Abstract
CD20 is a widely validated, B cell specific target for therapy in B cell malignancies. Rituximab is an anti-CD20 antibody that when combined with chemotherapy prolongs survival of CLL patients. Ofatumumab and GA101 (obinutuzumab) are CD20-directed antibodies now being developed as alternative agents to rituximab in CLL based upon different properties of enhanced direct cell death (DCD), NK cell-mediated antibody dependent cellular cytotoxicity (ADCC), or complement-dependent cytotoxicity (CDC). Despite wide spread study, ofatumumab and GA101 have not been directly compared to one another, nor studied for interaction with monocytes and macrophages that are critical to CD20-mediated antibody efficacy in murine models. In CLL cells, we show that DCD is greatest with GA101 and CDC with ofatumumab. GA101 promotes enhanced NK cell activation and ADCC at high antibody concentrations. Ofatumumab has superior antibody dependent cellular phagocytosis (ADCP) with monocyte derived macrophages (MDM). GA101 demonstrated reduced activation of monocytes with diminished pERK, TNF-α release, and FcγRIIa recruitment to lipid rafts. These data demonstrate GA101 and ofatumumab are superior to rituximab against CLL cells via different mechanisms of potential tumor elimination. These findings bear relevance to potential combination strategies with each of these anti-CD20 antibodies in the treatment of CLL.
INTRODUCTION
Expression of CD20 glycoprotein is tightly restricted to the surface of B cells, making it an ideal therapeutic target for antibody therapy. Over the past decade, CD20 has become a well-validated target for therapy in B cell malignancies, mainly due to the approval of rituximab for Non-Hodgkins Lymphoma in 1997. Rituximab is chimeric monoclonal antibody that has revolutionized therapy in a variety of B cell malignancies, including chronic lymphocytic leukemia (CLL). In CLL, rituximab was shown to have modest single agent activity (reviewed in(1, 2)) but has shown greatest promise in combination with chemotherapy (chemoimmunotherapy), where retrospective phase II comparison studies (3) (4) and a recent prospective phase III study demonstrated prolongation of survival(5). Despite its successes, not all patients respond to rituximab therapy and virtually all relapse. Improving the properties of rituximab to enhance its efficacy further is therefore highly desirable.
B cell depletion by rituximab and other anti-CD20 antibodies has been proposed to occur via several mechanisms. While many effector cells including Natural Killer (NK) cells, monocytes, macrophages, and granulocytes can mediate ADCC, several sentinel papers in mouse models have revealed that B cell depletion with anti-CD20 or anti-CD19 antibodies are predominantly dependent on monocytes and their expression of FcγRIIa, FcγRIIIa, and FcγRIV(6) (7) (8). Furthermore, others have shown that Tumor Necrosis Factor-α (TNF-α) secreted by monocytes activates NK cells and this crosstalk mediates enhanced ADCC (9) (10). In humans, NK cells have been suggested to be most important for rituximab tumor clearance based upon the FcγRIIIa single nucleotide polymorphisms (SNPs) expressed predominately in this cell type and result in a low or high affinity receptor that is highly predictive of antibody response (11) (12) and of normal B cell depletion(13). In CLL, these same FcγRIIIa SNPs have no correlation with response(14) (15) or extended progression free survival(16). The true importance of NK cells, monocytes, or other effector cells to CD20 antibody mediated killing in CLL remains controversial.
Other mechanisms of anti-CD20 mediated cytotoxicity including direct cell death and complement dependent cytotoxicity have also been documented. Direct cytotoxicity with Type I anti-CD20 antibodies such as rituximab generally require cross-linking with an anti-Fc directed antibody in vitro(17, 18), proposing to mimic in vivo binding to FcγR on effector cells. Evidence of in vivo apoptosis following rituximab treatment in CLL cells has supported this as a mechanism of action (19). However, a recent study has challenged this by using a novel mouse model with a FcγR lacking the active immune tyrosine activating motif (ITAM) that demonstrated little in vivo activity with CD20 antibodies (20). Type II anti-CD20 antibodies lack the need for cross linking and offer a potential advantage clinically by promoting homotypic adhesion and actin-dependent, lysosome-mediated cell death (21). Complement Dependent Cytotoxiciy (CDC) with rituximab occurs but the antigen density on CLL cells limits killing by this mechanism (22) (23). Additionally, up-regulation of complement protection antigens CD55 and CD59 may occur after rituximab based therapy (24) (25).
Based on the success of rituximab in NHL and CLL, the next generation of anti-CD20 therapeutic antibodies is emerging, intelligently engineered to enhance efficacy of anti-CD20 therapy via different mechanisms of action. Ofatumumab (Arzerra) is a human, Type I antibody that uniquely binds to the small and large extracellular loop of CD20 (26). It has been shown to induce potent CDC in vitro compared to rituximab at low concentrations and low antigen density (27) (26). Clinically, ofatumumab produced clinical responses in more than 50% of fludarabine and alemtuzumab refractory CLL patients with modest toxicity (28) (29) and is active in patients irrespective of prior treatment with rituximab (30). It is currently approved for this indication. GA101 (Obinutuzumab) is a Type II humanized anti-CD20 antibody that promotes direct killing without in vitro cross-linking and has an afucosylated Fc domain engineered for enhanced FcγRIIIa binding (31–36). Direct cell death and ADCC by NK cells is superior with GA101 as compared to rituximab against malignant B cells (31). A phase I study with GA101 in CLL showed favorable efficacy in relapsed/refractory patients with modest toxicity (37). The follow up phase II study of 20 relapsed and refractory CLL patients demonstrated promising blood clearance of tumor cells but only a 20% response by IWCLL 2008 response criteria (38). This response criterion has a 50% reduction of nodal mass by CT assessment for partial response, which was the most frequent reason for not attaining this. Prior studies with other therapeutic antibodies have not mandated CT for assessment of response but rather relied on physical exam measurements alone. Response in nodal disease may also have been influenced by dose of GA101 and larger studies will be required to ascertain the true efficacy of this antibody.
Whereas ofatumumab and GA101 have each been compared to rituximab in CLL, no study has compared these two antibodies or examined each ones interaction with monocytes or macrophages. Herein we report such a detailed analysis demonstrating ofatumumab and GA101 are both superior to rituximab but each has varied ability to activate different effector cell types that might bear relevance to combination based strategies in CLL.
MATERIALS & METHODS
Patient sample processing and cell culture
Blood was obtained from patients with informed consent in accordance with the Declaration of Helsinki and under a protocol approved by the Institutional Review Board of The Ohio State University (OSU). All patients examined in this series had immunophenotypically defined CLL and had been without prior therapy for a minimum of 30 days at the time of collection. CLL PBMC were isolated from freshly donated blood with Ficoll density gradient centrifugation (Ficoll-Plaque Plus, Amersham Biosciences, Piscataway, NJ). Enriched CLL fractions were prepared with the use of the “Rosette-Sep” kit from StemCell Technologies (Vancouver, British Columbia, Canada) according to the manufacturer’s instructions. Isolated cells were incubated in RPMI 1640 (Life Technologies, Grand Island, NY) media supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich, St. Louis, MO), 2mM L-glutamine (Life Technologies, Carlsbad, CA), and 56 U/mL penicillin/56 μg/mL streptomycin (Life Technologies) at 37°C in an atmosphere of 5% CO2. Normal cells were obtained from Red Cross partial leukocyte preparations, and natural killer (NK) cells were negatively selected with Rosette-Sep kits (StemCell Technologies) according to the manufacturer’s instructions. Monocytes were positively selected using MACS system (Miltenyi, Cambridge, MA). The purity of enriched populations of normal cells was routinely checked with the use of CD19, CD56, and CD14-PE staining by flow cytometry. NK cell and monocyte purity usually ranged from 75% to 90%, while purity of B cells was usually over 85% CD19+. Normal samples were from anonymous donors as part of a second exemption protocol approved by the institutional review board at OSU. The Raji and THP-1 cell lines were obtained from ATCC (Manassas, VA) and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. The NK92 CD16+ cells have been previously described (39).
In vitro treatment of cells with antibodies
Cells were suspended in complete media at a density of 1×107 cells/mL immediately after isolation. All therapeutic antibodies were used at 10 μg/mL unless otherwise noted. Where indicated, the Fc specific goat anti–human IgG crosslinker (Jackson Immunoresearch, West Grove, PA) was added to the cell suspension 5 minutes after adding the primary antibodies, at a concentration 5 times that of the primary antibodies (i.e., 50 μg/mL for 10 μg/mL). In addition, a group of samples with no treatment was collected as media control.
Assessment of apoptosis by flow cytometry
The apoptosis of cells was measured using annexin V-FITC/PI staining followed by FACS analysis according to the manufacturer’s protocol (BD Pharmingen, San Diego, CA) as described previously (40). Results are presented as percentage cytotoxicity, which is defined as (% annexinV± and/or PI± cells of treatment group)/(% annexin V± and/or PI± cells of media control) × 100. FACS analysis was performed using a Beckman Coulter FC500 cytometer (Beckman Coulter, Indianapolis, IN). Ten thousand events were collected for each sample and data were acquired in list mode.
Complement Dependent Cytotoxicity (CDC)
CLL B cells were suspended at 106/mL in RPMI 1640 media, media with 30% plasma from the patient blood samples, or media with 30% heat-inactivated (56°C, 30 minutes) plasma. Cells were then treated with antibodies and incubated at 37°C for 1 hour, pelleted and resuspended in 1% Formaldehyde with Live/Dead Stain (Sigma-Aldrich). The extent of CDC was measured by FACS analysis of percent staining for dead.
Antigen Quantification
Quantitative analysis of CD20 surface density was done using the Quantum Simply Cellular kit (Bangs Laboratories, Fishers, IN), according to the manufacturer’s instructions.
In vitro stimulation and cytokine assays
For in vitro NK cell stimulation experiments, wells of a 96-well flat-bottom plate were coated with 20 μg/mL of antibody in PBS. Freshly isolated NK cells were plated at 2×105 cells/well. CD107a-FITC or isotype control (BD Pharmingen) was added to the suspension at the start of the 4-hour incubation at 37°C. NK cells were harvested at the end of the 4-hour incubation period and stained with CD56-PE and analyzed by FACS for CD107a surface expression.
For NK cell cytokine experiments, cell-free culture supernatants were harvested after 4-hours of stimulation with immobilized antibody and analyzed for levels of IFN-γ by A Quantikine Human IFN-γ ELISA, performed according to the manufacturer’s instructions (R&D Systems). For experiments with monocytes and monocyte derived macrophages (MDM), cell- free culture supernatants were harvested after 24-hours of stimulation with immobilized antibody and analyzed for levels of TNF-α by A Quantikine Human TNF-α ELISA, performed according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Real-time reverse transcription–polymerase chain reaction
Monocytes were stimulated 18 hours with immobilized antibodies. Cells were collect and total RNA was extracted using TRIzol (Life Technologies). Real-Time PCR for TNF-α was performed using pre-designed TaqMan® Gene Expression Assays and ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA).
Antibody-dependent cellular cytotoxicity (ADCC) assay
ADCC activity was determined by standard 4-hour 51Cr-release assay. 51Cr-labeled target cells (5×103 cells/well of B CLL or Raji cells) were incubated 30 minutes with various concentrations of antibodies. Unbound antibodies were washed off and cells were placed in to 96-well plates. Effector cells (NK cells from healthy donors or CLL patients) were then added to the plates at the indicated effector-to-target (E:T) ratios. After 4 hours of incubation, supernatants were removed and counted on a Perkin Elmer (Waltham, MA) Wizard gamma counter. The percentage of specific cell lysis was determined by: % lysis = 100 × (ER − SR)/(MR − SR), where ER, SR, and MR represent experimental, spontaneous, and maximum release, respectively.
Antibody-dependent Cellular Phagocytosis (ADCP) Assay
Monocyte Derived Macrophages (MDMs) were derived from peripheral blood monocytes using Macrophage Colony-Stimulating Factor (M-CSF) (R&D Systems) at 20 μg/mL for 5 to 7 days. The MDM were fluorescently labeled with Min-Claret dye (Sigma-Aldrich). CLL cells were fluorescently labeled with PKH-67 (Sigma-Aldrich) and coated with antibody for one hour at 4°C. MDM and CLL cells were coincubated for 30 minutes at a 1:5 Effector: Target ratio, then colocalization of CLL with MDM was scored using flow cytometry and verified using microscopy.
Lipid Rafts and Immunoblot Analysis
Lipid raft fractions were isolated using a sucrose gradient, as previously described by our group (41). Purity of lipid raft fractions was determined by using cholera-toxin presence. Whole cell extracts were prepared as previously described by our group (42) with the addition of phosphatase inhibitor cocktail 1 and 2, protease inhibitor cocktail P8340 and 1 mM phenylmethylsulfonyl fluoride (all from Sigma-Aldrich) to the lysis buffer. Equivalent amounts of protein were separated on polyacrylamide gels and transferred onto nitrocellulose membranes. Following antibody incubations, proteins were detected with chemiluminescent substrate (Thermo Fisher Scientific, Rockford, IL). The following antibodies were used for detection: anti-phospho-ERK1/2 (Thr202/Tyr204) and anti-ERK1/2 (Cell Signaling Technologies, Danvers, MA); Anti-pan phosphor-tyrosine antibody 4G10 and anti- GAPDH (Millipore, Billerica, MA), Cholera-toxin (Sigma-Aldrich), and Actin (Santa Cruz, Santa Cruz, CA). The FcγRIIa and FcγRIIb antibodies have been previously described (43).
Statistics Methods
Considering each of the patient’s cells (CLL B cells, monocytes and MDM) were used under all indicated conditions of each experiment, linear mixed effect models were used to estimate unrestricted covariance structures and robust hypothesis tests (44).
RESULTS
GA101 mediates superior direct cytotoxicity with or without anti-Fc crosslinking antibody
Direct signaling to apoptosis has been shown to play a role in anti-CD20 therapy (19) (45). GA101, as a Type II antibody, promotes about 25% cell death of CLL B cells, with a range of 0 to 61%, in the absence of an Fc crosslinker. This is significantly more than the type I antibodies ofatumumab or rituximab in the absence of a crosslinking antibody (Figure 1A) (p = 0.0012 or 0.013, respectively). This direct cytotoxicity study was further extended to compare the three anti-CD20 antibodies along with the Fc cross-linking antibody in a larger sample of patients (n = 19) (Figure 1B). No enhanced cytotoxicity was observed with GA101 treatment when treated along with an Fc cross-linking antibody. GA101 mediated significantly greater cytotoxicity with an Fc cross-linking antibody than rituximab (p = 0.003) or ofatumumab (p = 0.03) (Figure 1B). The anti-CD52 antibody alemtuzumab is used here, as well as other experiments, as a positive control for antibody function in the assay. Collectively, these studies suggest the Type II antibody GA101 mediates superior direct cytotoxicty without requirement for an Fc cross-linking as compared to the Type I antibodies. However, ofatumumab mediated greater direct cytotoxicity with the Fc cross-linking antibody than rituximab (p = 0.0687).
Figure 1.
Anti-CD20 antibodies differentially mediate direct cytotoxicity and complement dependent cytotoxicity. (A.) GA101 mediates significantly greater direct cytotoxicity in CLL cells treated without a crosslinking antibody as compared to ofatumumab or rituximab (p = 0.0012 or 0.013, respectively) at 48 hours (n = 8). (B.) GA101 mediate significantly greater direct cytotoxicity on CLL cells with an Fc crosslinking antibody than rituximab (p = 0.003) or ofatumumab (p = 0.03). Flow cytometry results after treatment with 10 μg/ml of each antibody with 50 μg/ml of goat anti-human anti Fc crosslinking antibody for 48 hours (n = 19). (C.) Ofatumumab mediates enhanced Complement dependent Cytotoxicity (CDC) with human serum on previously frozen CLL B cells (p = 0.0001, n = 19). All antibodies used at 10 μg/ml. (D.) Quantification of CD20 on the surface of CLL cells by flow cytometry (n = 19). (E.) Ofatumumab shows a weak correlation between surface CD20 density and CDC (n = 19) as compared to GA101 and rituximab (r2 = 0.3, p = 0.026).
Ofatumumab mediates superior complement-dependent cytotoxicity
Initiation of complement can be a potent method of cytotoxicity for therapeutic antibodies (46) (47). In order to test the ability of the three CD20-directed antibodies to initiate complement, we examined CLL cells for sensitivity to complement-mediated killing. As shown in Figure 1C, ofatumumab demonstrates ~30% CDC in CLL cells, significantly greater than either rituximab or GA101 (p = 0.0001). This enhanced CDC with ofatumumab was maintained regardless whether fresh or frozen primary CLL samples were used as targests (data not shown). Quantification of CD20 antigen on CLL patient B cells showed a wide range of CD20 surface expression (from 17,000 to 600,000) (Figure 1D) and a weak correlation (r2 = 0.3, p = 0.026) between the level of CDC induced by ofatumumab and CD20 surface expression, as depicted in Figure 1E. There was no correlation between surface CD20 and CDC seen with rituximab (p = 0.96) or with GA101 (p = 0.836). Collectively, these data demonstrate that ofatumumab is superior to GA101 or rituximab in mediating CDC against CLL cells.
GA101 stimulates enhanced NK cell activation compared to ofatumumab or rituximab
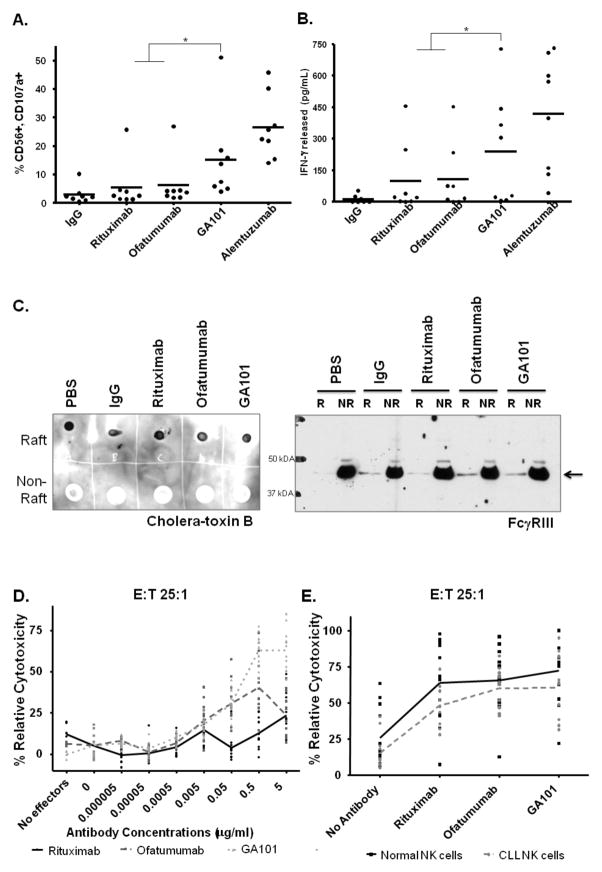
NK cells are the major effector cell population implicated in mediating ADCC. ADCC is initiated through FcγRIIIa (CD16) engagement (48) and engineered antibodies that are either mutated or lack a core fucosylation in the Fc region generally have enhanced affinity for this receptor. As an afucosylated antibody, GA101 has been reported to mediate enhanced NK cell ADCC (32, 34–36). In order to compare GA101 to the other anti-CD20 antibodies in terms of NK cell function, we first examined NK cell activation by looking at the activation marker CD107a. Normal donor NK cells were stimulated with immobilized antibody and CD107a expression was measured by flow cytometry. As shown in Figure 2A, GA101 significantly induces more CD107a expression on NK cells than ofatumumab (p = 0.02) or rituximab (p = 0.005). Furthermore, GA101 promoted a significant amount of IFN-γ release from stimulated normal donor NK cells as compared to ofatumumab and rituximab (p = 0.0008) (Figure 2B). There was no significant difference observed in IFN-γ production between ofatumumab and rituximab. Collectively, these data suggest GA101 is a better activator of NK cells as compared to ofatumumab or rituximab.
Figure 2.
NK cell function with anti-CD20 antibodies. (A.) GA101 stimulates significantly enhanced CD107a expression on primary NK cells than ofatumumab or rituximab (p = 0.02 or 0.005, respectively), as analyzed by flow cytometry (n = 8). (B.) Immobilized GA101 induces significantly more IFN-γ release from primary NK cells than ofatumumab or rituximab (p = 0.0008), as measured by ELISA (n = 8). (C.) GA101 and ofatumumab show recruitment of FcγRIII to lipid rafts. Immunoblot analysis for FcγRIII of lipid raft fraction made from stimulating NK92 CD16+ cells with immobilized antibodies for 5–7 minutes (results shown are representative of three independent experiments). Cholera-toxin blot on the right of panel 2C indicates purity of raft versus nonraft fractions. R = Raft, NR = NonRaft (D.) ADCC with normal NK cell effectors against CLL B cell targets in decreasing antibody concentrations (n = 4 CLL B cell targets with 3 NK cell effectors each). GA101 shows enhanced ADCC at higher concentrations as compared to ofatumumab or rituximab (p<0.0001). (E.) ADCC with CLL or normal NK cell effectors against Raji cell targets (n = 12) shows insignificant difference in ADCC between NK cells from CLL patients and normal donor NK cells with anti-CD20 antibodies (p = 0.141, 0.464, 0.085, for GA101, ofatumumab, and rituximab respectively), although the CLL cells have an overall trend toward decreased function.
Binding of FcγRs on immune cells and recruitment of these receptors into lipid rafts is necessary for signaling within effector cells. Therefore, we next evaluated the recruitment of FcγRIII to lipid rafts on NK cells post-stimulation with immobilized anti-CD20 antibody. In order to obtain sufficient cells to isolate lipid rafts, we utilized the NK92 cell line that has been stably transfected to express FcγRIII (39). These cells were briefly stimulated with immobilized antibodies and lipid raft fractions were isolated with subsequent assessment by immunoblot to study if FcγRIII was differentially recruited into the rafts. As seen in the representative blot in Figure 2C, FcγRIII was recruited equally into the raft fraction with GA101 and with ofatumumab. Rituximab showed recruitment to a lesser degree. Analysis of the lipid raft with cholera-toxin revealed the presence of GM1 ganglioside binding activity in the raft but not in the non-raft fractions demonstrating purity of the lipid raft preparations used in these studies.
GA101 mediates enhanced NK cell ADCC as compared to ofatumumab and rituximab
Activation of NK cells, as measured by CD107a and IFN-γ production, and recruitment of FcγRIII to lipid rafts often correlates with ADCC potential (49). To confirm superiority of GA101 in NK cell function, normal donor NK cells were used as effectors against CLL B cell targets in standard 51Cr release assays. As expected with an afucosylated Fc region engineered antibody, GA101 mediated significantly more ADCC than ofatumumab (p<0.0001) or rituximab (p<0.0001) at 5 μg/ml of antibody (Figure 2D). However, at decreasing concentrations of antibody, the enhanced ADCC seen with GA101 is no longer apparent. At concentrations of 0.05 μg/ml and less, GA101 is no longer significantly more effective than ofatumumab (p = 0.9761). In addition, at those lower antibody concentrations, ofatumumab is superior in ADCC function compared to rituximab (p<0.0001).
NK cells from CLL patients have been reported to have decreased effector function (50) and are most relevant to the studies pursued herein. To test these engineered antibodies with NK cells from CLL patients, ADCC assays were performed using NK cells from twelve patients with early stage disease as effectors against Raji cell targets (Figure 2E). At 10 μg/ml concentrations GA101, ofatumumab, and rituximab demonstrate insignificant difference in ADCC between NK cells from CLL patients and normal donor NK cells (p = 0.141, 0.464, 0.085, respectively), although the CLL cells have an overall trend toward decreased function. Collectively, these data support the findings of others that CLL patients NK cells may have a very modest defect in NK cell ADCC (51, 52) against CD20-targetted cells, however GA101 remains superior to ofatumumab or rituximab at NK cell-mediated ADCC.
The Anti-CD20 antibodies differentially stimulate monocytes
Monocytes have been implicated as being the most important effector cell in CD20-directed antibody efficacy in murine models (6) (8). Similar to the antibody effector function of NK cells, monocyte and monocyte derived macrophage (MDM) activation and phagocytosis is governed by the interplay between FcγRI, FcγRIIa, FcγRIIb and FcγRIIIa which are all expressed in these cells (53) (54). In order to compare GA101 and ofatumumab to rituximab in human monocytes and MDM, we tested production of TNF-α cytokine by monocytes from normal donors. Monocytes were stimulated with immobilized GA101, ofatumumab or rituximab and cells or supernatants were collected for mRNA and TNF-α analysis respectively. As seen in Figure 3A, monocytes have decreased mRNA levels of TNF-α following stimulation with GA101 as compared to rituximab or ofatumumab (p = 0.0018, <0.0001, respectively). Additionally, significantly less TNF-α is produced with GA101 as compared to rituximab (p = 0.018) (Figure 3B). GA101 also produces less TNF- α than ofatumumab, although this difference is not significant (p = 0.35) (Figure 3B).
Figure 3.
Monocyte and Monocyte Derived Macrophages function with CD20-directed antibodies (A.) GA101 stimulates less TNF-α messages levels in primary monocytes compared to ofatumumab or rituximab (p = 0.0018, <0.0001, respectively), as measured by RT-PCR. (n = 8). (B.) Immobilized GA101 induces significantly decreased TNF-α release from normal donor monocytes as compared to rituximab (n = 6) (p = 0.018). (C.) Immobilized GA101 induces decreased TNF-α release from normal donor Monocyte Derived Macrophages (MDM) than ofatumumab or rituximab (n = 5). (D.) Ofatumumab stimulates significantly more Antibody Dependent Phagocytosis by normal donor MDM of CLL B cells targets than GA101 or rituximab (p = 0.0036 or 0.03, respectively), as measured by flow cytometry (n = 6).
GA101 demonstrates inferior ADCP as compared to ofatumumab or rituximab
MDMs have also emerged as especially important in anti-CD20 antibody clearance of B cells, particularly in murine models (6). These macrophages have been demonstrated to phagocytose antibody-coated cells predominantly through FcγRIIa, with some contribution from FcγRI and FcγRIIIa (55) (48). We first examined the ability of immobilized GA101, ofatumumab, or rituximab to activate MDM, generated from normal human peripheral blood monocytes (PBM), as measured by TNF-α production. MDM cells exposed to plate-bound ofatumumab or rituximab produced higher levels of TNF-α, as compared to GA101 (p = 0.7, 0.34, respectively) (Figure 3C). These data support monocytes and MDM cells demonstrate inferior activation with GA101 as compared to ofatumumab or rituximab.
We next examined the ability of GA101, ofatumumab, or rituximab to mediate Antibody-dependent Cellular Phagocytosis (ADCP) by MDMs. Phagocytosis was tested by co-incubation experiments of membrane dyed MDM with antibody coated, membrane dyed CLL cells. The cells were analyzed by flow cytometry and percent phagocytosis was determined by double positivity for both dyes, indicating MDM that had ingested CLL cells. The results were validated by microscopy. Contrary to the TNF-α data, all three of the anti-CD20 antibodies show ADCP capability against CLL B cells (Figure 3D), with ofatumumab exhibiting the greatest ADCP (60% ± 8.8) as compared to rituximab (48% ± 17.8) and GA101 (41% ± 16.7). Ofatumumab is able to mediate significantly more ADCP as compared to GA101 (p = 0.0036) or rituximab (p = 0.03). Collectively, these data demonstrate that non-FcγR engineered ofatumumab and rituximab mediate superior MDM activation and phagocytosis as compared to GA101.
The anti-CD20 antibodies differentially signal in monocytes
We next sought to investigate the mechanism of differential TNF-α release and phagocytosis by the CD20-directed antibodies. Initial studies examined if there was differential global-tyrosine protein phosphorylation as measured by 4G10 immunoblots from monocytes stimulated with immobilized GA101, ofatumumab, or rituximab for 5 to 7 minutes. GA101 induced less pan tyrosine phosphorylation compared to the non Fc engineered ofatumumab or rituximab in the monocytic THP-1 cell line (Figure 4A) and in normal donor monocytes (data not shown). As the Fc binding region is responsible for binding to FcγR and ultimately recruitment to lipid rafts where activation signaling is mediated, we examined for differential phosphorylation and recruitment of FcγRIIa to lipid rafts by immobilized GA101, ofatumumab, and rituximab. Phosphorylation pattern of both the activating FcγRIIa (Figure 4B) and inhibitory FcγRIIb (Figure 4C) in monocytes exposed to the different immobilized CD20 antibodies does not clarify the differential TNF-α release.
Figure 4.
Anti-CD20 antibodies signal differentially in Monocytes. (A.) Immobilized GA101 induces decreased pan tyrosine-phosphorylated proteins response in THP-1 cells as compared to Ofatumuamb or rituximab (n = 4). (B.) There is no differential phosphorylation of FcγRIIb after stimulation with GA101 and ofatumumab. Primary monocytes stimulated 5–7 minutes on antibody coated plates and immunoblots tested for FcγRIIb (n = 3). (C.) There is no differential phosphorylation of FcγRIIa after stimulation with GA101 and ofatumumab. Pan phospho-tyrosine protein immunoprecipitation done on primary monocytes stimulated with plated antibodies for 5–7 minutes and immunoblots tested for FcγRIIa (n = 3). (D.) FcγRIIa is not recruited to lipid rafts after stimulating THP-1 cells with immobilized GA101 (representative of three independent experiments). FcγRIIa recruitment is seen with ofatumumab and rituximab. Cholera-toxin blot on the right of panel 4D indicates purity of raft versus nonraft fractions. R = Raft, NR = NonRaft (E.) The common γ-chain is not differentially recruited to lipid rafts in the same samples as 4D, indicating no differential recruitment of FcγRI or FcγRIII (representative of three independent experiments) in THP-1 cells with the anti-CD20 antibodies. (F.) Immunoblot analysis shows decreased phosphorylation of ERK in THP-1 cells after stimulation with immobilized GA101, as compared to ofatumumab and rituximab (results shown is a representative of three independent experiments).
Since phosphorylation of the receptor did not explain the differential activation of monocytes, we hypothesized potential differences in recruitment of the Fcγ Receptors into signaling lipid rafts. In Figure 4D we demonstrate that GA101 does not induce recruitment of the activating FcγRIIa to lipid rafts in the THP-1 cell line. This is in contrast to what is observed with the positive control (IgG), ofatumumab, and rituximab where robust recruitment of this ITAM containing FcγR occurs. Analysis of the lipid raft with cholera-toxin revealed the presence of GM1-ganglioside binding activity only in the raft, demonstrating purity of the lipid raft preparations (Figure 4D, left panel).
Next, to see if other receptors such as FcγRIIIa and FcγRI were differentially recruited we investigated the effect of CD20-directed antibodies on γ-chain recruitment to lipid rafts (Figure 4E). Whereas FcγRIIa contains its own cytosolic ITAM motif, the other activating FcγReceptors, namely Fc γRIIIa and FcγRI associate with an ITAM containing common γ-chain to initiate activating signals. There were no differences in recruitment of the common γ-chain between the three anti-CD20 antibodies tested, indicating no differences in FcγRIIIa and FcγRI recruitment. Therefore, GA101 lacks recruitment of only FcγRIIa into lipid rafts in monocytes.
To further explore if downstream signaling is differentially diminished in GA101 stimulated monocytes, we examined alteration of phospho-ERK following exposure to immobilized antibodies. Downstream phosphorylation of ERK following FcγR crosslinking has been demonstrated by others to be critical for the induction of transcription factors such as NFκBand c-fos and subsequent expression of cytokines (56) (57). In Figure 4F, we demonstrate that GA101 results in decreased phosphorylation of ERK (T202/Y204) as compared to ofatumumab or rituximab in THP-1 cells. This was further verified in primary monocytes (data not shown). These results collectively suggests that GA101 demonstrates diminished recruitment of FcγRIIa to lipid rafts and decreased signaling in monocytes as compared to ofatumumab or rituximab.
Combination of anti-CD20 antibodies with TLR-agonist R-848 enhances MDM cytokine production
Toll-like receptor (TLR) 7/8 agonists work predominantly through cytokine production (58). R-848, a TLR 7/8 agonist, has also been shown to increase expression of the activating Fc receptors FcγRI and FcγRIIa, as well as the common γ chain. Furthermore, monocytes treated with R-848 decreased expression of the inhibitory receptor, FcγRIIb (59). Given this positive link between the TLR7/8 and FcγR pathways, we set out to determine if the cytokine deficiency seen with the anti-CD20 antibodies could be enhanced with R-848. Monocytes or MDM were pretreated overnight with R-848 and subsequently stimulated for 24 hours with immobilized antibodies. TNF-α levels in the supernatant were measured by ELISA. Monocytes treated with R-848 followed by stimulation with GA101 showed no increase in TNF-α production, similar to R-848 alone stimulated cells (p = 0.92) (Figure 5A). In contrast, MDM pre-treated with R-848 showed significantly increased TNF-α produced by GA101 stimulated MDM over R-848 alone stimulation (p = 0.0071) (Figure 5B) to levels that were similar to the other anti-CD20 antibodies. This suggests that combination therapy of GA101 with R-848 may rescue the decreased cytokine release phenotype seen from MDM.
Figure 5.

Combination therapy of anti-CD20 antibodies and R-848. (A.) Pretreatment of primary monocytes with R-848 enhances overall production of TNF-α by rituximab, ofatumumab, or GA101 (n = 6), as measured by ELISA that is not significant over unstimulation monocytes (p = 0.49, 0.59, 0.92, respectively). (B.) Pretreatment of primary MDM with R-848 leads to increase in TNF-α production after stimulation with rituximab, ofatumumab, or GA101, which is significantly enhanced over unstimulated monocytes(p = 0.0005, 0.0005, 0.0071, respectively) (n = 6).
DISCUSSION
Herein, we have shown the divergent effector properties of three clinically relevant anti-CD20 antibodies. Both GA101 and ofatumumab are superior to rituximab in separate ways. GA101 displays superior direct cytotoxicity without an in vitro crosslinking antibody. Its engineered Fc region elicits enhanced NK cell stimulation and IFN-γ release, and subsequently superior NK cell-mediated ADCC. Conversely, ofatumumab exhibits superior complement activation against primary CLL cells, independent of CD20 antigen density. It mediates the greatest MDM-mediated ADCP. In addition, rituximab and ofatumumab elicited TNF-α release from both monocytes and MDM, while GA101 exhibited decreased response. Although high levels of TNF-α production post therapeutic antibody infusion may not be clinically desirable (60) (61) (62), TNF-α release from mononuclear cells is needed for death signal to target cells and for crosstalk to NK cells (9) (10). In addition, although GA101 was not able to elicit cytokine response from MDM, it had moderate ADCP function. This suggest that separate pathways may be involved for these two functions. Lastly, monocytes showed an overall decrease in tyrosine-phosphorylated proteins post stimulation with GA101 and decreased recruitment of FcγRIIa into lipid rafts.
The impact of Fc region afucosylation of antibodies on monocytes function has only been implied by studies looking at the binding affinity of these antibodies to FcγRII. Low-fucosylated antibodies have moderate to no enhanced binding to FcγRIIa, FcγRIIb, or FcγRIa compared to their high-fucosylated counterparts (63) (64). This would imply that monocytes, with their predominant expression of FcγRIIa and FcγRIIb, should not be differentially affected by glycoengineered antibodies. However, these systems only look at Fc region binding affinities to receptors and not how the receptors interact with the antibodies, i.e. recruitment to rafts and signaling. This process has not been described with Fc-engineered antibodies. Our results suggest that perhaps there may be differences in interaction with alternative FcγRs due to glycoengineering.
Lack of recruitment of FcγRIIa into lipid rafts has numerous implications for signaling in monocytes. FcγRIIa has a cytosolic ITAM motif, a sequence of conserved amino acids found on many immune receptors that contains a tyrosine that can be phosphorylated by activating kinases. Unlike GA101, the non-Fc engineered anti-CD20 antibodies are able to recruit FcγRIIa to the rafts, indicating that perhaps Fc engineering for enhanced binding to FcγRIII may affect IgG interactions with alternative FcγRs. This lack of recruitment to the rafts post GA101 treatment leads to decreased activation as reflected by a decrease in pan tyrosine phosphorylated proteins. This is further confirmed by diminished phosphorylation of ERK, a downstream target of FcγRIIa signaling, and functionally by decreased cytokine production.
Decreased activation of monocytes and MDM by GA101 may have clinical implication for therapy. Given the role of these cell types in anti-CD20 mediated B cell depletion in mouse models, this may explain the potential diminished efficacy seen with GA101 clinically in CLL and NHL as compared to trials done with other CD20-directed antibodies (65) (66, 67). GA101 showed lymphocyte depletion until week 25 but disease progression in patients with high tumor burden (68). Furthermore, our studies show that NK cell-mediated ADCC is not maintained at superior level with low antibody concentrations, making dosing schedules quite relevant. However, the deficiencies in monocyte and MDM cytokine production by GA101 can be overcome using combination therapy with TLR 7/8 agonists such as R-848. TLR 7 or 7/8 agonist have been successfully used in the clinic (reviewed in (69)) and have been shown to have antitumor responses in murine models (70).
The interaction of FcγRs on effector cells with engineered Fc portions is vital in antibody therapeutics. While engineered antibodies are designed to be effective activators of CLL NK cells in vitro (71), the in vivo role of these antibodies in activation of NK cells, monocytes, and MDM is controversial. Differential effects of the novel CD20 directed antibodies reported here underline the importance in choosing the ideal CD20 antibody as a single agent or in combination in the context of the functional competency of the relevant effector cell populations in CLL and other B cell malignancies.
Acknowledgments
We thank the patients for providing research samples used in this study and members of the CLL Experimental Therapeutics laboratory for critical comments.
Grant support: We are grateful for research support from The Leukemia and Lymphoma Society, P50-CA140158, PO1-CA95426, The Harry Mangurian Foundation, and The D. Warren Brown Foundation.
Footnotes
Authorship Contributions and Disclosure of Conflicts of Interest.
SR designed and performed experiments, wrote the first draft of the manuscript, contributed to revisions of the paper, and approved the final submitted version. JPB and CC provided input into experimental design, performed experiments, and reviewed and approved the final version of the manuscript. DJ and XM assisted in design of experiments, performed the statistical analysis reported, reviewed and approved the final version of the manuscript. RT, MC, and ST provided input into experimental design, and reviewed drafts of the manuscript and approved the final submitted version. NM and JCB obtained funding to perform the research, designed the experiments, participated in the analysis of the data, review of multiple drafts of the manuscript and approved the final version for submission.
References
- 1.Jaglowski SM, Alinari L, Lapalombella R, Muthusamy N, Byrd JC. The clinical application of monoclonal antibodies in chronic lymphocytic leukemia. Blood. 116:3705–3714. doi: 10.1182/blood-2010-04-001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaglowski SM, Byrd JC. Rituximab in chronic lymphocytic leukemia. Seminars in hematology. 47:156–169. doi: 10.1053/j.seminhematol.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Tam CS, O’Brien S, Wierda W, Kantarjian H, Wen S, Do KA, Thomas DA, Cortes J, Lerner S, Keating MJ. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wierda W, O’Brien S, Wen S, Faderl S, Garcia-Manero G, Thomas D, Do KA, Cortes J, Koller C, Beran M, Ferrajoli A, Giles F, Lerner S, Albitar M, Kantarjian H, Keating M. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 5.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, Hensel M, Hopfinger G, Hess G, von Grunhagen U, Bergmann M, Catalano J, Zinzani PL, Caligaris-Cappio F, Seymour JF, Berrebi A, Jager U, Cazin B, Trneny M, Westermann A, Wendtner CM, Eichhorst BF, Staib P, Buhler A, Winkler D, Zenz T, Bottcher S, Ritgen M, Mendila M, Kneba M, Dohner H, Stilgenbauer S. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 6.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst R, Wang Y, Gallagher S, Mittereder N, Kuta E, Damschroder M, Woods R, Rowe DC, Cheng L, Cook K, Evans K, Sims GP, Pfarr DS, Bowen MA, Dall’Acqua W, Shlomchik M, Tedder TF, Kiener P, Jallal B, Wu H, Coyle AJ. B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody. J Pharmacol Exp Ther. 2010;335:213–222. doi: 10.1124/jpet.110.168062. [DOI] [PubMed] [Google Scholar]
- 8.Tedder TF, Baras A, Xiu Y. Fcgamma receptor-dependent effector mechanisms regulate CD19 and CD20 antibody immunotherapies for B lymphocyte malignancies and autoimmunity. Springer Semin Immunopathol. 2006;28:351–364. doi: 10.1007/s00281-006-0057-9. [DOI] [PubMed] [Google Scholar]
- 9.Dalbeth N, Gundle R, Davies RJ, Lee YC, McMichael AJ, Callan MF. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol. 2004;173:6418–6426. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- 10.Welte S, Kuttruff S, Waldhauer I, Steinle A. Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat Immunol. 2006;7:1334–1342. doi: 10.1038/ni1402. [DOI] [PubMed] [Google Scholar]
- 11.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 12.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Anolik JH, Campbell D, Felgar RE, Young F, Sanz I, Rosenblatt J, Looney RJ. The relationship of FcgammaRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis and rheumatism. 2003;48:455–459. doi: 10.1002/art.10764. [DOI] [PubMed] [Google Scholar]
- 14.Farag SS, I, Flinn W, Modali R, Lehman TA, Young D, Byrd JC. Fc gamma RIIIa and Fc gamma RIIa polymorphisms do not predict response to rituximab in B-cell chronic lymphocytic leukemia. Blood. 2004;103:1472–1474. doi: 10.1182/blood-2003-07-2548. [DOI] [PubMed] [Google Scholar]
- 15.Woyach JA, Lin TS, Lucas MS, Heerema N, Moran ME, Cheney C, Lucas DM, Wei L, Caligiuri MA, Byrd JC. Aphase I/II study of rituximab and etanercept in patients with chronic lymphocytic leukemia and small lymphocytic lymphoma. Leukemia. 2009;23:912–918. doi: 10.1038/leu.2008.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dornan D, Spleiss O, Yeh RF, Duchateau-Nguyen G, Dufour A, Zhi J, Robak T, Moiseev SI, Dmoszynska A, Solal-Celigny P, Warzocha K, Loscertales J, Catalano J, Afanasiev BV, Larratt L, Rossiev VA, Bence-Bruckler I, Geisler CH, Montillo M, Wenger MK, Weisser M. Effect of FCGR2A and FCGR3A variants on CLL outcome. Blood. 116:4212–4222. doi: 10.1182/blood-2010-03-272765. [DOI] [PubMed] [Google Scholar]
- 17.Chow KU, Sommerlad WD, Boehrer S, Schneider B, Seipelt G, Rummel MJ, Hoelzer D, Mitrou PS, Weidmann E. Anti-CD20 antibody (IDEC-C2B8, rituximab) enhances efficacy of cytotoxic drugs on neoplastic lymphocytes in vitro:role of cytokines, complement, and caspases. Haematologica. 2002;87:33–43. [PubMed] [Google Scholar]
- 18.Pedersen IM, Buhl AM, Klausen P, Geisler CH, Jurlander J. The chimeric anti-CD20 antibody rituximab induces apoptosis in B-cell chronic lymphocytic leukemia cells through a p38 mitogen activated protein-kinase-dependent mechanism. Blood. 2002;99:1314–1319. doi: 10.1182/blood.v99.4.1314. [DOI] [PubMed] [Google Scholar]
- 19.Byrd JC, Kitada S, Flinn IW, Aron JL, Pearson M, Lucas D, Reed JC. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood. 2002;99:1038–1043. doi: 10.1182/blood.v99.3.1038. [DOI] [PubMed] [Google Scholar]
- 20.Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, Li Y, Pitti R, Totpal K, Yee S, Ross S, Vernes JM, Lu Y, Adams C, Offringa R, Kelley B, Hymowitz S, Daniel D, Meng G, Ashkenazi A. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 2011;19:101–113. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov A, Beers SA, Walshe CA, Honeychurch J, Alduaij W, Cox KL, Potter KN, Murray S, Chan CH, Klymenko T, Erenpreisa J, Glennie MJ, Illidge TM, Cragg MS. Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells. The Journal of clinical investigation. 2009;119:2143–2159. doi: 10.1172/JCI37884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellosillo B, Villamor N, Lopez-Guillermo A, Marce S, Esteve J, Campo E, Colomer D, Montserrat E. Complement-mediated cell death induced by rituximab in B-cell lymphoproliferative disorders is mediated in vitro by a caspase-independent mechanism involving the generation of reactive oxygen species. Blood. 2001;98:2771–2777. doi: 10.1182/blood.v98.9.2771. [DOI] [PubMed] [Google Scholar]
- 23.Golay J, Lazzari M, Facchinetti V, Bernasconi S, Borleri G, Barbui T, Rambaldi A, Introna M. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood. 2001;98:3383–3389. doi: 10.1182/blood.v98.12.3383. [DOI] [PubMed] [Google Scholar]
- 24.Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri GM, Bernasconi S, Tedesco F, Rambaldi A, Introna M. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95:3900–3908. [PubMed] [Google Scholar]
- 25.Takei K, Yamazaki T, Sawada U, Ishizuka H, Aizawa S. Analysis of changes in CD20, CD55, and CD59 expression on established rituximab-resistant B-lymphoma cell lines. Leuk Res. 2006;30:625–631. doi: 10.1016/j.leukres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Hagenbeek A, Gadeberg O, Johnson P, Pedersen LM, Walewski J, Hellmann A, Link BK, Robak T, Wojtukiewicz M, Pfreundschuh M, Kneba M, Engert A, Sonneveld P, Flensburg M, Petersen J, Losic N, Radford J. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trial. Blood. 2008;111:5486–5495. doi: 10.1182/blood-2007-10-117671. [DOI] [PubMed] [Google Scholar]
- 27.Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, van Meerten T, Ebeling S, Vink T, Slootstra JW, Parren PW, Glennie MJ, van de Winkel JG. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 28.Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A, Robak T, Furman RR, Hillmen P, Trneny M, Dyer MJ, Padmanabhan S, Piotrowska M, Kozak T, Chan G, Davis R, Losic N, Wilms J, Russell CA, Osterborg A. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keating MJ, Dritselis A, Yasothan U, Kirkpatrick P. Ofatumumab. Nat Rev Drug Discov. 2010;9:101–102. doi: 10.1038/nrd3100. [DOI] [PubMed] [Google Scholar]
- 30.Wierda WG, Padmanabhan S, Chan GW, Gupta IV, Lisby S, Osterborg A. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood. 2011;118:5126–5129. doi: 10.1182/blood-2011-04-348656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH, Shimada K, Chan CH, Tutt A, Beers SA, Glennie MJ, Cragg MS, Illidge TM. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood. 117:4519–4529. doi: 10.1182/blood-2010-07-296913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E, Ferrara C, Sondermann P, Jager C, Strein P, Fertig G, Friess T, Schull C, Bauer S, Dal Porto J, Del Nagro C, Dabbagh K, Dyer MJ, Poppema S, Klein C, Umana P. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pievani A, Belussi C, Klein C, Rambaldi A, Golay J, Introna M. Enhanced killing of human B-cell lymphoma targets by combined use of cytokine-induced killer cell (CIK) cultures and anti-CD20 antibodies. Blood. 117:510–518. doi: 10.1182/blood-2010-06-290858. [DOI] [PubMed] [Google Scholar]
- 34.Bologna L, Gotti E, Manganini M, Rambaldi A, Intermesoli T, Introna M, Golay J. Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol. 186:3762–3769. doi: 10.4049/jimmunol.1000303. [DOI] [PubMed] [Google Scholar]
- 35.Dalle S, Reslan L, Besseyre de Horts T, Herveau S, Herting F, Plesa A, Friess T, Umana P, Klein C, Dumontet C. Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Molecular cancer therapeutics. 10:178–185. doi: 10.1158/1535-7163.MCT-10-0385. [DOI] [PubMed] [Google Scholar]
- 36.Patz M, Isaeva P, Forcob N, Muller B, Frenzel LP, Wendtner CM, Klein C, Umana P, Hallek M, Krause G. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. British journal of haematology. 152:295–306. doi: 10.1111/j.1365-2141.2010.08428.x. [DOI] [PubMed] [Google Scholar]
- 37.Fc Morschhauser G, Lamy T, et al. Phase I Study of RO5072759 (GA101) in Relapsed/Refractory Chronic Lymphocytic Leukemia. American Society of Hematology 2009 [Google Scholar]
- 38.Cartron G, Morschhauser F, Thieblemont C, Lamy T, Milpied N, Tilly H, Birkett J, Salles G, Hallek M. Results from a Phase II Study of Obinutuzumab (GA101) Monotherapy in Relapsed/Refractory Chronic Lymphocytic Leukaemia (CLL) European Hematology Association 2011 [Google Scholar]
- 39.Trotta R, Dal Col J, Yu J, Ciarlariello D, Thomas B, Zhang X, Allard J, 2nd, Wei M, Mao H, Byrd JC, Perrotti D, Caligiuri MA. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J Immunol. 2008;181:3784–3792. doi: 10.4049/jimmunol.181.6.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X, Lapalombella R, Joshi T, Cheney C, Gowda A, Hayden-Ledbetter MS, Baum PR, Lin TS, Jarjoura D, Lehman A, Kussewitt D, Lee RJ, Caligiuri MA, Tridandapani S, Muthusamy N, Byrd JC. Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood. 2007;110:2569–2577. doi: 10.1182/blood-2006-12-062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mone AP, Cheney C, Banks AL, Tridandapani S, Mehter N, Guster S, Lin T, Eisenbeis CF, Young DC, Byrd JC. Alemtuzumab induces caspase-independent cell death in human chronic lymphocytic leukemia cells through a lipid raft-dependent mechanism. Leukemia. 2006;20:272–279. doi: 10.1038/sj.leu.2404014. [DOI] [PubMed] [Google Scholar]
- 42.Johnson AJ, Lucas DM, Muthusamy N, Smith LL, Edwards RB, De Lay MD, Croce CM, Grever MR, Byrd JC. Characterization of the TCL-1 transgenic mouse as a preclinical drug development tool for human chronic lymphocytic leukemia. Blood. 2006;108:1334–1338. doi: 10.1182/blood-2005-12-011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi T, Ganesan LP, Cao X, Tridandapani S. Molecular analysis of expression and function of hFcgammaRIIbl and b2 isoforms in myeloid cells. Mol Immunol. 2006;43:839–850. doi: 10.1016/j.molimm.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 44.Pan XLX, Jarjoura D. Testing with SmallSample Size without Restricting Mixed Model Covariance Structure. In: Yasmin Saulnier YLaHS., editor. ICSA 2012 Applied Statistics Symposium. International Chinese Statistical Association; Boston, MA: 2012. [Google Scholar]
- 45.Stolz C, Hess G, Hahnel PS, Grabellus F, Hoffarth S, Schmid KW, Schuler M. Targeting Bcl-2 family proteins modulates the sensitivity of B-cell lymphoma to rituximab-induced apoptosis. Blood. 2008;112:3312–3321. doi: 10.1182/blood-2007-11-124487. [DOI] [PubMed] [Google Scholar]
- 46.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, Botto M, Introna M, Golay J. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–1587. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 47.Golay J, Cittera E, Di Gaetano N, Manganini M, Mosca M, Nebuloni M, van Rooijen N, Vago L, Introna M. The role of complement in the therapeutic activity of rituximab in a murine B lymphoma model homing in lymph nodes. Haematologica. 2006;91:176–183. [PubMed] [Google Scholar]
- 48.Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs. 2010;2:181–189. doi: 10.4161/mabs.2.2.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer L, Penack O, Gentilini C, Nogai A, Muessig A, Thiel E, Uharek L. The anti-lymphoma effect of antibody-mediated immunotherapy is based on an increased degranulation of peripheral blood natural killer (NK) cells. Experimental hematology. 2006;34:753–759. doi: 10.1016/j.exphem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Ziegler HW, Kay NE, Zarling JM. Deficiency of natural killer cell activity in patients with chronic lymphocytic leukemia. Int J Cancer. 1981;27:321–327. doi: 10.1002/ijc.2910270310. [DOI] [PubMed] [Google Scholar]
- 51.Le Garff-Tavernier M, Decocq J, de Romeuf C, Parizot C, Dutertre CA, Chapiro E, Davi F, Debre P, Prost JF, Teillaud JL, Merle-Beral H, Vieillard V. Analysis of CD16+CD56dim NK cells from CLL patients: evidence supporting a therapeutic strategy with optimized anti-CD20 monoclonal antibodies. Leukemia. 25:101–109. doi: 10.1038/leu.2010.240. [DOI] [PubMed] [Google Scholar]
- 52.Moga E, Canto E, Vidal S, Juarez C, Sierra J, Briones J. Interleukin-15 enhances rituximab-dependent cytotoxicity against chronic lymphocytic leukemia cells and overcomes transforming growth factor beta-mediated immunosuppression. Experimental hematology. 39:1064–1071. doi: 10.1016/j.exphem.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 54.Tridandapani S, Siefker K, Teillaud JL, Carter JE, Wewers MD, Anderson CL. Regulated expression and inhibitory function of Fcgamma RIIb in human monocytic cells. J Biol Chem. 2002;277:5082–5089. doi: 10.1074/jbc.M110277200. [DOI] [PubMed] [Google Scholar]
- 55.Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther. 2008;7:2517–2527. doi: 10.1158/1535-7163.MCT-08-0201. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez-Mejorada G, Rosales C. Fcgamma receptor-mediated mitogen-activated protein kinase activation in monocytes is independent of Ras. J Biol Chem. 1998;273:27610–27619. doi: 10.1074/jbc.273.42.27610. [DOI] [PubMed] [Google Scholar]
- 57.Trotta R, Kanakaraj P, Perussia B. Fc gamma R-dependent mitogen-activated protein kinase activation in leukocytes: a common signal transduction event necessary for expression of TNF-alpha and early activation genes. J Exp Med. 1996;184:1027–1035. doi: 10.1084/jem.184.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol. 2002;27:571–577. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 59.Butchar JP, Mehta P, Justiniano SE, Guenterberg KD, Kondadasula SV, Mo X, Chemudupati M, Kanneganti TD, Amer A, Muthusamy N, Jarjoura D, Marsh CB, Carson WE, 3rd, Byrd JC, Tridandapani S. Reciprocal regulation of activating and inhibitory Fc{gamma} receptors by TLR7/8 activation: implications for tumor immunotherapy. Clin Cancer Res. 2010;16:2065–2075. doi: 10.1158/1078-0432.CCR-09-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8) Blood. 1999;94:2217–2224. [PubMed] [Google Scholar]
- 61.Byrd JC, Waselenko JK, Maneatis TJ, Murphy T, Ward FT, Monahan BP, Sipe MA, Donegan S, White CA. Rituximab therapy in hematologic malignancy patients with circulating blood tumor cells: association with increased infusion-related side effects and rapid blood tumor clearance. J ClinOncol. 1999;17:791–795. doi: 10.1200/JCO.1999.17.3.791. [DOI] [PubMed] [Google Scholar]
- 62.Wing MG, Moreau T, Greenwood J, Smith RM, Hale G, Isaacs J, Waldmann H, Lachmann PJ, Compston A. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J Clin Invest. 1996;98:2819–2826. doi: 10.1172/JCI119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 64.Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, Bleeker WW, Dechant M, Beyer T, Repp R, van Berkel PH, Vink T, van de Winkel JG, Parren PW, Valerius T. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood. 2008;112:2390–2399. doi: 10.1182/blood-2008-03-144600. [DOI] [PubMed] [Google Scholar]
- 65.Salles GAMF, Thieblemont C, et al. Promising efficacy with the new anti-CD20 antibody GA101 in heavily pre-treated patients: first results from a phase II study in patients with relapse/refractory indolent NHL. Program and abstracts of the 2010 EHA Congress; June 10–13, 2010; Barcelona, Spain. p. Abstract 0558 (oral). [Google Scholar]
- 66.Salles G, Morschhauser F, Lamy T, Milpied NJ, Thieblemont C, Tilly H, Bieska G, Asikanius E, Carlile D, Birkett J, Pisa P, Cartron G. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012 doi: 10.1182/blood-2012-01-404368. [DOI] [PubMed] [Google Scholar]
- 67.Sehn LH, Assouline SE, Stewart DA, Mangel J, Gascoyne RD, Fine G, Frances-Lasserre S, Carlile DJ, Crump M. A phase I study of obinutuzumab induction followed by two years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012 doi: 10.1182/blood-2012-02-408773. [DOI] [PubMed] [Google Scholar]
- 68.Salles G, MFTC, Solal-Celigny P, Lamy T, Tilly H, Feugier P, Le Gouill S, Gyan E, Bouabdallah R, Wenger MK, Wassner-Fritsch E, Asikanius E, Cartron G. Efficacy and Safety of Obinutuzumab (GA101) Monotherapy in Relapsed/Refractory Indolent Non-Hodgkin’s Lymphoma: Results From a Phase I/II Study (BO20999) Blood. ASH Annual Meeting Abstracts 2011 [Google Scholar]
- 69.Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 70.Sidky YA, Borden EC, Weeks CE, Reiter MJ, Hatcher JF, Bryan GT. Inhibition of murine tumor growth by an interferon-inducing imidazoquinolinamine. Cancer Res. 1992;52:3528–3533. [PubMed] [Google Scholar]
- 71.LeGarff-Tavernier M, Decocq J, de Romeuf C, Parizot C, Dutertre CA, Chapiro E, Davi F, Debre P, Prost JF, Teillaud JL, Merle-Beral H, Vieillard V. Analysis of CD16+CD56dim NK cells from CLL patients: evidence supporting a therapeutic strategy with optimized anti-CD20 monoclonal antibodies. Leukemia. 2011;25:101–109. doi: 10.1038/leu.2010.240. [DOI] [PubMed] [Google Scholar]