Abstract
AMP-activated protein kinase (AMPK) is a key energy-sensitive enzyme that controls numerous metabolic and cellular processes. Mammalian target of rapamycin (mTOR) is another energy/nutrient-sensitive kinase that controls protein synthesis and cell growth. In this study we determined whether older versus younger men have alterations in the AMPK and mTOR pathways in skeletal muscle, and examined the effect of a long term resistance type exercise training program on these signaling intermediaries. Older men had decreased AMPKα2 activity and lower phosphorylation of AMPK and its downstream signaling substrate acetyl-CoA carboxylase (ACC). mTOR phosphylation also was reduced in muscle from older men. Exercise training increased AMPKα1 activity in older men, however, AMPKα2 activity, and the phosphorylation of AMPK, ACC and mTOR, were not affected. In conclusion, older men have alterations in the AMPK-ACC and mTOR pathways in muscle. In addition, prolonged resistance type exercise training induces an isoform-selective up regulation of AMPK activity.
Keywords: Aging, Skeletal muscle, AMPK, mTOR, Resistance exercise
1. Introduction
AMP-activated protein kinase (AMPK) is an enzyme that functions as a metabolic master switch. AMPK is a heterotrimeric protein complex consisting of three subunits, α, β and γ. There are two α subunit isoforms, two β subunit isoforms and three γ subunit isoforms (Hardie and Carling, 1997; Stapleton et al., 1997; Steinberg and Kemp, 2009). The α subunit possesses the catalytic activity of the enzyme. AMPK complexes containing the α2 isoform are predominant in skeletal muscle, heart, and liver, while AMPKα1 containing complexes are ubiquitously expressed. Upon increases in the AMP/ATP ratio, the activity of AMPK increases through coordinated regulation of allosteric modification and αsubunit phosphorylation at site Thr172 by upstream kinases such as LKB1 and calcium–calmodulin dependent protein kinase kinase (CaMKK), and decreased dephosphorylation by phosphatases (Hardie and Carling, 1997; Kemp et al., 1999). In human muscle AMPK is phosphorylated and activated robustly by energy-consuming stimuli such as muscle contraction and hypoxia (Musi et al., 2001a; Wadley et al., 2006).
AMPK works to sustain cellular ATP, and it does so by modifying diverse metabolic and cellular pathways. For example, the increases in AMPK activity caused by contraction are thought to mediate, at least partially, exercise-induced increases in skeletal muscle glucose transport (Hayashi et al., 2000; Mu et al., 2001). Activation of AMPK also induces some of the adaptations to prolonged endurance type exercise training and increased activity of key mitochondrial enzymes (Frosig et al., 2004; Winder et al., 2000). Another important role of AMPK is to induce muscle fat oxidation through phosphorylation of acetyl CoA carboxylase (ACC) (Hardie and Carling, 1997). Considering the increasing evidence indicating that several energy-regulating pathways (anaerobic glycolysis, oxidative phosphorylation) may be altered in aging skeletal muscle (Russ and Lanza, 2011), and the key role that the AMPK-ACC pathway plays on metabolic regulation, it is important to establish whether human aging is associated with alterations in the AMPK-ACC axis. Indeed, animal studies have revealed decreased flux through the AMP-ACC pathway in muscle from old rodents (Qiang et al., 2007; Reznick et al., 2007), although there are limited data about this pathway in aging human muscle. Thus, one goal of this study was to determine whether older subjects have alterations in the AMPK-ACC axis in skeletal muscle.
Physical activity plays a fundamental role in promoting good health in older individuals. Resistance type exercise, in particular, stimulates protein metabolism(Yarasheski et al., 1993), which helps to increase muscle mass/strength and improve functional status. Yet, the molecular basis underlying the beneficial effects conveyed by resistance exercise in the older is not fully understood. The mammalian target of rapamycin (mTOR)is a serine/threonine kinase that integrates environmental stimuli such as nutrients (i.e. amino acids) and growth factors (i.e. insulin, insulin-like growth factor 1) to control protein synthesis (Raught et al., 2001). Regulation of protein synthesis by mTOR occurs through the phosphorylation of the downstream targets including initiation factor 4E binding protein 1 (4E-BP1) and S6 kinase (also known as p70S6K) (Dufner and Thomas, 1999; Jefferies et al., 1997; Raught and Gingras, 1999; Sonenberg and Gingras, 1998). Similar to AMPK, mTOR also responds to mechanical stimuli (i.e. muscle contraction). While some studies have reported that resistance type exercise acutely stimulates AMPK and mTOR (Dreyer et al., 2010; Koopman et al., 2006), it is not clear whether chronic resistance training leads to sustained activation of these signaling intermediaries. Therefore, another goal of this study was to investigate whether a long term resistance type exercise program stimulates the AMPK-ACC and mTOR pathways.
2. Materials and methods
2.1. Subjects
32 Younger (aged 19–41) and 32 older (aged 64–86) male subjects were recruited through advertisements in local newspapers and with flyers at the Maastricht University. Each subject underwent a medical history, physical examination, screening laboratory tests, and a 75-g oral glucose tolerance test (OGTT). Subjects with cardiac or peripheral vascular disease, orthopedic limitations and/or type 2 diabetes were excluded. All subjects were living independently, sedentary (not more than one session of exercise per week), and had no history of participating in any structured exercise training program for at least 5 years. Subjects did not report problems in activities of daily living (walking, climbing stairs, rising from a chair), and did not need any assistive equipment (e.g. using a cane) while walking. Eleven older subjects were taking anti-hypertensive medications, seven older individuals were taking lipid-lowering medications, and six older subjects were taking medications for benign prostate hypertrophy. The study was approved by the Medical Ethics Committee of the Maastricht University Medical Centre and all subjects gave written consent.
2.2. Exercise program and biopsy sampling
Twenty five older subjects were engaged in a supervised resistance type exercise training, which was performed 3 times a week (Mon–Wed–Fri) for a 12 week period. Exercise sessions were always performed in the morning, at the same time of day. Training consisted of a 5 min warm-up on a cycle ergometer, followed by 4 sets on both the leg press and leg extension machines (Technogym, Rotterdam), followed by a 5 min cooling-down period on the cycle ergometer. Workload was increased from 60% of 1RM (1-repetition maximum) in week 1 (10–15 repetitions per set) to 75% of 1RM in week 4 and after (8–10 repetitions per set). Resting periods of 90 s and 3 min were allowed between sets and between exercises, respectively. Workload intensity was adjusted based on 4-weekly 1RM testing (Verdijk et al., 2009b). In addition, workload was increased when more than 8 repetitions could be performed in 3 out of 4 sets. Post-training 1RM strength assessment was performed 2 days after the final training session.
After local anesthesia, percutaneous needle biopsies (50–80 mg) were taken from the vastus lateralis muscle, ~15 cm above the patella and 2–3 cm below entry through the fascia (Bergstrom, 1975). Any visible non-muscle tissue was removed immediately, and biopsy samples were frozen in liquid nitrogen and stored at −80 °C until further analyses. All biopsy samples were taken from the right leg in the morning, following an overnight fast. Subjects refrained from any heavy physical exercise/labor in the three days prior to muscle biopsy sampling. To assess the chronic effect of the training intervention in the older subjects post-training biopsies were taken 4 days after the post-intervention strength assessment (Verdijk et al., 2009a).
2.3. Western blotting
Muscle tissue was homogenized in cold lysis buffer containing 20 mmol/l Tris–HCl (pH 7.4), 1% Triton-X 100, 50 mmol/l NaCl, 250 mmol/l sucrose, 50 mmol/l NaF, 5 mmol/l sodium pyrophosphate, 2 mmol/l dithiothreitol (DTT), 4 mg/l leupeptin, 50 mg/l tripsin inhibitor, 0.1 mmol/l benzamidine, and 0.5 mmol/l PMSF, centrifuged at 14,000 g for 20 min at 4 °C, and soluble materials were collected. Proteins (40 μg) from muscle lysates were separated by 8% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked in Tris-buffered saline with 0.05% Tween 20 (TBST) and 5% nonfat milk or BSA for 1 h at room temperature. Immunoblotting was performed using the following primary antibodies: phospho-AMPK-Thr172, phospho-ACC-Ser221, phospho-mTOR-Ser2448, mTOR, phospho-4E-BP1-Thr37/46, 4E-BP1, phospho-S6K-Thr389, and S6K, from Cell Signaling (Beverly, MA), AMPK Pan α from Upstate Biotechnology (Lake Placid, NY), and LKB1 from Abcam (Cambridge, England). Anti-AMPKα1 and AMPKα2 antibodies were kindly provided by Dr. Laurie Goodyear. Bound primary antibodies were detected with anti-rabbit immunoglobulin–horseradish-peroxidase–linked antibodies. The membranes were washed with TBST then incubated with enhanced chemiluminescence reagents (NEN Life Science Products, Boston, MA) and exposed to film. Because ACC is a biotinylated protein, its content in muscle was measured using HRP-conjugated streptavidin (Pierce, Rockford, IL), which has high affinity for biotin. Bands were quantitated with ImageQuant software (Sunnyvale, CA). Two internal control samples were loaded on all the gels and the mean density from these samples was utilized to normalize the values from all the gels.
2.4. AMPK activity assay
The activities of AMPKα1 and AMPKα2 were measured after immunoprecipitating from muscle extracts (150 μg total protein) using antibodies against AMPKα1 (Upstate, Lake Placid, NY) and AMPKα2 (amino acid sequences 352–366), as previously described (Musi et al., 2001b).
2.5. Clinical laboratory assays
Plasma glucose concentrations were analyzed with a COBAS FARA analyzer (Uni Kit III; Roche, Basel, Switzerland), insulin was measured by radioimmunoassay (Insulin RIA Kit; LINCO Research Inc., St Charles, MO), and hemoglobin A1c content was analyzed by HPLC (Variant II; BioRad, Munich, Germany). The homeostatic model assessment index of insulin resistance (HOMA-IR) was calculated as described previously (Matthews et al., 1985).
2.6. Computer tomography (CT) scanning
To determine the effect of resistance training on vastus lateralis muscle mass in older subjects, CT scanning of the thigh was performed before and after completion of the exercise training program (3 days after the post-training strength assessment) using a Philips Medical System (IDT 8000; Philips, Best, The Netherlands) as described (Verdijk et al., 2009b).
2.7. Statistical analysis
All data are expressed as mean ± SE. Baseline data between younger and older groups were analyzed with an unpaired t-test. The effect of exercise within the older group (pre- and post-training) was analyzed with a paired t-test. Analyses were performed using SigmaStat software.
3. Results
3.1. Clinical characteristics
The baseline clinical characteristics of the subjects are shown in Table 1. There were no differences in body weight between younger and older men, however, because the older subject had a lower height, the body mass index (BMI) was higher in this group. Fasting plasma glucose, insulin, and hemoglobin A1c concentrations were elevated by 13% (P < 0.05), 27% (P < 0.05), and 9% (P < 0.05) in older versus younger, respectively. The HOMA index of insulin resistance also was significantly elevated in the older group (P < 0.05).
Table 1.
Baseline clinical characteristics.
| Younger | Older | |
|---|---|---|
| n | 32 | 32 |
| Age, yrs | 24 ± 1 | 73 ± 1* |
| Body weight, kg | 75.5 ± 1.6 | 77.7 ± 1.9 |
| Height, m | 1.81 ± 0.11 | 1.72 ± 0.10* |
| BMI, kg/m2 | 22.9 ± 0.5 | 26.2 ± 0.6* |
| FPG, mg/dl | 92 ± 1 | 104 ± 2* |
| HbA1c, % | 5.4 ± 0.1 | 5.9 ± 0.1* |
| FPI, mU/ml | 9.1 ± 0.5 | 11.6 ± 0.7* |
| HOMA-IR | 2.1 ± 0.1 | 3.0 ± 0.2* |
Data are means ± SE. BMI, body mass index; FPG, fasting plasma glucose; FPI, fasting plasma insulin.
P <0.05.
3.2. Clinical effects of resistance type exercise training
The older subjects performed resistance exercise training 3 times a week for 12 weeks. The resistance exercise training program led to significant increases in quadriceps cross-sectional area (9%) and 1RM muscle strength for leg extension (23%) and leg press (31%) (Table 2). Whole body lean mass increased, whereas whole body fat mass decreased after the resistance exercise training program. Glucose, insulin, and hemoglobin A1c concentrations did not change with exercise. The HOMA index also did not change significantly after resistance training. CT scanning did not reveal changes in lean tissue density after resistance training (not shown), suggesting that the exercise program did not affect intramyocellular lipid content.
Table 2.
Effects of resistance type exercise.
| Older
|
||
|---|---|---|
| Pre-training | Post-training | |
| n | 25 | 25 |
| Body weight, kg | 78.9 ± 2.1 | 78.7 ± 2.1 |
| BMI, kg/m2 | 26.7 ± 0.7 | 26.7 ± 0.7 |
| FPG, mg/dl | 104 ± 2 | 102 ± 2 |
| Hb A1c, % | 5.9 ± 0.1 | 5.8 ± 0.1 |
| FPI, mU/ml | 12.3 ± 0.7 | 12.4 ± 0.8 |
| HOMA-IR | 3.2 ± 0.2 | 3.2 ± 0.2 |
| Quadriceps CSA, cm2 | 74.4 ± 2.5 | 81 ± 2.5* |
| Whole body lean mass, kg | 56.6 ± 1.1 | 57.5 ± 1.1* |
| Whole body fat mass, kg | 19.3 ± 1.4 | 18.4 ± 1.4* |
| 1RM leg extension, kg | 85.6 ± 2.6 | 112.9 ± 3.4* |
| 1RM leg press, kg | 171.8 ± 5.5 | 213.9 ± 7.6* |
Data are means ± SE. BMI, body mass index; FPG, fasting plasma glucose; FPI, fasting plasma insulin; CSA, cross-sectional area; 1RM, one-repetition maximum strength.
P <0.05.
3.3. AMPK phosphorylation and enzymatic activity, and ACC phosphorylation
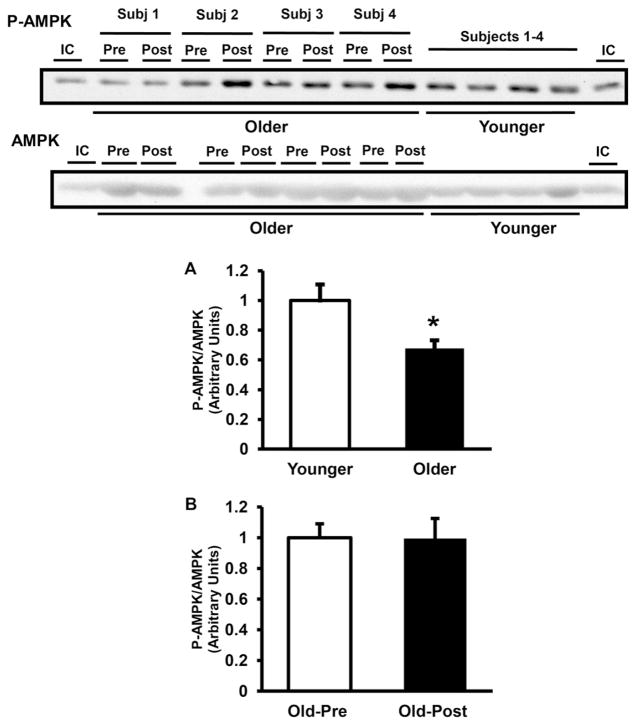
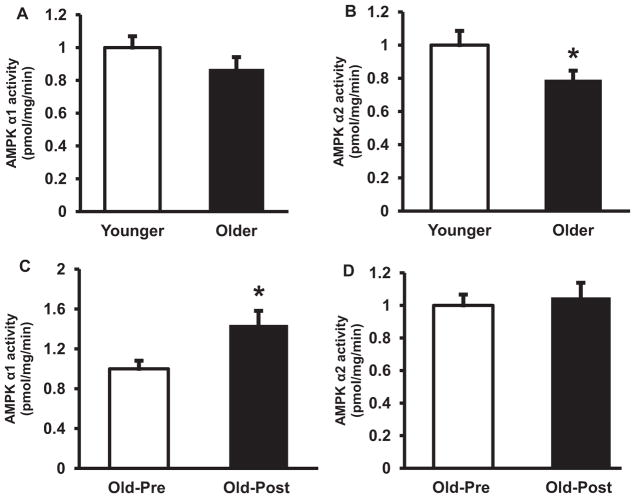
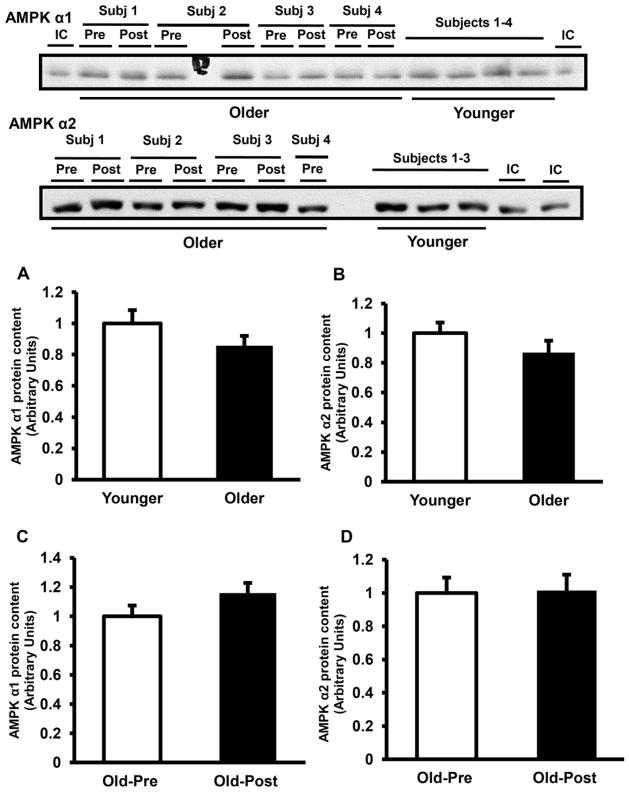
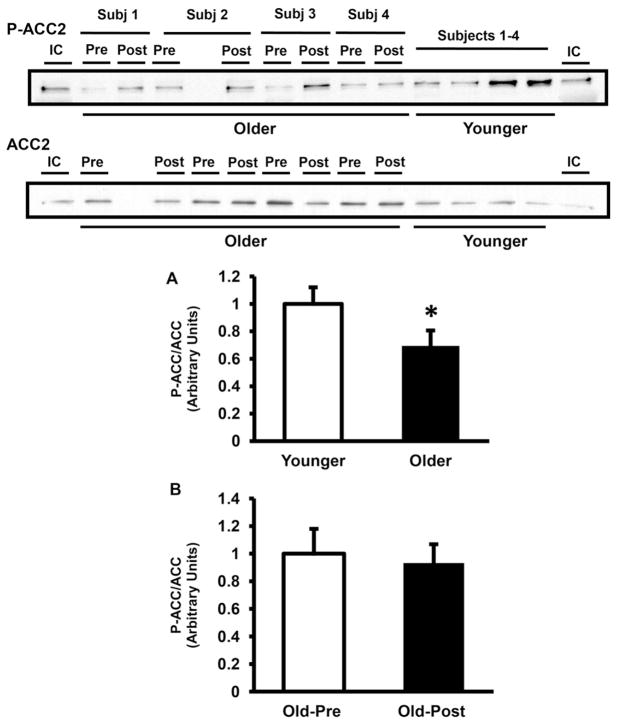
At baseline, AMPK Thr172 phosphorylation was significantly lower in elderly (68% of younger, P < 0.05) (Fig. 1A). Resistance exercise training did not affect AMPK phosphorylation (Fig. 1B). Consistent with the decrease in AMPK phosphorylation, the activity of AMPKα2-complexes also was lower in muscle from older subjects by 21% at baseline (P < 0.05 versus younger) (Fig. 2B), whereas AMPKα1 was not significantly different between groups (Fig. 2A). Resistance type exercise training significantly increased the activity of AMPKα1 (Fig. 2C) by 1.4-fold (P < 0.05), whereas the activity of AMPKα2 did not change with exercise training (Fig. 2D). The protein content of AMPKα1 and AMPKα2 was not different between older and younger (Fig. 3A and B) and resistance exercise training did not affect the content of these proteins (Fig. 3C and D). LKB1 protein content also was not different between groups and did not change with resistance training (not shown). In line with the decreases in AMPK phosphorylation and AMPKα2 activity, older subjects had a significant decrease in ACC2 Ser221 phosphorylation (Fig. 4A). Prolonged resistance training did not affect the phosphorylation of this enzyme (Fig. 4B).
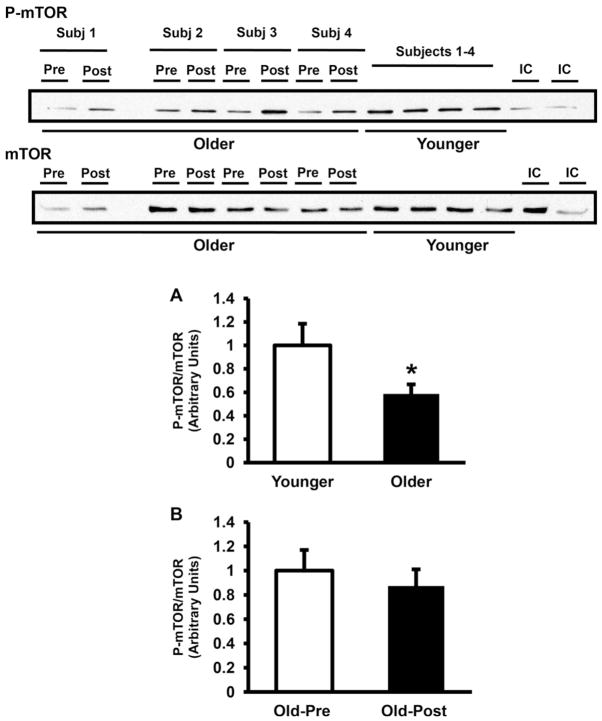
Fig. 1.
AMPK phosphorylation. AMPK phosphorylation and AMPK α subunit muscle protein content were measured in older versus younger subjects at baseline (A) and in older subjects pre- versus post-training (B). Data are means ± SE. n = 32/group in (A) and n = 25/group in (B). *P < 0.05 older versus younger group. Representative blots are shown for 4 younger and 4 older subjects. IC, internal control.
Fig. 2.
AMPK activity. AMPK α1 (A) and AMPK α2 (B) activities were measured in 32 older versus 32 younger subjects at baseline. AMPK α1 (C) and AMPK α2 (D) activities were also measured in 25 older subjects pre- versus post-training. Data are means ± SE. *P < 0.05 pre- versus post-exercise (B), older versus younger group (C). IC, internal control.
Fig. 3.
AMPK protein content. AMPK α1 (A) and AMPK α2 (B) protein content were measured in the skeletal muscle of 32 older versus 32 younger subjects at baseline. AMPK α1 (C) and AMPK α2 (D) protein content was measured in 25 older subjects pre- versus post-training. Data are means ± SE. Representative blots are shown for 4 younger and 4 older subjects for AMPK α1, and 3 younger and 4 older subjects for AMPK α2. IC, internal control.
Fig. 4.
ACC2 phosphorylation. ACC2 phosphorylation and protein content were measured in older versus younger subjects at baseline (A) and in older pre- versus post-training (B). Data are means ± SE. n = 32/group in (A) and n = 25/group in (B). *P < 0.05 older versus younger group. Representative blots are shown for 4 younger and 4 older subjects. IC, internal control.
3.4. mTOR signaling
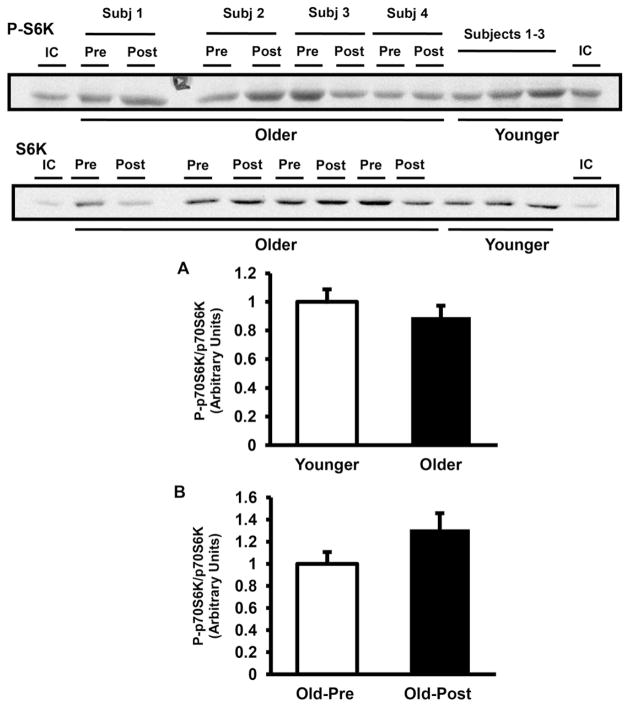
As shown in Fig. 5A, mTOR phosphorylation was significantly lower in muscle from older subjects by 41% (P < 0.05), but mTOR phosphorylation was not affected by exercise training (Fig. 5B). Consistent with the lower mTOR phosphorylation observed in older subjects, the phosphorylation of 4E-BP1 also was lower in this group by 30% (Fig. 6A). Resistance exercise training did not affect 4E-BP1 phosphorylation (Fig. 6B). In contrast to 4E-BP1, S6K phosphorylation was not lower in older subjects (Fig. 7A). Resistance exercise did not affect the phosphorylation of S6K (Fig. 7B).
Fig. 5.
mTOR phosphorylation. mTOR phosphorylation and protein content were measured in older versus younger subjects at baseline (A) and in older pre- versus post-training (B). Data are means ± SE. n = 32/group in (A) and n = 25/group in (B). *P < 0.05 older versus younger group. Representative blots are shown for 4 younger and 4 older subjects. IC, internal control.
Fig. 6.
4E-BP1 phosphorylation. 4E-BP1 phosphorylation and protein content were measured in older versus younger subjects at baseline (A) and in older pre- versus post-training (B). Data are means ± SE. n = 32/group in (A) and n = 25/group in (B). *P < 0.05 older versus younger group. Representative blots are shown for 3 younger and 4 older subjects. IC, internal control.
Fig. 7.
S6K phosphorylation. S6K phosphorylation and protein content were measured in older versus younger subjects at baseline (A) and in older pre- versus post-training (B). Data are means ± SE. n = 32/group in (A) and n = 25/group in (B). Representative blots are shown for 3 younger and 4 older subjects. IC, internal control.
4. Discussion
Reznick et al. reported that old rats have decreased AMPK activity in muscle upon acute stimulation with the AMPK-activating compound 5′-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) and treadmill exercise (Reznick et al., 2007). AMPK activity also was blunted in old rats after chronic stimulation with β-guanidinopropionic acid (β-GPA) (Reznick et al., 2007). Qiang et al. found that muscles from old rats have decreased basal AMPK and ACC phosphorylation, in association with a marked impairment in insulin-stimulated muscle glucose transport (Qiang et al., 2007). This is also consistent with a study performed in older mono- and dizygotic twins which showed that baseline AMPKγ3-associated activity is decreased in aging (Mortensen et al., 2009). Collectively, the results from these studies and the present findings indicate that aging leads to a down regulation of the AMPK-ACC axis in the basal state (Qiang et al., 2007; Mortensen et al., 2009) and in response to acute (Reznick et al., 2007) and chronic (Reznick et al., 2007) stimuli. Nonetheless, other studies have not observed a down regulation of the AMPK pathway with aging. Thompson et al. reported enhanced activation of AMPK after acute, electrically-stimulated contraction of extensor digitorum longus muscles in old versus young rats (Thomson et al., 2009) and Drummond et al. did not observe differences in baseline of AMPK phosphorylation between young and older subjects (Drummond et al., 2008). Drummond et al. reported that AMPK phosphorylation was accentuated in muscle from older subjects after ingestion of essential amino acids and a 1 h bout of resistance exercise, compared with younger individuals (Drummond et al., 2008). The contrasting results between studies regarding basal and stimulated AMPK activity could be explained by differences in experimental conditions, species, and populations studied (in case of the human studies).
In this study insulin sensitivity was lower in older versus younger men, based on the higher fasting plasma insulin concentrations and the higher HOMA index of insulin resistance. Of note, the older men had a higher BMI than the younger men, which likely contributed to the insulin resistant state in these individuals. We previously reported that obesity and type 2 diabetes actually up regulate AMPK activity in humans (Sriwi-jitkamol et al., 2007). Therefore, it is unlikely that the increased BMI in the older subjects would explain the down regulation in AMPK and ACC observed in older subjects in the present study.
The effect that prolonged resistance type exercise training has on AMPK and ACC is unclear. In previous studies, acute resistance exercise either stimulated (Dreyer et al., 2006) or did not affect AMPK (Harber et al., 2008). Wojtaszewski et al. evaluated the effect of strength training for 6 weeks in healthy volunteers and type 2 diabetic subjects, and did not observe changes in AMPK and ACC phosphorylation, although resistance exercise did cause a small increase in AMPKα1 protein (Wojtaszewski et al., 2005). In this study, we found that resistance training significantly increased the activity of α1 subunit-containing AMPK complexes, without affecting AMPKα2. This finding is consistent with the increasing evidence suggesting that α1- and α2-containing AMPK complexes have distinct regulatory properties and roles in the regulation of skeletal muscle adaptations. The AMPKα2 isoform is more sensitive to aerobic exercise (Fujii et al., 2000) and is thought to play a more predominant role in regulating adaptations to aerobic exercise training (Jorgensen et al., 2007). In contrast, muscle overload causes a marked activation of AMPKα1 (McGee et al., 2008) and this isoform plays an important role in limiting skeletal muscle overgrowth during overload-induced hypertrophy (Mounier et al., 2009).
AMPKα2-containing complexes are more abundant than AMPKα1 in human muscle (Musi et al., 2001a; Wojtaszewski et al., 2005), which is possibly why the increase in AMPKα1 activity after resistance training observed in this study was not reflected at the level of pan-αAMPK phosphorylation. Moreover, there is some evidence to suggest that sensitivity of the various AMPK complexes to different stimuli may depend on the specificity of upstream kinases for α subunit phosphorylation/activation. For example, in LKB1null mice, AMPKα2 activation is severely blunted after electrically-induced muscle contraction, whereas AMPKα1 activity increases significantly (Koh et al., 2006), suggesting that AMPKα2, but not α1 stimulation, depends on LKB1. On the other hand, chronic muscle overload causes a marked increase in the expression and the activity of CaMKKα and CaMKKβ (McGee et al., 2008), upstream AMPK kinases which also can activate AMPKα1- and α2-containing complexes (Witczak et al., 2007).
Some (Balagopal et al., 1997; Welle et al., 1993), albeit not all studies (Volpi et al., 2001), have shown a reduction in protein synthesis in muscle from older subjects. In this study we found a significant decrease in mTOR phosphorylation in the older subjects. In line with this, the phosphorylation of 4E-BP1 also was lower in the older group. 4E-BP1 inhibits protein translation by binding to eukaryotic initiation factor 4E (eIF4E). 4E-BP1phosphorylaiton leads to the release of eIF4E which functions to induce translation of capped cellular mRNAs (Raught and Gingras, 1999). Taken together, the reductions in mTOR and 4E-BP1 phosphorylation in the older group observed in this study suggest that down regulation of this signaling cascade might be implicated in age-related changes in protein metabolism reported by some groups (Balagopal et al., 1997; Welle et al., 1993). Furthermore, Cuthbertson et al. found that older subjects have significant decreases in mTOR, 4E-BP1 and S6K content, which should lead to a net decrease in the activity of these proteins (Cuthbertson et al., 2005) and Wu et al. showed that mTOR phosphorylation is reduced in muscle from very old (33 month old) rats (Wu et al., 2009). Notably, unlike the present findings, others have reported no age differences in mTOR (Drummond et al., 2008) and 4E-BP1 (Kumar et al., 2009; Mayhew et al., 2009) phosphorylation in muscle. More research in this area is needed to better clarify how age affects the mTOR axis in the basal and exercise-stimulated states and to define the physiological consequences of these changes.
Several studies have examined the effect of acute resistance exercise on mTOR signaling in human subjects. While these studies have reported variable responses, in general, mTOR signaling has been found to be enhanced when analyzed 0.5–2 h after a single session of acute resistance exercise (Dreyer et al., 2006; Fujita et al., 2009; Rommel et al., 2001; Terzis et al., 2010). Interestingly, acute resistance exercise had opposite effects on mTOR substrates; 4E-BP1 phosphorylation decreased, whereas phosho-S6K increased with exercise (Dreyer). In contrast, we did not observe differences on mTOR, 4E-BP1 or S6K after the 12 week resistance training program. Our goal was to evaluate the chronic effect of resistance training. Therefore, post-training muscle samples were obtained 4 days after the last bout of exercise, once the acute exercise effect had dissipated. We conclude that, under the experimental conditions tested in this study resistance exercise does not have long-lasting (i.e. chronic) effects on this signaling pathway, suggesting that during resistance exercise training the mTOR pathway induces muscle protein synthesis and enhances muscle mass through its repetitive activation, triggered by each successive exercise session.
In this study both AMPK and mTOR signaling were down regulated in muscle from older subjects. This finding contradicts the notion that AMPK has an inhibitory effect on the mTOR pathway. In corneal epithelial cells AICAR decreases mTOR complex (TORC)1 activity (Kimura et al., 2003), and this agent cannot suppress TORC1 in AMPKα1/α2 double-knockout fibro-blasts (Kalender et al., 2010). Moreover, in HEK293 cells AMPK physically associates with and phosphorylates tuberous sclerosis complex 2, reducing TORC1 activity (Inoki et al., 2003). While the findings from cell culture studies suggest that AMPK decreases mTOR signaling, there is limited evidence demonstrating that this phenomenon occurs in human subjects in vivo. AICAR administration to rats inhibits mTOR signaling (Bolster et al., 2002). Nonetheless, these experiments are limited by the non-specificity of AICAR. This compound has other non-AMPK mediated effects, including stimulation of adenosine receptors (Oei et al., 1991) and inhibition of gluconeogenic enzymes (Vincent et al., 1991). In addition, in vivo AICAR administration causes a whole host of systemic metabolic and hormonal alterations that indirectly could affect mTOR signaling, including lowering of plasma glucose and insulin concentration and increasing lactic and uric acid levels (Aschenbach et al., 2002). The data from the present study, along with other in vivo human investigations that have shown that acute resistance (Vissing et al., 2011) and aerobic (Supplementary Fig. 1) exercise simultaneously increase AMPK and mTOR phosphorylation in muscle, indicate that AMPK activity is not always inversely related to the function of mTOR. Clearly, more research is required to better understand the interaction between the AMPK and mTOR pathways in vivo, and to clarify what are the physiological consequences of such interactions.
One limitation of this study is that the cell signaling assays were carried out in muscle samples conformed of a mixture of muscle fiber types (glycolytic versus oxidative). Although care was taken to standardize the muscle biopsy procedure with respect to location and depth at which the biopsy was collected, each muscle biopsy procedure can yield samples with different mixtures of fiber types. In future studies it will be important to determine whether age-related differences in AMPK-mTOR signaling, and the effect of exercise, varies depending upon the fiber type, and whether these differences/changes are present across all fibers.
In summary, in this study we found that older men have a down regulation of both the AMPK and mTOR signaling pathways. Future studies will help to determine the physiologic relevance of these age-related differences. Also, we found that long-term resistance type exercise training increases the activity of AMPKα1-, but not AMPKα2-containing complexes, and does not lead to activation of the mTOR pathway beyond 96 h after the last exercise bout. This suggests that mTOR-regulated protein synthesis and hypertrophy resulting from resistance training is due to episodic, transient activation, rather than from sustained stimulation of this pathway.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (RO1-DK80157 and RO1-DK089229 to N.M). N.M. was the recipient of a Paul B. Beeson Career Development Award (K23-AG030979) from the American Federation for Aging Research and the National Institute on Aging. L.V. received support from the Anna Foundation (Leiden, the Netherlands) and K.S. from U.K. Medical Research Council.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.mad.2012.09.001.
References
- Aschenbach WG, Hirshman MF, Fujii N, Sakamoto K, Howlett KF, Good-year LJ. Effect of AICAR treatment on glycogen metabolism in skeletal muscle. Diabetes. 2002;51:567–573. doi: 10.2337/diabetes.51.3.567. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. American Journal of Physiology. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scandinavian Journal of Clinical and Laboratory Investigation. 1975;35:609–616. [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. Journal of Biological Chemistry. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB Journal. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. Journal of Physiology. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiologica (Oxford) 2010;199:71–81. doi: 10.1111/j.1748-1716.2010.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. Journal of Applied Physiology. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Experimental Cell Research. 1999;253:100–109. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. American Journal of Physiology –Endocrinology and Meatbolism. 2004;286:E411–E417. doi: 10.1152/ajpendo.00317.2003. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5′ AMP-activated protein kinase activity in human skeletal muscle. Biochemical and Biophysical Research Communications. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB. Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. Journal of Applied Physiology. 2009;106:1730–1739. doi: 10.1152/japplphysiol.90395.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber MP, Crane JD, Douglass MD, Weindel KD, Trappe TA, Trappe SW, Fink WF. Resistance exercise reduces muscular substrates in women. International Journal of Sports Medicine. 2008;29:719–725. doi: 10.1055/s-2007-989442. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The AMP-activated protein kinase—fuel gauge of the mammalian cell? European Journal of Biochemistry. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–531. doi: 10.2337/diabetes.49.4.527. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5′ TOP mRNA translation through inhibition of p70s6k. EMBO Journal. 1997;16:3693–3704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA. Role of AMPKalpha2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. American Journal of Physiology – Endocrinology and Meatbolism. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metabolism. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends in Biochemical Sciences. 1999;24:22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, Kemp BE, Witters LA, Mimura O, Yonezawa K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes to Cells. 2003;8:65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- Koh HJ, Arnolds DE, Fujii N, Tran TT, Rogers MJ, Jessen N, Li Y, Liew CW, Ho RC, Hirshman MF, Kulkarni RN, Kahn CR, Goodyear LJ. Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3. Molecular and Cellular Biology. 2006;26:8217–8227. doi: 10.1128/MCB.00979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman R, Zorenc AH, Gransier RJ, Cameron-Smith D, van Loon LJ. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. American Journal of Physiology – Endocrinology and Meatbolism. 2006;290:E1245–E1252. doi: 10.1152/ajpendo.00530.2005. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. Journal of Physiology. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. Journal of Applied Physiology. 2009;107:1655–1662. doi: 10.1152/japplphysiol.91234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SL, Mustard KJ, Hardie DG, Baar K. Normal hypertrophy accompanied by phosphoryation and activation of AMP-activated protein kinase alpha1 following overload in LKB1 knockout mice. Journal of Physiology. 2008;586:1731–1741. doi: 10.1113/jphysiol.2007.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen B, Poulsen P, Wegner L, Stender-Petersen KL, Ribel-Madsen R, Friedrichsen M, Birk JB, Vaag A, Wojtaszewski JF. Genetic and metabolic effects on skeletal muscle AMPK in young and older twins. American Journal of Physiology – Endocrinology and Meatbolism. 2009;297:E956–E964. doi: 10.1152/ajpendo.00058.2009. [DOI] [PubMed] [Google Scholar]
- Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Pende M, Daegelen D, Sakamoto K, Foretz M, Viollet B. Important role for AMPKalpha1 in limiting skeletal muscle cell hypertrophy. FASEB Journal. 2009;23:2264–2273. doi: 10.1096/fj.08-119057. [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Molecular Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Musi N, Fujii N, Hirshman MF, Ekberg I, Froberg S, Ljungqvist O, Thorell A, Goodyear LJ. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes. 2001a;50:921–927. doi: 10.2337/diabetes.50.5.921. [DOI] [PubMed] [Google Scholar]
- Musi N, Hayashi T, Fujii N, Hirshman MF, Witters LA, Goodyear LJ. AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. American Journal of Physiology – Endocrinology and Meatbolism. 2001;280:E677–E684. doi: 10.1152/ajpendo.2001.280.5.E677. [DOI] [PubMed] [Google Scholar]
- Oei HH, Burrier AC, Jeng AY. 5-Amino-4-imidazolecarboxamide riboside raises adenosine in perfused hypoxic rat heart. European Journal of Pharmacology. 1991;204:1–7. doi: 10.1016/0014-2999(91)90827-d. [DOI] [PubMed] [Google Scholar]
- Qiang W, Weiqiang K, Qing Z, Pengju Z, Yi L. Aging impairs insulin-stimulated glucose uptake in rat skeletal muscle via suppressing AMPKalpha. Experimental and Molecular Medicine. 2007;39:535–543. doi: 10.1038/emm.2007.59. [DOI] [PubMed] [Google Scholar]
- Raught B, Gingras AC. eIF4E activity is regulated at multiple levels. International Journal of Biochemistry and Cell Biology. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metabolism. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nature Cell Biology. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Russ DW, Lanza IR. The impact of old age on skeletal muscle energetics: supply and demand. Current Aging Science. 2011;4:234–247. doi: 10.2174/1874609811104030234. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Gingras AC. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Current Opinion in Cell Biology. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose–response study. Diabetes. 2007;56:836–848. doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton D, Woollatt E, Mitchelhill KI, Nicholl JK, Fernandez CS, Michell BJ, Witters LA, Power DA, Sutherland GR, Kemp BE. AMP-activated protein kinase isoenzyme family: subunit structure and chromosomal location. FEBS Letters. 1997;409:452–456. doi: 10.1016/s0014-5793(97)00569-3. [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE. AMPK in health and disease. Physiological Reviews. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- Terzis G, Spengos K, Mascher H, Georgiadis G, Manta P, Blomstrand E. The degree of p70 S6k and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. European Journal of Applied Physiology. 2010;110:835–843. doi: 10.1007/s00421-010-1527-2. [DOI] [PubMed] [Google Scholar]
- Thomson DM, Brown JD, Fillmore N, Ellsworth SK, Jacobs DL, Winder WW, Fick CA, Gordon SE. AMP-activated protein kinase response to contractions and treatment with the AMPK activator AICAR in young adult and old skeletal muscle. Journal of Physiology. 2009;587:2077–2086. doi: 10.1113/jphysiol.2008.166512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2009;64:332–339. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. American Journal of Clinical Nutrition. 2009;89:608–616. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- Vincent MF, Marangos PJ, Gruber HE, Van den Berghe G. Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes. 1991;40:1259–1266. doi: 10.2337/diab.40.10.1259. [DOI] [PubMed] [Google Scholar]
- Vissing K, McGee SL, Farup J, Kjolhede T, Vendelbo MH, Jessen N. Differentiated mTOR but not AMPK signaling after strength vs. endurance exercise in training-accustomed individuals. Scandinavian Journal of Medicine and Science in Sports. 2011 doi: 10.1111/j.1600-0838.2011.01395.x. http://dx.doi.org/10.1111/j.1600-0838.2011.01395.x. [Epub ahead of print] [DOI] [PubMed]
- Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadley GD, Lee-Young RS, Canny BJ, Wasuntarawat C, Chen ZP, Hargreaves M, Kemp BE, McConell GK. Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. American Journal of Physiology – Endocrinology and Meatbolism. 2006;290:E694–E702. doi: 10.1152/ajpendo.00464.2005. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Jozefowicz R, Statt M. Myofibrillar protein synthesis in young and old men. American Journal of Physiology. 1993;264:E693–E698. doi: 10.1152/ajpendo.1993.264.5.E693. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. Journal of Applied Physiology. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Ca2+/calmodulin-dependent protein kinase kinase-alpha regulates skeletal muscle glucose uptake independent of AMP-activated protein kinase and Akt activation. Diabetes. 2007;56:1403–1409. doi: 10.2337/db06-1230. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Birk JB, Frosig C, Holten M, Pilegaard H, Dela F. 5′ AMP activated protein kinase expression in human skeletal muscle: effects of strength training and type 2 diabetes. Journal of Physiology. 2005;564:563–573. doi: 10.1113/jphysiol.2005.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Katta A, Gadde MK, Liu H, Kakarla SK, Fannin J, Paturi S, Arvapalli RK, Rice KM, Wang Y, Blough ER. Aging-associated dysfunction of Akt/protein kinase B: S-nitrosylation and acetaminophen intervention. PLos One. 2009;4:e6430. doi: 10.1371/journal.pone.0006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. American Journal of Physiology. 1993;265:E210–E214. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.