Abstract
Older adults exhibit greater motor variability, which impairs their accuracy and function, compared with young adults. Low-intensity training that emphasizes muscle coordination reduces variability in older adults. Furthermore, low amount of visual feedback minimizes age-associated differences in variability. We hypothesize that an intervention that combines muscle coordination and reduced visual feedback would be advantageous to improve motor control in older adults.
Keywords: neuromuscular control, force variability, movement control, aging, intervention, training
INTRODUCTION
Motor output variability can be defined as the unintentional variations in the output of voluntary contractions and can be seen within a trial (e.g., movement trajectory) or from trial to trial (e.g., end point variability) (7). Older adults exhibit greater motor output variability than young adults, which often is associated with their inability to move smoothly and accurately (13). In addition to impairing motor performance, amplified motor output variability also may weaken the ability of older adults to learn new tasks (6). The amplified variability of voluntary contractions in older adults has been linked to altered activation of the involved muscles likely because of structural and neural changes that occur with aging at the higher centers (e.g., death of cortical neurons (17)) and spinal cord (e.g., motor unit reorganization (18)).
This age-associated augmentation in motor output variability can impair the ability even of healthy older adults to adapt to changing environments and consequently compromise their independence. Furthermore, the aging population will increase dramatically in the next 30 years and, in some developed nations (e.g., United States), may comprise 25% of the workforce (4). Minimizing motor output variability in older adults is therefore essential because it will improve their performance and improve their capability of learning new tasks (6,39). This paper will summarize scientific evidence that demonstrate that altered muscle activation amplifies motor output variability in older adults with functional consequences. With some of our recent findings as basis, we hypothesize that amplified motor output variability in older adults can be minimized to the level of young adults by using an intervention protocol that combines low-intensity training that emphasizes muscle coordination and low amount of visual feedback.
FUNCTIONAL SIGNIFICANCE OF AMPLIFIED MOTOR OUTPUT VARIABILITY
There is substantial experimental evidence that older adults exhibit greater motor output variability than young adults during isometric and anisometric voluntary contractions (Fig. 1). For example, older adults are more variable than young adults when they attempt to maintain a constant force (Fig. 1A) with various limbs including the finger (20), elbow (21), knee (37), and foot (27). Although these age-associated differences during constant force tasks are not always consistent in the literature (9), it is generally accepted that these differences occur reliably for very low force levels (<5% maximal voluntary contraction force) (13,18). Older adults also are more variable than young adults when they attempt to trace a line while lifting and lowering inertial loads (Fig. 1B) (13,14,18). This is demonstrated by greater variability in displacement and acceleration of the index finger (5,12) and the forearm (21). Controlling movements while lowering a load seems to be harder than controlling movements while lifting a load for older adults (14). Overall, when older adults attempt to maintain a constant force or to slowly control lifting and lowering of inertial loads, they exhibit greater within-trial variability than young adults, primarily for low-intensity contractions.
Figure 1.
Representative data for younger and older individuals performing three tasks. (A) Constant position task (positional variability) during a low-intensity contraction of the first dorsal interosseus muscle (10% 1-repetition maximum). Position (or force) variability during constant contractions is quantified from the detrended signal. (B) Tracing (position signal, gray line) of a slow sinusoidal task (0.1 Hz, black line) during shortening (ascending limb) and lengthening (descending limb) contractions while lifting a light load with the same muscle (10% 1-repetition maximum). Movement (or force) variability is quantified as the variations (SD) in the movement trajectory filtering out the frequency of the task (bottom trace of B). (C) A single trial during a goal-directed force task, where subjects attempt to match a force-time target by exerting 20% of their maximum force in 200 milliseconds (placing their peak force on the center of the force-time target). Each black dot represents their peak force for 20 different trials. Variability in force (or displacement) can be quantified as the SD of peak force across trials (y axis), whereas variability in time to peak force can be quantified as the SD of time to peak force across trials (x axis). End-point accuracy for force is quantified as the average of the shortest distance of the peak force across trials from the targeted force, whereas end-point accuracy for time is quantified as the average of the shortest distance of the exerted time to peak force across trials from the targeted time to peak force. Trajectory variability is quantified from the variations of the detrended force from the start to the peak force of each trial (bottom trace of C).
Augmentation in force and movement variability in older adults is presumably the consequence of differences in muscle activation. Because the motor unit is the final common pathway of the voluntary command to the muscle, these age-associated changes should be evident in the motor unit twitch force or discharge characteristics of motor units (18). The motor unit twitch force does not seem to explain differences in force variability between young and older adults, and this has been shown by a combination of simulations and experimental findings. During simulations, the amplitude of force variability did not vary significantly when the twitch force of the motor unit was increased (18). Furthermore, experimental findings suggest that, although older adults exhibited greater motor unit twitch force than young adults, training of the first dorsal interosseus muscle reduced their force variability without changing their motor unit twitch force (24). Thus, it is possible that either the motor unit size is not a significant contributor to force variability or other neural activation mechanisms are more relevant to force variability. Recent evidence suggests that force variability seems to be associated with low-frequency oscillations (common drive) in the discharge rate of multiple motor units (31). Nonetheless, because this study only examined the neural control of motor units in young adults, its findings cannot explain age-associated differences in motor output variability. One neural activation mechanism that is different (greater) in older adults and has been associated with their amplified variability when they attempt to maintain a constant force (18,28) or when they attempt to control lifting and lowering of inertial loads (26) is the variability of the motor unit discharge. Because the motor unit discharge rate variability has been associated with synaptic noise (30), these findings support the hypothesis (22) that part of motor output variability within a trial may be noise superimposed on the motor command at any level of the nervous system. Therefore, variability in such tasks demonstrates the impaired ability of older adults to control force or movement within a trial and suggests that these differences are amplified for low-intensity contractions likely because of a noisier activation of motor units.
The age-associated differences seem to be even greater and more consistent when motor output variability is examined from trial to trial (Fig. 1C). Typically, such tasks involve goal-directed contractions. For example, when young and older adults attempt to match a target with voluntary contractions of the first dorsal interosseus muscle (11) or knee extensors (9), they exhibit greater end point variability in force, space (position displacement), and temporal parameters. The differences seem to be greater for the temporal characteristics of movement, when subjects are required to lower an inertial load, and for lower contraction intensities (10,12,14). The activation of antagonistic muscles (e.g., observed triphasic pattern) seems to be the main neuromuscular mechanism that explains the age differences in end point variability. This altered activation of the antagonistic muscles in older adults can be observed as a change in the required triphasic pattern of the antagonistic muscles (11) or as increased coactivation (10,34). These findings demonstrate that older adults also exhibit greater end point variability, which seems to be related to an altered synergistic activation of the antagonistic muscles.
Theoretically, the functional significance of motor output variability is that it can impair the ability of humans to perform accurate movements (22). Recent experimental findings support that notion and provide evidence that both forms of variability (within trial and between trials) are strongly associated with greater end-point error in force, displacement, and time in older adults. For example, we have shown that end point accuracy of goal-directed force (11) and movement tasks (10) is influenced by greater trajectory variability and greater end point variability in older adults (Fig. 2). Therefore, these findings demonstrate that noisy trajectories and greater end point variability (10) can impair the ability of older adults to exert accurate single joint movements. Interestingly, trajectory variability and end point variability likely result from independent mechanisms because they contribute uniquely to end point accuracy in young and older adults (10).
Figure 2.
Associations between trajectory or end-point variability with force (top row) or movement (bottom row) error during goal-directed tasks. Trajectory variability refers to the fluctuations in position within a trial when the task frequency is filtered out (detrended). End-point variability refers to the variations (SD) in end point (force or time) across trials. The top row demonstrates that the amplified force error observed in older adults is strongly associated with greater trajectory (left) and end point (right) variability in force (data adapted from (11)). The bottom row demonstrates similar findings for movement (data adapted from (10)). Specifically, the amplified movement error observed in older adults is strongly associated with greater trajectory (left) and end-point (right) variability in movement. Overall, these results provide experimental evidence that amplified motor output variability in older adults impairs their end-point accuracy.
Recent experiments provide evidence that the amplified motor output variability observed in older adults also can impair their ability to perform functional movements. For example, the amplified force variability in older adults during low-intensity isometric plantar flexion contractions predicted their variability of center of pressure during quiet standing (27). In addition, there is a moderate correlation between the force variability exerted with abduction of the index finger or with a precision pinch task and the ability of older adults to perform functional tasks that require manual dexterity (29). Finally, these results are supported by training interventions in older adults. Specifically, older adults who participated in low-intensity training with the index finger for 2 weeks demonstrated not only improvements in the finger task but also improvements in a manual dexterity task (26). Overall, these findings suggest that the amplitude of motor output variability during tasks that require contractions (but not movement) with one joint (e.g., plantar flexion) can predict age-associated differences in function (such as standing) and manual dexterity. In summary, amplified motor output variability may predispose older adults to movement errors, which could have significant functional consequences. Therefore, it is important to devise interventions that minimize motor output variability in older adults.
TRAINING TO REDUCE MOTOR OUTPUT VARIABILITY IN OLDER ADULTS
The brain of older adults remain plastic, which suggests that training can potentially stimulate the brain of older adults to develop alternative strategies and minimize the age-associated impairments in motor performance that are caused by structural changes (16). On average, the impact of two types of interventions has been examined on reducing the amplified motor output variability of older adults, that of strength training and low-intensity training (controlled movements with light loads).
In the last decade, an important conclusion from studies that examined motor output variability before and after an intervention is that strength training does not necessarily reduce motor output variability in older adults. The disassociation between improvements in strength and improvements in variability is shown by the following findings: 1) older adults exhibit similar strength with young adults, especially for hand contractions, but greater motor output variability (Fig. 3). 2) Similar improvements in motor output variability can be achieved with low- or high-intensity practice of the first dorsal interosseous (26) and of the knee extensors (23). For example, when older adults trained for 2 weeks with a light load (10% maximum), they significantly reduced movement trajectory variability (26). This low-intensity training was followed with 4 weeks of heavy load training (70% of maximum), and although it further improved the strength of the first dorsal interosseus, it had no effect on motor output variability. 3) Furthermore, longitudinal studies found that in strength training, the knee extensors improved strength but did not improve fluctuations in motor output (2). 4) Some training interventions on older adults, such as practicing the martial art Taiji (15), clearly demonstrate that there was a dissociation between strength gains and reductions in motor output variability. Therefore, strength training or high-intensity protocols do not seem to be essential to reduce motor output variability in older adults.
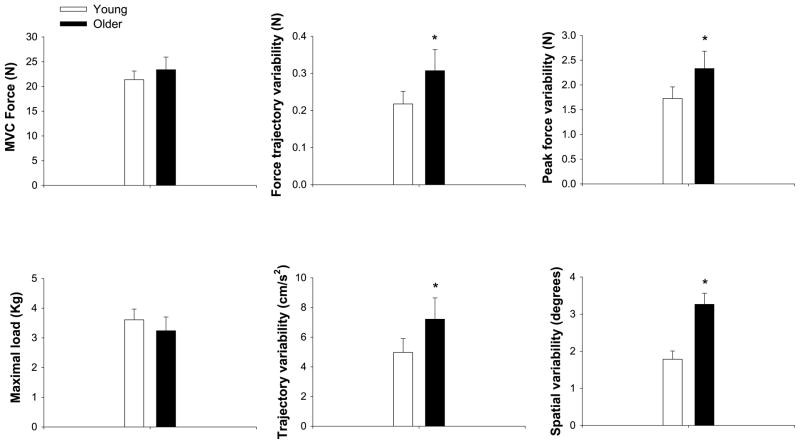
Figure 3.
Disassociation between strength and age-associated differences in motor output variability during hand contractions. The top row demonstrates isometric strength (maximal voluntary contraction force, left panel), force trajectory variability (middle panel), and end-point variability (peak force, right panel) during a goal-directed isometric force task with the index finger in young (white bars) and older (black bars) adults (data adapted from (11)). The bottom row demonstrates maximal load that can be lifted (1-repetition maximum, left panel), movement trajectory variability (middle panel), and end-point variability (spatial variability, right panel) during a goal-directed movement in young and older adults with the index finger (data adapted from (10)). Overall, the findings suggest that, although young and older adults exert similar maximal capabilities with the index finger, older adults exert greater trajectory and end-point variability than young adults. This suggests that the amplified motor output variability in older adults is not associated with reductions in muscle strength.
In contrast to strength training or high-intensity training protocols, interventions that emphasized smooth limb interaction (coordination) improved the consistency of motor output in various muscle groups (15,32). Although the exact neuromuscular mechanisms are not clear, it has been hypothesized that appropriate coactivation of the antagonistic muscles can improve movement accuracy (34). In one study, subjects learned to roll two metal balls clockwise and counterclockwise in their palm using independent and coordinated movements of their fingers (32). Although this training protocol did not improve strength, it reduced the fluctuations in force during a low-intensity pinch (<20% maximal voluntary contraction force) and improved the ability of individuals to accurately displace a small object with the hand. Furthermore, when older adults were trained with Taiji for 16 weeks, both muscle strength and fluctuations in force improved in the knee extensors (15). Nonetheless, strength improvements were not associated with improvements in fluctuations in force. These results suggest that low-intensity intervention protocols that emphasize muscle coordination seem to be effective in reducing motor output variability in older adults.
Nonetheless, some of our most recent data indicate that these improvements in motor output variability in older adults can occur within a single practice session (Fig. 4). For example, after approximately 40 trials of goal-directed contractions, older adults reduce trajectory variability and end-point variability to the level of young adults by changing the amplitude and timing of antagonistic muscles (11). In addition, within a session of tracing a continuous sinusoidal movement, when visual feedback is removed, older adults exhibit similar movement variability to young adults (8). It is possible that such fast improvements may be associated with how older adults use sensory information to organize the motor command. The amount of visual feedback provided to older adults seems to be critical on their ability to reduce motor output variability during voluntary contractions.
Figure 4.
Reduction in age-associated differences in motor output variability with short-term practice. The figure shows the short-term adaptations in end-point variability for young (white bars) and older (black bars) adults during a single session of practicing a goal-directed isometric force task (data adapted from (11)). It is clear that after four blocks of practice (~40 trials), older adults exhibited similar end-point variability in force with young adults, which they maintained throughout the session. These fast adaptations suggest that older adults adjust neural mechanisms (sensory or motor adaptations) to reduce motor output variability.
INTERACTION OF AGING AND AMOUNT OF VISUAL FEEDBACK
It has been proposed that the central nervous system attempts to reduce motor output variability through feedback control (33). Vision is one source of sensory information that can provide feedback to the central nervous system and consequently allow for motor corrections and adjustments (19). There are numerous changes that occur to the visual system with aging that can potentially alter the ability of older adults to perform visuomotor tranformations and corrections and thus exhibit greater motor output variability (35). These mechanisms will not be discussed because they are beyond the scope of this paper.
There is indirect and direct evidence that reducing the amount of visual feedback can minimize the age-associated differences in motor output variability. The indirect evidence comes from diverse findings in force variability. For example, force variability with the knee extensors is greater for older adults when they are provided with visual feedback of the task (37), whereas force variability is similar for young and older adults when the same task is performed under no visual feedback (9). The first direct evidence that greater amount of visual feedback amplifies force variability in older adults, however, was likely demonstrated in 2006 by Sosnoff and Newell (35). Specifically, their findings suggested that force variability in older adults was exacerbated when the visual gain increased from 2 to 512 p/N. Furthermore, some recent findings suggest that force variability exerted with the elbow flexors (36) or knee extensors (38) is reduced in older adults when visual feedback is removed. It seems that motor unit discharge rate variability decreases in older adults when visual feedback is removed (38).
A series of experiments in our laboratory confirm the above findings during constant isometric contractions and extend them to movements. These results suggest that one way to reduce the variability of motor output within a trial in older adults is to reduce the amount of visual feedback (1,25). For instance, when we decreased the amount of visual feedback of the task from 1.5 to 0.05 degrees (visual angle = 2 * tan−1 (height of the force signal/distance of the eye to the monitor)) during constant isometric contractions, older adults exerted similar force variability to young adults (Fig. 5A) (25). Our results suggest that the modulation of the agonist muscle activity with changes in visual feedback is different for young and older adults and can potentially explain the age-associated differences in motor output variability. The frequency band of interest was from 13 to 60 Hz, which has been associated with the cortical drive to modulate the motor neuron pool (3). Specifically, young adults were not to significantly influenced by variations in the amount of visual feedback because they increased the activation of the agonist muscle from 13 to 60 Hz. In contrast, older adults exhibited greater force variability with greater visual feedback because they did not modulate the agonist muscle at any frequency bands. These findings remain consistent when young and older adults maintain a constant position task with abduction of the index finger or with dorsiflexion of the foot (1). The visual gain was manipulated by changing the visual angle from 0.1 to 4 degrees. For older adults, positional variability increased with greater amount of visual feedback, whereas for young adults, positional variability did not change with manipulation of visual feedback (Fig. 5B).
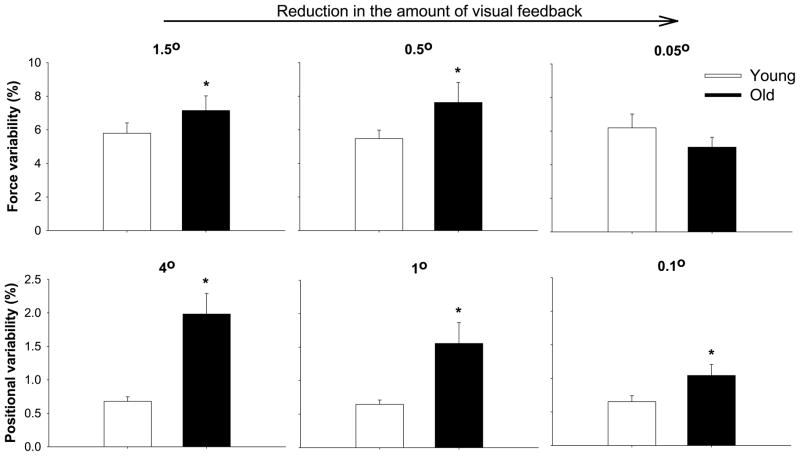
Figure 5.
Reduction in the amount of visual feedback minimizes age-associated differences in motor output variability during constant tasks. The top row demonstrates force variability at three different visual angles (lowest amount of visual feedback to the right) during constant isometric force tasks (data adapted from (25)). The bottom row demonstrates force variability at three different visual angles (lowest amount of visual feedback to the right) during constant position tasks (data adapted from (1)). Collectively, the findings suggest that lower amount of visual feedback minimizes (and in some cases, eliminates it — see lowest gain during the force task) the age-associated differences in force and positional variability during constant tasks.
Similar findings also are observed during sinusoidal movements. For example, when older adults practiced a slow sinusoidal task while lifting and lowering a light inertial load with abduction-adduction movement of the index finger, the trials that were produced with visual feedback induced significantly greater movement variability in older adults (8). However, when young and older adults exerted this movement out of memory (visual feedback was removed), there was no difference between young and older adults in movement variability (Fig. 6A). To determine whether the amount of visual feedback was responsible for eliminating the age-associated differences in movement variability, we manipulated the amount of visual feedback from 0.25 to 5.4 degrees in a follow-up experiment. When young and older adults performed slow sinusoidal movements with abduction-adduction of the index finger and with dorsiflexion-plantar flexion of the foot (1). Our findings indicate that age-associated differences in movement variability were minimized when young and older adults performed the task with the lowest visual gain (Fig. 7). Overall, these findings suggest that during constant contractions and movements, greater amount of visual feedback amplifies motor output variability in older adults and impairs their ability to control motor tasks.
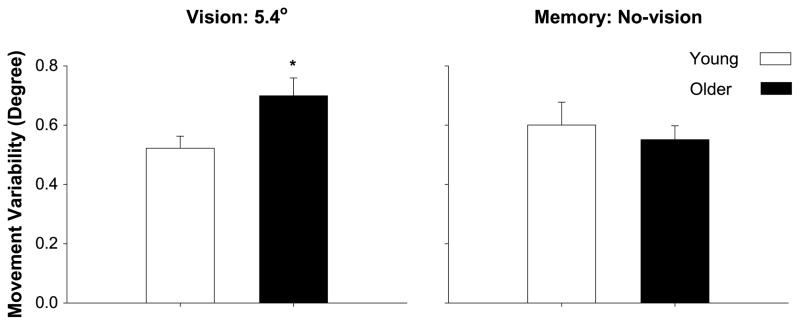
Figure 6.
Removal of visual feedback ameliorates age-associated differences in motor output variability during sinusoidal tasks. Young (white bars) and older (black bars) adults practiced sinusoidal movements with abduction-adduction of the index finger. The amount of visual feedback was 5.4 degrees. Following every block of five trials (a total of eight blocks in the practice session), young and older adults performed the sinusoidal task from memory (target was present but not their movement trace; thus, visual feedback was not available). During the practice trials with visual feedback (left panel), older adults exhibited greater movement variability than young adults (data adapted from (8)). In contrast, during the memory trials, movement variability in older adults decreased to the level of young adults. These results suggest that visual feedback is instrumental in amplifying motor output variability in older adults during movements.
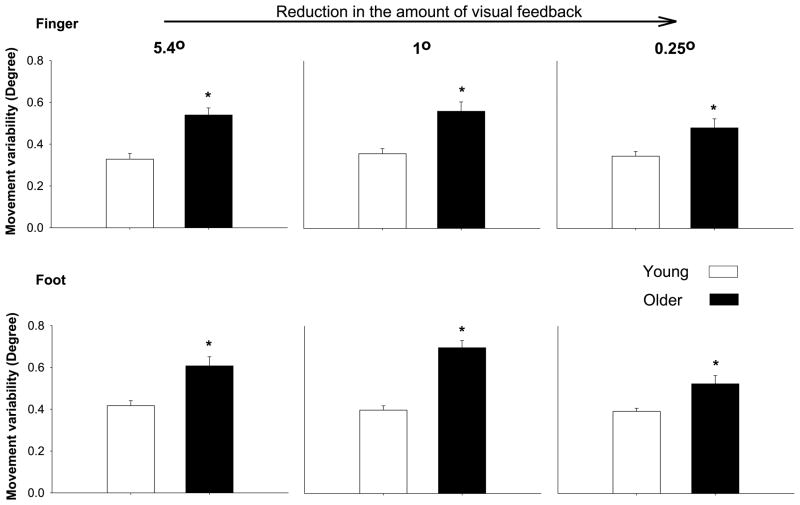
Figure 7.
Reduction in the amount of visual feedback minimizes age-associated differences in motor output variability during sinusoidal tasks. The top row demonstrates movement variability at three different visual angles (lowest amount of visual feedback to the right) when subjects attempt to match a slow sinusoidal task with the finger, whereas the bottom row demonstrates movement variability under the same conditions when the subjects attempt to match a slow sinusoidal movement task with the foot (dorsiplantar flexion; data adapted from (1)). Collectively, the findings suggest that lower amount of visual feedback minimizes the age-associated differences in movement variability during sinusoidal tasks.
SUMMARY
In summary, it is clear that older adults exert greater motor output variability, which impairs their functional capabilities, compared with young adults. This age-associated amplification in motor output variability seems to be related to the noisier activation of motor units or an altered synergistic activation of the antagonistic muscles. In addition, recent findings suggest that when older adults are given greater amount of visual feedback, they exhibit greater motor output variability than young adults. These results suggest that other mechanisms, such as impaired visuomotor transformations or visuomotor corrections, also may contribute to the amplified motor output variability in older adults.
Nonetheless, research suggests that older adults can decrease the amplified motor output variability with training. Evidence indicates that high-intensity training (e.g., strength training) is not beneficial in reducing motor output variability, whereas low-intensity training that emphasizes limb (muscle) coordination can decrease motor output variability in older adults. In addition, recent findings indicate that low amount of visual feedback minimizes and, in some cases, ameliorates the age-associated differences in motor output variability. Therefore, with our collective findings as basis, we propose that the optimal intervention to improve motor control in older adults should combine low-intensity training, muscle coordination, and low amount of visual feedback. However, further research is needed to determine whether a low amount of visual feedback would be optimal to maximize the benefits of low-intensity training that emphasizes muscle coordination on motor control and learning in older adults.
Acknowledgments
The author thanks Harsimran Baweja for helping with figure generation and comments on an earlier draft of this manuscript.
This study was supported by R01 AG031769.
References
- 1.Baweja HS, Kwon M, Glover SQ, Christou EA. Greater Amount of Visual Feedback Decreases Error But Not Variability During Movement and Positional Tasks with the Finger and Foot. San Diego (CA): Society for Neuroscience; 2010. p. 582.13. [Google Scholar]
- 2.Bellew JW. The effect of strength training on control of force in older men and women. Aging Clin Exp Res. 2002;14:35–41. doi: 10.1007/BF03324415. [DOI] [PubMed] [Google Scholar]
- 3.Brown P. Cortical drives to human muscle: the Piper and related rhythms. Prog Neurobiol. 2000;60:97–108. doi: 10.1016/s0301-0082(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 4.Brown SK. Staying Ahead of the Curve 2003: The AARP Working in retirement study. Washington (DC): 2003. [Google Scholar]
- 5.Burnett RA, Laidlaw DH, Enoka RM. Coactivation of the antagonist muscle does not covary with steadiness in old adults. J Appl Physiol. 2000;89:61–71. doi: 10.1152/jappl.2000.89.1.61. [DOI] [PubMed] [Google Scholar]
- 6.Christou EA. Aging and neuromuscular adaptations with practice. In: Shinohara M, editor. Advances in Neuromuscular Physiology of motor Skills and Muscle Fatigue. Kerala (India): Research Signpost; 2009. pp. 65–79. [Google Scholar]
- 7.Christou EA. Motor output variability. In: Kompoliti K, Verhagen Metman L, editors. Encyclopedia of Movement Disorders. Oxford (UK): Academic Press; 2010. pp. 202–4. [Google Scholar]
- 8.Christou EA, Baweja HS, Kennedy DM, Wright DL. Aging and Learning of Fine Sinusoidal Motor Tasks. San Diego (CA): Society for Neuroscience; 2010. p. 291.21. [Google Scholar]
- 9.Christou EA, Carlton LG. Old adults exhibit greater motor output variability than young adults only during rapid discrete isometric contractions. J Gerontol A Biol Sci Med Sci. 2001;56:B524–32. doi: 10.1093/gerona/56.12.b524. [DOI] [PubMed] [Google Scholar]
- 10.Christou EA, Enoka RM. Aging and movement errors when lifting and lowering light loads. Age. doi: 10.1007/s11357-010-9190-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christou EA, Poston B, Enoka JA, Enoka RM. Different neural adjustments improve endpoint accuracy with practice in young and old adults. J Neurophysiol. 2007;97:3340–50. doi: 10.1152/jn.01138.2006. [DOI] [PubMed] [Google Scholar]
- 12.Christou EA, Shinohara M, Enoka RM. Fluctuations in acceleration during voluntary contractions lead to greater impairment of movement accuracy in old adults. J Appl Physiol. 2003;95:373–84. doi: 10.1152/japplphysiol.00060.2003. [DOI] [PubMed] [Google Scholar]
- 13.Christou EA, Tracy BL. Aging and motor output variability. In: Davids K, Bennett S, Newell K, editors. Movement System Variability. Champaign (IL): Human Kinetics; 2005. pp. 199–215. [Google Scholar]
- 14.Christou EA, Tracy BL, Enoka RM. The steadiness of lengthening contractions. In: Latash ML, editor. Progress in Motor Control, Volume II: Structure-Function Relations in Voluntary Movements. Champaign (IL): Human Kinetics; 2002. pp. 195–207. [Google Scholar]
- 15.Christou EA, Yang Y, Rosengren KS. Taiji training improves knee extensor strength and force control in older adults. J Gerontol A Biol Sci Med Sci. 2003;58:763–6. doi: 10.1093/gerona/58.8.m763. [DOI] [PubMed] [Google Scholar]
- 16.Dinse HR. Cortical reorganization in the aging brain. Prog Brain Res. 2006;157:57–80. doi: 10.1016/s0079-6123(06)57005-0. [DOI] [PubMed] [Google Scholar]
- 17.Eisen A, Entezari-Taher M, Stewart H. Cortical projections to spinal motoneurons: changes with aging and amyotrophic lateral sclerosis. Neurology. 1996;46:1396–404. doi: 10.1212/wnl.46.5.1396. [DOI] [PubMed] [Google Scholar]
- 18.Enoka RM, Christou EA, Hunter SK, et al. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol. 2003;13:1–12. doi: 10.1016/s1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- 19.Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol. 1993;69:2108–15. doi: 10.1152/jn.1993.69.6.2108. [DOI] [PubMed] [Google Scholar]
- 21.Graves AE, Kornatz KW, Enoka RM. Older adults use a unique strategy to lift inertial loads with the elbow flexor muscles. J Neurophysiol. 2000;83:2030–9. doi: 10.1152/jn.2000.83.4.2030. [DOI] [PubMed] [Google Scholar]
- 22.Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394:780–4. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- 23.Hortobagyi T, Tunnel D, Moody J, Beam S, DeVita P. Low- or high-intensity strength training partially restores impaired quadriceps force accuracy and steadiness in aged adults. J Gerontol A Biol Sci Med Sci. 2001;56:B38–47. doi: 10.1093/gerona/56.1.b38. [DOI] [PubMed] [Google Scholar]
- 24.Keen DA, Yue GH, Enoka RM. Training-related enhancement in the control of motor output in elderly humans. J Appl Physiol. 1994;77:2648–58. doi: 10.1152/jappl.1994.77.6.2648. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy DM, Christou EA. Age-Associated Differences in the Control of Force and Modulation of Agonist Muscle Activity with Different Amounts of Visual Feedback. San Diego (CA): Society for Neuroscience; 2010. p. 893.5. [Google Scholar]
- 26.Kornatz KW, Christou EA, Enoka RM. Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. J Appl Physiol. 2005;98:2072–80. doi: 10.1152/japplphysiol.01149.2004. [DOI] [PubMed] [Google Scholar]
- 27.Kouzaki M, Shinohara M. Steadiness in plantar flexor muscles and its relation to postural sway in young and elderly adults. Muscle Nerve. 2010;42:78–87. doi: 10.1002/mus.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laidlaw DH, Bilodeau M, Enoka RM. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve. 2000;23:600–12. doi: 10.1002/(sici)1097-4598(200004)23:4<600::aid-mus20>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 29.Marmon AR, Pascoe MA, Schwartz RS, Enoka RM. Associations among strength, steadiness, and hand function across the adult life span. Med Sci Sports Exerc. doi: 10.1249/MSS.0b013e3181f3f3ab. in press. [DOI] [PubMed] [Google Scholar]
- 30.Matthews PB. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol. 1996;492:597–628. doi: 10.1113/jphysiol.1996.sp021332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negro F, Holobar A, Farina D. Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J Physiol. 2009;587:5925–38. doi: 10.1113/jphysiol.2009.178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranganathan VK, Siemionow V, Sahgal V, Liu JZ, Yue GH. Skilled finger movement exercise improves hand function. J Gerontol A Biol Sci Med Sci. 2001;56:M518–22. doi: 10.1093/gerona/56.8.m518. [DOI] [PubMed] [Google Scholar]
- 33.Scott SH. Optimal feedback control and the neural basis of volitional motor control. Nat Rev Neurosci. 2004;5:532–46. doi: 10.1038/nrn1427. [DOI] [PubMed] [Google Scholar]
- 34.Seidler-Dobrin RD, He J, Stelmach GE. Coactivation to reduce variability in the elderly. Motor Control. 1998;2:314–30. doi: 10.1123/mcj.2.4.314. [DOI] [PubMed] [Google Scholar]
- 35.Sosnoff JJ, Newell KM. Information processing limitations with aging in the visual scaling of isometric force. Exp Brain Res. 2006;170:423–32. doi: 10.1007/s00221-005-0225-5. [DOI] [PubMed] [Google Scholar]
- 36.Tracy BL, Dinenno DV, Jorgensen B, Welsh SJ. Aging, visuomotor correction, and force fluctuations in large muscles. Med Sci Sports Exerc. 2007;39:469–79. doi: 10.1249/mss.0b013e31802d3ad3. [DOI] [PubMed] [Google Scholar]
- 37.Tracy BL, Enoka RM. Older adults are less steady during submaximal isometric contractions with the knee extensor muscles. J Appl Physiol. 2002;92:1004–12. doi: 10.1152/japplphysiol.00954.2001. [DOI] [PubMed] [Google Scholar]
- 38.Welsh SJ, Dinenno DV, Tracy BL. Variability of quadriceps femoris motor neuron discharge and muscle force in human aging. Exp Brain Res. 2007;179:219–33. doi: 10.1007/s00221-006-0785-z. [DOI] [PubMed] [Google Scholar]
- 39.Wolpert DM, Ghahramani Z, Flanagan JR. Perspectives and problems in motor learning. Trends Cogn Sci. 2001;5:487–94. doi: 10.1016/s1364-6613(00)01773-3. [DOI] [PubMed] [Google Scholar]