Abstract
The purpose of this study was to compare force accuracy, force variability and muscle activity during constant isometric contractions at different force levels with and without visual feedback and at different feedback gains. In experiment 1, subjects were instructed to accurately match the target force at 2, 15, 30, 50, and 70% of their maximal isometric force with abduction of the index finger and maintain their force even in the absence of visual feedback. Each trial lasted 22 s and visual feedback was removed from 8–12 to 16–20 s. Each subject performed 6 trials at each target force, half with visual gain of 51.2 pixels/N and the rest with a visual gain of 12.8 pixels/N. Force error was calculated as the root mean square error of the force trace from the target line. Force variability was quantified as the standard deviation and coefficient of variation (CVF) of the force trace. The EMG activity of the agonist (first dorsal interosseus; FDI) was measured with bipolar surface electrodes placed distal to the innervation zone. Independent of visual gain and force level, subjects exhibited lower force error with the visual feedback condition (2.53 ± 2.95 vs. 2.71 ± 2.97 N; P < 0.01); whereas, force variability was lower when visual feedback was removed (CVF: 4.06 ± 3.11 vs. 4.47 ± 3.14, P < 0.01). The EMG activity of the FDI muscle was higher during the visual feedback condition and this difference increased especially at higher force levels (70%: 370 ± 149 vs. 350 ± 143 μV, P < 0.01). Experiment 2 examined whether the findings of experiment 1 were driven by the higher force levels and proximity in the gain of visual feedback. Subjects performed constant isometric contractions with the abduction of the index finger at an absolute force of 2 N, with two distinct feedback gains of 15 and 3,000 pixels/N. In agreement with the findings of experiment 1, subjects exhibited lower force error in the presence of visual feedback especially when the feedback gain was high (0.057 ± 0.03 vs. 0.095 ± 0.05 N). However, force variability was not affected by the vastly distinct feedback gains at this force, which supported and extended the findings from experiment 1. Our findings demonstrate that although removal of visual feedback amplifies force error, it can reduce force variability during constant isometric contractions due to an altered activation of the primary agonist muscle most likely at moderate force levels in young adults.
Introduction
Constant isometric contractions are often used to compare the control of force output between young and older adults (Enoka et al. 2003; Christou and Tracy 2005) and between Parkinsonian patients and healthy age-matched adults (Vaillancourt et al. 2001a, b). During such contractions, the force output always varies around a mean value and the amplitude of the force variability can be influenced by numerous factors including the force level (Christou et al. 2002), age of the subject (Enoka et al. 2003), fatigue (Hunter et al. 2004), and stress (Christou et al. 2004). Visual feedback has also been implicated with influencing the amplitude of force variability (Slifkin et al. 2000; Vaillancourt and Russell 2002; Sosnoff and Newell 2006a; Tracy et al. 2007), however, these findings remain mixed.
Studies that compared force variability with and without visual feedback show that removal of visual feedback does not significantly influence force variability (Vaillancourt and Russell 2002; Christou et al. 2004; Christou 2005) or reduces it (Tracy 2007a, b; Tracy et al. 2007; Welsh et al. 2007). One possible explanation for the mixed findings may be the limb used. The studies that showed no effect on force variability with removal of visual feedback were performed during dexterous contractions with the fingers (index finger flexion or pinch grip), whereas the studies that demonstrated lower force variability with removal of visual feedback were performed with larger upper (elbow flexion) or lower limb muscles (knee extension, plantarflexion and dorsiflexion). In addition, the findings from studies that exhibited reductions of force variability with removal of visual feedback may be confounded by an aging interaction (greater effects in older adults; Tracy et al. 2007). Therefore, it is still not clear how removal of visual feedback influences force variability during constant isometric contractions in young adults.
Other studies manipulated visual feedback by changing the amount or frequency of the visual feedback (Sosnoff and Newell 2006b; Sosnoff et al. 2006). For instance, Sosnoff et al. (2006), compared two visual feedback gains (128 vs. 2 pixels/N) at low force levels (0.4–4 N) and demonstrated that force variability was lower with greater visual feedback gain (Sosnoff et al. 2006). Interestingly, when the amount of visual information was manipulated by varying the visual gain from 2 to 512 pixels/N, force variability was highest at low visual gains and optimum at about 64 pixels/N for young adults (Sosnoff and Newell 2006b). Support to this finding also comes from studies that manipulated the frequency of visual feedback. In these studies, the visual feedback was provided to the subjects intermittently at frequencies ranging from 0.2 to 25.6 Hz (Slifkin et al. 2000; Sosnoff and Newell 2005). Force variability decreased with greater frequency of visual feedback. In general (c.f. gains higher than 256 pixels/N; Sosnoff and Newell 2006b), therefore, results from studies that manipulated the amount of visual feedback support the idea that greater amounts of visual feedback lead to lower force variability.
Nonetheless, the interactive effect of visual gain (amount of visual feedback), presence (or absence) of visual feedback, and force level on force variability is not well understood. The differential findings among studies that have compared force variability with and without visual feedback may depend on the amount of visual feedback prior to its removal. For example, the study by Vaillancourt and Russell (2002) used a constant gain of 20 pixels/N, whereas in the study by Tracy (2007a, b) visual gain decreased with force level. Although the above studies varied the force level significantly (up to 80%), they are limited by an order effect because visual feedback was always presented first. On the other hand, some of our previous studies that controlled for the order effect are limited to very low force levels and low visual gain (Christou 2005). The purpose of this study, therefore, was to compare force accuracy, force variability and muscle activity during constant isometric contractions at different force levels with and without visual feedback when the amount of visual feedback was varied. To account for some of the previous methodological limitations, we performed the following two experiments: In the first experiment, we examined abduction of the index finger from 2 to 70% of maximum while alternating visual feedback and no visual feedback conditions. The visual feedback condition was presented at two gain levels (51.2 and 12.8 pixels/N). In the follow-up experiment, we further examined the effect of visual feedback gain (3,000 and 15 pixels/N) at a force of 2 N. We hypothesized that greater amounts of visual feedback would improve accuracy and lower force variability by changing the activation of the single agonist muscle. Part of the findings have been reported in abstract form (Baweja et al. 2008).
Methods
Twenty young adults (20–32 years, 10 men and 10 women) volunteered to participate in experiment 1 and a separate group of fourteen young adults (20–34 years, 7 men and 7 women) participated in experiment 2. All subjects reported being healthy without any known neurological problems, were right-handed according to a standardized survey (Oldfield 1971), and had normal or corrected vision. The Institutional Review Board at Texas A&M University approved the procedures, and subjects provided written informed consent before participation in the studies.
Experimental arrangement
In both experiments, subjects were seated comfortably in an upright position facing a 22 in. computer screen (NEC MultiSync LCD 2180 UX, NEC Display Solutions, IL, USA) that was located 1 m away at eye level. The visual angle, therefore, varied with the visual gain for each subject and across subjects. The monitor was used to display the force produced by the abduction of the index finger. All subjects affirmed that they could see the display clearly. The left arm was abducted by 45° and flexed to ~90° at the elbow. The left forearm was pronated and secured in a specialized padding (Versa form™, AB Germa, Sweden). The thumb, middle, ring, and fifth fingers of the left hand were restrained with metal plates and there was approximately a right angle between the index finger and thumb. Only the left index finger was free to move. The left index finger was placed in an adjustable finger orthosis to maintain extension of the middle and distal interphalangeal joints (for a schematic see Taylor et al. (2003)). The left hand (non-dominant) was used so the results could be compared with previous studies (Enoka et al. 2003). This arrangement allowed abduction of the index finger about the metacarpophalangeal joint in the horizontal plane, a movement produced almost exclusively by contraction of the first dorsal interosseus (FDI) muscle (Chao et al. 1989; Li et al. 2003).
Force measurement
For experiment 1, the constant isometric force produced by the abduction of the index finger was recorded with a three-dimensional force transducer (JR3 Multi-Axis Force-Torque Sensor System, JR3 Inc., CA, USA). The focus of this study was the control of force exerted perpendicular to the force transducer (abduction force) and thus the other two force directions will be ignored. For experiment 2, the constant isometric force produced by the abduction of the index finger was recorded with a one-dimensional force transducer (FORT 100 rigid-lever force transducer, World precision Instruments Inc., FL, USA). The force signal was sampled at 1 kHz with a Power 1401 A/D board (Cam-bridge Electronic Design, UK) and stored on a personal computer.
EMG measurement
Abduction of the index finger is produced almost exclusively by the contraction of the FDI muscle (Chao et al. 1989; Li et al. 2003). For both experiments, the FDI muscle activity was recorded with gold disc electrodes (4 mm diameter, model F-E6GH, Grass Technologies, West Warwick, RI, USA) and taped on the skin distally to the innervation zone (Homma and Sakai 1991). The recording electrodes were placed in line with the muscle fibers. The center-to-center distance between the two electrodes was 5 mm. The reference electrode was placed over the ulnar styloid. The EMG signal was amplified (×2,000) and band pass filtered at 3–1,000 Hz (Grass Model 15LT system; Grass Technologies, West Warwick, RI, USA). The EMG signal was sampled at 2 kHz with a Power 1401 A/D board (Cambridge Electronic Design, UK) and stored on a personal computer.
MVC task
Subjects performed an MVC task only for experiment 1. Subjects were instructed to increase the force from baseline to maximum over a 2 s period and maintain the maximal force for about 4–7 s. Five such recordings were made or until two of the maximal trials were within 5% of each other. The maximal voluntary contraction (MVC) force was quantified as the average force over 3–6 s (constant part) of the highest trial. This procedure allows for the identification of a more conservative MVC that reflects the capability of the person to perform constant isometric contractions.
Constant isometric force task
A custom-written program in Matlab® (Math Works™ Inc., Natick, Massachusetts, USA) manipulated the targeted force level, visual feedback condition (presence or absence of visual feedback), and gain of visual feedback. The target force was provided as a red horizontal line in the middle of the monitor and the force exerted by the subjects as a blue line progressing with time from left to right. In experiment 1, each subject was presented with five constant force targets at 2, 15, 30, 50, and 70% MVC, in random order. The subjects were instructed to gradually push against the force transducer and increase their force to match the red line (target force) within 3 s. When the target was reached, subjects were instructed to maintain their force (blue line) on the target (red line) as accurate and as consistently as possible. The whole trial lasted 22 s and visual feedback was removed from 8–12 to 16–20 s (black bars, Fig. 1a, b). The gain of visual feedback was manipulated by changing the ordinate scale, while the abscissa remained the same. Because the resolution (number of pixels) of the computer screen remained the same throughout the experiment, manipulation of the ordinate scale resulted in two distinct visual feedback gains equal to 12.8 pixels/N (Fig. 1a, left column) and 51.2 pixels/N (Fig. 1a, right column). Subjects performed six trials at every force level, three at each visual gain. Within each force level (blocked), the rest time between each trial was 15 s and between visual feedback gains 30 s. To minimize the influence of muscle fatigue during higher force levels (50 and 70% or if needed by the subjects) the rest between trials increased to 45 s. The rest time between force levels was 3 min. In experiment 2, the subjects were given an absolute force of 2 N to perform the task. The whole trial lasted 45 s and visual feedback was removed from 25 to 30 s (black bar, Fig. 1c). The gain of visual feedback was manipulated like in experiment 1 and the two distinct visual feedback gains were 15 pixels/N and 3,000 pixels/N. Subjects performed five trials at each visual gain. Within each feedback gain (blocked), the rest time between each trial was 15 s and between the visual feedback gains 60 s. The order for the two visual feedback gain conditions was counterbalanced among subjects in both experiments.
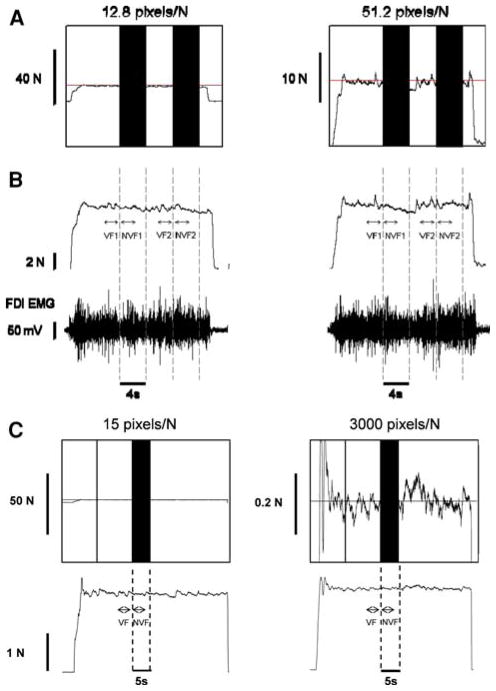
Fig. 1.
Constant isometric force task with the FDI muscle. a Representative trial from 1 subject when exerting a constant force at 30% MVC with a visual feedback gain of 12.8 pixels/N (left column) and 51.2 pixels/N (right column). Each subject was instructed to exert a force with abduction of the index finger against a force transducer and match the horizontal target line for 22 s. Visual feedback of the target line and exerted force was given to the subjects from 0–8 and 12–16 s (visual feedback condition), whereas visual feedback of the target and exerted force was removed (black bars) from 8–12 and 16–20 s (no visual feedback condition). b The force and EMG analysis was based on selected segments from each trial. The top row represents the force trace for the trials represented in A and the bottom row is the corresponding FDI EMG activity. The analysis was performed from 2.7 to 0.2 s prior to the removal of visual feedback (visual feedback condition; VF1 and VF2) and 0.2–2.7 s after the removal of the visual feedback (no visual feedback condition; NVF1 and NVF2). c Representative trial from 1 subject when exerting a constant force at 2 N with a visual feedback gain of 15 pixels/N (left column) and 3,000 pixels/N (right column). The visual feedback of the target line was given to the subjects from 0 to 25 s (visual feedback condition), whereas visual feedback of the target and the exerted force was removed from 25 to 30 s (no visual feedback condition)
Data analysis
Data were acquired with the Spike2 software (Version 6.02; Cambridge Electronic Design, Cambridge, UK) and analyzed off-line using custom-written programs in Matlab® (Math Works™ Inc., Natick, Massachusetts, USA). The force and surface EMG signals were analyzed in segments of 2.5 s. For the vision condition, segments were taken 2.7–0.2 s prior to the removal of visual feedback condition (VF1 and VF2 in Fig. 1b, VF in Fig. 1c), whereas for the no-vision condition segments were taken 0.2–2.7 s after the removal of visual feedback condition (NVF1 and NVF2 in Fig. 1b, NVF in Fig. 1c). Prior to data analysis, the force output was filtered with a fourth-order (bi-directional) Butterworth filter using a 20 Hz low-pass cut-off. The standard deviation and coefficient of variation of force was quantified from the detrended force output of the 2.5 s because any drift from the targeted force (especially during the absence of visual feedback condition) could influence the force variability. This was achieved by removing the linear trend from the force data. The dependent variables were the mean force, standard deviation (SD) of force, coefficient of variation of force (CV; (SD of force/mean force) × 100), error in force (root mean square error (RMSE) from targeted force; (Hong et al. 2008)), average drift of force from the target (mean force–targeted force; (Vaillancourt and Russell 2002)), and the amplitude of the EMG signal (RMS of interference signal; (Farina et al. 2004)).
In addition, a Fourier analysis was performed on the force signals (Christou 2005). Autospectral analysis of the force signals were obtained using Welch’s average period-ogram method with a nonoverlapping Hanning window (Matlab). The length of the data segment was 2.5 s and the sampling frequency was 1 kHz. The window size was 4,096, which gave a resolution of 0.244 Hz. For statistical comparisons, the frequency data of the force signal were divided into 0–1, 1–3, 3–7, and 7–10 Hz frequency bands (Slifkin et al. 2000). The dependent variable for the spectral analysis of the force signal was the percent peak power (%) in the above bins. The percent peak power was calculated as the relative power in each frequency band from the sum of peak powers from the selected bands (0–10 Hz).
Statistical analysis
Experiment 1
A three-way ANOVA (2 feedback conditions × 2 visual gains × 5 force levels) with repeated measures on all factors compared mean force, SD of force, CV of force, error in force, average drift, and EMG amplitude for the different force levels and visual feedback conditions. A four-way ANOVA (2 feedback conditions × 2 visual gains × 5 force levels × 4 frequency bins) with repeated measures on all factors compared the percent power in the force spectrum for the different force levels and visual feedback conditions.
Experiment 2
A two-way ANOVA (2 feedback conditions × 2 visual gains) with repeated measures on all factors compared mean force, SD of force, CV of force, error in force, average drift, and EMG amplitude for the different visual feedback conditions. A three-way ANOVA (2 feedback conditions × 2 visual gains × 4 frequency bins) with repeated measures on all factors compared the percent power in the force spectrum for the different visual feedback conditions.
Analyses were performed with the SPSS 16.0 statistical package (SPSS Inc., Chicago, IL.). Significant interactions from the ANOVA models were followed by appropriate post-hoc analyses. For example, differences among force levels were followed with one-way ANOVAs and paired t-tests. Differences between visual feedback conditions and gains were examined with paired t-tests. Multiple t-test comparisons were corrected using Bonferroni corrections. The alpha level for all statistical tests was 0.05. Data are reported as means ± SD within the text and as means ± standard error of the mean (SEM) in the figures. Only the significant main effects and interactions are presented, unless otherwise noted.
Results
Experiment 1
MVC force and EMG
To determine whether our experimental protocol induced muscle fatigue to our subjects we compared the MVC and EMG before and immediately after the experimental session. Both the MVC force and EMG amplitude did not significantly change (t > 0.3, P > 0.2). Specifically, the MVC force was 33.5 ± 14.2 N prior to the experimental protocol and 34.4 ± 17.1 N after the experimental protocol. The EMG amplitude was 452 ± 228 μV prior to the experimental protocol and 494 ± 222 μV after the experimental protocol. These findings demonstrate that the experimental protocol did not induce any fatigue to our subjects.
Force accuracy
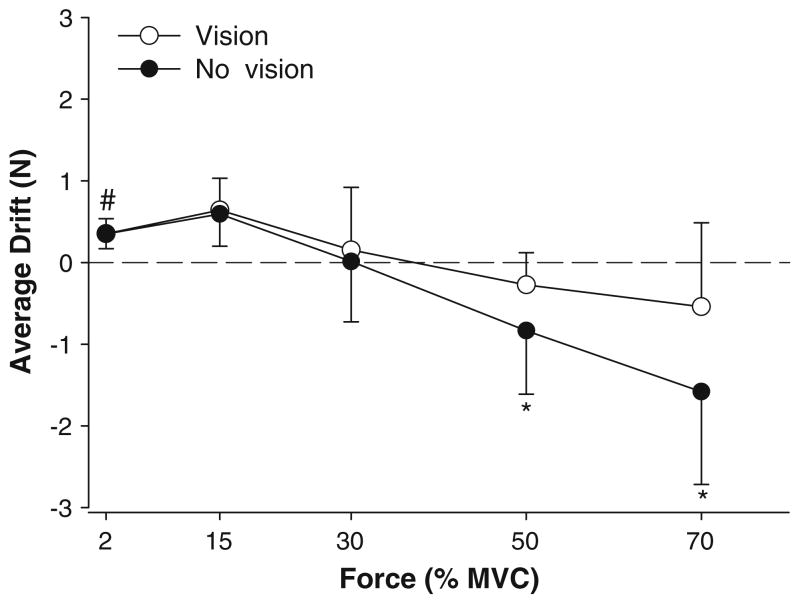
As expected, the mean force increased significantly with force level (F4,64 = 85.3, P < 0.001). There was a significant visual feedback condition × force interaction (F4,64 = 6.6, P < 0.001), indicating that the mean force was significantly greater in the presence of visual feedback only at 70% MVC (22.79 ± 8.84 vs. 21.8 ± 8.7 N; P < 0.01). The drift of force from the target was quantified as the average force away from the target. The drift was significantly smaller with visual feedback compared with no visual feedback (visual feedback condition main effect: F1,16 = 7.3, P = 0.016). The visual feedback condition × force interaction approached significance (F4,64 = 2.4, P = 0.057; Fig. 2), indicating that the drift of force was significantly greater at higher force levels, especially with the removal of feedback condition. Post-hoc analyses indicated that the drift was significantly greater only at 50 and 70% MVC without visual feedback. Furthermore, one sample t test indicated that the force drift was significantly (t > 1.9, P = 0.03) different from 0 (no drift) only for the lowest force output (2% MVC) for both visual feedback conditions. At this force level, force output drifted higher than the targeted force.
Fig. 2.
Average drift of the force output. The average drift was significantly higher (*) at 50 and 70% MVC in the absence of visual feedback (filled circles) compared with visual feedback (open circles). However, only at 2% MVC the average drift in force was significantly different from the targeted force (#). At 2% MVC force drifted higher than the targeted force and was similar in the presence and absence of visual feedback
Force accuracy was also quantified as the RMSE from the targeted force. The force error increased significantly with force level (F4,64 = 16.8, P < 0.001). The visual feed-back condition approached significance (F1,16 = 3.6, P = 0.078) and there was a significant visual feedback condition × gain interaction (F1,16 = 5.1, P = 0.04). Furthermore, there was a significant force × visual feedback × gain interaction (F4,64 = 2.9, P = 0.028; Fig. 3). Post-hoc analyses indicated that force accuracy was greater with the presence of visual feedback and that the greater differences between visual feedback conditions occurred with low gain at higher force levels (50 and 70% MVC). All other main effects and interactions were not significant.
Fig. 3.
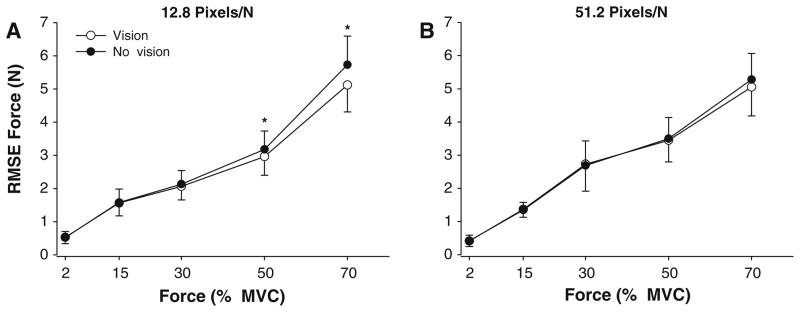
Force error as a function of visual feedback condition and gain. The RMSE of force increased with the force level for both 12.8 pixels/N (A) and 51.2 pixels/N(B) of visual feedback gain. The RMSE was similar with (open circles) and without (filled circles) visual feedback when the visual feedback gain was 51.2 pixels/N(B). However, the RMSE was significantly greater in the absence of visual feedback at 50 and 70% MVC at 12.8 pixels/N of visual feedback gain (A)
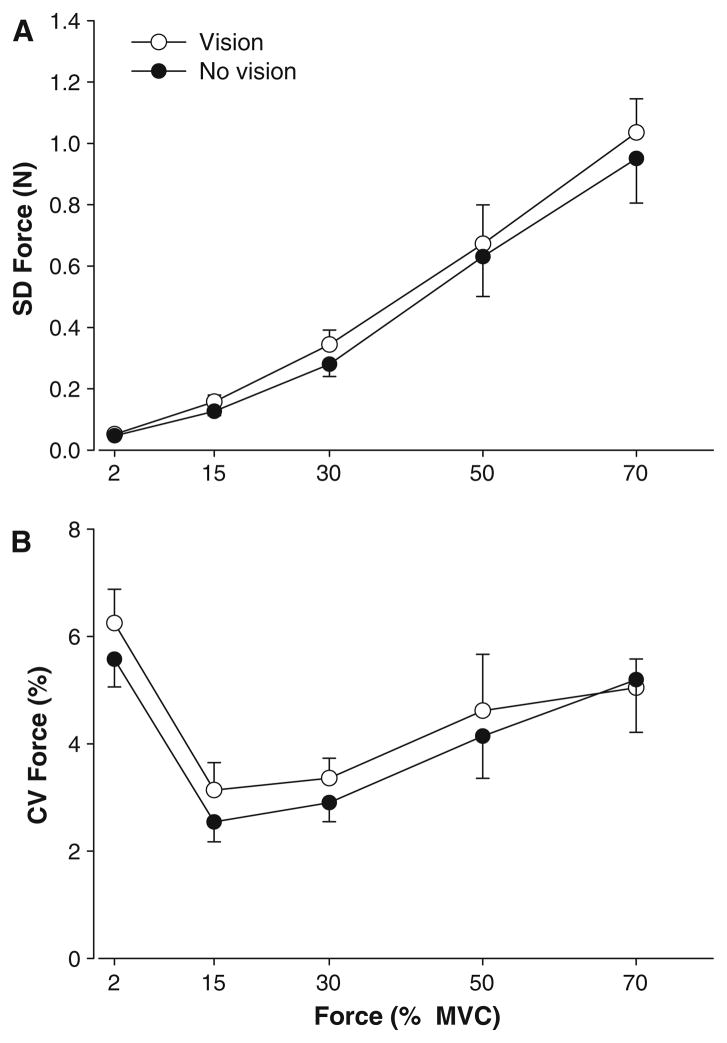
Force variability
Force variability was quantified as the SD of force and CV of force. The SD of force increased significantly with the force level (F4,64 = 51.9, P < 0.001) and the SD of force was higher in the presence of visual feedback (visual feedback condition main effect: F1,16 = 8.4, P = 0.010; Fig. 4a). Because the mean force was significantly different for the two visual feedback conditions, we also examined the CV of force. The results were similar to the SD of force. The CV of force varied significantly with force level (F4,64 = 12.8, P < 0.001) and was also significantly higher (visual feedback condition main effect: F1,12 = 4.6, P < 0.048; Fig. 4b) in the presence of visual feedback (4.48 ± 3.1 vs. 4.06 ± 3.1 N). All other main effects and interactions were not significant.
Fig. 4.
Force variability and visual feedback. a The SD of force increased with force level and on average was higher in the presence of visual feedback (open circles). b The CV of force varied significantly across the force levels and similar to the SD of force was higher with visual feedback (open circles) than without visual feedback (filled circles)
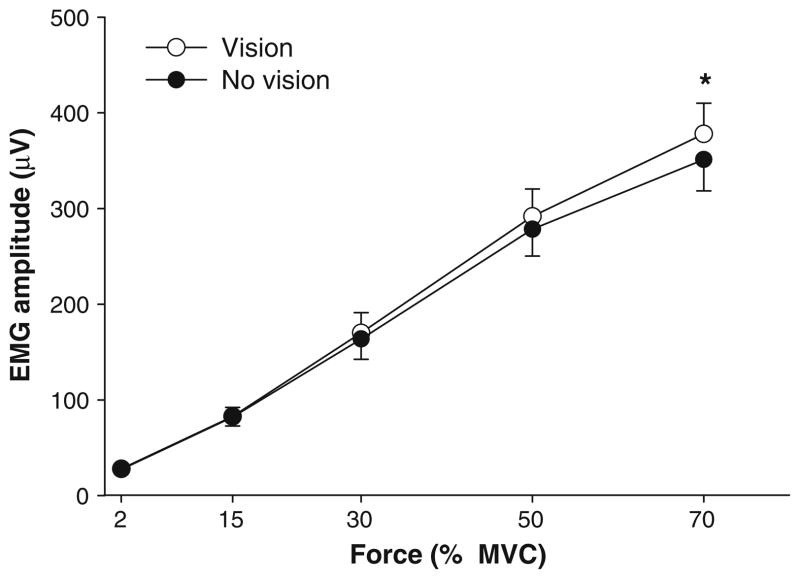
EMG amplitude
The amplitude of FDI EMG was quantified as the RMS of the interference signal. The EMG amplitude increased significantly with the force level (F4,64 = 1.31, P < 0.001). The FDI EMG amplitude was significantly greater in the presence of visual feedback (visual feedback condition main effect; F1,16 = 19.1, P < 0.001) and was greater with the higher visual feedback gain (visual feedback gain main effect: F1,16 = 3.5, P = 0.078). There was also a significant visual feedback condition × force interaction (F4,64 = 6.4, P < 0.002) indicating that the EMG activity was higher in the presence of visual feedback especially at 70% (380 ± 140 vs. 350 ± 140 μV; Fig. 5). The condition × gain × force level interaction approached significance (F4,64 = 2.8, P = 0.063). All other main effects and interactions were not significant.
Fig. 5.
FDI EMG activity and visual feedback. The FDI EMG activity increased with the force level. Muscle activity was higher with visual feedback (open circles) compared with no visual feedback (filled circles), especially at 70% MVC
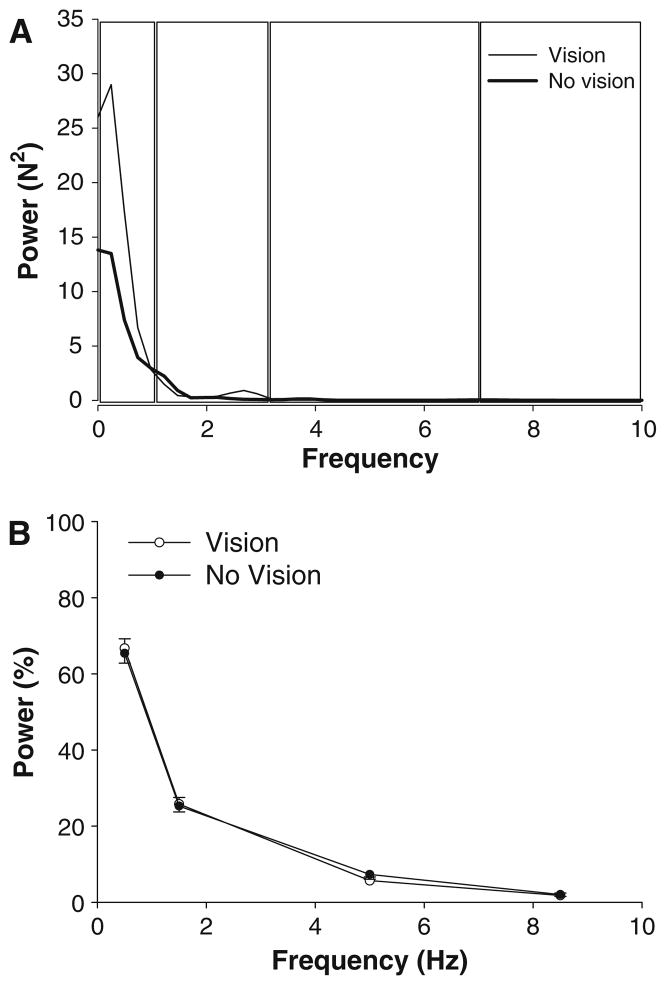
Force power spectrum
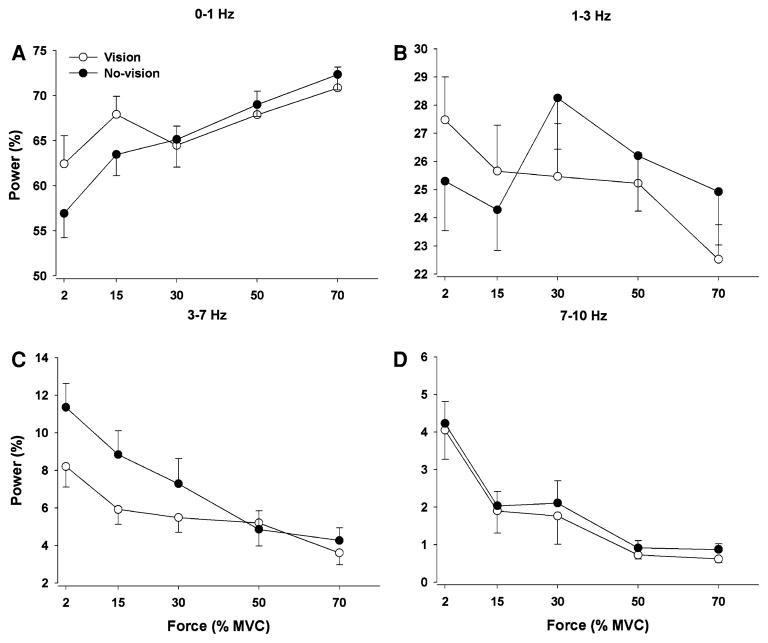
The structure of the force output during constant isometric contractions is typically evaluated with its power spectrum. The force spectrum was similar across visual feedback conditions and force levels (Fig. 6a). On average, ~66% of the power in the force spectrum occurred from 0 to 1 Hz, ~26% from 1 to 3 Hz, ~6% from 3 to 7 Hz, and ~2% from 7 to 10 Hz (frequency band main effect: F3,48 = 773.3, P < 0.001; Fig. 6b). The percent power from 0 to 1 Hz increased with force level, whereas the percent power in higher frequencies (3–10 Hz) decreased with force level (frequency band × force interaction: F12,192 = 3.3, P = 0.01). There was a significant visual feedback condition × frequency band × force interaction (F12,192 = 3.5, P = 0.009) and based on post-hoc analyses indicated the following: (1) the percent power from 0 to 1 Hz increased with force and was greater with visual feedback only for 2 and 15% MVC (Fig. 7a); (2) the percent power from 1 to 3 Hz decreased with force and was greater with visual feedback for 2 and 15% MVC and lower with visual feedback for 30, 50, and 70% MVC (Fig. 7b); 3) the percent power from 3 to 7 Hz decreased with force and was lower with visual feedback only for 2, 15, and 30% MVC (Fig. 7c); (4) the percent power from 7 to 10 Hz decreased with force and was similar in the presence and absence of visual feedback for all target forces (Fig. 7d). All other main effects and interactions were not significant.
Fig. 6.
Power spectrum of the force output. a Representative power spectrum of the force output in the presence (thin line) and absence of visual feedback (thick line) from one subject. The force spectrum was analyzed from 0–1 Hz, 1–3 Hz, 3–7 Hz, and 7–10 Hz (boxes). b Data from all subjects indicated that the structure of force output was similar in the presence (open circles) and absence of visual feedback (filled circles) conditions. On average, ~66% of the power in the force spectrum occurred in the 0–1 Hz bin, ~26% from 1–3 Hz, ~6% from 3 to 7 Hz and ~2% from 7 to 10 Hz
Fig. 7.
The interaction of vision, force, and frequency band of the force output spectrum. The relative power from 0 to 1 Hz increased with force level, whereas the relative power in all other frequency bins decreased with force level. The low-frequency oscillations (a 0–1 Hz and b 1–3 Hz) in force output were significantly greater with visual feedback only at 2 and 15% MVC. In contrast, force oscillations from 1 to 3 Hz (b) at higher force levels (30, 50, and 70% MVC) and oscillations from 3 to 7 Hz (c) from 2 to 30% MVC were significantly higher without visual feedback. Oscillations in the force output from 7 to 10 Hz (d) were similar with and without visual feedback
Experiment 2
The reasons for performing experiment 2 were: (1) to determine whether greater differences in the gain of visual feedback can influence motor performance and muscle activation; (2) to examine whether the decrease in force variability with removal of visual feedback was robust at low force levels.
Force accuracy
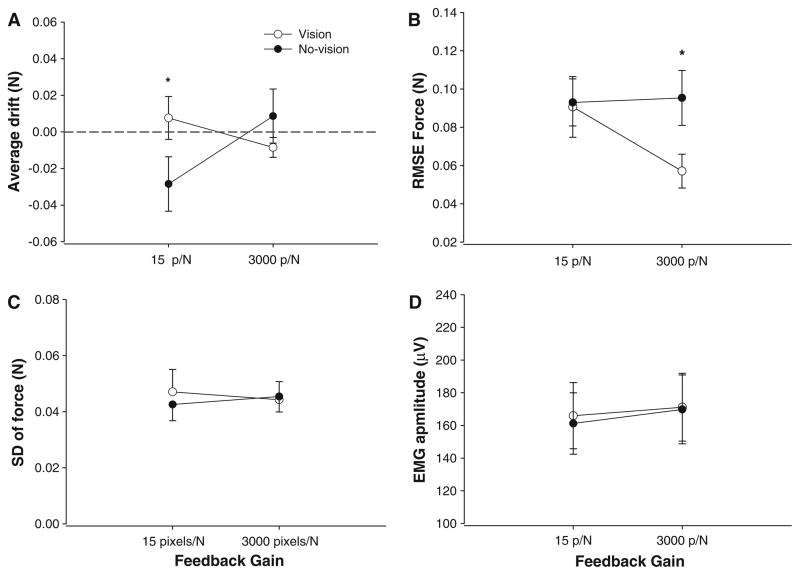
There was a significant visual feedback condition × gain interaction (F1,13 = 7.9, P = 0.015), indicating that the mean force was significantly higher in the presence of visual feedback only at 15 pixels/N (2 ± 0.04 vs. 1.97 ± 0.05 N; P = 0.0085). Similar to experiment 1, force accuracy was quantified as the average drift of force away from the target. There was a significant visual feedback condition × gain interaction (F1,13 = 7.9, P = 0.015), which indicated that the drift was significantly different between the visual feedback and no visual feedback conditions only when the gain was 15 pixels/N (Fig. 8a).
Fig. 8.
Force output and EMG activity for experiment 2. a The average drift from the targeted force was significantly greater at 15 pixels/N in the absence of visual feedback. b Force accuracy was greater in the presence of visual feedback with high gain visual feedback. c Differences in visual feedback gain did not influence force variability. d Differences in visual feedback gain did not influence the FDI EMG activity
Force accuracy was also quantified as the RMSE of the targeted force. The force error varied significantly with the visual feedback condition (condition main effect: F1,13 = 6.1, P = 0.028) and there was a significant visual feedback condition × gain interaction (F1,13 = 5.5, P = 0.035). Post-hoc analyses indicated that force accuracy was best in the presence of visual feedback and that the significant differences between visual feedback conditions occurred only at the 3,000 pixels/N gain (Fig. 8b). All other main effects and interactions were not significant.
Force variability and EMG amplitude
The SD and the CV of force did not change significantly with the visual feedback condition and gains (Fig. 8c). None of the main effects and interactions were significant. The amplitude of FDI EMG was not significantly affected by the visual feedback conditions and feedback gains (Fig. 8d).
Force power spectrum
The structure of the force spectrum was similar across visual feedback conditions and feedback gains. The percentage distribution of the force spectrum was similar to experiment 1 (frequency band main effect: F3,39 = 15.1, P < 0.0001). All other main effects and interactions were not significant.
Discussion
The influence of removing the visual feedback of force on the control of force during constant isometric contractions is unclear in the literature. The differential findings from previous studies (Vaillancourt and Russell 2002; Christou et al. 2004; Tracy 2007b; Tracy et al. 2007; Welsh et al. 2007) may depend on the amount of visual feedback prior to its removal, the force level, or the number and size of muscles involved in the task. In this paper, therefore, we compared force accuracy, force variability and muscle activity with and without visual feedback from 2 to 70% MVC during abduction of the index finger (primarily controlled by a single muscle) and when the gain of visual feedback was varied between 51.2 and 12.8 pixels/N. In addition, in a separate experiment, we further examined the influence of visual feedback gain at low force levels by comparing control of force with gains equal to 3,000 and 15 pixels/N. In contrast to our hypothesis, the findings of Experiment 1 indicated that in the presence of visual feedback, and independent of the amount of visual feedback, subjects exhibited greater force accuracy and amplified force variability at all force levels. Because these results are consistent with the findings from studies that performed such contractions with larger muscles in young and older adults (Tracy 2007b; Tracy et al. 2007; Welsh et al. 2007), they reveal that force variability decreases with removal of visual feedback independent of the joint used, the number and size of muscles that exert the contraction, and age of the subject. Furthermore, our findings from experiment 1 demonstrated that visual feedback influenced the oscillatory nature of the force output differently at each force examined and that the neural activation of the single agonist muscle required in this task was different with and without visual feedback. The findings from experiment 2 confirmed that even greater differences in the gain (amount) of visual feedback did not influence force variability, force structure, or muscle activity at low force levels. As expected, however, force accuracy was better with greater gain of visual feedback. Overall, these two experiments demonstrated the following novel findings: (1) although removal of visual feedback impaired force accuracy it reduced force variability especially at moderate force levels most likely by changing the activation of the agonist muscle; (2) the gain of visual feedback did not influence force variability when the constant isometric contraction was performed with or without visual feedback; (3) visuomotor corrections cannot fully explain the low-frequency oscillations in force during constant isometric contractions.
Force accuracy
In this study, we quantified force accuracy with the average drift from the target and the RMSE. As expected from previous findings (Vaillancourt and Russell 2002; Tracy 2007b; Tracy et al. 2007; Welsh et al. 2007), force error was lower in the presence of visual feedback, especially with the higher gain and higher force levels. On average, our results support previous findings that visual feedback improves force accuracy at higher force levels, however, some results appear inconsistent with the literature. For example, previous studies showed that subjects drifted significantly from the targeted force when visual feedback was removed. Our results show that the drift from the target was significant only at 2% MVC and the drift was similar during the presence and absence of visual feedback. Although the drift was greater during higher force levels, no other force level exhibited a significant drift from the target (see Fig. 2). The differential findings may be due to methodological differences. Previous studies always provide visual feedback first anywhere from 6 to 10 s followed by removal of visual feedback for the rest of the trial (Vaillancourt and Russell 2002; Tracy 2007b; Tracy et al. 2007; Welsh et al. 2007). For our first experiment, the removal of visual feedback was alternated with visual feedback. Furthermore, visual feedback was removed for 4 s at a time and only the initial 2.5 s were analyzed. The time segment used for analysis in the absence of visual feedback is close to the short-term memory (~1.5 s) estimates proposed by Vaillancourt and Russell (2002) and thus may limit a significant drift from the target. The longer time (2.5 instead of 1.5 s) for short-term memory capacity may be due to methodological differences in the presentation and removal of visual feedback. Further studies are needed to clarify the short-term memory capacity and its interaction with visual gain during constant isometric contractions.
Furthermore, our findings indicate that the force error was significantly greater with the 12.8 pixels/N gain compared with 51.2 pixels/N gain only at 50 and 70% MVC. In addition, the findings from experiment 2 demonstrated that larger differences in gain of visual feedback (3,000 vs. 15 pixels/N) influenced the accuracy of force even at low force levels (2 N). These findings are in contrast to the findings of Hong et al. (2008), which demonstrated that visual feedback gain did not have a significant effect on the accuracy of force during constant isometric contractions. The differences in findings may be due to the force levels used. Our paper examined force levels from 2 to 70% MVC and 2 N, whereas Hong et al. (2008) used a single absolute force of 6.1 N. Therefore, our findings contrast the findings by Hong et al. (2008) and indicate that greater amounts of visual feedback (as manipulated by the visual gain) improve force accuracy at very low and moderate-to-high force levels.
Force variability and removal of visual feedback
Previous studies that have compared force variability with and without visual feedback during constant isometric tasks exhibit the following discrepancy in findings: The studies that involved the use of fingers, and thus primarily small hand and forearm muscles, suggest that removal of visual feedback does not influence force variability (Vaillancourt and Russell 2002; Christou et al. 2004). In contrast, studies that involved primarily large muscles of the upper (no finger participation) and lower limbs, suggest that removal of visual feedback decreases force variability (Tracy 2007b; Tracy et al. 2007; Welsh et al. 2007). Furthermore, the discrepant findings could have been due to the gain of visual feedback, which was controlled for the small muscle studies (Vaillancourt and Russell 2002; Christou et al. 2004) but not for the larger muscle studies (Tracy 2007b; Tracy et al. 2007; Welsh et al. 2007).
The findings from our study, which was performed with the index finger and was controlled primarily by a single agonist muscle (Chao et al. 1989; Li et al. 2003), showed that force variability was significantly higher in the presence of visual feedback but this finding may have been driven primarily by moderate force levels. In experiment 1, on average, removal of visual feedback decreased force variability of the index finger by ~10% and thus it supports the studies by Tracy and colleagues (Tracy (2007b); Tracy et al. 2007; Welsh et al. 2007), which were performed with multiple muscles at the elbow, knee, and ankle joints. For that reason, the discrepancy in previous findings cannot be attributed to the limb used or the number of muscles involved. In contrast, the findings of experiment 2, which was performed at a very low force level (2 N; ~5%), demonstrated that force variability was not different with or without visual feedback. These findings support previous studies that examined contractions with hand muscles (Vaillancourt and Russell 2002; Christou et al. 2004) and arm and leg muscles (Tracy et al. 2007), which indicate that force variability is not altered in the absence of visual feedback at low force levels in young adults. Therefore, it appears that, on average, most studies demonstrate that removing visual feedback may not significantly influence force variability at low force levels in young adults.
The discrepancy in findings between our experiment 1 (support findings from Tracy 2007a, b; Tracy et al. 2007; Welsh et al. 2007) and experiment 2 (Vaillancourt and Russell 2002; Christou et al. 2004; Christou 2005), therefore, cannot be due to the limb used and the number of muscles involved. The only significant methodological difference between the two experiments is that in experiment 1 we altered the presentation and removal of visual feedback within a trial, whereas in experiment 2 we always provided visual feedback first and removal of visual feedback occurred only once. Even so, this methodological difference cannot explain the discrepant findings between the two studies because previous studies by Tracy and colleagues (Tracy 2007b; Tracy et al. 2007; Welsh et al. 2007) also presented the visual feedback condition first but their results and our findings from experiment 1 (alternating visual feedback) are similar.
It is possible; therefore, that the significant decrease in force variability with removal of visual feedback (main effect for visual feedback condition), as shown in experiment 1, may be primarily due to changes that occur primarily at moderate force levels (see Fig. 4a). This appears to be robust in analysis of the same data for a different experiment at 15 and 50% (Fulks et al. 2008). In the absence of visual feedback, the reduction in force variability is most likely due to the absence of visuomotor corrections. This finding is not only consistent with tasks that require individuals to exert constant isometric contractions with larger muscles (Tracy 2007b; Tracy et al. 2007; Welsh et al. 2007), but also supports findings from discrete tasks (Crossman and Goodeve 1983; Carlton 1992; Elliott et al. 2001) and tracking sinusoidal tasks (Miall et al. 1993). For example, removal of visual feedback reduces the number of submovements prior to reaching the target during goal-directed movements (Crossman and Goodeve 1983) and allows individuals to track sinusoidal force targets smoother (Miall et al. 1993). Therefore, it appears that in the absence of visual feedback, and independent of the motor task, the central nervous system exerts a smoother contraction but often less accurate.
Force variability and amount of visual feedback
We varied the mount of visual feedback in two experiment by varying the visual feedback gain. For both experiments, the amount of visual feedback did not influence force variability. Because the distance from the monitor was constant (within a subject and among subjects) our findings can also be compared with experiments that used variations of visual angle to manipulate the amount of visual feedback (Vaillancourt and Russell 2002). This finding, therefore, demonstrates that the decrease in force variability with removal of visual feedback is independent of the amount of visual feedback provided to the participant.
Furthermore, this result contrasts findings from previous studies which demonstrate significant variations in force variability with the amount of visual feedback provided to the subject (Vaillancourt and Russell 2002; Sosnoff and Newell 2006b; Sosnoff et al. 2006). The overall conclusion from those studies was that greater amount of visual information (e.g., greater visual gain or angle) resulted in lower force variability. However, it was clear that the relation between the amount of visual information and force variability was not linear but a weak U-shape function (Sosnoff and Newell 2006b). Specifically force variability appeared to be the greatest at very low amounts of visual feedback (2–4 pixels/N of visual gain), optimum at about 64 pixels/N, and then it either remained the same or slightly increased above 256 pixels/N (Sosnoff and Newell 2006b). It is possible, therefore, that the non-significant effects of visual gain on force variability in both of our experiments were due to the visual gains selected. For example, in the first experiment we compared 12.8 and 51.2 pixels/N, which were statistically not different most likely because the increases in force variability occur at very low visual angles (or gains <4 pixels/N; see Sosnoff and Newell 2006b). In the second experiment, however, we compared 15 and 3,000 pixels/N and still found no significant differences in force variability. This finding may suggest the following: (1) the relation between the amount of visual feedback and force variability cannot be precisely described by a U-shape function but rather an exponentially decreasing to a plateau function; (2) the relation between the amount of visual feedback and force variability is more complex than a quadratic function (e.g. cubic), where very high amounts of visual feedback decrease force variability. Clearly more research is needed to clarify the exact function that describes the relation between the amount of visual feedback and force variability. Future studies should incorporate not only very high amounts of visual feedback but also very low amounts of visual feedback. The reason for further examining very low amounts of visual feedback and their influence on force variability has to do with our findings when removing visual feedback. Specifically, we found that no visual feedback significantly reduces force variability and thus very low amounts of visual feedback may also reduce force variability.
Organization of force output and muscle activation
Our findings clearly demonstrate that the force output contains greater percent power at low frequencies (0–1 Hz) with visual feedback, which has been associated previously with visuomotor corrections (Miall et al. 1993; Slifkin et al. 2000). A novel finding of this study, however, is that the structure of force output appears to be influenced differently for various force levels. Only low force levels (2 and 15% MVC) appear to exhibit significantly lower percent power in low-frequency (0–3 Hz) oscillations without visual feedback. In contrast, moderate-to-high force levels exhibit greater percent power from 1 to 3 Hz with visual feedback. Finally, oscillations from 3 to 7 Hz appear to exhibit less percent power with visual feedback.
Another interesting finding was that even with the complete removal of visual feedback, low-frequency oscillations in force were still significant and the major contributor in force variability. Therefore, visuomotor corrections cannot completely explain the low-frequency oscillations in the force output. Factors that can induce such low-frequency oscillations in force in the absence of visual feedback include the coherent modulation of motor unit discharge at low frequencies (De Luca and Erim 1994; Brown 2000; Vaillancourt et al. 2003), variability in motor unit discharge due to synaptic noise (Taylor et al. 2003; Moritz et al. 2005), intrinsic neuronal properties such as active calcium conductances (Falcke 2003), heart rate (Hunter et al. 2007), and breathing (Turner 2002; Li and Yasuda 2007). Further research is needed to clarify the origins of low-frequency oscillations in force during constant isometric contractions.
In addition to the differential structure in force output during the presence and absence of visual feedback, our study also demonstrated that the neural activation of muscle was different during the two visual conditions. The control of force output for our study was primarily due to the activation of a single muscle, namely the first dorsal interosseus (Chao et al. 1989; Li et al. 2003). Previous studies have been performed at joints where multiple muscles contribute to the total force and thus their conclusions about neural activation may be limited (Tracy 2007b; Tracy et al. 2007; Welsh et al. 2007). The activation of the first dorsal interosseus muscle was significantly greater during visual feedback (main effect). This is interesting because subjects exhibited the same force output in the presence and absence of visual feedback (except 70% MVC). Furthermore, analysis of the same dataset at 15 and 50% for a different experiment shows similar findings (Fulks et al. 2008). This finding demonstrates that the descending input to the muscle must be greater in the presence of visual feedback. A possible explanation is that the antagonist activity, which was not measured in this experiment, is higher during visual feedback in anticipation for visuomotor corrections (Lee and Keller 2008).
More research is needed to determine the activation of the antagonist muscles when visual feedback is manipulated. It is possible that the descending drive to the muscle is different with and without visual feedback and can potentially contribute to the differences in force variability between the two visual feedback conditions. Recent findings provide such evidence and indicate that higher centers in humans are activated differently in the presence and absence of visual feedback. For example, Prodoehl et al. (2008) demonstrated that the internal globus pallidus and subthalamic nucleus, structures of the basal ganglia that have been shown to be active during the rate of change in force output (Vaillancourt et al. 2004), are activated differently with visual feedback of the force compared with auditory feedback (Prodoehl et al. 2008). Therefore, minimization of the fluctuating force output from visual information (visuomotor corrections) may include different activation of structures in higher centers and consequently different activation of the involved muscle from the motor cortex.
In summary, our findings demonstrate that removal of visual feedback amplifies force error but reduces force variability during constant isometric contractions most likely due to an altered activation of the primary agonist muscle. These results appear to be more robust at moderate force levels. The differences appear to be independent of the amount of visual feedback, at least for visual gains ranging from 12.5 to 3,000 pixels/N, and methodology in the presentation of visual feedback. These findings support and extend previous studies that used discrete tasks (Crossman and Goodeve 1983; Elliott et al. 2001), tracking sinusoidal tasks (Miall et al. 1993), and constant isometric tasks with larger muscles (Tracy 2007b; Tracy et al. 2007; Welsh et al. 2007). Finally, the findings demonstrate that visuomotor corrections may contribute to the low-frequency oscillations (0–3 Hz) only at low force levels (up to 15% MVC). Nonetheless, further research is needed to understand the mechanisms that may contribute to the low-frequency oscillations in force, which are significant even in the absence of visual feedback.
Acknowledgments
The authors would like to acknowledge the help of Meredith Smith for data collection and the help of Ankit Agrawal and Deepan Srinivasan for computer programming. Supported by R01 AG031769 to EA Christou.
References
- Baweja HS, Patel BK, Martinkewiz JD, Smith MA, Srinivasan D, Christou EA. Removal of visual feedback alters muscle activity and reduces force variability during constant isometric contractions. Society for Neuroscience; Washington DC: 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Cortical drives to human muscle: the Piper and related rhythms. Prog Neurobiol. 2000;60:97–108. doi: 10.1016/s0301-0082(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Carlton LG. Visual processing time and the control of movement. In: Proteau L, Elliott D, editors. Vision and motor control. Elsevier; Amsterdam: 1992. pp. 3–31. [Google Scholar]
- Chao EYS, An KN, Cooney WP, Linschied RL. A basic research study. World Scientific Publishing; Teaneack, NJ: 1989. Biomechanics of the hand. [Google Scholar]
- Christou EA. Visual feedback attenuates force fluctuations induced by a stressor. Med Sci Sports Exerc. 2005;37:2126–2133. doi: 10.1249/01.mss.0000178103.72988.cd. [DOI] [PubMed] [Google Scholar]
- Christou EA, Tracy BL. Aging and motor output variability. In: Davids K, Bennett S, Newell K, editors. Movement system variability. Human Kinetics; Champaign, IL: 2005. pp. 199–215. [Google Scholar]
- Christou EA, Grossman M, Carlton LG. Modeling variability of force during isometric contractions of the quadriceps femoris. J Mot Behav. 2002;34:67–81. doi: 10.1080/00222890209601932. [DOI] [PubMed] [Google Scholar]
- Christou EA, Jakobi JM, Critchlow A, Fleshner M, Enoka RM. The 1- to 2-Hz oscillations in muscle force are exacerbated by stress, especially in older adults. J Appl Physiol. 2004;97:225–235. doi: 10.1152/japplphysiol.00066.2004. [DOI] [PubMed] [Google Scholar]
- Crossman ER, Goodeve PJ. Feedback control of hand-movement and Fitts’ Law. Q J Exp Psychol A. 1983;35:251–278. doi: 10.1080/14640748308402133. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Erim Z. Common drive of motor units in regulation of muscle force. Trends Neurosci. 1994;17:299–305. doi: 10.1016/0166-2236(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Elliott D, Helsen WF, Chua R. A century later: Woodworth’s (1899) two-component model of goal-directed aiming. Psychol Bull. 2001;127:342–357. doi: 10.1037/0033-2909.127.3.342. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol. 2003;13:1–12. doi: 10.1016/s1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- Falcke M. Buffers and oscillations in intracellular Ca2+ dynamics. Biophys J. 2003;84:28–41. doi: 10.1016/S0006-3495(03)74830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- Fulks E, Baweja HS, Patel BK, Martinkewiz JD, Srinivasan D, Christou EA. Breathing amplitude influences force variability but not muscle activity during constant isometric contractions. Society for Neuroscience; Washington DC: 2008. [Google Scholar]
- Homma T, Sakai T. Ramification pattern of intermetacarpal branches of the deep branch (ramus profundus) of the ulnar nerve in the human hand. Acta Anat (Basel) 1991;141:139–144. doi: 10.1159/000147113. [DOI] [PubMed] [Google Scholar]
- Hong SL, Brown AJ, Newell KM. Compensatory properties of visual information in the control of isometric force. Percept Psychophys. 2008;70:306–313. doi: 10.3758/pp.70.2.306. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Shin IS, Enoka RM. Fatigability of the elbow flexor muscles for a sustained submaximal contraction is similar in men and women matched for strength. J Appl Physiol. 2004;96:195–202. doi: 10.1152/japplphysiol.00893.2003. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Taijin T, Patel B, Rodriguez TM, Christou EA. Heart rate contributes to the low-frequency oscillations in force. Society for Neuroscience; San Diego: 2007. [Google Scholar]
- Lee KM, Keller EL. Neural activity in the frontal eye fields modulated by the number of alternatives in target choice. J Neurosci. 2008;28:2242–2251. doi: 10.1523/JNEUROSCI.3596-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Yasuda N. Forced ventilation increases variability of isometric finger forces. Neurosci Lett. 2007;412:243–247. doi: 10.1016/j.neulet.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZM, Pfaeffle HJ, Sotereanos DG, Goitz RJ, Woo SL. Multi-directional strength and force envelope of the index finger. Clin Biomech (Bristol, Avon) 2003;18:908–915. doi: 10.1016/s0268-0033(03)00178-5. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Stein JF. Intermittency in human manual tracking tasks. J Mot Behav. 1993;25:53–63. doi: 10.1080/00222895.1993.9941639. [DOI] [PubMed] [Google Scholar]
- Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol. 2005;93:2449–2459. doi: 10.1152/jn.01122.2004. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Yu H, Wasson P, Corcos DM, Vaillancourt DE. Effects of visual and auditory feedback on sensorimotor circuits in the Basal Ganglia. J Neurophysiol. 2008;99:3042–3051. doi: 10.1152/jn.01108.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifkin AB, Vaillancourt DE, Newell KM. Intermittency in the control of continuous force production. J Neurophysiol. 2000;84:1708–1718. doi: 10.1152/jn.2000.84.4.1708. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Newell KM. Intermittent visual information and the multiple time scales of visual motor control of continuous isometric force production. Percept Psychophys. 2005;67:335–344. doi: 10.3758/bf03206496. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Newell KM. Aging, visual intermittency, and variability in isometric force output. J Gerontol B Psychol Sci Soc Sci. 2006a;61:P117–P124. doi: 10.1093/geronb/61.2.p117. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Newell KM. Information processing limitations with aging in the visual scaling of isometric force. Exp Brain Res. 2006b;170:423–432. doi: 10.1007/s00221-005-0225-5. [DOI] [PubMed] [Google Scholar]
- Sosnoff JJ, Valantine AD, Newell KM. Independence between the amount and structure of variability at low force levels. Neurosci Lett. 2006;392:165–169. doi: 10.1016/j.neulet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Christou EA, Enoka RM. Multiple features of motor-unit activity influence force fluctuations during isometric contractions. J Neurophysiol. 2003;90:1350–1361. doi: 10.1152/jn.00056.2003. [DOI] [PubMed] [Google Scholar]
- Tracy BL. Force control is impaired in the ankle plantarflexors of elderly adults. Eur J Appl Physiol. 2007a;101:629–636. doi: 10.1007/s00421-007-0538-0. [DOI] [PubMed] [Google Scholar]
- Tracy BL. Visuomotor contribution to force variability in the plantarflexor and dorsiflexor muscles. Hum Mov Sci. 2007b;26:796–807. doi: 10.1016/j.humov.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy BL, Dinenno DV, Jorgensen B, Welsh SJ. Aging, visuo-motor correction, and force fluctuations in large muscles. Med Sci Sports Exerc. 2007;39:469–479. doi: 10.1249/mss.0b013e31802d3ad3. [DOI] [PubMed] [Google Scholar]
- Turner DL. Expiratory resistive loaded breathing in humans increases fluctuations of force production in submaximal isometric quadriceps contractions. Neurosci Lett. 2002;328:13–16. doi: 10.1016/s0304-3940(02)00420-2. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Russell DM. Temporal capacity of short-term visuomotor memory in continuous force production. Exp Brain Res. 2002;145:275–285. doi: 10.1007/s00221-002-1081-1. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Slifkin AB, Newell KM. Intermittency in the visual control of force in Parkinson’s disease. Exp Brain Res. 2001a;138:118–127. doi: 10.1007/s002210100699. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Slifkin AB, Newell KM. Visual control of isometric force in Parkinson’s disease. Neuropsychologia. 2001b;39:1410–1418. doi: 10.1016/s0028-3932(01)00061-6. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Larsson L, Newell KM. Effects of aging on force variability, single motor unit discharge patterns, and the structure of 10, 20, and 40 Hz EMG activity. Neurobiol Aging. 2003;24:25–35. doi: 10.1016/s0197-4580(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Thulborn KR, Corcos DM. Sub-thalamic nucleus and internal globus pallidus scale with the rate of change of force production in humans. Neuroimage. 2004;23:175–186. doi: 10.1016/j.neuroimage.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Welsh SJ, Dinenno DV, Tracy BL. Variability of quadriceps femoris motor neuron discharge and muscle force in human aging. Exp Brain Res. 2007;179:219–233. doi: 10.1007/s00221-006-0785-z. [DOI] [PubMed] [Google Scholar]