Introduction
Insomnia and fatigue are among the earliest and most distressing complaints of people living with HIV, (K. Lee, C. Portillo, &, & H. Miramontes, 2001; Reid & Dwyer, 2005; Salahuddin, Barroso, Leserman, Harmon, & Pence; Vosvick, et al., 2004) who are more likely to experience insomnia symptoms than uninfected adults (Jean-Louis G, 2012). A systematic review found that 74% of people living with HIV have insomnia and that these disturbances occur at all stages of HIV disease. Increased sleep disturbances put them at increased risk for all-cause mortality.(Alvarez & Ayas, 2004; Patel, et al., 2004; Reid & Dwyer, 2005) Sleep is a complex biopsychosocial process several unique factors have been found to mediate and/or moderate sleep in people with HIV including medical variables including type of HIV medication, time since HIV diagnosis, HIV viral load, presence of other diseases (infections, depression); environmental and social variables including living conditions (noise, safety, private space) and social support; and personal characteristics such as employment status, illicit drug use, perceived stress, sleep habits, and history of incarceration.(K. A. Lee, C. J. Portillo, & H. Miramontes, 2001; Nokes, 2001; Phillips & Branson, 2009; Reid & Dwyer, 2005; Saberi, Neilands, & Johnson, In press) Among those living with HIV, poor sleep quality has been found to lead to negative health outcomes, HIV medication nonadherence, and diminished quality of life.(McDaniel, Buboltz, Chauvin, & Eddlemon, 2011; Reid & Dwyer, 2005; Saberi, et al., In press) Consequently, interventions to improve sleep may have a significant positive impact on health outcomes for people living with HIV.
Most interventions to improve sleep involve medical management strategies such as central positive airway pressure for sleep apnea,(Issa & Sullivan, 1986; Naughton, Liu, Bernard, Goldstein, & Bradley, 1995) prescribed and over-the-counter medications,(Morin, et al., 2009; NIH, 2005) and light therapy for other sleep problems.(Wilson, et al., 2010) These methods can be cumbersome and may have adverse effects. Behavioral interventions may also improve sleep but in a less burdensome and more efficient way. People living with HIV have unique barriers to proper sleep hygiene which are often overlooked in traditional medical management of sleep disturbances. Lee and colleagues (2012) found that, contrary to the accepted sleep paradigm, traditional risk factors for poor sleep (high BMI, anthropomorphic factors) were not associated with sleep disorders in people living with HIV.(K. Lee, et al., 2012) They conclude that targeted behavioral interventions focusing on sleep hygiene and environmental factors may be more effective than traditional medical interventions focusing on body weight to improve sleep in this population. This finding has been supported by research in other populations. (Haesler, 2004; K. A. Lee & Gay, 2011)
Previous research involving people living with HIV has found that sleep disturbances are among the most troubling aspects of HIV self-management, with 35% of subjects desiring interventions to improve sleep quantity and quality.(Webel, 2012) Nonetheless, few studies have examined how to improve healthy sleep in this group, particularly through behavioral sleep hygiene interventions. (Phillips & Branson, 2009; Reid & Dwyer, 2005) Limited research has been done in sleep behavior interventions. Dreher (2003) found that decreasing caffeine consumption led to increased sleep quality in those with HIV. (Dreher, 2003) Phillips and Skelton (2001) found that twice-weekly acupuncture improved subjective sleep quality in a one-group experimental study of 21 people living with HIV. (Phillips KD, 2001) Most recently, Hudson, et al.(2008) found that an individually tailored sleep hygiene intervention increased nighttime sleep in 30 women with HIV.(Hudson, Portillo, & Lee, 2008) Although promising, this work lacks a control group, has not been replicated or expanded to other HIV/AIDS populations and does not examine the sustained effect of the intervention beyond 1 week. These limitations were addressed in our pilot study examining the feasibility of the SystemCHANGETM-HIV intervention.
The objective of this investigation was to evaluate the feasibility and estimate the magnitude of the effect of a novel, evidence-based behavioral intervention, SystemCHANGE™-HIV on sleep outcomes and quality of life in people living with HIV compared with a usual care control group. We hypothesized that the SystemCHANGE™-HIV intervention would be a feasible intervention for HIV+ adults, as indicated by ratings of perceived usefulness, applicability, and sustainability and that participants in the intervention would have increased sleep quality, sleep quantity, and increased quality of life.
Methods
Participants
This study was approved by the Institutional Review Board at University Hospitals, Case Medical Center. Participants included 43 HIV+ adults in Northeast Ohio. Our sample size was pre-specified to be 20 participants per group. This follows the methodology of Hertzog (2008) which showed that samples of 10–40 participants per group are adequate for evaluating feasibility in a pilot study.(Hertzog, 2008) We anticipated approximately 10% attrition and recruited 45 participants, of whom 43 attended the first study appointment and were enrolled. Inclusion criteria were: >18 years of age, speaking fluent English, and having a confirmed HIV diagnosis. There were no additional exclusion criteria. Sample participants were recruited through Infectious Disease clinics, AIDS Service Organizations, and through an approved HIV research registry via flyers, letters, and physician referral. All participants provided informed consent.
Procedure
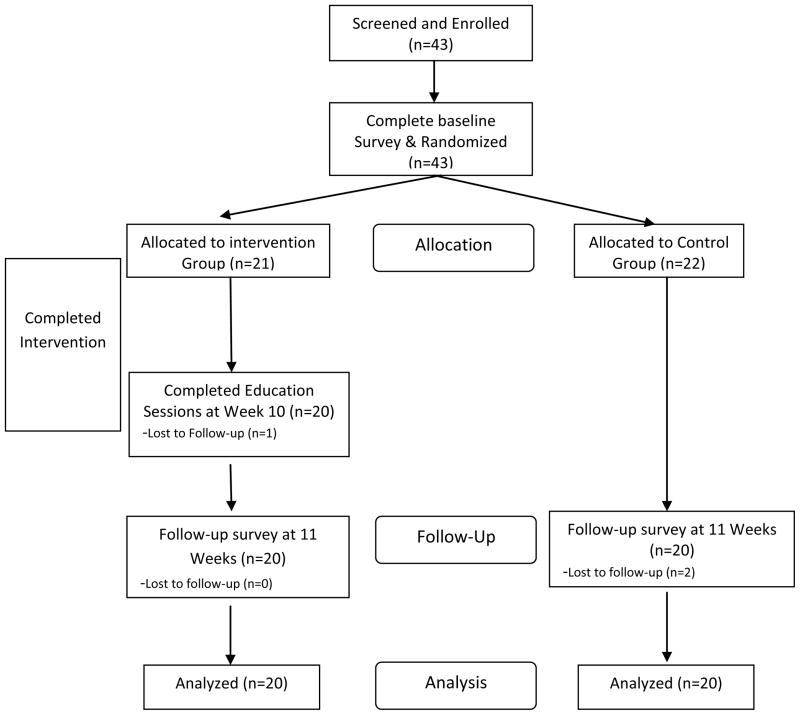
All interested participants called a screening line to ensure that they met the inclusion criteria between November 2010 and January 2011. Those meeting inclusion criteria were scheduled to attend an initial appointment with a research assistant. At this initial appointment, participants completed an informed consent document and received instructions on how to use an actigraph and sleep diary for the next seven days. An actigraph device is worn on the wrist and contains an accelerometer that detects movement from which sleep/wake state can be determined using an algorithm that has been validated against EEG measures of sleep and wake time.(T. Blackwell, 2005) Upon correct demonstration of each tool the participants were sent home the actigraph device to wear and the diary to complete. At the end of one week, the participants returned with the actigraphs, completed sleep diaries, and completed a pen and paper survey (described below). Following these baseline assessments, participants were randomized to either a behavior intervention or a standard treatment control group using block randomization with randomly chosen block sizes of 2 and 4. A flow chart of study procedures is provided in Figure 1. Participants were paid $50 for completion of their assessments (per assessment). Those randomized to the intervention group immediately began the first intervention session and those randomized to the control group received a copy of the HIV Symptom Management Strategies: A Manual for People Living with HIV/AIDS. The participants in the control group were orientated on how to use the manual by a research assistant, and then were instructed to return in 10 weeks. (Wantland, et al., 2008) The intervention group was reminded to come to each session by a weekly phone call from the study staff. After 10 weeks both groups were called and reminded to come to the follow up assessment. All study visits occurred at a convenient community-based setting and all study procedures occurred between February and June 2011. This study was registered at clinicaltrials.gov with the trial registration number NCT01256814.
Figure 1.
Flowchart of SystemCHANGE -HIV Participants
SystemCHANGE™-HIV Intervention
Those randomized to the intervention group were invited to return for 10 weekly sessions on different topics of HIV management, including sleep hygiene, and behavioral modification strategies. SystemCHANGE™-HIV is based on the successful SystemCHANGE™ intervention, grounded in a socioecologoical model. It represents a paradigm shift in behavioral interventions, by focusing on re-designing the system of the interpersonal environment and the daily routines linked to health behavior, rather than focusing on personal effort to change individual behaviors. (M. S. Alemi F, Baghi H., 2008; N. D. Alemi F, Ardito S, Headrick L, Moore S, Hekelman F, Norman L., 2000; Humpel, Owen, & Leslie, 2002) This approach was designed to assist individuals and their families to focus on changing the daily systems in their lives (routines, events, circumstances) that affect health behaviors. SystemCHANGE™ has been previously validated in numerous populations and its theoretical basis has been described elsewhere.(Moore, et al., 2006; Moore SM, 2002) Each of the 10 group sessions were taught by either a trained Health Educator or a Registered Nurse. These facilitators used a semi-scripted lesson plan to increase the fidelity of information delivered. These lessons were based on SystemCHANGE™ theory and therefore encouraged environmental and not cognitive changes to improve health-related behavior changes in the participants. The crux of SystemCHANGE™ approach is to use small environmental changes to make significant improvements in health. This was done each week by encouraging participants to attempt weekly small tests of change and to modify those changes to be the most beneficial in self-managing their HIV, focusing on sleep. Both fidelity of receipt of the intervention (dose) and perceived effectiveness and applicability of each intervention session were assessed at the end of each session with a participant feedback form. Participants in the intervention group received a $10 gift card at the end of each session were given a bus pass or parking pass to compensate them for time and travel expenses.
Measures
Sociodemographics and health history were assessed with a brief demographic survey and medical chart abstraction form. This instrument consisted of 26 items about demographics and illness characteristics. It included questions on age, gender, race, ethnicity, education, income level, and health insurance. Additionally, participants consented to allow the research team to abstract the data from their medical chart.
Feasibility of Intervention was assessed using a written qualitative assessment at the conclusion of the intervention. The purpose of this instrument is to examine the feasibility of this pilot intervention. Our operational definition of feasibility came from Grady, Cummings and Hulley (2007). Accordingly, we captured data on the following: perceived usefulness, applicability to the population, and sustainability; as well as time required and cost of recruitment, qualitative assessment of willingness to accept randomization in future interventions and comply with intervention procedures; acceptance of measurement procedures, problems with completion, and time to complete instruments (Grady, 2007).
Sleep duration and quality were measured using wrist actigraphy (Actiwatch Spectrum, Respironics, Inc.). Subjects were taught proper use of the actigraph and were asked to wear the device continuously for 1 week at baseline, and again for 1 week at 10 weeks later. Bedtime intervals were manually set in a standardized fashion by a technician who was blinded to treatment assignment and then quantitative sleep measures were obtained using a validated sleep scoring algorithm.(Kushida, et al., 2001)
Our primary measures of sleep quantity were mean sleep duration and of sleep quality, the sleep fragmentation index and sleep efficiency. All three are widely used and validated measures of sleep duration and quality.(Lauderdale, et al., 2006; Montgomery-Downs, Insana, Clegg-Kraynok, & Mancini, 2010) Mean sleep duration was defined as the average over 1 week of the total amount of sleep obtained in the main sleep period as defined by the subject. The sleep fragmentation index measured the degree to which sleep is fragmented by periods of wake. (McLean, et al., 2005; van den Berg, et al., 2008) Sleep efficiency is a measure of the proportion of time actually spent asleep between sleep onset and waking. Both sleep efficiency and sleep fragmentation index were calculated for the main sleep period of each 24-hour interval and then averaged for the week of recording.
Sleep disturbances and sleep-related impairments were assessed using the validated Patient Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance and Sleep-Related Impairments Scales.(Buysse, 2010) These scales measure self-reported sleep disturbances (trouble falling or staying asleep) and sleep-related daytime impairments (difficulty functioning due to poor sleep) in the past 7 days, and are normalized on a T distribution with a mean of 50 and standard deviation of 10. The Cronbach’s alphas at baseline were 0.90 and 0.92, respectively, indicating good reliability of the scales.
Quality of life was measured using the HIV/AIDS Targeted Quality of Life Instrument. This widely-used, 34-item instrument is a disease-specific measure assessing nine dimensions: Overall function, life satisfaction, health worries, financial worries, medication worries, HIV mastery, disclosure worries, provider trust, and sexual function(Sousa, 1999). Each dimension was scored and reported individually. Reliability coefficients ranged from 0.83 to 0.88 for all dimensions. (Holzemer W., 2000) For this study a select subset of the dimensions, requiring only 10 of the 34 items, was used to indicate quality of life (overall function and life satisfaction subscales). The Cronbach’s alphas at baseline 0.80 on the overall function subscale and 0.91 on the life satisfaction subscale.
Statistical Analyses
Descriptive statistics were used to summarize participant’s demographic and clinical characteristics. For continuous variables we reported means and standard deviations, and categorical variables were reported as frequencies with percentages. All analyses maintained subjects in the original treatment groups (intent to treat). The treatment effect (intervention group minus control group) on each endpoint was estimated using ANCOVA models looking at the difference between baseline and end-of-study responses. Unadjusted analyses included only the baseline value of the endpoint and the treatment assignment as covariates. Adjusted analyses included age, sex, and years since HIV diagnosis as additional covariates. These additional covariates were chosen a priori as they have been shown to impact sleep quality and quantity. (Dreher, 2003; Ohayon, Carskadon, Guilleminault, & Vitiello, 2004; Reid & Dwyer, 2005)
Results
We conducted a two-group randomized control study with repeated assessments at baseline and end-of treatment (week 10) with 43 HIV+ adults, of whom 40 completed all study procedures (7% attrition). Our sample was predominately male (58%), African-American (8%), single (79%), and unemployed (91%). Medically, most had health insurance (86%), on average, had been living with HIV for 13 years, most were taking HIV anti-retroviral medications (95%), and most had an undetectable HIV viral load (67%). Four percent of our sample had been diagnosed with sleep apnea (n=2; one participant in the intervention group and one participant in the control group). There were no statistically significant differences in baseline characteristics between participants in the intervention group and those in the control group. For more details on the demographic and medical characteristics, see Table 1.
Table 1.
Demographic and Medical Characteristics of Study Participants
| Control Group (n=22) | Intervention Group (n=21) | |
|---|---|---|
| Frequency (%)a | Frequency (%)a | |
| Mean Age (+/−SD), years | 47.8 (6.4) | 49.1 (7.4) |
| Female | 9 (40.9) | 9 (42.9) |
| Have Children | 11 (50.0) | 11 (52.3) |
| Number of children living with participant | ||
| 0 | 5 (22.7) | 5 (23.8) |
| 1 | 4 (18.2) | 1 (4.8) |
| 2 | 1 (4.5) | 3 (14.3) |
| Race | ||
| African American | 18 (90.0)b | 18(85.7) |
| White/Angelo | 2 (10.0)b | 2 (9.5) |
| Other | 0 | 1 (4.8) |
| Marital Status | ||
| Single | 17 (77.3) | 17 (81.0) |
| Divorced | 4 (18.2) | 2 (9.5) |
| Other | 1 (4.5) | 2 (9.5) |
| Education Level | ||
| 11th grade or less | 7 (31.8) | 6 (28.6) |
| High School or higher | 15 (68.2) | 15 (71.4) |
| Annual Income | ||
| No monthly income | 5 (22.7) | 3 (14.3) |
| $1–$599 | 6 (27.3) | 4 (19.1) |
| $600–$999 | 9 (40.9) | 13 (61.9) |
| $1000 or more | 2 (9.1) | 1 (4.8) |
| Currently Works for Pay | 3 (13.6)c | 1 (4.8) |
| Has Permanent Housing | 17 (77.3)d | 21 (100) |
| Has Health Insurance | 17 (77.3) | 20 (95.2) |
| Type of Health Insurance | ||
| Medicaid | 9 (40.9) | 11 (52.4) |
| Medicare | 2 (9.1) | 3 (14.3) |
| Private, not by work | 3 (13.6) | 2 (9.5) |
| Other | 1 (4.5) | 1 (4.8) |
| Medical Characteristicse | ||
| Mean Duration Diagnosed with HIV, years (+/−SD) | 13 (7.2) | 14 (5.8) |
| Currently Prescribed Anti-Retroviral Therapy (ART) | 21 (95.5) | 20 (95.3) |
| Mean Duration since ART Initiation, years (+/−SD) | 10 (6.0) | 9 (5.6) |
| Undetectable HIV Viral Load | 15 (68.2) | 14 (66.7) |
| Median HIV Viral Load for those with detectable values/mL (IQR) | 1,645 (780; 3,570) | 19,065 (2,816; 155,000) |
| Mean CD4 cells/μ1 (+/−SD) | 500 (261.1) | 529 (309.5) |
| Have Comorbidities | 18 (81.8) | 20 (95.2) |
| Admitted to Emergency Department in Past 12 months | 9 (42.9) | 8 (38.1) |
| Admitted to Hospital in Past 12 months | 4 (18.2) | 5 (23.8) |
| Mean Percent Missed HIV Primary Care Appointments in past 12 months (+/− SD) | 25.4 (27.2) | 44.6 (35.3) |
Descriptive statistics are reported as frequency and per cent of total sample, unless otherwise noted; no statistically significant differences were found between the intervention and control group at baseline
20 of 22 participants in the control group reported race;
19 of 22 participants in the control group reported employment status;
19 of 22 participants in the control group reported housing status;
1 of the 22 participants in the control group did have a regular HIV clinic and we were not able to abstract these variables from their medical chart. For these variables, the denominator in the control group was 21.; Figure calculated as the number of appointments missed/total number of the primary care appointments schedule in the previous 12 months.
Mean sleep duration and sleep efficiency for the members of the control group vs. the SystemCHANGE™-HIV intervention group (5.5 +/−2.1 hrs vs. 6.5+/−1.6 hrs; and 78.0 % +/− 13.1 vs. 82.0% +/− 8.7) tended to be lower, but not statistically significant, at baseline. Conversely, sleep fragmentation (33.9 % +/− 14.9 vs. 28.8% +/− 11.7), self-reported sleep disturbances (59.0 +/− 9.5 vs. 57.8 +/− 6.7), and self-reported sleep related impairments (55.8 +/− 8.1 vs. 53.0 +/−9.1) in members of the control group vs. the SystemCHANGE™-HIV intervention group tended to be greater, but not statistically significant, at baseline (Table 2).
Table 2.
Baseline and follow-up scores for outcomes by treatment assignment
| Endpoints | Control (n=20) | Intervention (n=20) | Control (n=20) | Intervention (n=20) |
|---|---|---|---|---|
| Baseline Sleep Outcomes | Follow-up Sleep Outcomes | |||
| Mean Sleep Duration in hours(+/− SD) | 5.5 (2.1) | 6.5 (1.5) | 5.6 ± 11.8 | 373 ± 74 |
| Mean Sleep Efficiency % (+/− SD) | 33.9 (14.9) | 28.8 (11.4) | 77.6 ± 11.7 | 82.1 ± 5.6 |
| Mean Sleep Fragmentation % (+/− SD) | 78.0 (13.1) | 82.0 (8.7) | 34.8 ± 16.3 | 29.5 ± 8.6 |
| Mean Sleep Disturbances (+/− SD) | 59.0 (9.5) | 57.8 (5.9) | 57.8 ± 5.9 | 57.4 ± 9.1 |
| Mean Sleep-related Impairments (+/− SD) | 55.8 (8.1) | 53.0 (9.1) | 50.4 ± 9.4 | 51.9 ± 9.0 |
| Baseline Quality of Life | Follow-up Quality of Life | |||
| Mean Overall Functioning (+/− SD) | 63.2 (18.7) | 55.2 (23.4) | 56.9 (19.4) | 56.9 (18.3) |
| Mean Life Satisfaction (+/− SD) | 73.8 (21.0) | 65.3 (27.6) | 71.6 (22.0) | 70.3 (26.0) |
18 of 20 participants in the control group had complete data;
19 of 20 participants in the control group had complete data
On average, SystemCHANGE™-HIV intervention participants attended 71% of all sessions (74% per protocol), rated the intervention as highly useful (77% rated it as superior or excellent), worth their time (76% rated it as superior or excellent), and all thought it would be useful in the future (94% rated it as superior). Overall intervention satisfaction ratings were high, with 44% rating it as a superior program, 25% as excellent, and 31% as a good program. Ninety-three percent of participants reported using the information that was taught during the 10-week program, and 86% applied the intervention content to other habits, including smoking cessation and searching for employment. The cost of the intervention was $187 per participant. When asked what they would pay for the intervention, the mean amount if the participant was self-paying was $177 (+/− $222) and the mean amount if insurance was paying was $479 (+/− $386). Participants also provided a number of brief qualitative comments expressing their level of interest in the SystemCHANGE™-HIV intervention and perceived effectiveness of the intervention Representative quotes from these comments, as well as additional intervention feasibility data, are presented in Table 3.
Table 3.
Measures of Feasibility of the SystemCHANGE -HIV Intervention (n=20)a
| Usefulness | Measure/Question | Result |
|---|---|---|
| Attendance Rate at Sessions | 71.4% of sessions attended | |
| Did you use what was taught? | Yes: 93% | |
| Was the program useful b | Good: 24% Excellent: 48% Superior: 29% |
|
| Did you think it was worth your time? b | Good: 24% Excellent: 35% Superior: 41% |
|
| Will you use the program in the future? b | Excellent: 6% Superior: 94% |
|
| Applicability | ||
| Overall satisfaction rating b | Good: 31% Excellent: 25% Superior: 44% |
|
| Did you apply it to other habits? | Yes: 86% Including smoking cessation and job search |
|
| What is the most important thing you learned?c | “[I really enjoyed] learning how to schedule time and apply myself more to do the things I have to do” “I learned that I can change and do better” “I can improve my health, sleep, and exercise” “Sometimes it’s not always motivation that keeps you from reaching your goals” |
|
| Sustainability | ||
| Intervention Cost d | Total: $3731 or $186.56/per participant | |
| What participant’s would pay for the intervention? | Mean if self-pay: $177 (+/− $222) Mean if insurance pay: $479 (+/− $386) |
17/20 Participants completed the final evaluation on which the self-report feasibility variables are based;
Self-assessment on a 5-point Likert Scale where 1=Poor, 2= Fair, 3=Good, 4=Excellent, 5=Superior;
Select representative quotes presented;
Intervention costs include: Staff time spent contacting each participant X $15/hr; Staff time spent at each group session X $15/hr X 2 staff members; Compensation for participant travel and time /session; Food costs ~$10/session (not included were intervention development and training costs, opportunity costs for participants, staff time for data collection and analysis)
At the end of the intervention, participants in the SystemCHANGE™-HIV intervention experienced a 10 minute/night increase in sleep time (t=0.38, p=0.71), a 2.3% absolute increase in sleep efficiency (t=0.98, p=0.33), a 2.0% absolute decrease in sleep fragmentation (t=−0.58, p=0.57), a 0.7%-point decrease in sleep disturbances (t=−0.27, p=0.79), and a 0.5%-point increase in sleep-related impairments (t=0.16, p=0.88) relative to the control group, based on the model estimates of the treatment effect adjusted for age, sex, and years since HIV diagnosis. Additionally, participants in the intervention group experienced a 2.1 point increase in life satisfaction (t=0.28, p=0.79) and 3.0 point increase in overall functioning (t=0.58, p=0.57) relative to control group based on the model estimates of the treatment effect adjusted for age, sex, and years since HIV diagnosis. Additional outcome statistics are reported in Table 4.
Table 4.
Mean change in outcome variables by treatment assignment (n=40)
| Control Group | Intervention Group | Adjusted for baseline score and group assignment | Further adjusted for demographic variablesb | |||||
|---|---|---|---|---|---|---|---|---|
| Endpoint | n | Mean Δ (+/− SD) a | n | Mean Δ (+/− SD) a | Intervention – Control | p-value | Intervention – Control | p-value |
| Sleep time in minutes per night | 20 | 6 (93) | 20 | −15 (94) | 7 (−44, 59) | 0.770 | 10 (−45, 65) | 0.706 |
| Sleep efficiency % | 20 | −0.3 (7.6) | 20 | 0.1(9.7) | 2.3% (−2.3, 7.0) | 0.316 | 2.3% (−2.5, 7.1) | 0.332 |
| Sleep fragmentation % | 20 | 0.9 (8.6) | 20 | 0.7 (13.7) | −2.2 (−9.0, 4.6) | 0.513 | −2.0% (−9.0, 5.0) | 0.568 |
| Self-reported sleep disturbances | 18 | −1.3 (9.2) | 20 | −0.4 (12.5) | −0.4 (−5.6, 4.8) | 0.880 | −0.7 (−6.2, 4.8) | 0.787 |
| Self-reported sleep impairments | 19 | −5.5 (15) | 20 | −1.1 (13.7) | 0.6 (−5.4,6.6) | 0.839 | 0.5 (−5.8, 6.7) | 0.876 |
| Quality of life overall functioning | 20 | −5.4 (18.2) | 20 | 01.7 (19.3) | 3.6 (−6.7, 13.8) | 0.486 | −3.0 (−7.6, 13.6) | 0.568 |
| Quality of life satisfaction | 19 | −2.2 (21.3) | 20 | 5.0 (32.5) | 1.7 (−13.1, 16.6) | 0.813 | 2.1 (−13.0, 17.2) | 0.778 |
Mean change from baseline to 10-week follow up;
adjusted for age, sex, and yrs since HIV diagnosis
Discussion
This is the first study of the SystemCHANGE™ approach to behavioral change in adults living with HIV. The findings from our pilot study suggest that the SystemCHANGE™-HIV intervention is a feasible behavioral intervention to improve sleep and quality of life in adults living with HIV. Using an intent-to-treat analysis, we found that participants attended, on average, 71% of all intervention sessions (74% per protocol). This high attendance rate supports the feasibility of the experimental intervention.(Atkins CJ, 1990) We found that participants believed the intervention was useful, both in the present and in the future; they perceived it has wide applicability to important behavioral outcomes; and that it was a program worth investing time and money in. As sleep has become recognized as an important factor in maintaining and improving health outcomes in disease conditions (Alvarez & Ayas, 2004; Durmer & Dinges, 2005), and especially in HIV (Reid & Dwyer, 2005), there has been a corresponding increase in behavioral interventions targeting sleep in this population (Dreher, 2003; Hudson, et al., 2008; Naughton, et al., 1995; Phillips & Branson, 2009; Phillips KD, 2001). Recent scholarship has highlighted the importance of focusing behavioral interventions on environmental and sleep hygiene factors, rather than traditional physical factors in people living with HIV(K. Lee, et al., 2012). As SystemCHANGE™ has its theoretical basis in the socioecological theory and emphasizes personal system improvement; it is uniquely situated to address these issues. Furthermore, it is a paradigm that has successfully improved behavior change in other populations and our data suggest that with further refinement and study, it could also be successful among people living with HIV.
Although the results were not statistically significant due to our small sample size, they suggest that a refined SystemCHANGE™-HIV intervention may improve objectively-measured sleep outcomes. An increase of 10 minutes per night is an improvement over a previous sleep hygiene intervention in our target population. Hudson, et al. (2008) reported no improvement in total sleep time for women living with HIV who underwent a tailored sleep improvement intervention.(Hudson, et al., 2008) We found improvement in both sleep efficiency (2.3%-point increase) and sleep fragmentation (2.0%-point decrease). Our evidence describing the effect of the intervention on self-reported sleep disturbances and impairments was mixed. While self-reported sleep disturbances decreased in the intervention compared to the control group, self-reported sleep-related impairments increased in the intervention group. This may be a function of the new PROMIS measures. Previously, these scales were rigorously validated in a large national study,(Buysse, 2010) but they have not been fully validated in a sample of HIV+ adults. This may have decreased the sensitivity of these measures to detect changes in perceived sleep quality. Future research should explore both questions in a larger sample of HIV+ adults.
An important part of feasibility of an intervention is whether it is sustainable. Accordingly, we tracked the cost of the intervention’s implementation in order to determine whether the SystemCHANGE™-HIV intervention could efficiently be delivered to its target population. We found a total cost of delivering the 10 week intervention to 20 people was $3,731 or $185.65 per participant, which is consistent with other group-based self-management programs in the United States (Gordon C, 2007). Most of our costs were associated with the $10 incentive given to each participant for each session attended ($2,000) - a cost that could be minimized if adapted to a community-based setting which do not customarily give incentives for programs. Whether or not the SystemCHANGE™-HIV intervention could be deemed cost-effective, will be dependent upon whether a refined intervention is effective in future research. However, participants of the intervention reported that they would be willing to pay, on average $177 (+/− $222) for the 10 week intervention. If insurance was paying for the intervention, the perceived average value of the 10 week intervention increased to $479 (+/− 386). This discrepancy between the value of the intervention based on who was paying was not surprising. The actual cost of implementing the intervention was close to the average value if participants were self-paying (+ $9) and was well below the average reported value if insurance was paying (-$293). Taken together, the SystemCHANGE™-HIV appears to be a sustainable intervention which enhances the feasibility of the intervention.
While the results of this pilot study suggest that the SystemCHANGE™-HIV intervention is a feasible, and possibly effective intervention, our study has several limitations. First, our control condition was not a time-matched, attention-matched control condition. Those randomized to the control group received an effective HIV self-management manual and were oriented to how to appropriately use the manual, but did not attend weekly group sessions (as the intervention group did). Second, recruiting from multiple sites may allow for more generalizable findings. Third, our sample size (while appropriate for a pilot study) was too small to conclusively determine the efficacy of the intervention. It is possible that the apparent efficacy is an artifact of random chance and small sample size, but the direction and size of the results are consistent with a benefit that is worth pursuing in this at-risk population.
Notwithstanding these limitations, our pilot study provides preliminary data that support the possibility that a refined SystemCHANGE™-HIV intervention may efficiently improve sleep and quality of life in adults living with HIV—outcomes greatly desired by this population. Our data suggest that a larger randomized control trial of a refined intervention, with an appropriate time-matched attention control condition, in a larger sample of HIV-infected adults, examining the efficacy of the intervention is warranted.
Acknowledgments
The authors gratefully acknowledge the support of the women and men who participated in this study, our clinical colleagues including Jane Baum, Robert Bucklew, Sheila Garven, Barbara Gripsholver, Isabel Hilliard, Jason McMinn, and Julie Ziegler. We also acknowledge the contributions of Jen Smith, Diana Thomas, and Sarah Arbuckle for their input and insight. The project described was supported by the National Institute for Allergy and Infectious Disease through Grant P30AI36219; National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grants 5KL2RR024990 and UL1RR024989; and the Association of Nurses in AIDS Care/Sigma Theta Tau. The contents of this article are solely the views of the authors and do not represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Allison R. Webel, Email: allison.webel@case.edu, Clinical Research Scholar, Frances Payne Bolton School of Nursing Case Western Reserve University, 10900 Euclid Avenue Cleveland, OH 44106-4904, USA, Fax: 216-368-3542, Phone: 216-368-3939.
Shirley M. Moore, Email: Shirely.moore@case.edu, Frances Payne Bolton School of Nursing, Case Western Reserve University, Frances Payne Bolton School of Nursing Case Western Reserve University, 10900 Euclid Avenue Cleveland, OH 44106-4904, USA, Phone: 216-368-3939.
Jan E. Hanson, Email: jan.hanson@case.edu, Department of Anthropology and Public Health, Case Western Reserve University, Frances Payne Bolton School of Nursing Case Western Reserve University, 10900 Euclid Avenue Cleveland, OH 44106-4904, USA
Sanjay R. Patel, Email: SPatel@Partners.org, Brigham and Women’s Hospital, Division of Sleep Medicine, 221 Longwood Avenue, Room 225-C, Boston, MA 02115, Tel: 857-307-0347.
Brian Schmotzer, Email: brian.schmotzer@case.edu, Center for Clinical Investigation, Case Western Reserve University, 10900 Euclid Avenue Cleveland, OH 44106-4904, USA
Robert A. Salata, Email: Robert.salata@case.edu, Professor and Executive Vice-Chair, Department of Medicine, Division of Infectious Diseases and HIV Medicine, Case Western Reserve University, Case Western Reserve University, 10900 Euclid Avenue Cleveland, OH 44106-4904, USA.
References
- Alemi F, MS, Baghi H. Self-experiments and analytical relapse prevention. Quality Management in Health Care. 2008;17(1):53–65. doi: 10.1097/01.QMH.0000308638.04850.48. [DOI] [PubMed] [Google Scholar]
- Alemi F, ND, Ardito S, Headrick L, Moore S, Hekelman F, Norman L. Continuous self-improvement: systems thinking in a personal context. The Joint Commission journal on quality improvement. 2000;26(2):74–86. doi: 10.1016/s1070-3241(00)26006-9. [DOI] [PubMed] [Google Scholar]
- Alvarez GG, Ayas NT. The Impact of Daily Sleep Duration on Health: A Review of the Literature. Progress in Cardiovascular Nursing. 2004;19(2):56–59. doi: 10.1111/j.0889-7204.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- Atkins CJ, SK, Rupp J, Kaplan RM, Patterson TL, Sallis JF, Jr, Nader PR. Attendance at health promotion programs: baseline predictors and program outcomes. Health Education Quarterly. 1990;17(4):417–428. doi: 10.1177/109019819001700406. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian Integration of Metabolism and Energetics. Science. 2010;330(6009):1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28(12):1599. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, et al. Poor Sleep Is Associated With Impaired Cognitive Function in Older Women: The Study of Osteoporotic Fractures. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61(4):405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- Buysse DJ. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33(6):781. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Umanski G, Blank A, Meissner P, Grossberg R, PAS Comorbidity-Related Treatment Outcomes among HIV-Infected Adults in the Bronx, NY. Journal of Urban Health. 2011 doi: 10.1007/s11524-010-9540-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew MA, Hoch CC, Buysse DJ, Monk TH, Begley AE, Houck PR, et al. Healthy Older Adults’ Sleep Predicts All-Cause Mortality at 4 to 19 Years of Follow-Up. Psychosomatic Medicine. 2003;65(1):63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- Dreher HM. The effect of caffeine reduction on sleep quality and well-being in persons with HIV. Journal of Psychosomatic Research. 2003;54(3):191–198. doi: 10.1016/s0022-3999(02)00472-5. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive Consequences of Sleep Deprivation. Semin Neurol. 2005;25(01):117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. International Journal of Cancer. 2008;123(1):187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- Gordon CGT. Review of findings on Chronic Disease Self-Management Program (CDSMP) outcomes: Physical, emotional, & health-related quality of life, healthcare utilization and costs. 2007 Retrieved from http://health.utah.gov/arthritis/pdf/Review_Findings_CDSMP_Outcomes1.8.08.pdf.
- Grady D, Cummings S, Hulley S, editors. Alternative trial designs and implementation issues. 3. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- Haesler EJ. Effectiveness of strategies to manage sleep in residents of aged care facilities. JBI Reports. 2004;2(4):115–183. doi: 10.1111/j.1479-6988.2004.00010.x. [DOI] [PubMed] [Google Scholar]
- Hertzog MA. Considerations in determining sample size for pilot studies. Research in Nursing & Health. 2008;31(2):180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- Holzemer W, SH, Portillo C, Miramontes H. The client adherence profiling-intervention tailoring (CAP-IT) intervention for enhancing adherences to HIV/AIDS medications: A pilot study. Journal of the Association of Nurses in AIDS care. 2000;11(1):36–44. doi: 10.1016/s1055-3290(06)60420-2. [DOI] [PubMed] [Google Scholar]
- Hudson AL, Portillo CJ, Lee KA. Sleep Disturbances in Women With HIV or AIDS: Efficacy of a Tailored Sleep Promotion Intervention. Nursing Research. 2008;57(5):360–366. doi: 10.1097/01.NNR.0000313501.84604.2c. 310.1097/1001.NNR.0000313501.0000384604.0000313502c. [DOI] [PubMed] [Google Scholar]
- Humpel N, Owen N, Leslie E. Environmental factors associated with adults’ participation in physical activity: A review. American Journal of Preventive Medicine. 2002;22(3):188–199. doi: 10.1016/s0749-3797(01)00426-3. [DOI] [PubMed] [Google Scholar]
- Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain, Behavior, and Immunity. 2004;18(4):349–360. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Issa FG, Sullivan CE. Reversal of central sleep apnea using nasal CPAP. Chest. 1986;90(2):165–171. doi: 10.1378/chest.90.2.165. [DOI] [PubMed] [Google Scholar]
- Jean-Louis G, WK, Aouizerat BE, Levine AM, Maki PM, Liu C, Anastos KM, Milam J, Althoff KN, Wilson TE. Insomnia symptoms and HIV infection among participants in the Women’s Interagency HIV Study. Sleep. 2012;35(1):131–137. doi: 10.5665/sleep.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-Sectional Associations Between Measures of Sleep and Markers of Glucose Metabolism Among Subjects With and Without Diabetes. Diabetes Care. 2011;34(5):1171–1176. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiology of Disease. 2010;37(3):542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep medicine. 2001;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Rathouz PJ, Hulley SB, Sidney S, et al. Objectively Measured Sleep Characteristics among Early-Middle-Aged Adults. American Journal of Epidemiology. 2006;164(1):5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- Lee K, Gay C, Portillo CJ, Coggins T, Davis H, Pullinger CR, et al. Types of Sleep Problems in Adults Living with HIV/AIDS. Journal of Clinical Sleep Medicine. 2012;8(1):67–75. doi: 10.5664/jcsm.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Portillo C, Miramontes H. The Influence of Sleep and Activity Patterns on Fatigue in Women With HIV/AIDS. Journal of the Association of Nurses in AIDS care. 2001;12(Supplement 1):19–27. doi: 10.1177/105532901773742257. [DOI] [PubMed] [Google Scholar]
- Lee KA, Gay CL. Can modifications to the bedroom environment improve the sleep of new parents? Two randomized controlled trials. Research in Nursing & Health. 2011;34(1):7–19. doi: 10.1002/nur.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA, Portillo CJ, Miramontes H. The Influence of Sleep and Activity Patterns on Fatigue in Women With HIV/AIDS. Journal of the Association of Nurses in AIDS care. 2001;12(Supplement 1):19–27. doi: 10.1016/s1055-3290(06)60154-4. [DOI] [PubMed] [Google Scholar]
- Lescure F-X, Omland LH, Engsig FN, Roed C, Gerstoft J, Pialoux G, et al. Incidence and Impact on Mortality of Severe Neurocognitive Disorders in Persons With and Without HIV Infection: A Danish Nationwide Cohort Study. Clinical Infectious Diseases. 2011;52(2):235–243. doi: 10.1093/cid/ciq041. [DOI] [PubMed] [Google Scholar]
- McDaniel J, Buboltz W, Chauvin I, Eddlemon O. Sleep Quality and Habits of Adults with Human Immunodeficiency Virus. International Journal of Humanities and Soical Sciences. 2011;1(7):23–27. [Google Scholar]
- McLean SA, Williams DA, Harris RE, Kop WJ, Groner KH, Ambrose K, et al. Momentary relationship between cortisol secretion and symptoms in patients with fibromyalgia. Arthritis & Rheumatism. 2005;52(11):3660–3669. doi: 10.1002/art.21372. [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, Insana SP, Clegg-Kraynok MM, Mancini LM. Normative longitudinal maternal sleep: the first 4 postpartum months. American Journal of Obstetrics and Gynecology. 2010;203(5):465.e461–465.e467. doi: 10.1016/j.ajog.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Charvat J, Gordon N, Roberts B, Pashkow F, Ribisl P, et al. Effects of a CHANGE intervention to increase exercise maintenance following cardiac events. Annals of Behavioral Medicine. 2006;31(1):53–62. doi: 10.1207/s15324796abm3101_9. [DOI] [PubMed] [Google Scholar]
- Moore SM, CJ Using the CHANGE intervention to enhance long-term exercise. The Nursing clinics of North America. 2002;37(2):273–283. doi: 10.1016/s0029-6465(01)00008-1. [DOI] [PubMed] [Google Scholar]
- Morin CM, Vallières A, Guay B, Ivers H, Savard J, Mérette C, et al. Cognitive Behavioral Therapy, Singly and Combined With Medication, for Persistent Insomnia. JAMA: The Journal of the American Medical Association. 2009;301(19):2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosomatic Medicine. 2005;67(2):187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- Naughton MT, Liu PP, Bernard DC, Goldstein RS, Bradley TD. Treatment of congestive heart failure and Cheyne-Stokes respiration during sleep by continuous positive airway pressure. American Journal of Respiratory & Critical Care Medicine. 1995;151(1):92–97. doi: 10.1164/ajrccm.151.1.7812579. [DOI] [PubMed] [Google Scholar]
- NIH. NIH State-of-the-Science Conference Statement on manifestations and management of chronic insomnia in adults. NIH consensus and state-of-the-science statements. 2005;22(2):1–30. [PubMed] [Google Scholar]
- Nokes K. Correlates of sleep quality in persons with HIV disease. Journal of the Association of Nurses in AIDS Care. 2001;12(1):17. doi: 10.1016/S1055-3290(06)60167-2. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep (Rochester) 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Opp MR, Born J, Irwin MR. Sleep and the Immune System. In: Ader R, editor. Psychoneuroimmunology. 4. Vol. 1. Oxford: Elsevier Academic Press; 2007. pp. 579–618. [Google Scholar]
- Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, et al. A prospective study of sleep duration and mortality risk in women. Sleep (Rochester) 2004;27(3):440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- Phillips KD, Branson S. Insomnia and HIV/AIDS. Sleep Review. 2009 Jan-Feb; Retrieved from http://www.sleepreviewmag.com/issues/articles/2009-01_01.asp.
- Phillips KD, SW Effects of individualized acupuncture on sleep quality in HIV disease. Journal of the Association of Nurses in AIDS care. 2001;12:27–39. doi: 10.1016/S1055-3290(06)60168-4. [DOI] [PubMed] [Google Scholar]
- Reid S, Dwyer J. Insomnia in HIV Infection: A Systematic Review of Prevalence, Correlates, and Management. Psychosom Med. 2005;67(2):260–269. doi: 10.1097/01.psy.0000151771.46127.df. [DOI] [PubMed] [Google Scholar]
- Saberi P, Neilands TB, Johnson M. Quality of Sleep: Associations with Antiretroviral Nonadherence. AIDS PATIENT CARE and STDs. 25 doi: 10.1089/apc.2010.0375. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin N, Barroso J, Leserman J, Harmon JL, Pence BW. Daytime Sleepiness, Nighttime Sleep Quality, Stressful Life Events, and HIV-Related Fatigue. Journal of the Association of Nurses in AIDS care. 20(1):6–13. doi: 10.1016/j.jana.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa K, Holzemer W, Bakken Henry S, Slaughter RR. Dimensions of health-related quality of life in persons living with HIV disease. Journal of Advanced Nursing. 1999;29(1):178–187. doi: 10.1046/j.1365-2648.1999.00877.x. [DOI] [PubMed] [Google Scholar]
- van den Berg JF, Miedema HME, Tulen JHM, Neven AK, Hofman A, Witteman JCM, et al. Long Sleep Duration is Associated With Serum Cholesterol in the Elderly: The Rotterdam Study. Psychosomatic Medicine. 2008;70(9):1005–1011. doi: 10.1097/PSY.0b013e318186e656. [DOI] [PubMed] [Google Scholar]
- von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association Between Polysomnographic Measures of Disrupted Sleep and Prothrombotic Factors*. Chest. 2007;131(3):733–739. doi: 10.1378/chest.06-2006. [DOI] [PubMed] [Google Scholar]
- Vosvick M, Gore-Felton C, Ashton E, Koopman C, Fluery T, Israelski D, et al. Sleep disturbances among HIV-positive adults: The role of pain, stress, and social support. Journal of Psychosomatic Research. 2004;57(5):459–463. doi: 10.1016/j.jpsychores.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Wantland DJ, Holzemer WL, Moezzi S, Willard SS, Arudo J, Kirksey KM, et al. A Randomized Controlled Trial Testing the Efficacy of an HIV/AIDS Symptom Management Manual. Journal of Pain and Symptom Management. 2008;36(3):235–246. doi: 10.1016/j.jpainsymman.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Webel AR, Dolansky M, Henry A, Salata R. Women’s Self-Management of HIV: Context, Strategies and Considerations. Journal of the Association of Nurses in AIDS care. 2012 doi: 10.1016/j.jana.2011.09.002. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. Journal of Psychopharmacology. 2010;24(11):1577–1601. doi: 10.1177/0269881110379307. [DOI] [PubMed] [Google Scholar]