Abstract
The purpose of this study was to compare control of force and modulation of agonist muscle activity of young and older adults when the amount of visual feedback was varied at two different force levels. Ten young adults (25 years ± 4 years, 5 men and 5 women) and ten older adults (71 years ± 5 years, 4 men and 6 women) were instructed to accurately match a constant target force at 2 and 30% of their maximal isometric force with abduction of the index finger. Each trial lasted 35 s, and the amount of visual feedback was varied by changing the visual angle at 0.05, 0.5, and 1.5°. Each subject performed three trials for each visual angle condition. Force variability was quantified as the standard deviation and coefficient of variation (CV) of force. Modulation of the agonist muscle activity was quantified as the normalized power spectrum density of the EMG signal recorded from two pairs of bipolar electrodes placed on the first dorsal interosseus muscle. The frequency bands of interest were between 5 and 100 Hz. There were significant age-associated differences in force control with changes in the amount of visual feedback. The CV of force did not change with visual angle for young adults, whereas it increased for older adults. Although older adults exhibited similar CV of force to young adults at 0.05° (5.95 ± 0.67 vs. 5.47 ± 0.5), older adults exhibited greater CV of force than young adults at 0.5° (8.49 ± 1.34 vs. 5.05 ± 0.5) and 1.5° (8.23 ± 1.12 vs. 5.49 ± 0.6). In addition, there were age-associated differences in the modulation of the agonist muscle activity. Young adults increased normalized power in the EMG signal from 13 to 60 Hz with an increase in visual angle, whereas older adults did not. These findings suggest that greater amount of visual information may be detrimental to the control of a constant isometric contraction in older adults, and this impairment may be due to their inability to effectively modulate the motor neuron pool of the agonist muscle.
Keywords: Visual gain, EMG, Aging, Force variability
Introduction
Numerous studies suggest that the control of force output is impaired in older adults, especially at very low force levels (Enoka et al. 2003; Christou and Tracy 2005). The impaired force control in older adults may be due to numerous neural changes that occur with healthy aging and include neuronal death in higher centers, death of alpha motor neurons at the spinal cord (sarcopenia), and sensory changes (Schiffman 2007). Recently, the processing of visual information (visuomotor integration) has been implicated as a key contributing factor to the impaired force control in older adults during constant isometric contractions (Sosnoff and Newell 2006a, b, 2007; Tracy 2009). Nonetheless, these findings are not consistent in the literature and thus require further investigation. In addition, understanding the interactive effect of aging and amount of visual information has functional significance as it is important for fall prevention (Lord 2006) and fitness to drive automobiles (Kotecha et al. 2008).
Most studies that manipulated the amount of visual information and compared force control between young and older adults demonstrate that in older adults force variability increases with greater amount of visual information. For example, when the amount of visual feedback increased more than 250-fold (2–512 pixels/N) at two force levels (5 and 25% MVC), older adults exhibited greater force variability with the index finger compared with young adults at greater visual gains (Sosnoff and Newell 2006b). Another study manipulated the type of visual display (compensatory or pursuit) and demonstrated that the type of visual display influences the control of force output for older adults (Ofori et al. 2010). Furthermore, studies from Tracy’s group demonstrate that removal of visual feedback decreases force variability of larger limb actions (elbow flexion and knee extension) in older adults to the level of variability exerted by young adults (Tracy et al. 2007) and sometimes even lower (Welsh et al. 2007a, b). Further, indirect support comes from our studies (Christou and Carlton 2001) that demonstrated that young and older adults exhibited similar force variability with the knee extensors when contractions were exerted without any visual feedback.
The notion that greater amount of visual information would exacerbate force variability in older adults, however, is not consistent in the literature. For example, when the intermittency rate of visual feedback increased 100-fold (0.21–20 Hz) at two force levels (5 and 25% MVC), older adults exhibited greater variability of force than young adults independent of the amount of visual feedback provided (Sosnoff and Newell 2006a). Furthermore, visual feedback under stressful conditions equally decreased pinch-grip force variability in both young and older adults at low force levels (2% MVC), which demonstrates that visual feedback is not always detrimental to the performance of older adults (Christou 2005).
In addition to the contradicting literature, it remains unclear whether the amount of visual information exacerbates force variability in older adults during constant isometric contractions because of methodological limitations in previous studies. For example, the study by Sosnoff and Newell (2006b) was based on the manipulation of the visual gain and not visual angle, which has been argued as the appropriate way to manipulate the amount of visual feedback (Vaillancourt et al. 2006). Sosnoff and Newell (2006b) manipulated visual gain by manipulating the ordinate scale, which resulted in distinct visual gain conditions (pixels/N). However, the gain of visual feedback is dependent upon the mean force exerted. In their study, older subjects exhibited lower absolute force levels at 5 and 25% MVC than young adults, which may have resulted in significantly different amount of visual feedback relative to the exerted force for the two groups. Furthermore, studies that have examined the influence of the removal of visual feedback on force variability (Tracy 2007a, b; Tracy et al. 2007; Vaillancourt and Russell 2002; Welsh et al. 2007a, b) did not manipulate the amount of visual information prior to its removal. Therefore, it is not clear whether the amount of visual feedback that is provided prior to its removal influences the findings. Finally, previous research that has manipulated the amount of visual information and compared force control in young and older subjects did not measure the neural activation of the involved muscles. This is important because it may provide information about age-associated differences in the neural control of constant contractions with changes in visual information.
Therefore, the interactive effect of age, visual angle (amount of visual feedback), presence (or absence) of visual feedback and force level on force variability and muscle activity is not well understood. The purpose of this study, therefore, was to compare force variability and agonist muscle activity during constant isometric contractions at different force levels with and without visual feedback when the amount of visual feedback was varied. We hypothesized that greater amounts of visual feedback would increase force variability in older adults but not in young adults and that the neural activation of muscle will be different for young and older adults as a function of visual angle. Part of the findings have been reported in abstract form (Kennedy et al. 2009).
Methods
Ten young adults (25 years ± 4 years, 5 men and 5 women) and ten older adults (71 years ± 5 years, 4 men and 6 women) volunteered to participate in this study. All subjects reported being healthy without any known neurological problems were right-handed according to a standardized survey (Oldfield 1971) and had normal or corrected vision. The Institutional Review Board at Texas A&M University approved the procedures, and subjects provided written informed consent before participation in the studies.
Experimental arrangement
Subjects were seated comfortably in an upright position facing a 27-inch computer screen (Samsung Syncmaster™ 275T+, Samsung Electronics America, NJ, USA) with a resolution of 1,920 × 1,200 pixels, which was located 1.25 m away at eye level. The monitor was used to display the force produced by the abduction of the index finger. All subjects affirmed that they could see the display clearly. The left arm was abducted by 45° and flexed to ~90° at the elbow. The left forearm was pronated and secured in a specialized padding (Versa form™, AB Germa, Sweden). The thumb, middle, ring, and fifth fingers of the left hand were restrained with metal plates, and there was approximately a right angle between the index finger and thumb. Only the left index finger was free to move. The left index finger was placed in an adjustable finger orthosis to maintain extension of the middle and distal interphalangeal joints [for a schematic see Taylor et al. (2003)]. The left hand (non-dominant) was used so the results could be compared with previous studies (Enoka et al. 2003). This arrangement allowed abduction of the index finger about the metacarpophalangeal joint in the horizontal plane, a movement produced almost exclusively by contraction of the first dorsal interosseus (FDI) muscle (Chao et al. 1989; Li and Yasuda 2007).
Visual feedback manipulation
Previous experiments that have manipulated the amount of visual feedback provided to young and older adults have done so by manipulating visual gain. The gain of visual feedback was manipulated by changing the ordinate scale. This type of manipulation resulted in distinct visual feedback gains. However, this may not be the most appropriate method to manipulate visual feedback conditions when you do a between-group comparison because the mean force may influence the gain of visual feedback. For example, if an older adult exerts half the force than a young adult (e.g. 5 N instead of 10 N; see paper by Sosnoff and Newell 2006b), but the manipulation of the Y axis is constant (e.g. 20 pixels/N), then the two age-groups will receive significantly different amounts of visual feedback relative to their exerted force. Specifically, in this example, the young adult will receive twice the amount of visual information relative to the older adult (200 pixels vs. 100 pixels). This methodological detail may be important for age-associated comparisons as it can alter the findings. Therefore, it is important to normalize the amount of visual feedback provided to the subjects. According to Vaillancourt et al. (2006), this can be accomplished by manipulating the visual angle. Thus, we used visual angle to manipulate visual gain, ensuring the amount of feedback provided to be normalized across subjects. We used the following formula to manipulate the visual angle:
| (1) |
with a = visual angle, h1 = height of character (force fluctuations), and d = distance of the eye to the computer screen. Therefore, the visual angle can be manipulated by changing the distance of the eye to the computer screen or by changing the height of the character (amount of force fluctuations) viewed by the subject on the computer screen. Because the distance of the computer screen remained the same throughout our experiment, we varied visual angle by manipulating the height of the character. As in the study by Vaillancourt et al. (2006), we estimated the height of the character to be six times the variability of the force output. Based on previous studies (Christou and Carlton 2001; Taylor et al. 2003; Baweja et al. 2009), force variability was estimated to be 3% of targeted force (CV of mean force) for 0–5% MVCs and 0.5% of targeted force (CV of mean force) for 5–50% MVCs.
Force measurement
The constant isometric force produced by the abduction of the index finger was recorded with a one-dimensional force transducer (Futek LRF400 (L2338) Futek Advanced Sensor Technology Inc. CA, USA). The force signal was sampled at 1 kHz with a Power 1401 A/D board (Cambridge Electronic Design, UK) and stored on a personal computer.
EMG measurement
Abduction of the index finger is produced almost exclusively by the contraction of the FDI muscle (Chao et al. 1989; Li et al. 2003; Li and Yasuda 2007). The FDI muscle activity was recorded with Ag–AgCl-sintered fixed-wire electrodes (4 mm diameter, model E220N-LS, In Vivo Metric, Healdsburg, CA, USA) and taped on the skin distally to the innervation zone (Homma and Sakai 1991). The recording electrodes were placed in line with the muscle fibers. The center-to-center distance between the two electrodes was 5 mm. The reference electrode was placed over the ulnar styloid. The EMG signal was amplified (×2,000) and band-pass filtered at 3–1,000 Hz (Grass Model 15LT system; Grass Technologies, West Warwick, RI, USA). The EMG signal was sampled at 2 kHz with a Power 1401 A/D board (Cambridge Electronic Design, UK) and stored on a personal computer.
MVC task
Subjects were instructed to increase the force from baseline to maximum over a 2-s period and maintain the maximal force for about 4–7 s. Five such recordings were made or until two of the maximal trials were within 5% of each other. The force produced was displayed on the computer monitor to provide visual feedback. The maximal voluntary contraction (MVC) force was quantified as the average force over 3–6 s (constant part) of the highest trial. This procedure allows for the identification of a more conservative MVC that reflects the capability of the person to perform constant isometric contractions.
Constant isometric force task
A custom-written program in Matlab®(Math Works™ Inc., Natick, Massachusetts, USA) manipulated the targeted force-level visual feedback condition (presence or absence of visual feedback), and gain of visual feedback. The target force was provided as a red horizontal line in the middle of the monitor and the force exerted by the subjects as a blue line progressing with time from left to right (Fig. 1). Each subject was presented with two constant force targets at 2 and 30% MVC in counterbalanced order. These forces were chosen because they represent a low force level (2% MVC) where age-associated differences in variability consistently occur and a moderate force level (30%) where force variability is typically similar for young and older adults (Christou and Tracy 2005). The subjects were instructed to gradually push against the force transducer and increase their force to match the red line (target force) within 5 s. When the target was reached, subjects were instructed to maintain their force (blue line) on the target (red line) as accurate and as consistently as possible. The whole trial lasted 35 s, and visual feedback was removed after 15 s (gray bars Fig. 1). To accomplish the visual angle, the gain of visual feedback was manipulated by changing the ordinate scale. Within each force level (blocked), the rest time between each trial was 15 s, and between visual feedback gains, 30 s. The rest time between force levels was 3 min. Subjects performed three trials at each visual angle. The order for the three visual feedback gain conditions was randomly presented.
Fig. 1.
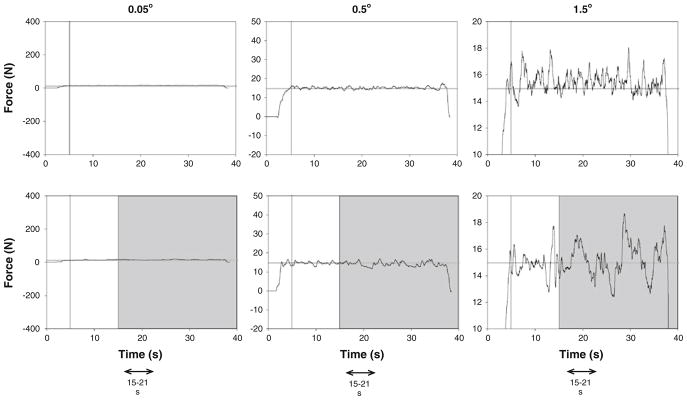
Representative trials from one subject when exerting a constant force at 30% MVC with a visual feedback angle of 0.05° (left column), 0.5° (center column), and 1.5° (right column). Each subject was instructed to exert a force with abduction of the index finger against a force transducer and match the horizontal target line for 35 s. Visual feedback of the target line and exerted force was given to the subjects throughout the trial (visual feedback condition/ top row), whereas visual feedback of the target and exerted force was removed (gray bars) from 15 to 35 s (no visual feedback condition/ bottom row). The force and EMG analysis was based on the 15–21-s segments from each trial
Data analysis
Data were acquired with the Spike2 software (version 6.02; Cambridge Electronic Design, Cambridge, UK) and analyzed off-line using custom-written programs in Matlab® (Math Works™ Inc., Natick, Massachusetts, USA). The force and surface EMG signals were analyzed in segments of 6 s. For both vision conditions, segments were taken from 15 to 21 s (Fig. 1). Prior to data analysis, the force output was filtered with a fourth-order (bidirectional) Butterworth filter using a 20-Hz low-pass cut-off. The standard deviation (SD) and coefficient of variation of force (CV) was quantified from the detrended force output of the 6 s because any drift from the targeted force (especially during the absence of visual feedback condition) could influence force variability. This was achieved by removing the linear trend from the force data. The dependent variables were the mean force, standard deviation (SD) of force, coefficient of variation of force [CV; (SD of force/mean force) × 100], and the amplitude of the EMG signal [RMS of interference signal; (Farina et al. 2004)]. The independent variables were age of the subjects (young and older), the force (2 and 30% MVC), feedback condition (vision (VF) and no vision (NVF)), and resolution of visual feedback (0.05, 0.5, and 1.5°).
In addition, a Fourier analysis was performed on the force and EMG signals (Christou 2005). Autospectral analysis of the force signals were obtained using Welch’s average periodogram method with a non-overlapping Hanning window. The length of the data segment was 6 s, and the sampling frequency was 1 kHz. The window size was 4,096, which gave a resolution of 0.244 Hz. For statistical comparisons, the frequency data of the force signal were divided into 0–1, 1–3, 3–7, and 7–10 Hz frequency bands (Slifkin et al. 2000). Additionally, the EMG signals were divided into 5–13, 13–30, 30–60, and 60–100 Hz frequency bands. We used sub-100-Hz EMG frequency because power in the EMG signal from 5 to 100 Hz reflects the modulation of the motor neuron pool with voluntary effort and it is not associated with the shape of the motor unit action potential (Myers et al. 2003; Neto and Christou 2010; Christou and Neto 2010). The EMG signal was divided into the four frequency bands (5–13, 13–30, 30–60, and 60–100 Hz) because they have been previously associated with specific cortical drives (Brown 2000). Some of these bands (13–30 and 30–60 Hz) have been recently associated with changes in voluntary effort (Neto and Christou 2010). The dependent variable for the spectral analysis of the force and EMG signals was the percent power (%) in the above bins. The normalized power was calculated as the relative power in each frequency band to the total power of the signal (5–100 Hz for the EMG signal and 0–10 Hz for the force signal).
Statistical analysis
A mixed ANOVA (2 age-groups × 3 visual angles × 2 visual feedback conditions × 2 force levels) with repeated measures on force (2 and 30% MVC), resolution of visual feedback (0.05, 0.5, and 1.5°), and visual feedback condition (VF, NVF) compared mean force, SD of force, CV of force, and EMG amplitude for the different force levels and visual feedback conditions. A five-way ANOVA (2 age × 2 feedback conditions × 3 visual angles × 2 force levels × 4 frequency bins) with repeated measures on all factors except age compared the percent power in the force spectrum for the different force levels and visual feedback conditions.
Additionally, stepwise multiple linear regression models were used to establish statistical models that could predict the change in CV of force (criterion variable) from the changes in normalized EMG power (predictor variable). The goodness-of-fit of the model, which indicates how well the linear combination of the variables predicted the change in CV of force, was given by the squared multiple correlation (R2) and the adjusted squared multiple correlation (adjusted R2). The adjusted R2 is reported because the R2 can overestimate the percentage of variance in the criterion variable that can be accounted for by the linear combination of the predictor variables, especially when the sample size is small and the number of predictors is large (Green and Salkind 2002).
Analyses were performed with the PASW Statistics 18.0 statistical package (SPSS Inc., Chicago, IL). Significant interactions from the ANOVA models were followed by appropriate post hoc analyses. For example, age-associated differences were followed with independent t tests, whereas differences between visual feedback conditions were examined with paired t tests. Multiple t test comparisons were corrected using Tukey’s HSD corrections. The alpha level for all statistical tests was 0.05. Data are reported as means ± SD within the text and as means ± standard error of the mean (SEM) in the figures. The interest in this study was the interactive effects of aging with the amount of visual feedback, and thus, only the main effects and age-associated interactions are reported, unless otherwise noted.
Results
Strength and fatigue
To determine whether there were any strength differences between the two age-groups and whether our experimental protocol induced muscle fatigue to our subjects, we compared the MVC before and immediately after the experimental session. Young and older adults exhibited similar MVC before (young: 25.0 N ± 12.0 N; older: 25.0 N ± 4.1 N; P = 0.99) and after (young: 24.8 N ± 10.7 N; older: 25.3 N ± 4.7 N; P>0.88) the experimental session. These findings, therefore, demonstrate that the experimental protocol did not induce any fatigue to our subjects.
Mean force and constant error
There was a significant age × visual angle × force interaction on the mean force (F2,34 = 4.6, P = 0.016). The interaction indicated that at 2% MVC older adults exerted lower force only for the 0.5 and 1.5° visual angles, whereas at 30% MVC, older adults exhibited lower mean force at the lowest gain (0.05°).
There was a significant age × visual angle × force interaction on the constant error of force (F2,36 = 4.8, P = 0.014). The interaction indicated that at 2% MVC, young and older adults overshot the target, but this overshoot was lesser in older adults at the higher visual angles (0.5 and 1.5°). At 30% MVC, young and older adults were close to the targeted force for all visual angles, except older adults undershot the target force at the lowest visual angle (0.05°; Table 1).
Table 1.
Constant error of the force produced by young and older adults at 2 and 30% MVC with a visual feedback angle of 0.05, 0.5, and 1.5°
| 0.05° | 0.5° | 1.5° | |
|---|---|---|---|
| 2% MVC | |||
| Young | 0.656 ± 0.270 | 0.623 ± 0.255 | 0.678 ± 0.266 |
| Older | 0.515 ± 0.142 | 0.326 ± 0.105 | 0.414 ± 0.135 |
| 30% MVC | |||
| Young | −0.132 ± 1.618*+ | 0.228 ± 1.637 | 0.304 ± 1.642 |
| Older | −1.216 ± 0.580*+ | −0.004 ± 0.497 | −0.108 ± 0.497 |
At 2% MVC, on average, young and older adults exerted slightly greater force than the target. At 30% MVC, older adults undershot the target at the lowest visual feedback angle (0.05°), whereas young adults were, on average, close to the targeted force for all visual angles
Significantly different (P<0.05) from 0.5°
Significantly different (P<0.05) from 1.5°
Force variability and structure
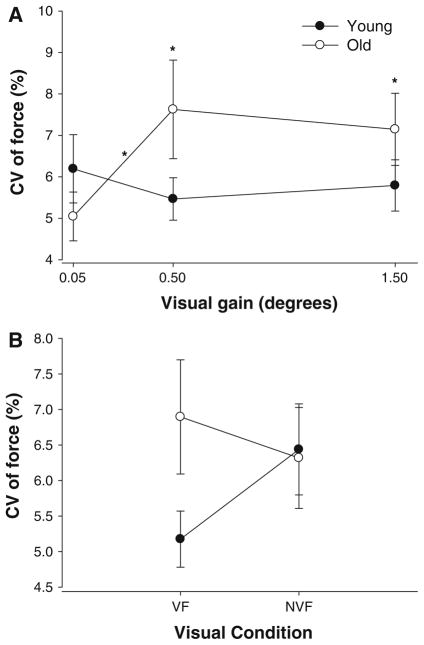
There were no differences in overall strength (MVC) between young and older subjects; however, the mean force produced by the two groups was different in the three visual angle conditions as it is evident from differences in constant error (Table 1). Because the mean force was different between young and older adults during the various conditions, we used the CV of force as our measure of force control. There was a significant age × visual angle interaction (F2,34 = 4.9, P = 0.013). Post hoc analyses indicated that older adults exhibited similar CV of force with young adults at 0.05° but greater CV of force at 0.5 and 1.5° (Fig. 2a). In addition, the CV of force for young adults did not change with increases in visual angle, whereas the CV of force for older adults increased with visual angle. Furthermore, the age × visual feedback condition interaction approached significance (F1,17 = 3.7, P = 0.07) and indicated that older adults exhibited greater CV of force only during the visual feedback condition (Fig. 2b). During the no visual feedback condition, young and older adults exhibited similar CV of force.
Fig. 2.
The CV of force for young (filled circles) and older (open circles) adults as a function of visual angle (a) and visual feedback conditions (b). a Older adults exhibited greater CV of force at 0.5 and 1.5° compared with young adults. The CV of force increased at higher visual angles for older adults, whereas it remained the same for young adults. b The age × visual feedback condition interaction approached significance (P = 0.07) and suggests that the CV of force was greater in older adults compared with young adults only during the visual feedback condition. Asterisk above the symbols indicate age differences, while asterisk above the line indicate visual angle differences
We used the normalized power spectrum of force to determine whether the force structure was similar for young and older adults during the different visual conditions. Because there was no age main effect (P = 0.26) or age interaction with visual angle or feedback (P>0.4), the results demonstrate that young and older adults structured their force output similarly. Specifically, they exhibited most of the power from 0 to 1 Hz (82%) and lesser power at other frequency bands (1–3 Hz: 13.8%; 3–7 Hz: 3.6%; 7–13 Hz: 0.6%).
Agonist muscle activity
Overall EMG amplitude was quantified as the RMS of the interference signal. The amplitude of the EMG increased significantly with force level (F1,17 = 37.39, P<.001). All other main effects and interactions were not significant (P>0.05). Additionally, we quantified muscle activity with the normalized power spectrum density of the muscle activity from 5 to 100 Hz (see Methods for reasoning). There was an age × force interaction (F1,17 = 9.1, P = 0.008). The age × vision × gain × force approached significance (F2,34 = 2.9, P = 0.07). Most importantly, there was an age × gain × force × frequency interaction (F12,204 = 1.9, P = 0.02), which indicated that at higher visual angles young adults modulated the activity of the first dorsal interosseus muscle from 13 to 30 and 30 to 60 Hz, whereas older adults did not. These age-associated differences in modulation of the agonist muscle were greater for the 30% MVC (Fig. 3). Additionally, older adults decreased their power in the 5–13-Hz frequency bands with an increase in visual angle only for 2% MVC, while young adults did not demonstrate a statistically significant change in power in the 5–13-Hz frequency bands (Fig. 3).
Fig. 3.
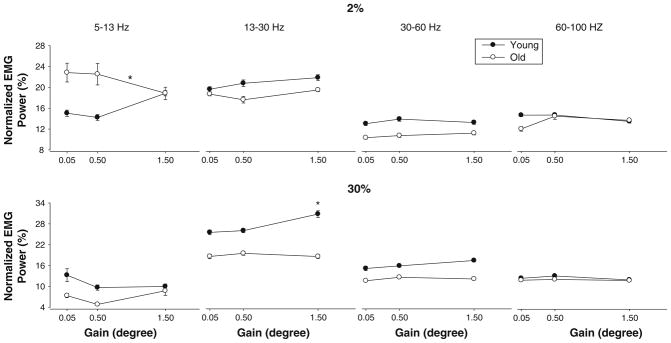
Normalized first dorsal interosseus EMG power across four different frequency bands (5–13, 13–30, 30–60, and 60–100 Hz), three different visual angles (0.05, 0.5, and 1.5°), and two force levels (2% and 30% MVC). It is evident that visual angle influenced EMG power only from 13 to 30 Hz. This finding was consistent for young and older adults at both force levels. In addition, there were some age-associated differences as a function of frequency band. Specifically, at 2% MVC, older adults exhibited greater normalized power from 5 to 13 Hz at the lowest visual angle, whereas at 30% MVC, young adults exhibited greater normalized power from 5 to 13 Hz at the lowest visual angle. Also, at 30% MVC, older adults exhibited greater normalized power from 13 to 30 Hz at the lowest visual angle. Asterisk above the symbols indicate age differences, while asterisk above the line indicate visual angle differences
Change in modulation of agonist muscle activity and CV of force
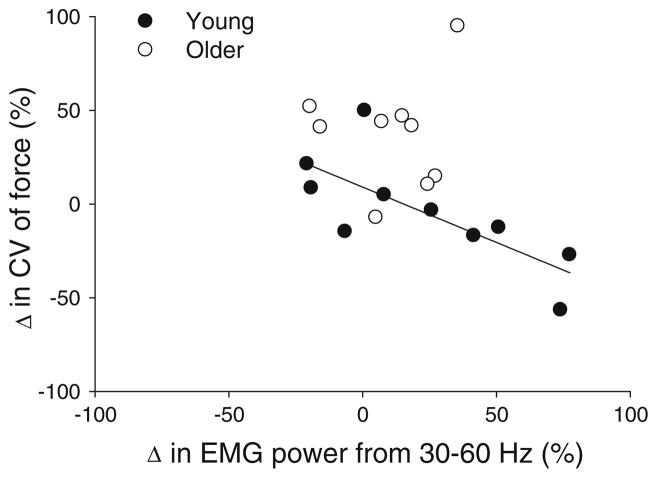
The most important finding was that increases in visual angle amplified the CV of force in older adults but not in younger adults and that only younger adults appear to have modulated the activity of the first dorsal interosseus muscle. To determine whether there was a direct association between the amount of modulation in muscle activity and the increase in CV of force, we performed a stepwise regression analysis. Specifically, we examined whether changes in the normalized power from 5 to 100 Hz (5–13, 13–30, 30–60, and 60–100 Hz) in the first dorsal interosseus muscle were able to predict the change in CV of force from the lowest gain (0.05°; no age differences) to the moderate visual angle (0.5°; largest age difference). The stepwise regression model was significant (adjusted R2 = 0.3, P = 0.007, Durbin Watson = 2.1) and indicated that amplification of the normalized power from 30 to 60 Hz was associated with smaller increases in the CV of force with visual angle (r = −0.581; Fig. 4). The amplification in EMG power from 13 to 30 Hz was also associated with smaller increases in the CV of force (r = −0.56 P<0.05) but was not an independent contributor to the CV of force and thus was excluded from the stepwise regression model. The modulation of all other frequency bands was not associated with changes in the CV of force.
Fig. 4.
Association between the changes in CV of force and 30–60- Hz EMG power. The changes were quantified as the percent change from 0.05 to 1.0°, where the biggest change occurred in the CV of force (young adults in filled circles). The increase in CV of force from 0.05 to 1.0° was predicted with lesser changes in normalized EMG power from 30 to 60 Hz (R2 = 0.3, P<0.01). A single point from an older adult is omitted for presentation clarity (point coordinates are −44, 327)
Discussion
The integration of visual information has been recently implicated as a key contributing factor to the impaired control of force in older adults during constant isometric contractions (Tracy 2009; Sosnoff and Newell 2006b). Furthermore, previous research that has manipulated the amount of visual feedback and compared force control in young and older adults has not examined changes to the neural activation of the involved muscles. In this paper, therefore, we compared the force variability and activity of the single agonist muscle (FDI) when young and older adults exerted constant isometric contractions at different force levels and when the amount of visual feedback was varied. The findings of this study indicate that older adults exhibit greater force variability than young adults at higher visual angles (0.5 and 1.5°) but not during the low visual angle condition (0.05°). Our results, therefore, support previous findings by Sosnoff and Newell (2006b) that greater amount of visual feedback impairs the force control of older adults compared with young adults. We extend the previous findings by providing evidence that the exacerbation of force variability in older adults with greater visual angle is due to their inability to modulate the agonist muscle activity from 30 to 60 Hz.
Aging, amount of visual feedback, and force variability
Older adults exhibited greater variability in force compared with young adults only at visual angles equal to 0.5 and 1.5° but not at 0.05°. These age-associated differences were consistent for low (2% MVC) and moderate levels of force (30% MVC). This result supports previous findings by Sosnoff and Newell (2006b). Nonetheless, this replication is important because previous studies increased gain of the visual feedback simply by manipulating the scale of the Y axis (manipulation of pixels/N) rather than manipulating the visual angle (as we performed in this study). Manipulation of the amount of visual feedback via changes in the visual angle is critical (Vaillancourt et al. 2006). Furthermore, in this study, we demonstrate that when the gain of visual feedback is low (visual angle = 0.05°) the variability of force is similar for young and older adults. This finding supports previous studies, which demonstrate that the force control in older adults is equal to that of young adults when the gain of visual feedback is low (Christou et al. 2004; Vaillancourt et al. 2002; Tracy 2007a, b).
The most novel finding of our paper is that the exacerbation of force variability in older adults with greater visual angle is associated with their inability to modulate the single agonist muscle in this task. Specifically, we found that 13–60 Hz was lower for older adults compared with young adults; especially at 30% MVC (see Fig. 3). The changes in both 13–30- and 30–60-Hz bands were negatively associated with the change (increase) in force variability from 0.05 to 0.5°. This indicates that subjects who increased the power in their agonist muscle activity from 13 to 60 Hz with increases in visual angle exhibited the lowest increase in variability of force. The stepwise regression indicated that the change in 30–60-Hz band in the FDI muscle activity was a significant predictor of the changes in force variability (Fig. 4). It is clear from Fig. 4 that older adults did not modulate the 30–60-Hz band as well as young adults with changes in visual angle (see spread of observations in X axis). In addition, this figure suggests that almost all older adults increased force variability with visual angle, whereas most young adults decreased force variability with visual angle, especially the ones that modulated the 30–60-Hz band in their muscle activity. There is evidence that the modulation of 30–60- Hz band in the agonist muscle may be associated with the readiness of an individual to respond to visual feedback (Schoffelen et al. 2005). This may suggest that the neural mechanisms that are involved with the control of constant force in older adults may be impaired or different compared with young adults.
Potential mechanisms that may contribute to the impaired ability of older adults to control force during constant contractions when the amount of visual information increases include the following: (1) aging changes the integration of visual information with motor execution that may be caused by a delay in the visual perception or transmission of visuomotor information to the motor cortex (van der Lubbe and Verleger 2002). (2) Increased amount of visual feedback requires greater attention. It is believed that older adults have a reduced capability to allocate attentional control and cortical resources (Prakash et al. 2009), which may be caused by their inability to efficiently and flexibly activate the prefrontal cortex in attention-demanding tasks (Milham et al. 2002; Prakash et al. 2009). (3) Additionally, tasks that require greater attentional demands are more stressful. There is evidence that when older adults were stressed with noxious electrical stimuli, they exhibited greater impairments in force variability than young adults (Christou et al. 2004). In summary, it is possible that older adults exhibit an impaired ability to control force during constant contractions when the amount of visual feedback is highly likely due to impaired visuomotor transformations or reduced attentional capacity. Further, research is needed to determine the exact neural mechanisms that explain the exacerbation in force variability with greater amount of visual feedback.
Force variability and removal of visual feedback
Previous studies that have compared force variability with the removal of visual feedback during constant isometric tasks have reported inconsistent findings. Results of such studies have demonstrated that the removal of visual feedback can reduce force variability (Tracy 2007b; Tracy et al. 2007; Welsh et al. 2007a, b), can increase force variability (Slifkin et al. 2000; Baweja et al. 2009), or has no effect on force variability (Vaillancourt and Russell 2002; Christou et al. 2004; Baweja et al. 2010). The differential findings may be due to methodological issues. One methodological difference is that the gain of visual feedback was not controlled in some of the studies (Tracy 2007a, b; Tracy et al. 2007; Welsh et al. 2007a, b; Slifkin et al. 2000). In other studies, the gain was not controlled by manipulating the visual angle (Vaillancourt and Russell 2002; Christou et al. 2004; Baweja et al. 2009, 2010), which may be problematic. Another methodological issue that may account for the differential findings reported in the literature may be the amount of visual feedback given to the subjects before its removal. In our study, we manipulated the amount of visual feedback (with using visual angle) before its removal, in order to understand the effect of varying amount of visual information prior to its removal on force variability.
The findings from our study demonstrate that on average the CV of force became similar for young and older adults when visual feedback was removed (age × visual condition interaction; P = 0.07; see Fig. 2b). This was caused primarily from a slight decrease in the CV of force for older subjects and an increase in the CV of force for young adults when visual feedback was removed. Nonetheless, neither change was statistically significant. This finding partially supports previous studies that have reported that force variability can decrease with the removal of visual feedback for older adults and that age-associated differences in force variability are evident in the presence of visual feedback (Tracy et al. 2007; Welsh et al. 2007a, b; Christou et al. 2004). Our results indicate that force variability does not significantly change with removal of visual feedback in both young and older adults (Schiffman et al. 2002; Vaillancourt et al. 2002; Baweja et al. 2009). Another interesting finding was that the gain of visual feedback prior to its removal did not influence force variability.
The decrease (~10%) in force variability with removal of visual feedback for older subjects is most likely due to the absence of visuomotor corrections. This finding is not only consistent with tasks that require individuals to exert constant isometric contractions with larger muscles (Tracy 2007a, b; Tracy et al. 2007; Welsh et al. 2007a, b), but also supports findings from discrete tasks (Crossman and Goodeve 1983; Carlton 1992; Elliott et al. 2001) and tracking sinusoidal tasks (Miall et al. 1993). For example, removal of visual feedback reduces the number of sub-movements prior to reaching the target during goal-directed movements (Crossman and Goodeve 1983) and allows individuals to track sinusoidal force targets smoother (Miall et al. 1993). Another possibility that may account for the decrease in force variability with removal of visual feedback for older adults may be their inability to effectively use feedback during visual feedback trials (Ofori et al. 2010; Welsh et al. 2007b). Therefore, it appears that in the absence of visual feedback, and independent of the motor task, the central nervous system of older subjects exerts a smoother contraction. In contrast, for young adults, it is possible that removal of visual feedback can impair force variability. Previous studies from Vaillancourt’s laboratory (Coombes et al. 2010; Prodoehl and Vaillancourt 2010) and our laboratory (Baweja et al. 2009) suggest that young adults can incorporate visual feedback to decrease force variability. These findings suggest that the neural mechanisms that control force during steady contractions may be different for young and older adults in the presence or absence of visual feedback. More research is needed to identify the neural pathways that can explain these behavioral differences.
In summary, our findings demonstrate that older adults exhibit greater force variability than young adults with greater amount of visual information. The exacerbation in force variability in older adults with visual angle appears to be related to their inability to modulate the agonist muscle activity from 13 to 60 Hz. Specifically, young adults increase the power in muscle activity from 13 to 60 Hz with more visual information of the task and variability in force remains constant, whereas older adults do not change the power in muscle activity from 13 to 60 Hz with more visual information of the task and variability in force increases. Further, research is needed to understand the neural mechanisms that may contribute to the increased force variability observed in older adults at higher visual resolutions and lower force levels.
Acknowledgments
The authors would like to acknowledge the help of Jonathan Leake with computer programming, Harsimran Baweja with data collection, and Osmar Pinto Neto with data analysis. Supported by R01 AG031769 to E. A. Christou.
References
- Baweja HS, Patel BK, Martinkewiz JD, Vu J, Christou EA. Removal of visual feedback alters muscle activity and reduces force variability during constant isometric contractions. Exp Brain Res. 2009;197:35–47. doi: 10.1007/s00221-009-1883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baweja HS, Kennedy DM, Vu J, Vaillancourt DE, Christou EA. Greater amount of visual feedback decreases force variability by reducing force oscillations from 0 to 1 and 3 to 7 Hz. Eur J Appl Physiol. 2010;108:935–943. doi: 10.1007/s00421-009-1301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Cortical drives to human muscle: the piper and related rhythms. Prog Neurobiol. 2000;60:97–108. doi: 10.1016/s0301-0082(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Carlton LG. Visual processing time and the control of movement. In: Proteau L, Elliott D, editors. Vision and motor control. Elsevier; Amsterdam: 1992. pp. 3–31. [Google Scholar]
- Chao E, An KN, Cooney WP, Linschied R. Biomechanics of the hand. A basic research study. World Scientific Publishing; Teaneack: 1989. [Google Scholar]
- Christou EA. Visual feedback attenuates force fluctuations induced by a stressor. Med Sci Sports Exerc. 2005;37:2126–2133. doi: 10.1249/01.mss.0000178103.72988.cd. [DOI] [PubMed] [Google Scholar]
- Christou EA, Carlton LG. Old adults exhibit greater motor output variability than young adults only during rapid discrete isometric contractions. J Gerontol A Biol Sci Med Sci. 2001;56:B524–B532. doi: 10.1093/gerona/56.12.b524. [DOI] [PubMed] [Google Scholar]
- Christou EA, Neto O. Identification of oscilations in muscle activity from surface EMG: reply to Halliday and Farmer. J Neurophysiol. 2010;103:3548–3549. [Google Scholar]
- Christou EA, Tracy BL. In movement system variability. Human Kinetics; Champaign: 2005. Aging and motor output variability. [Google Scholar]
- Christou EA, Jakobi JM, Critchlow A, Fleshner M, Enoka RM. The 1–2 Hz oscillations in muscle force are exacerbated by stress, especially in older adults. J Appl Physiol. 2004;97:225–235. doi: 10.1152/japplphysiol.00066.2004. [DOI] [PubMed] [Google Scholar]
- Coombes SA, Corcos DM, Sprute L, Vaillancourt DE. Selective regions of the visuomotor system are related to gain-induced changes in force error. J Neurophysiol. 2010;103:2114–2123. doi: 10.1152/jn.00920.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman ER, Goodeve PJ. Feedback control of hand-movement and Fitts’ law. Q J Exp Psychol A. 1983;35:251–278. doi: 10.1080/14640748308402133. [DOI] [PubMed] [Google Scholar]
- Elliott D, Helsen WF, Chua R. A century later: Woodworth’s (1899) two-component model of goal-directed aiming. Psychol Bull. 2001;127:342–357. doi: 10.1037/0033-2909.127.3.342. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol. 2003;13:1–12. doi: 10.1016/s1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- Green SB, Salkind NJ. Using SPSS for the windows and macintosh: analyzing and understanding data. Prentice Hall; Upper Saddle River: 2002. [Google Scholar]
- Homma T, Sakai T. Ramification pattern of intermetacarpal branches of the deep branch (ramus profundus) of the ulnar nerve in the human hand. Acta Anat (Basel) 1991;141:139–144. doi: 10.1159/000147113. [DOI] [PubMed] [Google Scholar]
- Kennedy DM, Baweja HS, Vaillancourt DE, Christou EA. Time onset and amplitude of force drift varies with force level during low-intensity constant isometric contractions. Proceedings of the society of neuroscience; Chicago. 2009. [Google Scholar]
- Kotecha A, Spratt A, Viswanathan A. Visual function and fitness to drive. Br Med Bull. 2008;87:163–174. doi: 10.1093/bmb/ldn028. [DOI] [PubMed] [Google Scholar]
- Li S, Yasuda N. Forced ventilation increases variability of isometric finger forces. Neurosci Lett. 2007;412:243–247. doi: 10.1016/j.neulet.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZM, Pfaeffle HJ, Sotereanos DG, Goitz RJ, Woo SL. Multidirectional strength and force envelope of the index finger. Clin Biomech (Bristol, Avon) 2003;18:908–915. doi: 10.1016/s0268-0033(03)00178-5. [DOI] [PubMed] [Google Scholar]
- Lord SR. Visual risk factors for falls in older people. Age Ageing. 2006;35:42–45. doi: 10.1093/ageing/afl085. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Intermittency in human manual tracking tasks. J Mot Behav. 1993;25:53–63. doi: 10.1080/00222895.1993.9941639. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Myers LJ, Lowery M, O’Malley M, Vaughan CL, Heneghan C, ClairGibson A, Harley YX, Sreenivasan R. Rectification and non-linear preprocessing of EMG signals for cortico-muscular analysis. J Neurosci Methods. 2003;124:157–165. doi: 10.1016/s0165-0270(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Neto OP, Christou EA. Rectification of the EMG signal impairs the identification of oscillatory input to the muscle. J Neurophysiol. 2010;103:1093–1103. doi: 10.1152/jn.00792.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofori E, Samson JM, Sosnoff JJ. Age-related differences in force variability and visual display. Exp Brain Res. 2010;203:299–306. doi: 10.1007/s00221-010-2229-z. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Erickson KI, Colcombe SJ, Kim JS, Voss MW, Kramer AF. Age-related differences in the involvement of prefrontal cortex in attentional control. Brain Cogn. 2009;71:328–335. doi: 10.1016/j.bandc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J, Vaillancourt DE. Effects of visual gain on force control at the elbow and ankle. Exp Brain Res. 2010;200:67–79. doi: 10.1007/s00221-009-1966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS. Critical illness and changes in sensory perception. Proc Nutr Soc. 2007;66:331–345. doi: 10.1017/S0029665107005599. [DOI] [PubMed] [Google Scholar]
- Schiffman JM, Luchies CW, Richard LG, Zebas CJ. The effects of age and feedback on isometric knee extensor force control abilities. Clin Biomech. 2002;6:486–493. doi: 10.1016/s0268-0033(02)00041-4. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Oostenveld R, Fries P. Neuronal coherence as a mechanism of effective corticospinal interaction. Science. 2005;308:111–113. doi: 10.1126/science.1107027. [DOI] [PubMed] [Google Scholar]
- Slifkin AB, Vaillancourt DE, Newell KM. Intermittency in the control of continuous force production. J Neurophysiol. 2000;84:1708–1718. doi: 10.1152/jn.2000.84.4.1708. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Newell KM. Aging, visual intermittency, and variability in isometric force output. J Gerontol B Psychol Sci Soc Sci. 2006a;61:P117–P124. doi: 10.1093/geronb/61.2.p117. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Newell KM. Information processing limitations with aging in the visual scaling of isometric force. Exp Brain Res. 2006b;170:423–432. doi: 10.1007/s00221-005-0225-5. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Newell KM. Are visual feedback delays responsible for aging-related increases in force variability? Exp Aging Res. 2007;33:399–415. doi: 10.1080/03610730701525311. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Christou EA, Enoka RM. Multiple features of motor-unit activity influence force fluctuations during isometric contractions. J Neurophysiol. 2003;90:1350–1361. doi: 10.1152/jn.00056.2003. [DOI] [PubMed] [Google Scholar]
- Tracy BL. Force control is impaired in the ankle plantarflexors of elderly adults. Eur J Appl Physiol. 2007a;101:629–636. doi: 10.1007/s00421-007-0538-0. [DOI] [PubMed] [Google Scholar]
- Tracy BL. Visuomotor contribution to force variability in the plantarflexor and dorsiflexor muscles. Human Mov Sci. 2007b;26:796–807. doi: 10.1016/j.humov.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy BL. Contributions of visuomotor processing to force variability in human aging. In: Shinohara M, editor. Advances in neuromuscular physiology of motor skills and muscle fatigue. Research Signpost; Kerala: 2009. pp. 39–63. [Google Scholar]
- Tracy BL, Dinenno DV, Jorgensen B, Welsh SJ. Aging, visuomotor correction, and force fluctuations in large muscles. Med Sci Sports Exerc. 2007;39:469–479. doi: 10.1249/mss.0b013e31802d3ad3. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Haibach PS, Newell KM. Visual angle is the critical variable mediating gain-related effects in manual control. Exp Brain Res. 2006;173:742–750. doi: 10.1007/s00221-006-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Larsson L, Newell KM. Time-dependent structure in the discharge rate of human motor units. Clin Neurophysiol. 2002;13:1325–1338. doi: 10.1016/s1388-2457(02)00167-0. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Russell DM. Temporal capacity of short-term visuomotor memory in continuous force production. Exp Brain Res. 2002;145:275–285. doi: 10.1007/s00221-002-1081-1. [DOI] [PubMed] [Google Scholar]
- Van der Lubbe R, Verleger R. Aging and the Simon task. Psychophysiology. 2002;39:100–110. doi: 10.1017/S0048577202001221. [DOI] [PubMed] [Google Scholar]
- Welsh SJ, Dinenno DV, Tracy BL. Variability of quadriceps femoris motor neuron discharge and muscle force in human aging. Exp Brain Res. 2007a;179:219–233. doi: 10.1007/s00221-006-0785-z. [DOI] [PubMed] [Google Scholar]
- Welsh TN, Higgins L, Elliott D. Are there age-related differences in learning to optimise speed, accuracy and energy expenditure? Hum Mov Sci. 2007b;26:892–912. doi: 10.1016/j.humov.2007.04.004. [DOI] [PubMed] [Google Scholar]