- Zannettino, A.C., Buhring, H.J., Niutta, S., Watt, S.M., Benton, M.A., and Simmons, P.J. (1998). The sialomucin CD164 (MGC-24v) is an adhesive glycoprotein expressed by human hematopoietic progenitors and bone marrow stromal cells that serves as a potent negative regulator of hematopoiesis. Blood 92, 2613-2628. [PubMed] [Google Scholar]