Abstract
Rationale and objectives
Previously, Albu-CocH, a cocaine hydrolase derived from human butyrylcholinesterase, blocked cocaine-induced reinstatement of drug seeking in rats. In the present study, rats were treated with Albu-CocH while self-administering cocaine under a progressive ratio (PR) schedule during 2-h sessions and under a fixed-ratio 1 (FR 1) schedule during 6-h sessions.
Methods
In experiment 1, rats were treated with saline or Albu-CocH (2 or 4 mg/kg) before a single 2-h cocaine (0.2 mg/kg) self-administration (PR) session. In experiment 2, rats were treated with Albu-CocH or saline for the first seven of the 21-day 6-h sessions prior to cocaine (0.2 or 0.4 mg/kg) self-administration sessions (FR 1).
Results
In experiment 1, Albu-CocH (vs saline) reduced cocaine infusions immediately following treatment compared with sessions pretreatment and posttreatment. In experiment 2, the Albu-CocH-treated groups (vs saline) showed an initial twofold to threefold increase in 0.2 and 0.4 mg/kg cocaine infusions over the 7 days of treatment, but they decreased to the infusion levels of saline controls by day 7. Cocaine (0.4 mg/kg) intake in the saline-treated group was elevated during the last 3 days of 6-h access compared with the first 3 days, indicating an escalation effect. Responding for 0.4 mg/kg (but not 0.2 mg/kg) cocaine during 2-h sessions after the 21 days of 6-h access was elevated in the saline groups (compared with 2-h sessions before long access) but not in the Albu-CocH-treated groups.
Conclusions
Albu-CocH decreased cocaine infusions under the PR schedule, indicating a reduced reward value of cocaine (experiment 1). However, Albu-CocH, compared with saline, temporarily increased cocaine infusions during long access. The post-long access 2-h cocaine intake was not increased in the Albu-CocH-treated groups as it was in the saline-treated groups. Albu-CocH is an effective agent for reducing cocaine reward under conditions of low cocaine exposure and chronic treatment.
Keywords: Cocaine self-administration, Cocaine hydrolase, Escalation, Maintenance, PR schedule, Rats
Introduction
Cocaine abuse and dependence and its toxic sequelae continue to result in adverse social and medical consequences (Devlin and Henry 2008; Substance Abuse and Mental Health Services Administration 2009). A number of creative approaches to this devastating disorder are in progress to solve these problems, including novel immunotherapies and medications (e.g., Dickerson and Janda 2005; Sofuoglu and Kosten 2005; Vocci et al. 2005); however, currently, there are no approved effective treatments for cocaine abuse. One strategy under consideration is to use protein-based agents that alter the pharmacokinetics of cocaine in a favorable manner (Collins et al. 2009; Gao et al. 2008; Gao and Brimijoin 2009; Gorelick 2008). Following that approach, enzymes that rapidly metabolize cocaine have been shown to reduce cocaine’s entry into the brain and prevent cocaine-primed drug seeking (Brimijoin et al. 2008) as well as toxicity (Collins et al. 2009; Gao et al. 2008; Ko et al. 2009).
In previous studies from our laboratories, a cocaine hydrolase engineered from human butyrylcholinesterase (BChE), Albu-CocH, selectively protected rats from cocaine toxicity (seizures) (Gao et al. 2008), rescued them from ongoing seizures, and blocked cocaine-induced reinstatement of drug seeking in rats (Brimijoin et al. 2008). Blocking reinstatement of cocaine-seeking behavior by Albu-CocH was selective for cocaine-primed reinstatement, as the treatment did not alter spontaneous locomotor behavior, food-reinforced operant behavior, or the reinstatement of cocaine seeking after injection of amphetamine which is not metabolized by the enzyme (Brimijoin et al. 2008). Similar protective and rescuing abilities have been reported for “CocE”, a bacterial-based cocaine esterase (Bresler et al. 2000; Collins et al. 2009; Cooper et al. 2006; Ko et al. 2009) that also suppressed cocaine-reinforced responding in a dose-dependent manner (Collins et al. 2009).
The present study examined the effects of Albu-CocH on other forms of cocaine-seeking behavior, such as cocaine self-administration under a progressive ratio (PR) schedule and the escalation of cocaine intake when long access to the drug is allowed. Behavioral models were selected for the present study to evaluate the effect of Albu-CocH on two aspects of drug-seeking behavior that have not yet been examined. First, we used a PR schedule that is thought to provide an unambiguous determination of effects on the motivation to self-administer a drug such as cocaine (e.g., Paterson and Markou 2003; Wee et al. 2008). With simple fixed ratio (FR 1) schedules, decreased responding can indicate that reinforcing effectiveness (motivation) has decreased or increased (less drug needed to achieve a desired effect), but in animals it is difficult to determine which effect is in place. A PR schedule imposes an increased response requirement for each successive infusion until responses cease, and the final response output is used as an index of the drug’s reinforcing effectiveness.
The second model assessed the escalation of cocaine self-administration using a long-access FR 1 schedule. The escalation procedure is used to model the transition from steady to dysregulated drug consumption that characterizes a critical phase of the drug abuse process (Ahmed and Koob 1998, 1999, 2005; Kalivas and Volkow 2005). In humans, escalation is a process that leads to bingeing on drugs and is linked to overdose and death (Kalivas and Volkow 2005). In animal models, escalation of intake has been shown to occur with several drugs of abuse (Ahmed and Koob 1998, 1999; Carroll et al. 2005; Kalivas and Volkow 2005; Kitamura et al. 2006; Roth and Carroll 2004). This effect is typically achieved by changing the time of drug availability from short (e.g., 1–3 h) to long (e.g., 6–12 h) access (Anker et al. 2009, 2010; Carroll et al. 2005; Fitch and Roberts 1993; Larson et al. 2007; Lenoir and Ahmed 2008; Lynch et al. 2000; Lynch and Carroll 2001; Morgan and Roberts 2004; Perry et al. 2006; Roth and Carroll 2004). Self-administration of drugs such as cocaine (Ahmed and Koob 1999), heroin (Lenoir and Ahmed 2008), or methamphetamine (Kitamura et al. 2006) during long access (e.g., 6–12 h) tends to increase over time. When escalation of drug intake occurs during long access, the elevation in drug intake usually persists later when short access is resumed, compared with intake when drug was available during short access before the long-access phase (e.g., Paterson and Markou 2003; Wee et al. 2008). Thus, after long access in the present study (exp 2), the rats were returned to 2-h access, and infusions were compared to the initial infusions during short access.
Initially, a 0.4 mg/kg cocaine self-administration dose was used, as work with the long-access procedure in this and other laboratories (Ahmed and Koob 1999) indicated there was a narrow dose range for modeling escalation of cocaine intake in rats, and this dose produced escalation without toxic effects. However, the experiment was subsequently repeated with a lower cocaine dose (0.2 mg/kg), as initial results suggested that rats attempted to surmount the effect of Albu-CocH by self-administering more of the 0.4 mg/kg cocaine dose. Thus, the purpose of using a lower self-administration dose was to determine whether Albu-CocH would suppress escalation of cocaine intake when there was less cocaine available to counter the enzyme’s effects.
Methods
Subjects
Thirty-eight experimentally naïve female Wistar rats (Harlan-Sprague Dawley, Madison, WI) (>90 days old), weighing an average of 250 g at onset of the study, were used. Female rats were used to compare results with previous studies because our initial work on the effects of Albu-CocH on cocaine seeking was conducted with females (Brimijoin et al. 2008), and the supply of enzyme was too limited to compare sexes. The rats were divided into two experiments; experiment 1 (n=6) was a within-subjects PR study, and experiment 2 (n=32) was a between-subjects long-access (6 h) cocaine self-administration (FR 1) study. Before surgical cannulation, the rats were pair-housed in plastic cages in a vivarium with access to food (Purina Laboratory Chow, Purina Mills, Minneapolis, MN) and water. The housing rooms were maintained at 24°C and 40–50% humidity under a 12/12-h light/dark cycle with lights on at 6:00 a.m. The experimental protocol (0708A15263) was approved by the University of Minnesota Animal Care and Use Committee. The experiment was conducted in accordance with the Principles of Laboratory Animal Care (National Research Council 2003), and laboratory facilities were accredited by the American Association for the Accreditation of Laboratory Animal Care.
Apparatus
Rats were housed in octagonal operant conditioning chambers with alternating sides of Plexiglas and stainless steel. Each chamber contained two levers (Coulbourn Instruments, Allentown, NJ) located approximately 2.5 cm above the wire mesh floor, and a set of three multi-colored stimulus lights was located above each lever. A house light (4.76 W) was attached to the top of the chamber, and a spout from a drinking bottle was accessible from the chamber. Each experimental chamber was enclosed in a sound-attenuating, melamine-coated wooden cabinet that contained a ventilation fan. An infusion pump was mounted in the cabinet outside each chamber, and Tygon tubing connected the pump to a swivel (1050-0022; Alice King Chatham, Hawthorne, CA, USA) that protruded inside the top of the chamber and was connected to the rat by a spring-covered cannula. A syringe pump (model PHM-100, Med Associates, Inc., St. Albans, VT, USA) was used for the PR study, and an infusion pump with a larger reservoir (RHSYOCKC, Fluid Metering, Oyster Bay, NY, USA) was used for the escalation study. Experimental programming and data collection were accomplished with PC-compatible computers and Med-PC interfacing (Med Associates, St. Albans, VT, USA).
Drugs
Cocaine HCl was obtained from the National Institute on Drug Abuse (Research Triangle Institute, NC, USA) and dissolved in a solution of sterile saline (0.9%) at a concentration of 0.8 mg/ml for the 0.2 mg/kg testing dose and 1.6 mg/ml for the 0.4 mg/kg testing dose. Heparin (1 ml/200 ml saline; 190 USP units/kg) was added as an anticoagulant to the cocaine solution. The cocaine solution was injected at a volume of 0.025 ml/100 g of body weight after completion of the FR or PR reinforcement schedule requirement, and the duration of each infusion was 1 s/100 g of body weight (the average infusion time was 2.5 s). Albu-CocH was obtained from CoGenesys, Inc. (Rockville, MD, USA). It is a fusion of truncated (E1-V529) mutant BChE (accession number gi:116353) to the N terminus of albumin (gi:28592). As described fully elsewhere (Gao et al. 2008), monomeric protein was expressed in Chinese hamster ovary cells and purified to near homogeneity (>95%) using blue affinity and ion exchange chromatography. Each batch of enzyme was titrated by incubation for 24 h with varying amounts of the irreversible inhibitor, diisopropyl fluorophosphate (Sigma-Aldrich, St. Louis, MO, USA), followed by determination of residual enzyme activity (Sun et al. 2002).
Albu-CocH was developed by fusing human serum albumin to the C terminus of a BChE modified by successive rational mutations that selectively enhanced catalytic efficiency in hydrolyzing cocaine (Pan et al. 2005; Pancook et al. 2003; Sun et al. 2001, 2002). The albumin–cocaine hydrolase fusion (Albu-CocH) retains a catalytic efficiency with cocaine (kcat=2,700 min−1, Km=2 μM) that is 1,000-fold higher than natural human BChE, and it exhibits a half-life of 8 h after i.v. injection in rats (Gao et al. 2008).
General methods
Each rat was surgically implanted with a polyurethane catheter (MRE-040, Braintree Scientific, Inc., Braintree, MA, USA) into the right jugular vein following a procedure reported by Carroll and Boe (1982) and modified by Lynch et al. 2000. The rats were first anesthetized with a combination of ketamine (60 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), and they were administered with doxapram (5 mg/kg, i.p.) and atropine (0.4 mg/ml, 0.15 ml, s.c.) to facilitate respiration. Next, an incision was made lateral to the trachea, and a beveled end of the catheter was inserted into the vein and secured with silk sutures. The free end of the catheter was channeled subcutaneously to the midscapular region where it exited the skin through a small incision. The catheter was attached to needle tubing extending from a cannula connector (C3236, Plastics One, Roanoke, VA, USA) that was embedded in the infusion harness (Instech Laboratories, Plymouth Meeting, PA, USA). After catheter implantation, rats were allowed a 3-day recovery period when they were given antibiotic (gentamicin) and analgesic (buprenorphine) medications.
All rats were then trained to self-administer cocaine (0.4 mg/kg) which was then reduced to 0.2 mg/kg in experiment 1 and in two groups in experiment 2. During training, rats received three experimenter-delivered priming infusions of cocaine (0.4 mg/kg) at 9:00 a.m. Rats were considered to have achieved stable performance under this training procedure after meeting three criteria: (1) no steadily increasing or decreasing trend in number of infusions, (2) at least 30 infusions per day over three consecutive days, and (3) a 2:1 active/inactive lever response ratio. A house light was illuminated from 9:00 to 11:00 a.m. to signal session duration. A response on the left (active) lever resulted in a cocaine infusion, and stimulus lights above the lever were illuminated for the duration of each infusion. Responses during infusions were counted but had no programmed consequences. Responses on the right lever did not result in an infusion, but they were counted and resulted in illumination of the stimulus lights above the inactive lever for a time that was equivalent to the infusion duration. These responses were considered to be an estimation of nonspecific lever pressing. Approximately every 7 days at 3:00 p.m., the rats’ body weights were recorded, and catheter patency was checked by injecting a 0.1-ml solution containing ketamine (60 mg/kg), midazolam (3 mg/kg), and saline. If a loss of the righting reflex was not manifest upon this catheter check, a second catheter was implanted in the left jugular vein by the surgical methods described above. After a 3-day recovery period, behavior was allowed to stabilize before the experiment resumed.
Experiment 1: maintenance of cocaine self-administration under a PR schedule
Rats were trained to acquire cocaine self-administration during 2-h sessions using a 0.4-mg/kg dose according to the “General methods.” Once they had acquired stable behavior with the 0.4-mg/kg cocaine dose under the FR 1 schedule (see “General methods”), a PR schedule replaced the FR 1 schedule, and the dose of cocaine was changed to 0.2 mg/kg, the lowest dose that maintained self-administration. Due to difficulties in maintaining catheter life over the prolonged period required for the above procedures and training, two daily sessions (9:00–11:00 a.m. and 1:00–3:00 p.m.) were used to facilitate rapid stabilization under the PR schedule, although data from only the first session of the day were used. The PR schedule was similar to that used by Roberts et al. (1989) in which the lever press response requirement for each infusion increased according to the following sequence: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 188, etc. This procedure has been previously used with female rats in our laboratory (Anker et al. 2009; 2010; Larson et al. 2007). After the rats exhibited stable responding for six consecutive sessions (no increasing or decreasing trend in responses or infusions), saline or Albu-CocH (2 or 4 mg/kg) was given 30 min prior to the next morning session. These treatments were given 30 min before the session onset by administering the treatment through the i.v. catheter at the swivel and subsequently flushing the cannula with 0.2 ml saline. Behavior was allowed to stabilize for at least another six sessions before saline or another Albu-CocH injection was given. Stability was defined as no steadily increasing or decreasing trend in responses or infusions and a change of no more than two infusions over the 3 days of stability. Saline and Albu-CocH doses were given in nonsystematic order.
Experiment 2: escalation of cocaine self-administration during long access
Once rats were trained to acquire cocaine (0.4 mg/kg) self-administration according to procedures described in the “General methods,” they were allowed access to cocaine infusions (0.2 or 0.4 mg/kg) under a FR 1 schedule. After they stabilized during 2-h sessions (9:00–11:00 a.m.), session length was changed to 6-h each day from 9:00 a.m. to 3:00 p.m. Under the FR 1 schedule, responses during an infusion did not result in another infusion; however, they were counted. Thus, the number of responses generally exceeded infusions. These long-access (6 h) sessions continued for 21 days, and they were followed by 2-h short-access sessions (9:00–11:00 a.m.) until behavior stabilized. Initially, three groups that self-administered 0.4 mg/kg cocaine were randomly assigned to saline or two doses of Albu-CocH (2 or 4 mg/kg), and subsequently, two groups were added that self-administered 0.2 mg/kg cocaine and were randomly assigned to saline or 2 mg/kg Albu-CocH. Treatments were administered 30 min before the sessions. An additional five rats that had neither Albu-CocH or saline but 21 days of 6-h access to cocaine (0.4 mg/kg) were added to the saline group, as their data did not differ from the saline rats’ data. The i.v. infusions of Albu-CocH were administered for the first 7 days of the 21-day long-access period in all rats, except one rat that received 0.4 mg/kg cocaine was administered the 2 mg/kg dose of Albu-CocH for 14 days. This rat was included in the analyses for only the first 7 days of treatment, as that portion of the procedure was the same for all rats in the group; however, data from this rat were excluded from the second and third 7-day blocks and the subsequent 2-h post long-access period. Limited enzyme supply precluded using the extended-treatment condition on additional rats; thus, it was an exploratory investigation in one rat. After the treatments, rats continued to self-administer 0.2 or 0.4 mg/kg cocaine for the remaining 14 days (or seven for the rat treated for 14 days) under the 6-h sessions for a total of 21 days. Session length was subsequently reduced to 2 h (9:00–11:00) until behavior again reached stability criteria for at least 3 days. Behavior was considered stable when there was no steadily increasing or decreasing trend in the number of responses or infusions over a series of three consecutive days. This was attained in 3 days for most rats and within 5 days for all rats. These stability criteria were selected, as they were consistent with those used in previous studies using similar procedures (Larson et al. 2007).
Data analysis
In experiment 1 (PR), infusions and lever responses on the active (left) and inactive (right) sides were analyzed using a two-factor, repeated-measures ANOVA with Albu-CocH dose (0, 2, or 4 mg/kg) and sequential measures. The ANOVA compared the three morning sessions before Albu-CocH treatment, the treatment session, and the three morning sessions after treatment. Responses during infusions were counted as part of total responses, although they did not result in additional infusions. Responses during infusions are counted because they may indicate impulsive or anticipatory behavior that would reflect individual differences. Although that was not a factor considered in the present study, they were counted and presented for comparison with previous work using similar procedures. Responses during infusions were counted as part of total responses. In experiment 2 (6 h), a two-factor mixed ANOVA was used with Albu-CocH dose as the between-subjects factor and individual animals’ means of three 7-day blocks of cocaine (0.4 mg/kg) self-administration sessions (6 h) as the within-subjects factor. The rat that had 14 days of Albu-CocH treatment was included in the analysis only during its first 7-day block, as it did not differ from the other rats in the 2 mg/kg group that had 7 days of treatment. Responses and infusions were compared across groups, across the 21 long-access self-administration days, by 7-day blocks of the 21 days for the doses of cocaine, and by treatment. This analysis was also conducted for the initial hour of the 6-h sessions, as others have indicated that escalation is evident in the first hour of long access (Specio et al. 2008). Infusions during each hour of the 6-h session for the saline group were compared to the 2 and 4 mg/kg Albu-CocH groups that were combined due to no significant differences between the Albu-CocH groups. Repeated-measures ANOVAs were conducted with group and hour as the factors on days 1, 4, 7, and 10 of the 6-h access sessions. This analysis was repeated for the two groups self-administering 0.2 mg/kg cocaine condition. Responses and infusions during the 2-h sessions before and after the 21-days of long access were analyzed using repeated-measures ANOVAs comparing saline with the combined 2 and 4 mg/kg Albu-CocH conditions. After a significant interaction, post hoc tests were conducted using Fisher’s LSD-protected t tests. Statistical analyses were conducted using GB Stat (Dynamic Microsystems, Inc., Silver Spring, MD, USA), and results were considered statistically significant at p<0.05.
Results
Experiment 1: maintenance of cocaine self-administration under a PR schedule
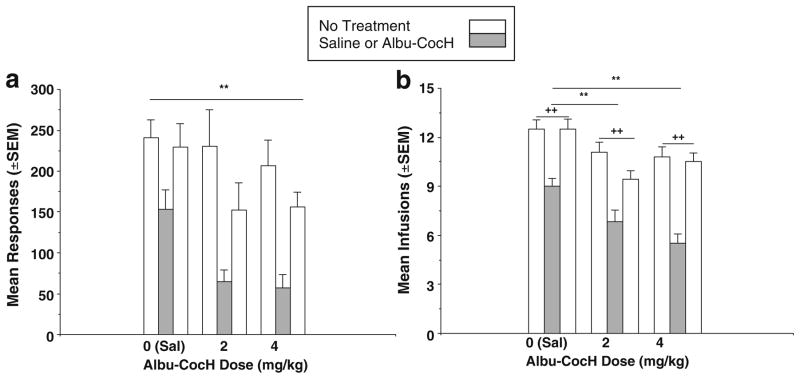
Figure 1 shows the responses (a) and infusions (b) earned under the PR schedule. Each Albu-CocH or saline injection was preceded and followed by six sessions of stable cocaine self-administration when Albu-CocH was not administered. Data from the last three sessions before and the first three sessions after the treatments were averaged to compare the treatment conditions with pretreatment and posttreatment baselines. In Fig. 1, means of three pre-Albu-CocH sessions, the treatment session, and three post-Albu-CocH treatment sessions are presented. For lever response (Fig. 1a), there was a significant effect of dose (F2, 53 = 6.79, p<0.005) and time (before, during, and after treatment) (F2, 53 =20.40, p <0.0001), but there was no dose × time interaction.
Fig. 1.
Mean (±SEM) responses (a) and cocaine infusions (b) under a PR schedule during 2-h sessions are presented for the mean of three morning sessions before Albu-CocH (2 or 4 mg/kg) or saline (Sal, 0) treatment (open bars), for the first session after Albu-CocH or Sal treatment (filled bars), and for three morning sessions after treatment (open bars). Plus signs indicate significant differences in two-group contrasts between the 3-day pre- and post-treatment periods and the treatment (Sal or Albu-CocH) session. The asterisks refer to significant differences between the saline (0) and Albu-CocH treatment effects
For infusions, Fig. 1b indicates that there was also a significant effect of dose (F2, 53=31.72, p <0.0001), time (F2, 53=42.34, p <0.0001), and a significant dose × time interaction (F4, 53 =3.02, p<0.05). Post hoc analyses indicated that there were significantly fewer infusions after the 2 mg/kg (p<0.01) and 4 mg/kg (p<0.001) Albu-CocH treatment than when saline was injected. Thus, both Albu-CocH doses significantly reduced response compared with saline treatment.
Experiment 2: escalation of cocaine self-administration during long access
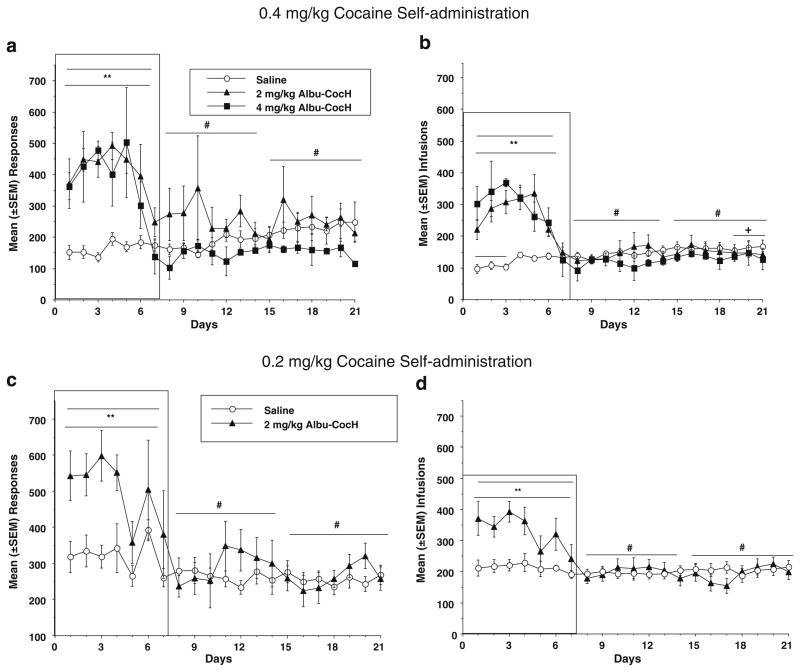
Figure 2a shows the responses for the saline-treated group and the two groups treated with Albu-CocH (2 and 4 mg/kg) during the first 7 days of the 21-day long-access period in rats self-administering 0.4 mg/kg cocaine. The data were divided into three 7-day blocks and compared across the three groups using a mixed-factors ANOVA. There was a significant effect of group (F2, 62=15.60, p<0.0001), 7-day block (F2, 62=30.85, p<0.001), and a group × block interaction (F4, 62=15.74, p<0.001). Post hoc comparisons indicated that the 2 mg/kg Albu-CocH and 4 mg/kg Albu-CocH groups responded significantly more during the first 7-day block than the saline group (p<0.01); however, the 2 and 4 mg/kg Albu-CocH groups did not differ from each other. There were no group differences during the second and third 7-day blocks in the rats treated with Albu-CocH for only the first 7 days.
Fig. 2.
Mean (±SEM) responses (a and c) and cocaine infusions (b and d) are presented for groups self-administering 0.4 mg/kg cocaine (a and b) and treated with saline (open circles), 2 mg/kg Albu-CocH (triangles), or 4 mg/kg Albu-CocH (squares) and groups (c and d) self-administering 0.2 cocaine and treated with saline (open circles) or 2 mg/kg Albu-CocH (triangles). Albu-CocH or saline treatment was given on the first 7 (shown in box) of the 21 days. The asterisks indicate a significant group difference in responses (a and c) and infusions (b and d) during the first 7 days when Albu-CocH vs saline pretreatment occurred daily before the sessions. Pound signs indicate significant within-group differences in the first 7-day block vs the second and/or third 7-day blocks. The plus sign indicates significant increase (escalation) of cocaine infusions in the saline group when the first three and last three sessions were compared in the group self-administering 0.4 mg/kg cocaine (b)
The number of infusions for the groups self-administering the 0.4 mg/kg cocaine dose is presented in Fig. 2b by group and block. Results of the mixed-factors ANOVA revealed a significant main effect for group (F2, 62=4.84, p<0.05) and block (F2,62=91.00, p <0.0001) and a significant group × block interaction (F4, 62=39.54, df =4, 62, p < 0.0001). Post hoc tests indicated that the 2 and 4 mg/kg Albu-CocH groups exceeded the saline group in infusions during the first 7 days (p<0.01), but the Albu-CocH-treated groups did not differ from each other. There were no significant group differences during the second and third 7-day blocks. Overall, the Albu-CocH treatment elevated response and cocaine infusions, but this effect was not demonstrably sensitive to dose over the tested range. Responding and infusions rapidly returned to levels of the saline controls after the 7 days of treatment.
Figure 2c shows responses for the groups self-administering the 0.2 mg/kg cocaine dose. Results of the mixed-factors ANOVA indicated that there was a significant effect of group (F1, 47 =4.90, p <0.05), 7-day block (F2, 47 =16.59, p <0.0001), and a group × block interaction (F2, 47 =7.41, p <0.005). Post hoc comparisons indicated that the 2 mg/kg Albu-CocH-treated 0.2 mg/kg cocaine self-administering group responded significantly more than the saline group during the first 7-day block (p< 0.01) but not the second and third 7-day blocks. This group also responded more during the first 7-day block than the second and third blocks (ps<0.01). A comparison between responses in the groups self-administering 0.2 and 0.4 mg/kg (Fig. 2) cocaine while pretreated with saline or Albu-CocH resulted in no significant group differences due to cocaine dose. Thus, Albu-CocH had the same effect and time course in rats self-administering 0.2 and 0.4 mg/kg cocaine.
Figure 2d shows infusions for the 0.2 mg/kg cocaine dose condition and the saline-treated control group. The mixed-factors ANOVA results indicated that there was no significant effect of group, but there was a significant 7-day block effect (F2, 47,=13.84, p <0.0001) and a significant group × block interaction (F2, 47=9.71, p <0.001). Post hoc comparisons indicated that the 2 mg/kg Albu-CocH group responded significantly more than the saline group during the first 7-day block and more than the Albu-CocH and saline groups during blocks 2 and 3 (p<0.01). A comparison between the groups self-administering 0.2 and 0.4 mg/kg cocaine while pretreated with 2 mg/kg Albu-CocH or saline resulted in a significant difference between the 0.2 and 0.4 mg/kg cocaine groups (F1, 56 =7.60, p<0.05), no significant block effect, and a significant group × block interaction (F2, 56=4.83, p <0.05). Post hoc analyses showed differences between the 0.2 and 0.4 mg/kg cocaine groups during blocks 1 and 2.
To establish whether the saline group escalated their 0.4 mg/kg cocaine intake as previously reported in similar experiments (Anker et al. 2009; 2010; Larson et al. 2007; Perry et al. 2006; Roth and Carroll 2004), individual rats’ mean responses and infusion data from the first 3 (1–3) long-access days were compared with the means during the last 3 (19–21) long-access days. Paired t tests indicated no significant escalation effect for responses, but infusions were significantly higher over the last 3 days (days 19–21) compared with the first 3 days (t9=3.29, p <0.01) in the saline group. This comparison made at the 0.2 mg/kg cocaine dose did not reveal a significant increase in responses or infusions from the first 3 to the last 3 days of the long-access period. A similar comparison was not made in the Albu-CocH-treated groups due to the treatment occurring during the first 3 days and not the last 3 days. The 2 and 4 mg/kg Albu-CocH treatment groups were combined due to lack of a significant difference between them, and a similar analysis of groups (saline vs Albu-CocH) × block of days was conducted for the first hour of the 6-h sessions. The results and the statistical analyses (not shown) were the same as shown for the 6-h sessions in Fig. 2.
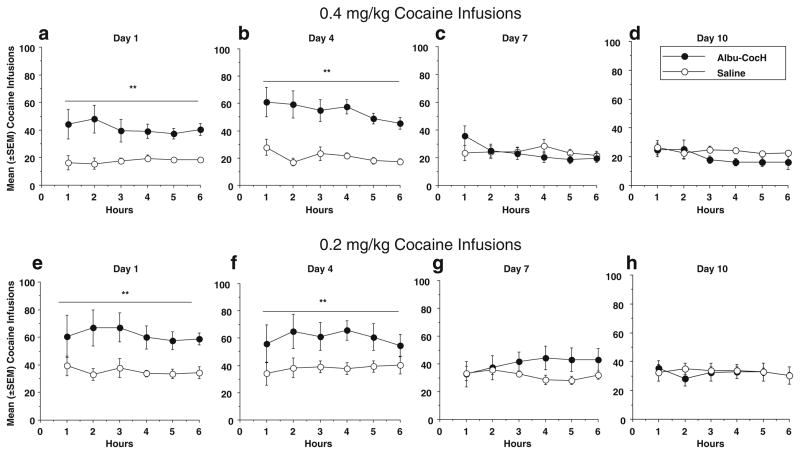
Figure 3 summarizes the total hourly infusions over the 6-h sessions for the Albu-CocH-and saline-treated groups on days 1, 4, and 7 of Albu-CocH or saline treatment and on day 10, 3 days after treatment had stopped. For this analysis, the two and four Albu-CocH groups were combined due to lack of a significant difference between them, and a similar analysis of group (saline vs Albu-CocH) × block of days was conducted for the first hour of the 6-h session, and results and statistical analyses were the same as shown in Fig. 2 (data not shown). Days 7 and 10 were representative of the other days (8–21) after treatment ended; thus, only selected days were presented. For the groups self-administering 0.4 mg/kg cocaine (Fig. 3a–d), since there were no differences in the hourly or total infusions between the two Albu-CocH groups (2 or 4 mg/kg), data were combined. On days 1 (a) and 4 (b) of treatment, the Albu-CocH-treated rats had significantly more infusions than saline controls (F1, 89 =11.00, p <0.01; F1,89 =43.61, p<0.0001), respectively. There were no differences due to hour or group or a group × hour interaction. However, on day 7 (Fig. 3c), the last day of treatment, and on day 10 (Fig. 3d), which was 3 days after treatment, there were no significant differences in hourly infusions across groups or hours and no significant group × hour interactions. A similar analysis was performed for the groups self-administering 0.2 mg/kg cocaine (Fig. 3e–h), and similar results were obtained. On days 1 (Fig. 3e) and 4 (Fig. 3f) of treatment, the Albu-CocH-treated rats had significantly more infusions than saline controls (F1, 95 =8.24, p <0.05; F1, 95 =6.98, p <0.05), respectively. There were no differences due to hour or a group × hour interaction. On days 7 (Fig. 4g) and 10 (Fig. 4h), there were no significant differences in infusions across groups or hours and no significant interactions.
Fig. 3.
Mean (±SEM) cocaine infusions are presented for each hour of the 6-h session for the Albu-CocH-treated (filled circles) and saline-treated (open circles) rats for days 1 (a and e), 4 (b and f), and 7 (c and g) of Albu-CocH or saline treatment, and day 10 (d and h) of the 21-day 6-h cocaine access period. Note that Albu-CocH or saline was given only on days 1–7, and there was no treatment on days 8–21. In the upper frames, the 2 and 4 mg/kg Albu-CocH groups were combined as they did not differ on hourly or total cocaine infusions. In the lower frames, the group was treated with 2 mg/kg Albu-CocH. The asterisks and horizontal lines on a, b, e, and f indicate a significant treatment group difference across the 6 h
Fig. 4.
Mean (±SEM) responses (a and c) and cocaine infusions (b and d) are presented for a mean of 3 short-access sessions before (open bars) and after (filled bars) the 21-day long-access period for the three groups self-administering 0.4 mg/kg cocaine, Sal (0), 2, and 4 mg/kg Albu-CocH (a and b) and the two groups self-administering 0.2 mg/kg cocaine, Sal (0), and 2 mg/kg Albu-CocH (c and d). Plus signs refer to a significant increase in responses and infusions after long-access compared with the initial short-access (2 h) condition in the 0.4 mg/kg cocaine group treated with saline (a and b). Asterisks refer to significant differences between the saline and Albu-CocH-treated groups in post-long access responses and infusions. These results indicate an escalation effect only in the saline group
Figure 4 compares mean responses (Fig. 4a, c) and infusions (Fig. 4b, d) during short-access sessions when 0.4 mg/kg cocaine (a and b) or 0.2 mg/kg cocaine (c and d) was available under an FR 1 schedule for 3 stable days before and 3 stable days after the 21-day long-access period. When responses were compared before and after long access to 0.4 mg/kg cocaine (Fig. 3a, b) there was a significant group (saline, 2 or 4 mg/kg Albu-CocH) effect (F1, 29=6.28, p<0.05) and a significant group × time (before vs after long access) interaction (F2, 29=4.00, p<0.05). Post hoc comparisons indicated that the saline-treated group had significantly more responses (p<0.01) during short-access sessions after long access compared with those before long access. However, the other two groups (2 and 4 mg/kg Albu-CocH) did not differ in responses before vs after long access. The saline group also had more responses than the 4 mg/kg Albu-CocH group (p<0.01) after the long-access period. Figure 4b shows that the number of infusions post- vs pre-long-access was affected by saline vs Albu-CocH in a manner similar to the effect on responses. The ANOVA resulted in a significant interaction between group and time (before vs after long access) (F2, 29=4.73, p<0.05), but there was no significant main effect. Again, post hoc tests showed significantly more infusions after (vs before) long access in the saline group but not in the Albu-CocH-treated groups. Similar analyses of responding (4c) and infusions (4d), comparing short access sessions before and after long access when 0.2 mg/kg cocaine was available, revealed no significant effects of treatment (Albu-CocH vs saline).
Discussion
In a model of cocaine self-administration in which rats earned a moderate number of infusions (e.g., 12 per session), Albu-CocH had a limited but significant effect on reducing cocaine intake. In particular, although the data did not demonstrate a relationship with enzyme dose, overall comparison indicated a temporary reduction in responses, while infusions under the PR schedule were significantly fewer after Albu-CocH than after saline treatment. The PR schedule used is considered an index of a self-administered drug’s reinforcing effectiveness and an estimate of the animals’ motivation to seek cocaine (Paterson and Markou 2003; Wee et al. 2008). Therefore, we conclude that Albu-CocH was mildly effective in reducing the rewarding value of cocaine infusions, likely by reducing the effective unit dose or amount of cocaine reaching the brain and available to the animal. However, drug-seeking behavior was not eliminated, as in the next session after treatment it returned to pre-Albu-CocH treatment levels, where it remained for six subsequent sessions. Thus, Albu-CocH effectively reduced the response and infusions only during the session in which it was administered. The injection procedure itself (e.g., saline injection) may have accounted for some of the reduction in cocaine-rewarded behavior, possibly due to the stress of handling/injection, as infusions were also decreased after saline treatment. On the other hand, Albu-CocH treatment produced a greater reduction in cocaine infusions than saline injections, indicating the effectiveness of Albu-CocH vs vehicle. At the doses tested, Albu-CocH had only a mild effect, as it did not completely eliminate responding, and there was no enduring effect that persisted into subsequent sessions.
These results contrast with the nearly complete elimination of cocaine-seeking that we reported previously using a cocaine-primed reinstatement procedure (Brimijoin et al. 2008). One important difference in the experimental paradigms is that the reinstatement study involved only one experimenter-administered cocaine injection, while in the current work several infusions of cocaine were self-administered. Thus, the overall magnitude of the reinforcing stimulus may have been greater in the present studies and hence more difficult to antagonize. A second procedural difference is that cocaine-priming injections in the reinstatement model were given i.p., while self-administered cocaine was delivered directly into the superior vena cava. Again, the latter may be more difficult to antagonize because such delivery prevents drug mixing before reaching the brain. The behavioral results obtained with Albu-CocH thus far, including the present study and Brimijoin et al. (2008), suggest that the enzyme treatment specifically reduces the effective dose of cocaine, as other behaviors such as food and water intake and locomotor activity were not affected, and response after a priming dose of amphetamine in cocaine-trained animals was not reduced by Albu-CocH (Brimijoin et al. 2008).
Others working with a bacterial-based cocaine-metabolizing enzyme, “CocE,” have reported a similar reduction in cocaine self-administration under an FR 5 schedule in rats (Collins et al. 2009). That reduction, however, occurred only when the animals received a very small dose of cocaine (0.1 mg/kg) delivered through the tail vein (allowing considerable mixing in the peripheral circulation). Interestingly, a larger dose of cocaine (1 mg/kg) in the same study caused an opposite effect, namely increased responding (Collins et al. 2009). It is not known whether this difference might have been related to rewarding effects of a smaller dose and/or aversive effects of a larger dose. Unfortunately, our short supply of the enzyme precluded a full-scale chronic treatment experiment or a complete dose–response analysis with cocaine and/or Albu-CocH. Nonetheless, our findings and those of Collins et al. (2009) are consistent, and together they suggest that ample and sustained delivery of cocaine hydrolase (at the doses tested) would be needed to suppress ongoing cocaine-motivated behavior, and sufficient quantities of cocaine could overwhelm even such treatments. As will be discussed further below, this conclusion does not imply that enzyme-based therapies are unsuitable for therapy of cocaine abuse.
The second experiment extended Albu-CocH treatment to 7 days and increased cocaine access to 6 h per day. The reinforcement schedule also changed from a challenging one yielding few infusions (PR) to a simple one allowing nearly free access to cocaine (FR 1). In the saline control group, the FR 1 long-access condition generated an escalation of cocaine (0.4 mg/kg) intake comparable to that previously reported using this long-access procedure and similar doses (Anker et al. 2009; 2010; Larson et al. 2007; Perry et al. 2006; Roth and Carroll 2004). More specifically, a comparison of infusions during the first 3 days and the last 3 days of the 21 days of long access in the saline-treated groups indicated that there was a significant increase over time that is indicative of escalation. However, lowering the self-administered cocaine dose to 0.2 mg/kg, and consequently the total intake, eliminated this escalation effect. While the rats responded more and received more infusions than the group self-administering 0.4 mg/kg cocaine, the 0.2 mg/kg group’s total cocaine intake was less.
The effect of subchronic treatment (7 days) with Albu-CocH for both doses of self-administered cocaine was opposite to the one seen on the PR schedule and to what we reported previously for cocaine-primed reinstatement (Brimijoin et al. 2008). Instead of reducing cocaine-seeking behavior (Experiment 1) or eliminating it (Brimijoin et al. 2008), treatment with Albu-CocH during long access increased initial levels of responding more than threefold at both the low (0.2 mg/kg) and moderate (0.4 mg/kg) doses of cocaine. By the last 2 days of the 7-day treatment period, however, responding began to decline. Responding during the second 7-day block in the rat treated for 14 days continued to remain slightly elevated comparable to day 6 or 7 of the groups treated for only 7 days. At the end of its 14 days of treatment, this rat’s response returned to levels comparable to the other groups at the end of their 7 days of treatment.
The analysis of hourly data during the 6-h sessions on selected days revealed that infusions were nearly evenly spread across hours. There did not seem to be a front-loading effect during hour 1 of Albu-CocH treatment to counteract the effect of reduced efficacy of cocaine. Instead, there was a steady and sustained higher rate of responding throughout the 6 h, and when the attempts to surmount Albu-CocH treatment ended (e.g., days 6–7), again there was a similar pattern of even responding over the 6 h in both Albu-CocH- and saline-treated groups. Thus, the dramatic attempts to surmount Albu-CocH’s blockade of cocaine rewarding effects diminished after 4 or 5 days, suggesting a loss of efficacy of Albu-CocH to diminish cocaine’s effects or tolerance to the effects of CocH.
In all Albu-CocH-treated rats, at both cocaine doses, responses and infusions reached the level of the saline-treated controls shortly after treatment ended. However, in contrast to controls, the Albu-CocH-treated groups did not escalate their responding during the 14 days remaining posttreatment. This may have been due to the treatment and/or time needed for increased responding to emerge. That the temporal aspects of treatment may interfere with the escalation process is an area that has seldom been investigated. We attempted to do that in a previous study in which our treatment (agmatine) began after the escalation of cocaine and fentanyl self-administration had started, and we found that to prevent escalation the treatment has to start at the beginning of long access (Morgan et al. 2002). However, we are not aware of the effects of early discontinuation of treatment on escalation of cocaine intake. This is an important area for further study that we were not able to explore in the present experiments.
It was also interesting to note that the cocaine or Albu-CocH dose did not have a predictable pharmacological effect on the self-administration behavior during long access. If Albu-CocH was functioning to reduce the effective dose of cocaine, it would have been expected that lowering the cocaine dose or increasing the Albu-CocH dose would have diminished the intake of cocaine during 6-h sessions. However, while lowering the cocaine dose from 0.4 to 0.2 mg/kg reduced the total cocaine intake, the pattern of responses and infusions was similar in both the 0.2 and 0.4 mg/kg cocaine groups. We also did not find a predicted increase in the suppressant effects of Albu-CocH on intake of 0.4 mg/kg cocaine when the dose was increased from 2 to 4 mg/kg. Both doses substantially increased cocaine intake during the first 4–5 days of treatment.
An analysis across groups self-administering 0.4 mg/kg cocaine during short access for 3 stable days before and 3 stable days after the 21-day long-access period showed that response and infusions did not differ in either Albu-CocH-treated group. In contrast, the saline-treated rats showed significant increases in cocaine intake post-long-access when their 3 short access days before and after the long-access period were compared. The magnitude of these increases was similar to results reported previously from studies using a similar dose (0.5 mg/kg) and escalation procedure (Larson et al. 2007; Roth and Carroll 2004; Carroll et al. 2005). However, in the 0.2 mg/kg saline-treated group, there was no escalation effect during long access, and correspondingly no post-escalation elevation in short access cocaine self-administration. Apparently escalation (0.4 mg/kg cocaine group) was key to producing this post-long-access elevation of responding for cocaine. Inspection of the saline group’s hourly data during the last few days of the long-access period (compared with the initial days) revealed an overall increase over each of the 6-h sessions, suggesting possible development of tolerance that would explain the escalation effect and the elevation in subsequent short-access intake. In contrast, while the Albu-CocH-treated groups showed higher responding than the saline control group during treatment (Fig. 2), there was no enduring elevation in post long-access responding under short-access conditions (Fig. 4) that would be indicative of escalation of cocaine intake. This might suggest that enzyme treatment partially blocked cocaine’s effects on the progression of elevated intake during long access. Thus, while Albu-CocH increased daily cocaine self-administration during long access, Albu-CocH treatment blocked the persistent increase in short-access intake that is a characteristic after-effect of the escalation model.
The lack of escalated long-access intake in the Albu-CocH groups may have been due to the short time they had left after treatment (14 days) to express this effect. Alternatively, Albu-CocH may have been weakened or aborted by other mechanisms, escalation, and its subsequent increased motivation for cocaine self-administration during short access (Paterson and Markou 2003; Wee et al. 2008) that are arguably central to the evolving syndrome of drug abuse. In contrast to these results, the groups self-administering 0.2 mg/kg cocaine did not show a post-long-access elevation in short-access cocaine responses or infusions. At this dose, intake escalated over the 21 days of long access (defined as an elevation in the last 3 vs the first 3 of the 21 days of long access), and these results suggested that the post-long-access elevation in short-access intake may be dependent on escalation of intake during the long-access period. The escalated long-access intake and post- vs pre-increases in short-access intake after the long-access period were similar to those found in another drug, phencyclidine, in rhesus monkeys using an oral route of self-administration (Carroll et al. 2005), suggesting a general phenomenon that exists across drugs, species, and routes of self-administration. Overall, the results of several studies agree that short-access responding may result from escalation during long access.
In summary, the present results produced mixed effects —increases during long access, while subsequently reducing post-long-access short-access intake. The opposite effects of Albu-CocH on cocaine self-administration in experiments 1 and 2 illustrate the importance of the reinforcement schedule used, the cocaine dose, amount of cocaine available, and the duration of Albu-CocH treatment. It is worthwhile to consider in more depth the “perverse” increased responding and infusions when Albu-CocH was given to rats in the long-access protocol with its FR 1 schedule and when a bacterial cocaine esterase was given by Collins et al. (2009) to a high-dose cocaine group (1 mg/kg) under a PR schedule. We would argue that a perceived reduction in drug delivery to reward centers explains increased responding in both cases. This effect was especially strong during the early portion of our escalation paradigm, as Albu-CocH-treated rats self-administered nearly three times more cocaine than the saline-treated controls. Since the elevated responding extinguished after about 5 days, it is likely that even the increased drug intake did not completely surmount the blockade of cocaine’s effects. The rats treated with Albu-CocH did not appear more intoxicated; in fact, the higher unit dose of cocaine per infusion (0.4 mg/kg) caused only mild behavioral signs of psychomotor stimulation both during and after Albu-CocH treatment, and there were no differences in responses or infusions as a function of Albu-CocH dose. Altogether, the findings suggest that Albu-CocH was functioning as an antagonist. Although the antagonism was clearly incomplete in a setting with essentially unlimited drug access, it may have altered the in vivo fate of cocaine to an important degree.
We previously demonstrated that administration of cocaine hydrolase not only protects rats from cocaine toxicity but also greatly reduces cocaine uptake into brain (Brimijoin et al. 2008; Gao and Brimijoin 2009). In particular, we found that the brains of rats pretreated with Albu-CocH (3 mg/kg, i.v.) accumulated only 20% as much cocaine as unprotected rats when the two groups were given identical drug challenges (3 mg/kg, i.v.). That amounts to a fivefold reduction in drug uptake. Thus, it is likely that the rats treated with Albu-CocH in our present long-access experiment (experiment 2), despite a twofold to threefold increase in drug infusions, were actually experiencing less drug delivery to reward centers in the brain. This hypothesis can explain why responding remained elevated for just a few days and then returned to baseline. This possibility deserves to be tested experimentally even though large amounts of enzyme would be required. It should also be worthwhile to investigate events at the molecular level in the brains of rats that self-administer cocaine in the escalation paradigm. Meanwhile, it is encouraging to note the absence of apparent adverse side effects from Albu-CocH treatment in the present study. This outcome is in line with our previous work showing that similar doses of Albu-CocH did not alter locomotor activity or operant behavior reinforced by food (Brimijoin et al. 2008). Altogether, the results support our view that enzyme treatment, delivered directly or by other means such as gene transfer (Gao and Brimijoin 2009), could eventually serve as a benign and effective therapeutic intervention for cocaine abuse.
Acknowledgments
The authors are grateful to Luke Gliddon, Nathan Holtz, Amy Saykao, Rachael Turner, and Natalie Zlebnik for their excellent technical assistance. This research was supported by the National Institute on Drug Abuse grants R01 DA023979-03 and 03S1 (SB, PI, MEC, subcontractor) and K05 DA 015267-07 (MEC).
Contributor Information
Marilyn E. Carroll, Email: mcarroll@umn.edu, Department of Psychiatry, University of Minnesota, MMC 392, Minneapolis, MN 55455, USA
Yang Gao, Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA.
Stephen Brimijoin, Email: brimijoi@mayo.edu, Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA.
Justin J. Anker, Department of Psychiatry, University of Minnesota, MMC 392, Minneapolis, MN 55455, USA
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Carroll ME. Differential effects of allopregnanolone on the escalation of cocaine self-administration and sucrose intake in female rats. Psychopharmacology. 2010;212 (3):419–429. doi: 10.1007/s00213-010-1968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresler M, Rosser S, Basran A, Bruce N. Gene cloning and nucleotide sequencing and properties of a cocaine esterase from Rhodococcus sp. strain MB1. Appl Environ Microbiol. 2000;66:904–908. doi: 10.1128/aem.66.3.904-908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimijoin S, Gao Y, Anker JJ, Gliddon LA, LaFleur D, Shah R, Zhao Q, Singh M, Carroll ME. A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuro-psychopharmacology. 2008;27:15–25. doi: 10.1038/sj.npp.1301666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Boe IN. Increased intravenous drug self-administration during deprivation of other reinforcers. Pharmacol Biochem Behav. 1982;17:563–567. doi: 10.1016/0091-3057(82)90319-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Batulis DA, Landry KL, Morgan AD. Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology. 2005;180:414–426. doi: 10.1007/s00213-005-2182-x. [DOI] [PubMed] [Google Scholar]
- Collins GT, Brim RL, Narasimhan D, Ko MC, Sunahara RK, Zhan CG, Woods JH. Cocaine esterase prevents cocaine-induced toxicity and the ongoing intravenous self-administration of cocaine in rats. J Pharmacol Exp Ther. 2009;331:445–455. doi: 10.1124/jpet.108.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Narasimhan D, Sunahara RK, Mierrzejewski P, Jutkiewicz EM, Larsen NA, Wilson IA, Landry DW, Woods JH. Rapid and robust protection against cocaine-induced lethality in rats by the bacterial cocaine esterase. Mol Pharmacol. 2006;70:1885–1891. doi: 10.1124/mol.106.025999. [DOI] [PubMed] [Google Scholar]
- Devlin RJ, Henry JA. Clinical review: major consequences of illicit drug consumption. Crit Care. 2008;12:202. doi: 10.1186/cc6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson TJ, Janda KD. Recent advances for the treatment of cocaine abuse: central nervous system immunopharmacotherapy. AAPS J. 2005;7:E579–E586. doi: 10.1208/aapsj070359. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fitch TE, Roberts DC. The effects f dose and access restrictions on the periodicity of cocaine self-administration in the rat. Drug Alcohol Depend. 1993;33:119–128. doi: 10.1016/0376-8716(93)90053-s. [DOI] [PubMed] [Google Scholar]
- Gao Y, Brimijoin S. Lasting reduction of cocaine action in neostriatum—a hydrolase gene therapy approach. J Pharmacol Exp Ther. 2009;330:449–457. doi: 10.1124/jpet.109.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, LaFleur D, Shah R, Zhao Q, Singh M, Brimijoin S. An albumin-butyrylcholinesterase for cocaine toxicity and addiction: catalytic and pharmacokinetic properties. Chem-Biol Interact. 2008;175:83–87. doi: 10.1016/j.cbi.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA. Pharmacokinetic approaches to treatment of drug addiction. Exp Rev Clin Pharmacol. 2008;1:277–290. doi: 10.1586/17512433.1.2.277. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose–effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Ko MC, Narasimhan D, Berlin A, Lukacs N, Sunahara R, Woods J. Effects of cocaine esterase following its repeated administration with cocaine in mice. Drug Alcohol Depend. 2009;101:202–209. doi: 10.1016/j.drugalcdep.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Supply of a nondrug substitute reduces escalated heroin consumption. Neuropsychopharmacology. 2008;33:2272–2282. doi: 10.1038/sj.npp.1301602. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Morgan D, Roberts DC. Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev. 2004;27:803–812. doi: 10.1016/j.neubiorev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Morgan AD, Campbell UC, Fons RD, Carroll ME. Effects of agmatine on the escalation of intravenous cocaine an fentanyl self-administration in rats. Pharmacol Biochem Behav. 2002;72:873–880. doi: 10.1016/s0091-3057(02)00774-8. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. The National Academies; Washington DC: 2003. p. 209. [PubMed] [Google Scholar]
- Pan Y, Gao D, Yang W, Cho H, Yang G, Tai HH, Zhan CG. Computational redesign of human butyrylcholinesterase for anti-cocaine medication. Proc Natl Acad Sci U S A. 2005;102:16656–16661. doi: 10.1073/pnas.0507332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancook JD, Pecht G, Ader M, Mosko M, Lockridge O, Watkins JD. Application of directed evolution technology to optimize the cocaine hydrolase activity of human butyrylcholinesterase. FASEB J. 2003;17:565. [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. NeuroReport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Perry JL, Dess NK, Morgan AD, Carroll ME. Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats selectively bred for high and low saccharin intake. Psychopharmacology. 2006;186:235–24. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to self-administration. Pharm Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Kosten TR. Novel approaches to the treatment of cocaine addiction. CNS Drugs. 2005;19:13–25. doi: 10.2165/00023210-200519010-00002. [DOI] [PubMed] [Google Scholar]
- Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology. 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) NSDUH Series H-32 DHHS Publication No SMA 07-4293 SAMHSA. Office of Applied Studies; Rockville, MD: 2009. Results from the 2008 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- Sun H, El Yazal J, Brimijoin S, Pang Y-P. Predicted Michaelis–Menten complexes of cocaine-butyrylcholinesterase: engineering effective butyrylcholinesterase mutants for cocaine detoxification. J Biol Chem. 2001;276:9330–9336. doi: 10.1074/jbc.M006676200. [DOI] [PubMed] [Google Scholar]
- Sun H, Pang YP, Lockridge O, Brimijoin S. Re-engineering butyrylcholinesterase as a cocaine hydrolase. Mol Pharmacol. 2002;62:220–224. doi: 10.1124/mol.62.2.220. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Wee S, Mandymam CD, Lekic DM, Koob GF. Noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]