Abstract
Background
Family history of coronary heart disease (CHD) has been well studied as an independent risk factor for CHD events in the short term (<10 years). However, data are sparse on the association between family history and risk for CHD across long-term follow-up.
Methods and Results
We included 49 255 men from the Cooper Center Longitudinal Study. Premature family history of CHD was defined as the presence of angina, myocardial infarction, angioplasty, or bypass surgery in a relative <50 years of age. Cause-specific mortality was obtained from the National Death Index. The association between premature family history and cardiovascular disease (CVD) or CHD death was compared across 3 unique follow-up periods (0–10, >10–20, and >20 years). Lifetime risk was estimated by use of a modified survival analytic technique adjusted for competing risk with non-CVD death as the competing event. After 811 708 person-years of follow-up, there were 919 CHD deaths and 1456 CVD deaths. After adjustment for traditional risk factors, premature family history was associated with CHD mortality >10 to 20 years (1.59; 95% confidence interval, 1.14–2.22) and >20 years (1.43; 95% confidence interval, 1.05–1.95) with wider confidence intervals at 0 to 10 years (1.32; 95% confidence interval, 0.76–2.31). Similar findings were observed for CVD mortality. Compared with men without a family history of coronary artery disease, premature family history was associated with an ≈50% higher lifetime risk for both CHD and CVD mortality (13.7% versus 8.9% and 21% versus 14.1%, respectively).
Conclusion
Premature family history was associated with a persistent increase in both CHD and CVD mortality risk across long-term follow-up, resulting in significantly higher lifetime risk estimates.
Keywords: cardiovascular diseases, coronary disease, heredity, risk factors
Family history of coronary heart disease (CHD) is a well-recognized risk factor, with multiple prospective studies demonstrating a consistent, independent association with CHD.1–3 Although definitions vary, it is well established that the strength of the association between family history and CHD is greatest with earlier age of presentation of CHD in the family member (ie, premature family history [pre-FHx]).4–6 Current prevention guidelines recommend that pre-FHx be incorporated into the risk estimation process that guides treatment decisions,5 and family history can be easily and systematically queried in the clinical setting.7 However, family history is included in some,8 but not all,9,10 short-term risk prediction equations because of its relatively modest contribution to short-term risk.4,9,10
Clinical Perspective on p 3098
Although risk in the short term (ie, 10 years) represents a well-established approach to guide treatment decisions, it may not completely reflect the burden of CHD risk across the lifespan.11–14 Specifically, a risk factor that promotes a small but consistent increase in CHD risk can translate into substantial differences in risk across the lifespan despite a more modest increase in short-term risk.15
To the best of our knowledge, the association between a family history of CHD and long-term risk for CHD has not been studied. Therefore, we sought to determine the association between the presence of a family history of CHD and both CHD and cardiovascular disease (CVD) mortality across short-term (0–10 years), intermediate-term (>10–20 years), and long-term (>20 years) follow-up. We also sought to determine the association between the presence of a family history of CHD and the lifetime risk for both CHD and CVD mortality.
Methods
Study Sample and Definitions
The Cooper Center Longitudinal Study (CCLS) is an ongoing, prospective study at the Cooper Clinic in Dallas, TX, that began over 40 years ago.16–18 The Cooper Center is a preventive medical practice that focuses on periodic health examinations. Patients come from all 50 states and are referred by their employer or personal physician or are self-referred. Fewer than 5% of patients are nonwhite. For the present study, we included all men between 20 and 90 years of age who underwent a complete clinical examination and completed a family history questionnaire at the Cooper Center between 1970 and 2006 (n=49 956). After excluding 701 men with a prior myocardial infarction, we had a final study sample of 49 255. Women were excluded from the present study because of the small number of CHD end points in each decade of follow-up (6 CHD deaths at 0–10 years, 27 CHD deaths at >10–20 years, and 32 CHD deaths at >20 years), particularly when stratifying by family history status.
The CCLS undergoes annual review by the Institutional Review Board of the Cooper Institute. The present study was approved by the Institutional Review Board of University of Texas Southwestern Medical Center at Dallas.
Measurements
All participants underwent a comprehensive clinical examination that included self-reported personal medical history and smoking habits, a physical examination, and measurement of blood pressure, fasting blood glucose, and cholesterol. Details of anthropometric and laboratory measurements and other variable definitions have been given previously.17 Diabetes mellitus was defined by self-report or a fasting blood glucose >125 mg/dL. Smoking habits (current smoker or not) were obtained from a standardized questionnaire. Systolic and diastolic blood pressures were measured in standard fashion with a sphygmomanometer.
Definition of Family History
Participants completed a standardized questionnaire on their family history status. A positive family history was defined as the presence of angina, myocardial infarction, angioplasty, or coronary artery bypass surgery in a sibling, aunt or uncle, parent, or grandparent (excluding cousins, relatives by marriage, and half-relatives). If a patient had a family member with a history of CHD, the patient was asked to indicate whether the event occurred before 50 years of age (pre-FHx) or thereafter (late-FHx); if not, the patient was defined as having no family history (no-FHx). The 3 categories of family history (no-FHx, pre-FHx, and late-FHx) were mutually exclusive. No specific information was recorded in the questionnaire on the type of CHD event or family member.
Outcome
Participants were followed up from the date of initial examination until death or the end of follow-up on December 31, 2006 (range of follow-up period, 0.01–36 years), through the use of data from the National Death Index. CHD mortality was defined as the primary cause of death indicated by International Classification of Diseases, ninth revision (ICD-9), codes 410 to 414 or equivalent codes from ICD-8 or ICD-10. CVD mortality was defined as ICD-9 codes 390.0 to 458.9 or their equivalents from ICD-8 or ICD-10.
Statistical Analysis
The follow-up period was partitioned into 3 unique, mutually exclusive time periods: 0 to 10 inclusive, >10 to 20 inclusive, and >20 years. For example, a participant surviving for 25 years provided 10 years of follow-up for the first follow-up interval (0–10 years), 10 years of follow-up for the second interval (>10–20 years), and 5 years of follow-up for the third interval (>20 years). Family history was characterized as a single categorical variable with 3 levels (no-FHx, pre-FHx, and late-FHx), and a Cox proportional hazards model was constructed for each follow-up period with no-FHx as the referent group. The model was multivariable and included age, systolic blood pressure, serum total cholesterol, body mass index, smoking, and diabetes mellitus. Secondary analyses with further adjustment for fitness were also performed.
Finally, to estimate lifetime risk for CHD mortality, we applied a modified survival analytic technique that has been described previously.12,19 In this type of analysis, participants contributed information on CHD death and death free of CHD for each age attained during follow-up. Because the Kaplan-Meier cumulative incidence does not reflect the competing risk for death from other causes before the development of CHD, adjustment was made for this competing risk to yield a true remaining lifetime risk for CHD. Lifetime risk estimates were calculated separately for each family history category (no-FHx, pre-FHx, and late-FHx) beginning at 45 and 55 years of age. Similar analyses were performed to estimate the lifetime risk for CVD mortality. All statistical analyses were performed with SAS for Windows (release 9.2; SAS Institute, Inc, Cary, NC).
Results
Baseline Characteristics
Among 49 255 men in the study sample, 7832 (16%) had a late-FHx and 3203 (6.5%) had a pre-FHx. Baseline characteristics of the study sample are shown in Table 1, demonstrating similar overall levels of traditional risk factors with and without a family history of CHD. After a median follow-up of 16 years, there were 919 CHD deaths and 1456 CVD deaths across 811 708 person-years of follow-up, with a large number of person-years and events across all periods of follow-up (Table 2).
Table 1.
Participant Characteristics in the Cooper Center Longitudinal Study
| Characteristic | No Family History (n=38 220) |
Late-Onset Family History (n=7832) |
Premature Family History (n=3203) |
|---|---|---|---|
| Baseline age, y | 45.1 (9.8) | 43.2 (9.6) | 40.5 (8.7) |
| SBP, mm Hg | 122.8 (13.7) | 120.4 (13.5) | 121.0 (13.2) |
| DBP, mm Hg | 82.1 (9.7) | 80.3 (9.5) | 80.7 (9.4) |
| Total cholesterol, mg/dL | 206.6 (40.2) | 212.5 (40.1) | 214.3 (40.4) |
| Triglycerides, mg/dL | 139.2 (120.3) | 139.2 (112.9) | 145.1 (107.2) |
| BMI, kg/m2 | 27.0 (4) | 26.1 (3.5) | 26.1 (3.6) |
| Diabetes mellitus, n (%) | 1740 (4.6) | 320 (4.1) | 161 (5) |
| Smoking, n (%) | 6361 (16.6) | 1623 (20.7) | 702 (21.9) |
| CHD deaths, n | 582 | 230 | 107 |
| Age-adjusted rate per 1000 person-y |
1.0 | 1.3 | 1.9 |
| CVD deaths, n | 905 | 387 | 387 |
| Age-adjusted rate per 1000 person-y |
1.6 | 2.2 | 2.9 |
| All-cause deaths, n | 2657 | 1057 | 407 |
| Age-adjusted rate per 1000 person-y |
4.6 | 6.0 | 7.0 |
SBP indicates systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; CHD, coronary heart disease; and CVD, cardiovascular disease. Data presented as mean (SD) unless otherwise noted. Late-onset family history indicates a diagnosis of coronary artery disease after 50 years of age in a family member; premature family history indicates a diagnosis of coronary artery disease before 50 years of age in a family member.
Table 2.
Mortality Rates by Cause of Death for 0 to 10, >10 to 20, and >20 Years of Follow-Up Among 49 255 Men in the Cooper Center Longitudinal Study
| Follow-Up of 0–10 y (n=49 255) |
Follow-Up of >10–20 y (n=32 789) |
Follow-Up of >20 y (n=20 282) |
|
|---|---|---|---|
| Person-y | 411 648 | 270 036 | 130 024 |
| CHD deaths, n | 203 | 356 | 360 |
| Rate (per 1000 person-y) | 0.49 | 1.32 | 2.77 |
| CVD deaths, n | 281 | 540 | 635 |
| Rate (per 1000 person-y) | 0.68 | 2.00 | 4.88 |
| All-cause deaths, n | 810 | 1499 | 1812 |
| Rate (per 1000 person-y) | 1.97 | 5.55 | 13.94 |
CHD indicates coronary heart disease; CVD, cardiovascular disease.
Family History of CHD and Risk at 0 to 10, >10 to 20, and >20 Years of Follow-Up
The associations between late-FHx and CHD, CVD, and all-cause death across short-term, intermediate-term, and long-term follow-up are shown in Table 3. The association between late-FHx and CHD mortality was strongest in the short term (0–10 years) with no apparent association across intermediate- and long-term follow-up. Findings were similar in both age-adjusted and multivariable-adjusted models and for both CHD and all-cause mortality.
Table 3.
Multivariable- and Age-Adjusted Hazard Ratios (95% Confidence Intervals) for Premature Family History, Late-Onset Family History, and Coronary Heart Disease Mortality, Cardiovascular Disease Mortality, and All-Cause Mortality Among Men in the Cooper Center Longitudinal Study*
| Variable | Follow-Up of 0–10 y |
Follow-Up of >10–20 y |
Follow-Up of >20 y |
|---|---|---|---|
| CHD mortality | |||
| Age-adjusted | |||
| No-FHx | 1 | 1 | 1 |
| Late-FHx | 1.25 (0.89–1.76) | 1.10 (0.87–1.39) | 0.99 (0.76–1.27) |
| P, late-FHx vs no-FHx | 0.196 | 0.436 | 0.910 |
| pre-FHx | 1.41 (0.81–2.45) | 1.59 (1.14–2.22) | 1.46 (1.07–1.99) |
| P, pre-FHx vs no-FHx | 0.226 | 0.006 | 0.017 |
| Multivariable | |||
| No-FHx | 1 | 1 | 1 |
| Late-FHx | 1.25 (0.88–1.76) | 1.15 (0.91–1.46) | 1.00 (0.78–1.29) |
| P, late-FHx vs no-FHx | 0.210 | 0.250 | 0.984 |
| pre-FHx | 1.32 (0.76–2.31) | 1.59 (1.14–2.22) | 1.43 (1.05–1.95) |
| P, pre-FHx vs no-FHx | 0.324 | 0.006 | 0.025 |
| Log-rank P | 0.644 | 0.001 | <0.0001 |
| CVD mortality | |||
| Age-adjusted | |||
| No-FHx | 1 | 1 | 1 |
| Late-FHx | 1.42 (1.07–1.88) | 1.15 (0.95–1.39) | 1.10 (0.91–1.32) |
| P, late-FHx vs no-FHx | 0.016 | 0.155 | 0.345 |
| pre-FHx | 1.60 (1.02–2.53) | 1.44 (1.09–1.92) | 1.47 (1.16–1.86) |
| P, pre-FHx vs no-FHx | 0.042 | 0.011 | 0.002 |
| Multivariable | |||
| No-FHx | 1 | 1 | 1 |
| Late-FHx | 1.40 (1.06–1.86) | 1.20 (0.99–1.45) | 1.11 (0.92–1.33) |
| P, late-FHx vs no-FHx | 0.019 | 0.065 | 0.299 |
| pre-FHx | 1.51 (0.96–2.39) | 1.46 (1.10–1.94) | 1.43 (1.13–1.82) |
| P, pre-FHx vs no-FHx | 0.078 | 0.009 | 0.003 |
| Log-rank P | 0.347 | <0.0001 | <0.0001 |
| All-cause mortality | |||
| Age-adjusted | |||
| No-FHx | 1 | 1 | 1 |
| Late-FHx | 1.32 (1.11–1.56) | 1.09 (0.97–1.22) | 1.01 (0.90–1.13) |
| P, late-FHx vs no-FHx | 0.001 | 0.132 | 0.858 |
| pre-FHx | 1.26 (0.95–1.67) | 1.21 (1.02–1.44) | 1.14 (0.98–1.32) |
| P, pre-FHx vs no-FHx | 0.115 | 0.033 | 0.098 |
| Multivariable | |||
| No-FHx | 1 | 1 | 1 |
| Late-FHx | 1.30 (1.10–1.54) | 1.10 (0.98–1.24) | 0.99 (0.89–1.11) |
| P, late-FHx vs no-FHx | 0.002 | 0.094 | 0.887 |
| pre-FHx | 1.19 (0.90–1.58) | 1.20 (1.01–1.43) | 1.09 (0.94–1.27) |
| P, pre-FHx vs no-FHx | 0.228 | 0.040 | 0.248 |
| Log-rank P | 0.091 | <0.0001 | <0.0001 |
CHD indicates coronary heart disease; CVD, cardiovascular disease; no-FHx, no family history of CHD; late-FHx, family history of late-onset CHD; and pre-FHx, family history of premature CHD.
Pre-FHx indicates a diagnosis of coronary artery disease before 50 years of age in a family member. Late-onset family history indicates a diagnosis of coronary artery disease occurring at or after 50 years of age in a family member.
The associations between pre-FHx and CHD, CVD, and all-cause death across the short-term, intermediate-term, and long-term follow-up are also shown in Table 3. pre-FHx was associated with both CVD and CHD mortality across intermediate-term (>10–20 years) and long-term (>20 years) follow-up in both age-adjusted and multivariable-adjusted models, with a similar pattern of results in short-term follow-up but with wider confidence intervals. Secondary analyses with additional adjustment for cardiorespiratory fitness levels demonstrated similar results (data not shown). In addition, we stratified our analyses by high (10-year risk ≥10%) and low (10-year risk <10%) Framingham Risk Score groups. The magnitude of the association between pre-FHx and CHD mortality at >20-year follow-up was similar in the groups with high (hazard ratio, 1.45; 95% confidence interval, 0.89–2.34) and low (hazard ratio, 1.37; 95% confidence interval, 0.91–2.06) Framingham Risk Score but with wider confidence intervals as expected.
Family History and Lifetime Risk of CVD Mortality
For men 55 years of age, the presence of a pre-FHx was associated with a 6.9% higher lifetime risk for CVD mortality (Figure 1). For men 45 years of age, pre-FHx was associated with a 4.7% higher lifetime risk for CVD mortality (Figure 2). Similar findings were observed for men at both ages for lifetime risk for CHD mortality (at 55 years of age, 13.7% with pre-FHx versus 8.9% with no family history; at 45 years of age, 8.1% versus 5.5%). In contrast, there was no apparent difference in the lifetime risk for either CHD or CVD mortality between the no-FHx and late-FHx groups (data not shown).
Figure 1.
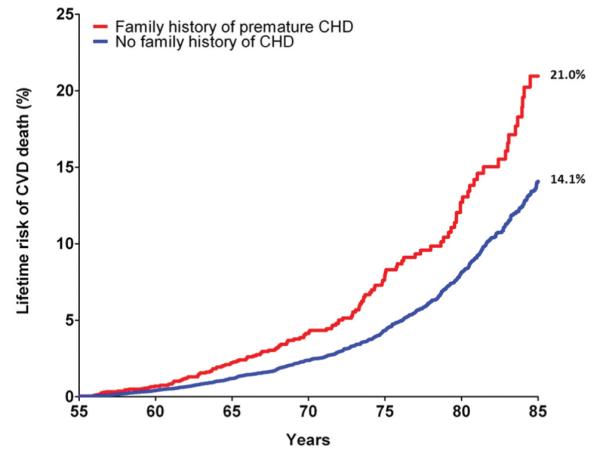
Cumulative incidence of cardiovascular disease (CVD) death adjusted for competing risk (lifetime risk) for men according to family history status at 55 years of age. Family history of premature coronary heart disease (CHD) denotes a CHD event in a first-degree family member before 50 years of age. n=26 447 for patients with attained age ≥55 years.
Figure 2.
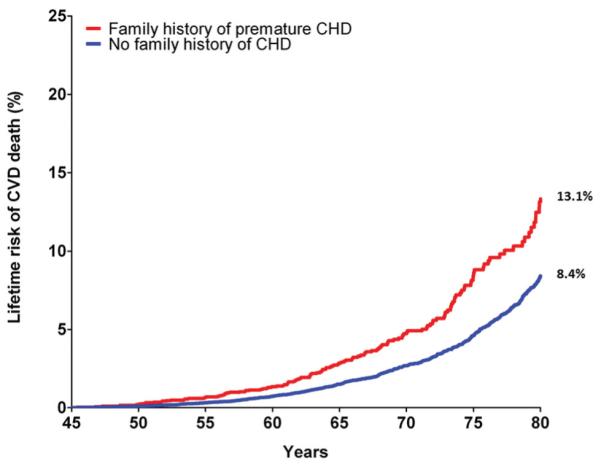
Cumulative incidence of cardiovascular disease (CVD) death adjusted for competing risk (lifetime risk) for men according to family history status at 45 years of age. Family history of premature coronary heart disease (CHD) denotes a CHD event in a first-degree family member before 50 years of age. n=37 036 for patients with attained age ≥45 years.
Discussion
In the present study, we observed several important findings. First, the association between pre-FHx and both CHD and CVD mortality relative to overall mortality was consistent across short-term (0–10 years), intermediate-term (>10–20 years), and long-term (>20 years) follow-up. In contrast, the presence of late-FHx was associated with an increased risk in the short term with no apparent association across later follow-up periods. In addition, the presence of pre-FHx was associated with an ≈5% absolute and 50% relative difference in the lifetime risk for CVD and CHD mortality. These findings suggest that the presence of a pre-FHx of CHD represents a clinically significant increase in CHD and CVD risk across the lifespan.
Current prevention guidelines recommend the use of short-term risk estimation (ie, 10 years) to guide treatment decisions (ie, statin therapy).5,20–25 Although these risk prediction algorithms have some limitations, the ability to improve their performance through the addition of novel risk markers has had only limited success.26 In particular, the addition of pre-FHx to a traditional risk factor model was not associated with a significant improvement in risk classification.4
In contrast, lifetime risk estimation has been proposed as a novel strategy to provide clinically meaningful improvements in risk prediction.12,14,27 Most US adults ≤50 years of age are at low short-term risk for CHD (ie, <10%); however, more than one half have a high lifetime risk.12,28 This discordance reflects the dominant effects of age in short-term risk equations and the sustained contribution of modest risk factor levels on CHD risk across the remaining lifespan.13,29,30 Therefore, extending the time horizon for risk estimation beyond the 10-year window to include the remaining lifespan represents a novel approach to risk estimation that could have important clinical implications.
In the present study, we extend our prior work in long-term risk estimation to include family history, demonstrating that pre-FHx is associated with a 5% absolute and 50% relative difference in the lifetime risk for CVD mortality. Earlier literature from the CCLS11 and from other data sets13,14 suggests that this increment represents a clinically significant difference in lifetime risk and is similar to the effect of a major risk factor. For example, we recently observed that the presence of a major risk factor was associated with a lifetime risk for CVD mortality of 23% compared with a lifetime risk of just 17% among participants without a major risk factor.11 Similarly, compared with participants without a family history, the presence of a pre-FHx was associated with an increased lifetime risk of CVD death (8.4% versus 13.1% at 45 years of age). Thus, when making treatment decisions, clinicians could consider the contribution of a pre-FHx to the lifetime risk of CVD as similar to a major risk factor.
The discordance between the clinical impact on short-term and long-term risk is not unexpected. For example, nonsense mutations in PCSK9 resulting in a 15% reduction in low-density lipoprotein cholesterol were associated with a 47% reduction in the risk of CHD.15 Thus, the more typical 1:1 relationship between cholesterol and CHD risk in clinical trials was increased 3-fold, consistent with the cumulative effects of lower low-density lipoprotein cholesterol across the lifespan. Similarly, sustained exposure to the genetic factors involved in a pre-FHx could be expected to result in a significant, cumulative CHD risk over the lifespan.
Although the association between family history and CHD risk in the short term (ie, ≤10 years) has been well studied,1,6,31–40 relatively limited data are available on the association between family history and long-term risk. In 1 study from the Swedish Twin Registry, the heritability of CHD death was assessed across different follow-up periods.41 An additional 10 years of follow-up was associated with a small but statistically insignificant decrease in the heritability of CHD death (0.66 versus 0.57 in men; 0.44 versus 0.38 in women). These data are consistent with our observations that a pre-FHx was associated with CHD and CVD death across longer-term follow-up.
In contrast to the results for pre-FHx, the association between late-FHx and CHD mortality attenuated over time. This is consistent with prior studies4,6 and likely reflects the heterogeneous nature of self-reported family history, which influences CHD risk through both genetic and environmental mechanisms.33,37,42 Although premature CHD events likely have a greater genetic component, late CHD events may reflect a greater contribution of environmental factors and behaviors that are less heritable. Thus, the risk associated with the likely stronger genetic component of pre-FHx appeared to persist with time, in contrast to the attenuation in risk observed with late-FHx.
Several limitations of our study should be acknowledged. First, the definition of family history of CHD in our study was broad (including grandparents, aunts, and uncles) and was acquired within the context of a clinical study. However, these effects would tend to attenuate the observed association between family history and CHD risk, and a more rigorous definition of family history would be expected to have an even larger effect size. In addition, family history of CHD was obtained through patient report and was not validated. However, in the National Heart, Lung, and Blood Institute Family Heart Study and other cohorts, self-report of a family history of premature CHD has a sensitivity of >80% and a specificity approaching 90%.43–45 In the Newcastle Family History Study, the net bias in recall of family history of CHD was toward the null.46 Therefore, at a minimum, we believe that our data represent conservative estimates on the association between family history and long-term risk.
Second, the prevalence of pre-FHx and late-FHx is lower than in comparative studies4 and likely reflects multiple factors, including the age restrictions in our definition of family history. Data on family size and the specific age at which a family member’s CHD or CVD event occurred were not available in the CCLS database, preventing us from exploring more traditional age cut points. As a result, we were not able to use sex-specific age restrictions with higher age thresholds in women. Although our definition of family history may have resulted in some misclassification, the large sample size and long-term follow-up provided adequate statistical power to assess the association between family history and long-term CHD risk.
Third, the CCLS is a unique cohort of predominantly white participants with a lower risk factor burden and higher socioeconomic status than the general population. Although the prevalence of prior CHD and traditional risk factors is lower,47 the effect of these risk factors is quite similar. For example, we have recently shown that the presence of a major risk factor in middle age in the CCLS is associated with a comparable lifetime risk for CVD in other cohorts within the Lifetime Risk Pooling Project, a pooled analysis of 18 cohorts.11,14 Moreover, we believe that the overall healthy nature of the cohort represents an important strength, illustrating the contribution of family history to an otherwise low-risk population. Finally, our analyses were restricted to men because of the overall low prevalence of family history and the small number of events in women across the different follow-up periods.
Conclusions
We observed a persistent association between pre-FHx and both CHD and CVD mortality across intermediate-term (>10–20 years) and long-term (>20 years) follow-up. We also observed that pre-FHx was associated with a clinically significant increase in the lifetime risk for both CVD and CHD mortality. We believe these findings could be useful to clinicians, providing a broader context with which to consider the effects of a pre-FHx on the lifetime risk of CVD.
CLINICAL PERSPECTIVE.
Sustained exposure to the genetic factors involved in a family history of premature coronary heart disease (CHD) can be expected to result in a significant accumulation of CHD risk over a patient’s lifetime. Although a family history of CHD is a well-described short-term risk factor, data are sparse on the association between family history and long-term risk. Accordingly, current prevention guidelines recommend the use of short-term risk estimation to guide treatment decisions such as statin therapy. In this study involving 49 255 men from the Cooper Center Longitudinal Study and more than 800 000 person-years of follow-up, we investigated the association between premature family history and CHD mortality. After adjusting for traditional risk factors, we observed a persistent association between premature family history and both CHD and cardiovascular disease mortality across short-term (0–10 years), intermediate-term (>10–20 years), and long-term (>20 years) follow-up. We also observed that premature family history was associated with a 50% increase in the lifetime risk for both cardiovascular disease and CHD mortality, approaching that of a major CHD risk factor. We believe that these findings are useful for clinicians and provide a broader context with which to consider the effects of premature family history on the lifetime risk of CHD.
Acknowledgments
We thank Dr Kenneth H. Cooper for establishing the Cooper Center Longitudinal Study, the Cooper Center staff for collecting clinical data, and the Cooper Institute for maintaining the database.
Sources of Funding Dr Berry receives funding from the Dedman Family Scholar in Clinical Care endowment at University of Texas–Southwestern Medical Center; grant K23 HL092229 from the National Heart, Lung, and Blood Institute; and grant 10BG1A4280091 from the American Heart Association. Dr Berry had full access to all the data in the study and had the final responsibility for the decision to submit for publication. All authors have read and agree to the manuscript as written.
Footnotes
Disclosures Dr Berry is a member of the Speaker’s Bureau for Merck & Co. The other authors report no conflicts.
Contributor Information
Justin M. Bachmann, Division of Cardiology, Department of Internal Medicine University of Texas Southwestern Medical Center Dallas, TX.
Benjamin L. Willis, Cooper Institute University of Texas Southwestern Medical Center Dallas, TX.
Colby R. Ayers, Reynolds Cardiovascular Clinical Research Center University of Texas Southwestern Medical Center Dallas, TX.
Amit Khera, Division of Cardiology, Department of Internal Medicine University of Texas Southwestern Medical Center Dallas, TX.
Jarett D. Berry, Division of Cardiology, Department of Internal Medicine University of Texas Southwestern Medical Center Dallas, TX.
References
- 1.Lloyd-Jones DM, Nam BH, D’Agostino RB, Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O’Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291:2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 2.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 3.Andresdottir MB, Sigurdsson G, Sigvaldason H, Gudnason V. Fifteen percent of myocardial infarctions and coronary revascularizations explained by family history unrelated to conventional risk factors: the Reykjavik Cohort Study. Eur Heart J. 2002;23:1655–1663. doi: 10.1053/euhj.2002.3235. [DOI] [PubMed] [Google Scholar]
- 4.Sivapalaratnam S, Boekholdt SM, Trip MD, Sandhu MS, Luben R, Kastelein JJ, Wareham NJ, Khaw KT. Family history of premature coronary heart disease and risk prediction in the EPIC-Norfolk prospective population study. Heart. 2010;96:1985–1989. doi: 10.1136/hrt.2010.210740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 6.Sesso HD, Lee IM, Gaziano JM, Rexrode KM, Glynn RJ, Buring JE. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 2001;104:393–398. doi: 10.1161/hc2901.093115. [DOI] [PubMed] [Google Scholar]
- 7.Qureshi N, Armstrong S, Dhiman P, Saukko P, Middlemass J, Evans PH, Kai J. Effect of adding systematic family history enquiry to cardiovascular disease risk assessment in primary care: a matched-pair, cluster randomized trial. Ann Intern Med. 2012;156:253–262. doi: 10.7326/0003-4819-156-4-201202210-00002. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2251. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 10.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 11.Berry JD, Willis B, Gupta S, Barlow CE, Lakoski SG, Khera A, Rohatgi A, de Lemos JA, Haskell W, Lloyd-Jones DM. Lifetime risks for cardiovascular disease mortality by cardiorespiratory fitness levels measured at ages 45, 55, and 65 years in men: the Cooper Center Longitudinal Study. J Am Coll Cardiol. 2011;57:1604–1610. doi: 10.1016/j.jacc.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry JD, Liu K, Folsom AR, Lewis CE, Carr JJ, Polak JF, Shea S, Sidney S, O’Leary DH, Chan C, Lloyd-Jones DM. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the coronary artery risk development in young adults study and multi-ethnic study of atherosclerosis. Circulation. 2009;119:382–389. doi: 10.1161/CIRCULATIONAHA.108.800235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd-Jones DM, Leip EP, Larson MG, D’Agostino RB, Beiser A, Wilson PW, Wolf PA, Levy D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 14.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 16.Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, Blair SN. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality: a prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 18.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 19.Beiser A, D’Agostino RB, Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham study: the practical incidence estimators (PIE) macro. Stat Med. 2000;19:1495–1522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.McPherson R, Frohlich J, Fodor G, Genest J, Canadian Cardiovascular Society Canadian Cardiovascular Society position statement: recommendations for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease. Can J Cardiol. 2006;22:913–927. doi: 10.1016/s0828-282x(06)70310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D’Agostino RB, Levy D. Framingham Risk Score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94:20–24. doi: 10.1016/j.amjcard.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 22.de Visser CL, Bilo HJ, Thomsen TF, Groenier KH, Meyboom-de Jong B. Prediction of coronary heart disease: a comparison between the Copenhagen Risk Score and the Framingham Risk Score applied to a Dutch population. J Intern Med. 2003;253:553–562. doi: 10.1046/j.1365-2796.2003.01137.x. [DOI] [PubMed] [Google Scholar]
- 23.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ. 2007;335:136. doi: 10.1136/bmj.39261.471806.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodward M, Brindle P, Tunstall-Pedoe H. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN Score from the Scottish Heart Health Extended Cohort (SHHEC) Heart. 2007;93:172–176. doi: 10.1136/hrt.2006.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tunstall-Pedoe H. Cardiovascular risk and risk scores: ASSIGN, Framingham, QRISK and others: how to choose. Heart. 2011;97:442–444. doi: 10.1136/hrt.2010.214858. [DOI] [PubMed] [Google Scholar]
- 26.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pencina MJ, D’Agostino RB. Evaluation of the Framingham Risk Score in the European Prospective Investigation of Cancer-Norfolk Cohort: invited commentary. Arch Intern Med. 2008;168:1216–1218. doi: 10.1001/archinte.168.11.1216. [DOI] [PubMed] [Google Scholar]
- 28.Marma AK, Lloyd-Jones DM. Systematic examination of the updated Framingham Heart Study general cardiovascular risk profile. Circulation. 2009;120:384–390. doi: 10.1161/CIRCULATIONAHA.108.835470. [DOI] [PubMed] [Google Scholar]
- 29.Cavanaugh-Hussey MW, Berry JD, Lloyd-Jones DM. Who exceeds ATP-III risk thresholds? Systematic examination of the effect of varying age and risk factor levels in the ATP-III risk assessment tool. Prev Med. 2008;47:619–623. doi: 10.1016/j.ypmed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry JD, Lloyd-Jones DM, Garside DB, Greenland P. Framingham Risk Score and prediction of coronary heart disease death in young men. Am Heart J. 2007;154:80–86. doi: 10.1016/j.ahj.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nadir MA, Struthers AD. Family history of premature coronary heart disease and risk prediction. Heart. 2011;97:684. doi: 10.1136/hrt.2011.222265. [DOI] [PubMed] [Google Scholar]
- 32.Pohjola-Sintonen S, Rissanen A, Liskola P, Luomanmaki K. Family history as a risk factor of coronary heart disease in patients under 60 years of age. Eur Heart J. 1998;19:235–239. doi: 10.1053/euhj.1997.0543. [DOI] [PubMed] [Google Scholar]
- 33.Colditz GA, Rimm EB, Giovannucci E, Stampfer MJ, Rosner B, Willett WC. A prospective study of parental history of myocardial infarction and coronary artery disease in men. Am J Cardiol. 1991;67:933–938. doi: 10.1016/0002-9149(91)90163-f. [DOI] [PubMed] [Google Scholar]
- 34.Schildkraut JM, Myers RH, Cupples LA, Kiely DK, Kannel WB. Coronary risk associated with age and sex of parental heart disease in the Framingham study. Am J Cardiol. 1989;64:555–559. doi: 10.1016/0002-9149(89)90477-3. [DOI] [PubMed] [Google Scholar]
- 35.Colditz GA, Stampfer MJ, Willett WC, Rosner B, Speizer FE, Hennekens CH. A prospective study of parental history of myocardial infarction and coronary heart disease in women. Am J Epidemiol. 1986;123:48–58. doi: 10.1093/oxfordjournals.aje.a114223. [DOI] [PubMed] [Google Scholar]
- 36.Shea S, Ottman R, Gabrieli C, Stein Z, Nichols A. Family history as an independent risk factor for coronary artery disease. J Am Coll Cardiol. 1984;4:793–801. doi: 10.1016/s0735-1097(84)80408-8. [DOI] [PubMed] [Google Scholar]
- 37.Barrett-Connor E, Khaw K. Family history of heart attack as an independent predictor of death due to cardiovascular disease. Circulation. 1984;69:1065–1069. doi: 10.1161/01.cir.69.6.1065. [DOI] [PubMed] [Google Scholar]
- 38.Heller RF, Kelson MC. Family history in “low risk” men with coronary heart disease. J Epidemiol Community Health. 1983;37:29–31. doi: 10.1136/jech.37.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sholtz RI, Rosenman RH, Brand RJ. The relationship of reported parental history to the incidence of coronary heart disease in the Western Collaborative Group Study. Am J Epidemiol. 1975;102:350–356. doi: 10.1093/oxfordjournals.aje.a112171. [DOI] [PubMed] [Google Scholar]
- 40.Slack J, Evans KA. The increased risk of death from ischaemic heart disease in first degree relatives of 121 men and 96 women with ischaemic heart disease. J Med Genet. 1966;3:239–257. doi: 10.1136/jmg.3.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zdravkovic S, Wienke A, Pedersen NL, Marenberg ME, Yashin AI, De Faire U. Heritability of death from coronary heart disease: a 36-year follow-up of 20 966 Swedish twins. J Intern Med. 2002;252:247–254. doi: 10.1046/j.1365-2796.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- 42.Hippe M, Vestbo J, Bjerg AM, Borch-Johnsen K, Appleyard M, Hein HO, Andersen PK, Jensen G, Sorensen TI. Cardiovascular risk factor profile in subjects with familial predisposition to myocardial infarction in Denmark. J Epidemiol Community Health. 1997;51:266–271. doi: 10.1136/jech.51.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murabito JM, Nam BH, D’Agostino RB, Sr, Lloyd-Jones DM, O’Donnell CJ, Wilson PW. Accuracy of offspring reports of parental cardiovascular disease history: the Framingham Offspring Study. Ann Intern Med. 2004;140:434–440. doi: 10.7326/0003-4819-140-6-200403160-00010. [DOI] [PubMed] [Google Scholar]
- 44.Li R, Bensen JT, Hutchinson RG, Province MA, Hertz-Picciotto I, Sprafka JM, Tyroler HA. Family risk score of coronary heart disease (CHD) as a predictor of CHD: the Atherosclerosis Risk in Communities (ARIC) study and the NHLBI Family Heart Study. Genet Epidemiol. 2000;18:236–250. doi: 10.1002/(SICI)1098-2272(200003)18:3<236::AID-GEPI4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 45.Kee F, Tiret L, Robo JY, Nicaud V, McCrum E, Evans A, Cambien F. Reliability of reported family history of myocardial infarction. BMJ. 1993;307:1528–1530. doi: 10.1136/bmj.307.6918.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silberberg JS, Wlodarczyk J, Fryer J, Ray CD, Hensley MJ. Correction for biases in a population-based study of family history and coronary heart disease: the Newcastle Family History Study I. Am J Epidemiol. 1998;147:1123–1132. doi: 10.1093/oxfordjournals.aje.a009410. [DOI] [PubMed] [Google Scholar]
- 47.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]


