Abstract
Influenza infection following allogeneic hematopoietic cell transplantation (allo-HCT) can result in severe complications. The effectiveness of the annual vaccine depends on age, immune competence and the antigenic potential of the 3 strains included (1). . We hypothesized that a second vaccine dose, the standard of care for vaccine-naïve children, might improve post-HCT immune responses. Patients >60 days post-HCT were randomized to receive either 1 (n=33) or 2 (n=32) influenza vaccine doses separated by one month. The primary endpoint was whether two vaccinations induced superior immunity, however, we found no difference. Secondary endpoints were to identify variables associated with responses. Both hemagglutination inhibition (HAI) (p<0.005) and ELISpot responses (p=0.03) were greater for patients vaccinated ≥1 year post transplant. UCB recipients showed less IFN-γ responses (p=<0.001). Interestingly, there was a positive correlation between the total number of CD19+ cells prior to vaccination and seroconversion (p=0.01) and an inverse correlation for IFN-γ responses (p=0.05). Variables not associated with vaccine responses included: pre-vaccine CD4+ cell counts (total, naïve or memory), steroid usage at vaccination, age, or conditioning intensity. Time from transplantation to vaccination and absolute CD19+ cell counts were the strongest predictors of vaccine responses. Methods to improve influenza vaccine responses after allo-HCT are needed.
Keywords: BMT, UCB Transplantation, Vaccine, Influenza
Introduction
Early after allogeneic hematopoietic cell transplantation (allo-HCT), influenza infection causes an illness that ranges from a mild viral syndrome to a severe life threatening illness (2). The incidence of influenza infection in the early transplant period ranges from 14–20% in adult allo-HCT patients with a respiratory illness (3, 4). Mortality rates following influenza infection in transplant recipients in the 1980s–90s have been reported to be as high as 50%–85% (5–7). However, more recent data suggest an overall decrease in mortality. For instance, Nichols et al noted a 10% mortality within 30 days of influenza infection after HSCT (8), likely related to improved supportive care measures (9). While encouraging, the recent development of antiviral resistant strains of influenza, that have been reported in HSCT patients, clearly have the potential to increase overall mortality(10).
Severe, life threatening infections, including influenza, are more common in the immediate post-transplant period when lymphopenia is prevalent and marked (4, 8, 11). Vaccination against influenza has the potential to provide life-saving immunity to the virus (12). However, influenza vaccination early after transplantation results in suboptimal responses (8, 13, 14) and the ideal post-transplantation vaccine schedule has yet to be established. Newer ways to improve immune responses to vaccinations after HSCT are being explored, and include vaccinating the donor prior to harvest. However this is not always possible, especially with umbilical cord blood (UCB) graft sources. Previous ASBMT and EBMT guidelines have been consolidated into CIBMTR guidelines and recommend yearly influenza vaccinations starting between 4 and 6 months after HSCT, as well as providing a yearly vaccination for household contacts (15). Vaccination guidelines for healthy children, recommended by the Centers for Disease Control, state that those <9 years of age who have never been immunized against influenza should receive 2 doses separated by 4 weeks (16). This is supported, in part, by an efficacy study in healthy vaccine naïve children (17).
The current practice is to vaccinate patients against influenza early after allo-HCT with a single vaccination. However, in previously unvaccinated children, two vaccinations invoke better immune responses (17, 18)_ENREF_16. In 1993, the approach of using two vaccine doses was tested in a small cohort of patients after allo-HCT. While T cell responses were not tested, the investigators were unable to show any improvement in humoral immune responses (19). Importantly, this study was performed following severe immunodepletion (by adding alemtuzumab in the preparative regimen), thus the issue of whether two vaccine doses enhance influenza specific immunity following allo-HCT is unresolved. Here, in a randomized study, we tested the hypothesis that allo-HCT recipients who receive a second influenza vaccine will have stronger vaccine specific immune responses. Secondary goals of this study were to identify other variables associated with the likelihood of influenza vaccination responses.
Methods
Study design and procedures
Patients were screened from September 2010 to February 2011 for study eligibility during routine post-transplant clinic appointments. To be eligible patients had to be >60 days after allo-HCT, have neutrophil recovery, be in remission and assessed to be well enough to receive the vaccine. Patients were ineligible if they had received any of the following: intravenous immune globulin (IVIG) within the previous three months, alemtuzumab within six months, or the influenza vaccine within four months of study entry. Written informed consent was obtained from patients (or parents if <18 years old) for this institutional review board approved study (study # NCT01215981). Blood was collected at the time of enrollment, prior to the first vaccination (Fluzone, Sanofi-Pasteur, PA according to the manufactures recommendations). Four weeks after vaccination, patients were randomized to receive a second vaccine dose and blood was again collected. If applicable, a second vaccine dose was then administered. Finally, patients returned at eight weeks following enrollment for blood collection. From the blood samples sera and peripheral blood mononuclear cells (PBMCs) were isolated and stored at −20C or liquid nitrogen, respectively. The randomization between one or two vaccine doses was stratified by age (≥18 and <18 years) and steroid use (present or absent). As the primary endpoint of this study was to determine whether two vaccine doses resulted in superior immune responses compared to a single vaccine, we used immune response to power enrollment. Since viral strains in the vaccine potentially vary from year to year, and antibody titers may differ, we focused on increases in T cell based (ELIspot) responses. Assuming a baseline proportion of patients responding to a single vaccine dose of 40%, our sample was sufficient to detect a 30% increase in response rate.
Transplantation procedures
Patients enrolled on this trial received either myeloablative preparative regimens (n=39) or reduced intensity conditioning (n=26). TBI was used in 86% of the conditioning regimens. GVHD prophylaxis consisted of CSA and MMF (n=33), another CSA containing regimen (n=19) or other combinations (n=10) (table 1). The presence or history of GVHD, whether acute or chronic, was not an exclusion criteria, and patients on steroids at the time of enrollment (n=22) were stratified to be evenly distributed between the two randomization groups. Detailed information about steroid dosing is listed in supplemental table 1.
Table 1.
Patient Characteristics Between One and Two Vaccine Dose Groups. Patients were randomized to receive one or two vaccine doses......
| Randomization Group | ||||
|---|---|---|---|---|
| Factors | Single Shot | Double Shot | Total | p |
| Total | 33 | 32 | 65 | |
| Patient/Donor CMV Serostatus | 0.53 | |||
| Negative/negative | 12 (40%) | 11 (34%) | 23 | |
| Negative/positive | 1 (3%) | 0 | 1 | |
| Positive/ | 17 (57%) | 21 (66%) | 38 | |
| Conditioning | 0.77 | |||
| Cy/TBI | 14 (42%) | 12 (38%) | 26 | |
| Cy/Flu/TBI | 12 (36%) | 15 (47%) | 27 | |
| Bu/Cy Containing | 4 (12%) | 2 (6%) | 6 | |
| Other | 23(9%) | 3 (9%) | 6 | |
| Non-myelablative Conditioning | 12 (36%) | 14 (44%) | 26 | 0.54 |
| GvHD Prophylaxis | 0.68 | |||
| T-deplete | 0 | 1 (3%) | 1 | |
| CsA/MMF | 16 (48%) | 17 (53%) | 33 | |
| CsA containing | 11(33%) | 8 (25%) | 19 | |
| Other | 6 (18%) | 6 (19%) | 12 | |
| Age | ||||
| ≥18 | 25 (76%) | 23 (72%) | 48 | 0.72 |
| <9 | 3 (4%) | 5 (7%) | 8 | 0.26 |
| Median (range), (interquartile range) | 39 (8–62), (32–50) | 42 (4–68), (15–52) | 40 (4–68), (16–51) | 0.62 |
| Donor Type | 0.77 | |||
| Sibling | 16 (48%) | 19 (59%) | 35 | |
| Mismatched URD | 4 (12%) | 3 (9%) | 7 | |
| UCB | 13 (39%) | 10 (31%) | 23 | |
| Gender: Male | 20 (61%) | 19 (59%) | 39 | 0.92 |
| Race: White | 26 (79%) | 27 (84%) | 53 | 0.75 |
| Years from Tx to vaccination | ||||
| Median (range), (interquartile range) | 0.7 (0.2–19.7) (0.2–2.0) | 1.0 (0.2–7.0) (0.3–2.0) | 0.9 (0.2–19.7) (0.2–2.0) | 0.40 |
Determination of lymphocyte subsets prior to vaccination
Prior to vaccination, PBMCs were obtained and cryopreserved. T and B cell immunophenotyping was performed in bulk. For T cells, antibodies against CD3, CD4, and CD8 were used to identify the percentages of total T cells (CD3+) and CD4+ and CD8+ T cell fractions (CD3+CD4+ and CD3+CD8+, respectively). CD4 and CD8 T cell subsets were distinguished using: CD45RA and CD27 to identify naïve (CD45RA+CD27+), central memory (CD45RA−CD27+), effector memory (CD45RA−CD27−) and effector memory RA+ cells (CD45RA+CD27−). For B cells: CD19, IgD and CD27 were used to identify naïve (CD19+IgD+CD27−), unswitched memory (CD19+IgD+CD27+), switched memory (CD19+IgD−CD27+) and double negative cells (CD19+IgD−CD27−). NK cells were identified by gating on the CD3- fraction and then by using a CD56+CD16− and CD56+CD16+ phenotype. The total number of each cell population was determined using the following formula:
Antibody responses
The hemagglutin inhibition (HI) assay was conducted as described previously (20). Briefly, human sera samples were treated with receptor destroying enzyme (RDE) (Denka Seiken Co.) to remove non-specific inhibitors. Three volumes of RDE was added to 1 volume of human sera and incubated overnight at 37°C. Samples were then heated to 56°C for 30 minutes and six volumes of physiological saline (Gibco) was added, resulting in a 1:10 dilution of each serum sample. The sera were serially diluted two-fold up to a final dilution of 1:5,120. Antigens used in the hemagglutination inhibition assay were beta-propiolactane inactivated influenza A viruses: A/Wisconsin/15/2009 X-183 (H3N2) and A/California/07/2009 NYMC X-179A (H1N1pdm09), and the ether-treated influenza B antigenB/Brisbane/60/2009 (Victoria lineage) (available at Influenza Reagent Resource, http://www.influenzareagentresource.org). Each antigen was standardized to have 4 HA units/25 μl and then 25 μl was added to all wells containing diluted sera. The plates were mechanically shaken for 10 seconds, and incubated at room temperature for 15 minutes. 50 μl of a 0.5% solution of turkey red blood cells (University of Georgia) was added to each well, plates were mechanically shaken for 10 seconds, and incubated at room temperature for 30 minutes to allow RBCs to settle. After 30 minutes, the plates were tilted at 45–60° angle and observed for the presence or absence of hemagglutination. The HI titer was calculated as the reciprocal of the highest dilution of antiserum that completely inhibits hemagglutination. Positive seroconversion was defined as a ≥ 4 fold rise of HI antibody titer in post vaccination sera compared with prevaccination.
IFN-γELISpot responses
ELISpot was performed using cryopreserved PBMCs collected at 8 weeks after vaccination. PBMCs were thawed using standard techniques and rested overnight at 37°C in RPMI1640 with 10% fetal bovine serum (FBS). Following this, cells were washed with PBS and resuspended in RPMI. 0.25 ×106 cells were plated on Millipore's Multiscreen filter plates and stimulated with a 0.8 ug/mL concentration of the 2010 influenza vaccine for 15–20 hours overnight at 37°C. Poke Weed Mitogen (PWM, 20 ng/mL), a known stimulator of IFN-γ, was used as a positive control for each sample and resting cells were used as a negative control. After the overnight stimulation with vaccine, the cells were washed three times with a 0.05% PBS Tween-20 solution. IFN-γ was detected and quantitated using sandwich immune-enzyme technology (biotinylated antibody with detection using streptavidin-alkaline phosphatase) according the manufactures recommendations (R&D systems). Filter plates were then developed using a BCIP/NBT substrate, which resulted in spot formation. Responses were considered positive if they were 4× above the background (resting control) and if the patient showed response to the PWM stimulation.
Statistical Analysis
Factors were compared across randomization group using the Chi-square test or Fisher's exact test when appropriate. Univariate comparison of factors by serological response defined as H3N1, H1N1, b/Vic at 8 weeks by a 4 fold increase or ELISpot at 8 weeks defined as a positive response were carried out by the Chi-square test or Fisher's exact test when expected cell counts were too small. Logistic regression analysis was employed to look at the independent effect on response by the randomization group (one vs. two vaccinations) controlling for the following factors: years from transplant to infusion (continuous per year or categorical (< 1 year versus > 1 year depending on frequencies), gender (male versus female), age (<18 versus ≥18), use of TBI in the conditioning regimen, disease risk (standard versus high risk versus non-malignant disease), donor type (cordblood versus other), use of steroids (no versus yes), B and T cell subsets and cytomegalovirus (CMV) serostatus (patient/donor both negative versus other).
Results
Study Population
In all, 73 allo-HCT patients were enrolled in the study between September 2010 and March 2011. However, due to relapse (n=1), desire to be withdrawn from the study (n=2), missed follow-up visits (n=4) and physician discretion to not give a second vaccine dose (n=1), a total of 65 patients were evaluable, with blood samples available for correlative studies. Demographic data on the study population are listed in table 1. Patients randomized to receive either one vs. two vaccine doses did not differ in age, gender, time from transplant, conditioning intensity, GVHD prophylaxis, or stem cell source. Prior to randomization patients were stratified based on age and current steroid use, so each group contained equal numbers of pediatric patients and those on varying doses (and schedules) of prednisone at the time of study enrollment, (n=22, supplemental table 1).
Vaccine Associated Antibody Responses
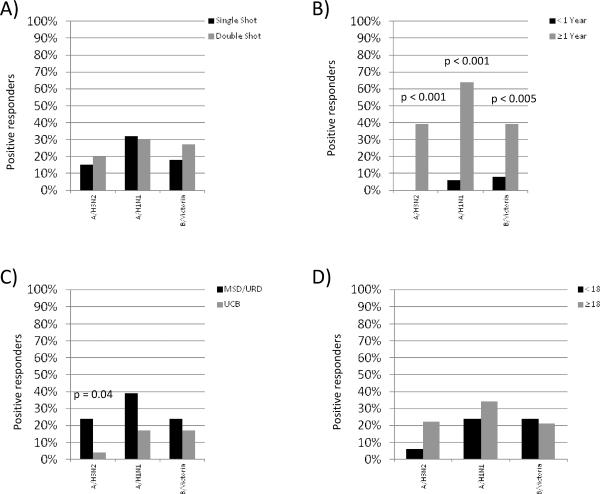
Prior to vaccination and at 4 and 8 weeks after vaccination, the HIA assay was used to detect influenza specific antibody titers. Two different measure of vaccine specific response were measured including seroprotection and seroconversion. Comparing patients who received a single vaccine dose vs. those that received two doses, there were no significant differences in the rates of seroprotection (vaccine titer of >1:40 at 8 weeks) for influenza H3 (19% vs. 19%), H1N1 (32% vs. 32%) and (32% vs. 23%). Similarly, the rate of seroconversion following vaccination (>4× increase in antibody titers). At week 8 after vaccination, 13% of patients randomized to the single vaccine dose arm and 22% randomized to receive two vaccines showed seroconversion to the A/H3N2 strain (p=0.32). The percentages were similar for the A/H1N1 (31% vs. 31%, p=0.99) and the B/Victoria strains (16% vs. 25%, p=0.55), respectively (figure 1a).
Figure 1. Variables Associated With Serological Vaccine Immune Responses.
A) Number of vaccines delivered, B) Time from transplantation to time of vaccination, C) Donor source (UCB vs. MSD/URD) and D) Recipient age.
There was a higher likelihood of vaccine seroconversion in patients that were ≥1 year from transplant (n=29) compared to those who are <1 year (n=36) (figure 1b). In fact, none of the patients vaccinated <1 year from transplant showed seroconversion to the A/H3N2 virus versus 39% of patients vaccinated ≥1 year (p=0.001). Similarly, only 6% and 8% of patients in the <1 year group seroconverted to the A/H1N1 and B/Victoria, respectively, while 64% (p=0.001) and 39% (p=0.003) seroconverted in the ≥1 year group, respectively (figure 1b). While the seroconversion rates were low for patients vaccinated <1 year after transplant, the response to any one of the three vaccine strains did not differ for patients vaccinated 2–6 months after transplantation compared to those vaccinated ≥6–12 months after transplantation (12% vs. 30%, p=0.43). Conversely, since responses were more robust in patients vaccinated >1 year after transplant, subgroup analysis was performed to determine if there was a differences in seroconversion rates (to any one of the 3 vaccine strains) for patients randomized to receive 1 vs. 2 vaccines, however, no differences were observed (75% vs. 63%, p=0.48).
In univariate analysis, stem cell source was also associated with seroconversion since MSD/MUD recipients were more likely to show A/H3N2 responses compared to UCB recipients (24% vs. 4%, p=0.04). Analogous trends were observed with responses to A/H1N1 where 39% vs. 17%, p=0.07), respectively. There was no difference between the two stem cell sources in the B/Victoria group (figure 1c). Other parameters not associated with antibody responses included recipient age, conditioning intensity (RIC vs. MA), disease risk group or use of steroids (not shown).
Prior to vaccination, blood was collected for immunophenotyping to examine B, T, and NK cells and their subsets. As shown in table 2, higher numbers of B cells (total CD19+ fraction, unswitched memory B cells and naïve B cells) at the time of first vaccination were more likely to develop antibody responses (p=0.03, p=0.03, and p=0.03, respectively). There was no correlation between antibody responses and the total numbers of CD3, CD4 and CD8 cells prior to vaccination (p=0.26, p=0.07 and p=0.19, not shown). For CD4+ T cells, patients in the highest tertile (>347 cells/m2) were more likely to develop influenza antibodies following vaccination (p=0.04), but the absolute numbers of naïve, effector memory or effector memory CD45RA+ cells prior to vaccination were not associated with antibody responses (p=0.26, p=0.14, p=0.95, not shown). Likewise, there was no correlation between the absolute numbers of any of the CD8 subpopulations (naïve, central memory, effector memory or effector memory CD45RA+) or NK cells prior to transplantation and vaccine responses (p=0.26, p=0.32, p=0.23, p=0.67, and p=0.23, respectively).
Table 2.
B Cell Subsets and Vaccine Specific Immune Responses. The number of B cells (and subset) in the blood prior to vaccination were divided into tertiles and the percentage of patients with either antibody or ELISPOT response are shown. Differences in the groups were determined using Fischer's exact test.
| Factors | 4x Antibody Response | P | Elispot Response | P |
|---|---|---|---|---|
| Total CD19 | 0.03 | <0.01 | ||
| 1stTertile(<71.4) | 3 (15%) | 13 (65%) | ||
| 2nd Tertile (71.4–560) | 9 (43%) | 12 (57%) | ||
| 3rd Tertile (560+) | 11 (55%) | 3 (15%) | ||
| Switched Mem | 0.12 | 0.22 | ||
| 1st Tertile | 4 (20%) | 11 (55%) | ||
| 2nd Tertile | 9 (43%) | 11 (52%) | ||
| 3rd Tertile | 10 (50%) | 6 (30%) | ||
| Unswitched Mem | 0.03 | <0.01 | ||
| 1st Tertile (<0.92) | 3 (15%) | 14 (70%) | ||
| 2nd Tertile (0.92–6.2) | 9 (43%) | 10 (48%) | ||
| 3rd Tertile (6.2+) | 11 (55%) | 4 (20%) | ||
| Naive | 0.03 | 0.02 | ||
| 1st Tertile (<61.2) | 3 (15%) | 14 (70%) | ||
| 2nd Tertile (61.2–480) | 9 (43%) | 9 (43%) | ||
| 3rd Tertile (480+) | 11 (55%) | 5 (25%) | ||
| Double Neg | 0.14 | <0.01 | ||
| 1st Tertile (<7) | 4 (20%) | 15 (75%) | ||
| 2nd Tertile (7–35) | 10 (48%) | 8 (38%) | ||
| 3rd Tertile (35+) | 9 (45%) | 5 (25%) |
In multivariate analysis antibody responses to any one of the 3 vaccine strains were significantly higher in patients who were ≥1 year from transplantation at time of vaccination (RR-15.5, 95% CI [3.2–76], p<0.01, table 3). Patients who had higher numbers of CD19+ B cells prior to vaccination were more likely to have antibody responses (table 3). However, due to the correlation between the various B cell subpopulations, we were not able to independently investigate the impact of these subpopulations on vaccine responses. Vaccine specific immune responses did not differ between the randomization groups (one vs. two vaccines), steroid usage, or the numbers of T cells (or the T cell subsets) in the blood prior to vaccination (not shown).
Table 3.
Factors Associated with Serological Vaccine Response. Multiple variable analysis models were constructed as described in the methods taking into account the variables described in the methods.
| Factor | Odds Ratio of Responding | 95% CI | P-value |
|---|---|---|---|
| Randomization Group | |||
| Single shot* | 1.0 | ||
| Double shot | 1.0 | (0.2–4.4) | 0.96 |
| Years from transplant to Infusion | |||
| <1 year* | 1.0 | ||
| ≥1 year | 15.5 | (3.2–76.4) | <0.001 |
| Steroid Use | |||
| No* | 1.0 | ||
| Yes | 0.9 | (0.7–1.2) | 0.52 |
| CD19 | |||
| 1stTertile(<71.4)* | 1.0 | ||
| 2nd Tertile (71.4–560) | 5.0 | (0.9–28.8) | 0.07 |
| 3rd Tertile (560+) | 18.0 | (1.9–169.7) | 0.01 |
| CD4+ | |||
| 1st Tertile (<255) | 1.0 | ||
| 2nd Tertile (255–669) | 1.1 | (0.2–6.2) | 0.88 |
| 3rd Tertile (669+) | 1.4 | (0.2–10.7) | 0.77 |
T cell Vaccine Associated-INF-γ Responses
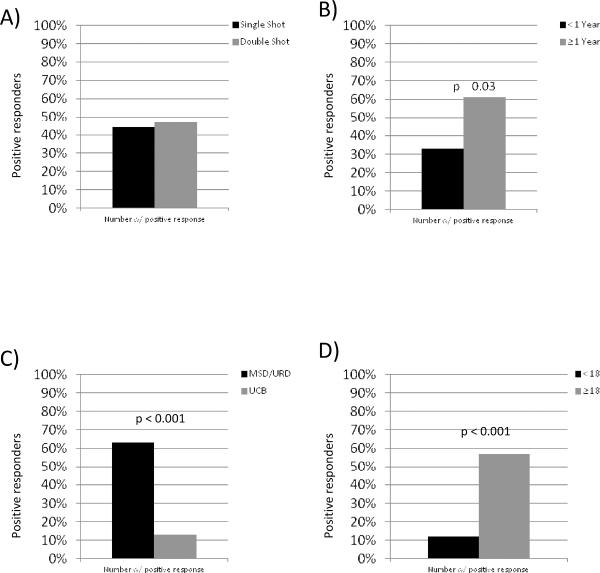
A total of 64 patients had 8 week post-vaccination samples evaluable for IFN-γ ELISpot testing (n=32 in each randomized group). Similar to vaccine associated antibody responses, there was no statistical differences in responses between recipients that received one or two vaccinations (44% vs. 47%, p=0.84) (figure 2a).
Figure 2. Variables Associated With IFN-γ Vaccine Immune Responses.
A) Number of vaccines delivered, B) Time from transplantation to time of vaccination, C) Donor source (UCB vs. MSD/URD) and D) Recipient age.
Again, the time from transplant to vaccination was associated with vaccine response since patients ≥1 year from transplant were more likely to show vaccine-induced IFN-γ production (61% vs. 33%, p=0.03) (figure 2b). Of the patients <1 year from transplantation, there were no differences in ELISpot responses for those vaccinated 2–6 months vs. ≥6–12 months (28% vs. 40%, p=0.36). As ELISpot responses were better in patient vaccinated > 1 year after transplant, subgroup analysis was performed to determine whether a second vaccine impacted responses, but there were no differences between patients that received one vs. two vaccines (67% vs. 59%, p=0.58). Additionally, the stem cell source was also significantly associated with IFN-γ vaccine responses, as 63% of MSD/MUD recipients showed IFN-γ production, compared to only 13% of UCB recipients (p=<0.001) (figure 2c).
In contrast to the antibody responses, T cell based vaccine responses (IFN-γ) were associated with recipient age in the univariate analysis. Patients who were ≥18 years old at time of vaccination were more likely to have a positive ELISpot. In fact, 57% of patients in this group showed IFN-γ response whereas only 12% of those aged <18 years old did so (p=0.001) (figure 2d). While adult patients were more likely to have received RIC conditioning, there was no association between IFN-γ responses between MA or RIC conditioning regimens (41% vs. 53%, p=0.53).
Similar to antibody responses, the absolute numbers of B cells, T cells and NK cells (and their subpopulations) were stratified into tertiles. As shown in table 2, the absolute numbers of CD19+ B cells prior to vaccination were inversely correlated with IFN-γ production (p<0.01). Patients who had lower numbers of B cell subpopulations (naïve, unswitched memory and double negative B cells) at the time of first vaccination were more likely to develop IFN-γ responses (table 2). There was no correlation between the prevaccination numbers of CD3, CD4 and CD8 cells (p=0.36, p=0.8 and p=0.16, data not shown). Likewise, no differences were noted for IFN-γ responses in the CD4 or CD8 subpopulations (naïve, central memory, or effector memory or effector memory CD45RA+) or in the NK cell fraction (data not shown).
Multivariate analysis confirmed the association of stem cell source for vaccine associated T cell IFN-γ response as responses for UCB were less likely than MSD/MUD (RR=0.1, 95% CI=0.02–0.5, p=0.004, table 4). Time from transplantation and steroid use were not significant variables in the multivariate analysis. As in the univariate analysis, there was a significant inverse correlation between the number of CD19 cells prior to vaccination and IFN-γ responses (table 4).
Table 4.
Factors Associated with IFN-γ Vaccine Response. Multiple variable analysis models were constructed as described in the methods taking into account the variables described in the methods.
| Factor | Odds Ratio of Responding | 95% CI | P-value |
|---|---|---|---|
| Randomization Group | |||
| Single shot | 1.0 | ||
| Double shot | 0.6 | (0.2–2.3) | 0.50 |
| Donor Type | |||
| MSD/URD | 1.0 | ||
| UCB | 0.1 | (0.02–0.5) | 0.004 |
| Years from transplant to Infusion | |||
| <1 year | 1.0 | ||
| ≥1 year | 2.7 | (0.7–10.6) | 0.17 |
| Steroid Use | |||
| No | 1.0 | ||
| Yes | 1.1 | (0.7–1.5) | 0.79 |
| CD19 | |||
| 1stTertile (<71.4)* | 1.0 | ||
| 2nd Tertile (71.4–560) | 0.3 | (0.1–1.2) | 0.09 |
| 3rd Tertile (560+) | 0.1 | (0.02–1.0) | 0.05 |
Discussion
We tested the hypothesis that a second vaccination would bolster influenza specific immune responses by performing a randomized trial in allo-HCT patients where patients received either one or two influenza vaccine doses. There was no evidence for increased serologic or T cell mediated immune responses for patients who received a second, “booster” vaccine compared to those that received one vaccine dose. Secondary aims of this study were to determine whether clinical and biological parameters predicted vaccine responses. Clinical variables associated with responses included the time from transplantation to vaccination, where patients further from the transplantation were more likely to have a protective rise in antibody titers and IFN-γ production. Another significant variable was the stem cell source (UCB vs. PBSCs/BM), where UCB recipients showed less vaccine associated IFN-γ production, but no difference in antibody production in multivariate analysis. We also found that, in general, higher numbers of B cells (and B cell subsets) at the time of vaccination were associated with antibody responses and lower numbers of B cells predicted IFN-γ production. Surprisingly, the numbers of T cells (or their subsets) were not correlated with vaccine responses.
In 1993, Engelhard first examined serological responses after a two dose regimen of the influenza vaccine (19). While they found no efficacy for this approach, this study was performed in the context of in vivo lymphodepletion (i.e., alemtuzumab). Given that that T and B cells are important for vaccine-associated responses (21), we tested this hypothesis in lymphocyte replete allo-HCT recipients. Similar to Englehard, (19) we found that a second vaccine dose was not beneficial, at least when given 4 weeks apart, as suggested by the CDC pediatric guidelines for vaccine naïve children (16). While not tested, Ljungmann proposed that a second vaccine dose might be given to patients initially vaccinated <6 months from transplant during an influenza outbreak or upcoming influenza season (22). Given that a significant proportion of patients in our study fell into this category, our data does not support this approach. In contrast others such as De Lavallade et al have recently demonstrated a benefit to a booster dose of A/H1N1 vaccine among 97 adult patients with hematologic malignancies receiving chemotherapy and in a smaller number after allogeneic allo-HCT. Importantly, only 2 allo-HCT patients in this series were receiving immune suppressive therapy and neither responded to the vaccine (23). A considerable proportion of patients in our cohort were vaccinated either early after transplant and were still on immune suppression (26%) or were UCB recipients (39%); making it difficult to compare the two studies.
Similar to previous studies of influenza vaccination in allo-HCT patients (12, 13, 19, 21) our seroconversion rates were higher among patients who were farther from the time of HCT. Such findings are not entirely surprising knowing that post-HCT immune reconstitution is a protracted process. While immunoglobulin responses are believed to be the integral component in protective influenza-specific immunity (24), we observed measurable and significant T cell responses in some patients vaccinated as early as 60 days after transplantation. In fact, we could detect no difference in either antibody or IFN-γ responses when comparing patients vaccinated 2–6 months after transplantation to those vaccinated ≥6 –12 months. While the numbers of patients were small, these results might suggest that vaccination earlier than suggested by CIBMTR guidelines (15) may be efficacious, but further studies are needed to confirm these findings.
Interestingly, we found that the number of CD19+ cells were directly correlated to the ability to seroconvert, and inversely correlated to the ability to produce IFN-γ. Taken at face value, these findings appear to be logical (more B cells are associated with serological responses, while less B cells may be associated with T cells responses), however, the exact explanation for these findings are not entirely clear. To our knowledge, these findings represent novel data, however few transplant studies have addressed role of B cell reconstitution on vaccine associated responses and perhaps B cell recovery as measured here is a surrogate for more complete immune recovery and reflective of the likelihood of vaccine responses. Related to this, we have recently demonstrated that in the marrow early after transplantation, B cell precursors (hematogones) are associated with less GVHD and improved UCB transplant outcomes (25), while others have recently hypothesized that B cells regulate/attenuate T cell responses in the setting of transplantation (26). Somewhat suprisingly, the CD4+ count was not associated with the likelihood of seroconversion, This is in contrast to Mohty et al who noted a significant naïve CD4+ count of >150/m3 with seroconversion (27) Importantly, pre-vaccination numbers of B cells were not assessed in the above study. For other vaccines, such as the 7-protein-valent-pneumococcal vaccine, Pao et al demonstrated that recovery of CD4+ cells > 200/m3 was associated with a response (11/19 patients vs 0/8), however above that level there was no association with increased response (28). In our study, greater than 30% of patients vaccinated early after transplantation showed IFN-γ responses, thus, the close proximity of vaccination from transplant suggests that this approach may provide some benefit to allo-HCT recipients early after transplantation. Likewise, Avetisyan, et al demonstrated similar results in patients 3 months after transplant(13) Thus, we conclude that it is safe and potentially effective to use influenza vaccination as early as 2 months from transplant. It is important to note that in our study a majority of patients who were vaccinated less than 1 year from their transplant were clustered around Day 60–100 at study entry (21/31 patients), making it impossible to examine whether other time points after transplantation were associated with vaccine responses.
Interestingly, we observed a significant difference in the immune responses of marrow/PBSCT recipients compared with UCB recipients. Data are scarce regarding UCB transplant recipients' immune response to the influenza vaccine (29). Avetisyan et al included only 3 UCB recipients in their study, but did not specify specifically their results (13). Issa et al also had only 3/82 patients having received UCB transplants (30). Given that 39% of patients in our study were UCB recipients, we were able to separate responses based on stem cell source, although still small sample numbers. UCB recipients were less likely to show a positive ELISpot assay. Similar findings have been noted for polyclonal mitogens (SEB) and CMV peptide responses in UCB vs. bone marrow recipients (31). These findings are consistent with prior laboratory studies showing a reduced capacity of UCB T cells to produce IFN-γ relative to PB T cells, due to a reduction in NFAT associated responses (32, 33).
Another seemingly surprising finding in our study was that corticosteroid usage did not negatively impact the probability of immune response to the influenza vaccine. Nichols et al previously showed that corticosteroids were a protective factor in evaluating for progression of upper respiratory tract infection (URI) symptoms to lower respiratory tract (8) Additionally, Machado et al demonstrated that steroid use and influenza vaccine were independent factors associated with not developing influenza (12). In multivariate analysis, we found that the use of steroids did not prevent either B or T cell specific influenza responses. This is in line with Issa et al results, which also did not observe a difference in response to the A/H1N1 vaccine in participants who were on steroids for aGVHD or cGVHD vs. those that were not (30).
In this randomized clinic trial, we found that two doses of influenza vaccine, separated by one month, did not confer better vaccine associated T or B cell responses. In contrast, allo-HCT recipient who were >1 year from the time of transplant and those who received MRD/URD were more likely to response. Given the evidence that time from transplant is the most significant variable in the likelihood of an immune response to the influenza vaccine (14, 21), the timing of vaccination seems to be the easiest way to manipulate a response. Traditionally, our center has taken the approach of administering the first opportunity after day +60 to vaccinate transplant recipients once the seasonal influenza vaccine is available, usually starting in early October each year. Perhaps consideration should be given to defer vaccination, until December or January, thus allowing patients to progress further from their transplant so as to increase the likelihood of vaccine responses. This approach might be influenced by an early epidemic of influenza or predicted vaccine shortages. Unfortunately, we were not able to correlate these results with actual acquired influenza infections, given that this study was not powered to detect such differences and that many patients were not primarily followed at our center. However, as there was some evidence of immune response in our patients and no adverse effects, it is reasonable to vaccinate at day +60, with the advisory given to this population that the vaccination is unlikely to be sufficient. Still other approaches that might be useful to induce vaccine specific immune responses are the high dose vaccine that is now approved for elderly patients. To date this vaccine has not been tested and might provide better influenza specific immunity, but randomized clinical trials are needed.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Curr Top Microbiol Immunol. 2009;333:43–82. doi: 10.1007/978-3-540-92165-3_3. [DOI] [PubMed] [Google Scholar]
- 2.Lee I, Barton TD. Viral respiratory tract infections in transplant patients: epidemiology, recognition and management. Drugs. 2007;67:1411–1427. doi: 10.2165/00003495-200767100-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ljungman P, Ward KN, Crooks BN, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28:479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 4.Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11:781–796. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowden RA. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102:27–30. doi: 10.1016/s0002-9343(97)00007-7. discussion 42–23. [DOI] [PubMed] [Google Scholar]
- 6.Champlin RE, Whimbey E. Community respiratory virus infections in bone marrow transplant recipients: the M.D. Anderson Cancer Center experience. Biol Blood Marrow Transplant. 2001;7(Suppl):8S–10S. doi: 10.1053/bbmt.2001.v7.pm11777103. [DOI] [PubMed] [Google Scholar]
- 7.Whimbey E, Elting LS, Couch RB, et al. Influenza A virus infections among hospitalized adult bone marrow transplant recipients. Bone Marrow Transplant. 1994;13:437–440. [PubMed] [Google Scholar]
- 8.Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39:1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 9.Ljungman P. Prevention and treatment of viral infections in stem cell transplant recipients. Br J Haematol. 2002;118:44–57. doi: 10.1046/j.1365-2141.2002.03515.x. [DOI] [PubMed] [Google Scholar]
- 10.Mohty B, Thomas Y, Vukicevic M, et al. Clinical features and outcome of 2009-influenza A (H1N1) after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2012;47:236–242. doi: 10.1038/bmt.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ljungman P. Respiratory virus infections in stem cell transplant patients: the European experience. Biol Blood Marrow Transplant. 2001;7(Suppl):5S–7S. doi: 10.1053/bbmt.2001.v7.pm11777102. [DOI] [PubMed] [Google Scholar]
- 12.Machado CM, Cardoso MR, da Rocha IF, Boas LS, Dulley FL, Pannuti CS. The benefit of influenza vaccination after bone marrow transplantation. Bone Marrow Transplant. 2005;36:897–900. doi: 10.1038/sj.bmt.1705159. [DOI] [PubMed] [Google Scholar]
- 13.Avetisyan G, Aschan J, Hassan M, Ljungman P. Evaluation of immune responses to seasonal influenza vaccination in healthy volunteers and in patients after stem cell transplantation. Transplantation. 2008;86:257–263. doi: 10.1097/TP.0b013e3181772a75. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi MK, Egner W, Sizer L, et al. Antibody responses to vaccinations given within the first two years after transplant are similar between autologous peripheral blood stem cell and bone marrow transplant recipients. Bone Marrow Transplant. 2001;28:775–781. doi: 10.1038/sj.bmt.1703239. [DOI] [PubMed] [Google Scholar]
- 15.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Control CfD . Pediatric Vaccination Schedule. 2010. [Google Scholar]
- 17.Hurwitz ES, Haber M, Chang A, et al. Studies of the 1996–1997 inactivated influenza vaccine among children attending day care: immunologic response, protection against infection, and clinical effectiveness. J Infect Dis. 2000;182:1218–1221. doi: 10.1086/315820. [DOI] [PubMed] [Google Scholar]
- 18.Block SL, Toback SL, Yi T, Ambrose CS. Efficacy of a single dose of live attenuated influenza vaccine in previously unvaccinated children: a post hoc analysis of three studies of children aged 2 to 6 years. Clin Ther. 2009;31:2140–2147. doi: 10.1016/j.clinthera.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Engelhard D, Nagler A, Hardan I, et al. Antibody response to a two-dose regimen of influenza vaccine in allogeneic T cell-depleted and autologous BMT recipients. Bone Marrow Transplant. 1993;11:1–5. [PubMed] [Google Scholar]
- 20.Manual for the Laboratory diagnosis and virological surveillance of influenza. 2011. [Google Scholar]
- 21.Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant. 2008;42:637–641. doi: 10.1038/bmt.2008.264. [DOI] [PubMed] [Google Scholar]
- 22.Ljungman P, Cordonnier C, Einsele H, et al. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2009;44:521–526. doi: 10.1038/bmt.2009.263. [DOI] [PubMed] [Google Scholar]
- 23.de Lavallade H, Garland P, Sekine T, et al. Repeated vaccination is required to optimize seroprotection against H1N1 in the immunocompromised host. Haematologica. 2011;96:307–314. doi: 10.3324/haematol.2010.032664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox RJ, Brokstad KA. The postvaccination antibody response to influenza virus proteins. APMIS. 1999;107:289–296. doi: 10.1111/j.1699-0463.1999.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 25.Honebrink T, Dayton V, Burke MJ, et al. Impact of bone marrow hematogones on umbilical cord blood transplantation outcomes in patients with acute myeloid leukemia. Biol Blood Marrow Transplant. 2012;18:930–936. doi: 10.1016/j.bbmt.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaudette-Zlatanova BC, Le PT, Knight KL, et al. A potential role for B cells in suppressed immune responses in cord blood transplant recipients. Bone Marrow Transplant. 2012 doi: 10.1038/bmt.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohty B, Bel M, Vukicevic M, et al. Graft-versus-host disease is the major determinant of humoral responses to the AS03-adjuvanted influenza A/09/H1N1 vaccine in allogeneic hematopoietic stem cell transplant recipients. Haematologica. 2011;96:896–904. doi: 10.3324/haematol.2011.040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pao MPE, Chou J, Glenn H, Castro-Malaspina H, Jakubowski AA, Kernan NA, Perales MA, Prokop S, Scaradavou A, VanDenBrink MR, Young JW, O'Reilly RJ, Small TN. Response to pneumococcal (PNCRM7) and haemophilus influenzae conjugate vaccines (HIB) in pediatric and adult recipients of an allogeneic hematopoietic cell transplantation (alloHCT) Biology of Blood and Marrow Transplantation. 2008;14:1022–1030. doi: 10.1016/j.bbmt.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Small TN, Cowan MJ. Immunization of hematopoietic stem cell transplant recipients against vaccine-preventable diseases. Expert Rev Clin Immunol. 2011;7:193–203. doi: 10.1586/eci.10.103. [DOI] [PubMed] [Google Scholar]
- 30.Issa NC, Marty FM, Gagne LS, et al. Seroprotective titers against 2009 H1N1 influenza A virus after vaccination in allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2011;17:434–438. doi: 10.1016/j.bbmt.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadereit S, Mohammad SF, Miller RE, et al. Reduced NFAT1 protein expression in human umbilical cord blood T lymphocytes. Blood. 1999;94:3101–3107. [PubMed] [Google Scholar]
- 33.Kaminski BA, Kadereit S, Miller RE, et al. Reduced expression of NFAT-associated genes in UCB versus adult CD4+ T lymphocytes during primary stimulation. Blood. 2003;102:4608–4617. doi: 10.1182/blood-2003-05-1732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.