Abstract
Children born preterm are at risk for deficits in language and reading. They are also at risk for injury to the white matter of the brain. The goal of this study was to determine whether performance in language and reading skills would be associated with white matter properties in children born preterm and full-term. Children born before 36 weeks gestation (n=23, mean±SD age 12.5±2.0 years, gestational age 28.7±2.5 weeks, birth weight 1184±431 g) and controls born after 37 weeks gestation (n=19, 13.1±2.1 years, 39.3±1.0 weeks, 3178±413 g) underwent a battery of language and reading tests. Diffusion Tensor Imaging (DTI) scans were processed using Tract-Based Spatial Statistics to generate a core white matter skeleton that was anatomically comparable across participants. Fractional anisotropy (FA) was the diffusion property used in analyses. In the full-term group, no regions of the whole FA-skeleton were associated with language and reading. In the preterm group, regions of the FA-skeleton were significantly associated with verbal IQ, linguistic processing speed, syntactic comprehension, and decoding. Combined, the regions formed a composite map of 22 clusters on 15 tracts in both hemispheres and in the ventral and dorsal streams. ROI analyses in the preterm group found that several of these regions also showed positive associations with receptive vocabulary, verbal memory, and reading comprehension. Some of the same regions showed weak negative correlations within the full-term group. Exploratory multiple regression in the preterm group found that specific white matter pathways were related to different aspects of language processing and reading, accounting for 27–44% of the variance. The findings suggest that higher performance in language and reading in a group of preterm but not full-term children is associated with higher fractional anisotropy of a bilateral and distributed white matter network.
Keywords: Language, Reading, Prematurity, Preterm, DTI, White matter
1. Background
Approximately 13% of the children in the United States are born prematurely (Allen, 2008), and these children are at elevated risk of neurodevelopmental disability (Fletcher et al., 1997; Hack, 2006; Msall & Tremont, 2002; Ornstein, Ohlsson, Edmonds, & Asztalos, 1991; Rose & Feldman, 1996; Rose et al., 2009; Strang-Karlsson et al., 2010). Among those born before 32 weeks gestation or weighing less than 1500 g, major disabilities, including cerebral palsy, sensory impairments, and/or intellectual disability affect approximately 20% of survivors (Mikkola et al., 2005), and less severe disabilities, including language-based learning disabilities affect approximately 50% of survivors (Aylward, 2002; Grunau, Whitfield, & Davis, 2002; Hille et al., 1994). Children born late preterm, between 32 and 36 weeks gestation, also have poorer school outcomes than children born at term (Chyi et al., 2008).
Many studies report that children born preterm have deficits in the domains of language and reading. For example, compared to full-term children, those born preterm have shown deficits in (1) vocabulary and semantics, including vocabulary size and quality of word use (Foster-Cohen, Friesen, Champion, & Woodward, 2010; Guarini et al., 2009; Van Lierde, Roeyers, Boerjan, & De Groote, 2009); (2) morpho-syntax, including morphological and syntactic complexity (Foster-Cohen et al., 2010; Guarini et al., 2009) and comprehension of ‘wh’-questions and passive sentences (Van Lierde et al., 2009); (3) verbal processing speed (Anderson et al., 2003; Lee, Yeatman, Luna, & Feldman, 2011b); and (4) verbal memory (Caldu et al., 2006; Foster-Cohen et al., 2010;Lee, Yeatman, Luna, & Feldman, 2011a; Van Lierde et al., 2009). In addition, in comparison to full term peers, children born preterm have poorer scores on tests of reading (Anderson et al., 2003; Andrews et al., 2010; Hack et al., 1994; O'Callaghan et al., 1996) and slower reading speed (Guarini et al., 2009). Recent meta-analyses find that differences between preterm and full-term children range from .38 to .77 standard deviations (Barre, Morgan, Doyle, & Anderson, 2011), persist after controlling for socioeconomic status, and increase between ages 3 and 12 years (van Noort-van der Spek, Franken, & Weisglas-Kuperus, 2012).
The overall goal of the current study was to determine whether performance on assessments of language and reading would be associated with properties of white matter as measured by Diffusion Tensor Imaging (DTI) in a sample of older children and adolescents born preterm and full-term. Children born pre-term are at risk for injury to the white matter of the brain (Back, Riddle, & McClure, 2007; Volpe, 2009). Using modern neuroima-ging techniques, properties of white matter in children born preterm have been associated with neurodevelopmental outcomes (Ment, Hirtz, & Huppi, 2009). Using DTI, recent studies have found that in both adults and typically developing children, specific white matter connections are associated with language (Friederici & Friederici, 2009) and reading abilities (Ben-Shachar, Dougherty, & Wandell, 2007). Below we will first review the literature on white matter in the preterm population and then turn to findings and theories of white matter characteristics in relation to language and reading skills in full-term individuals. From this literature review, we will formulate specific hypotheses for the analyses in this study.
1.1. White matter characteristics after preterm birth
Adverse outcomes in children born prematurely, particularly those born between 24 and 32 weeks gestation, have been attributed to injury to the cerebral white matter and associated neuronal and axonal abnormalities (Back et al., 2007; Fletcher, Bohan, Brandt, & al, 1992; Kinney, 2006; Luciana, 2003; Nagy et al., 2003; Soria-Pastor et al., 2008; Vangberg et al., 2006; Volpe, 2009; Yung et al., 2007). The explanation for the injury is that in the early third trimester of pregnancy, white matter is populated by precursors of the oligodendrocytes, the cells that in their subsequent maturity produce myelin. These precursors are especially vulnerable to hypoxia, ischemia, and inflammation, complications of preterm birth (Peterson, 2003). Injury to the precursors appears to cause irreparable damage to the oligodendrocyte cell line and ultimately to the amount or to health of white matter tracts.
Until the modern era, white matter injuries after prematurity were only identified on post-mortem histology and consisted of large or multiple cysts in periventricular regions. With the advent of magnetic resonance imaging (MRI), the injuries could be detected in vivo. Improvements in neonatal care have reduced the prevalence of cystic injuries (Volpe, 2009). Nonetheless, subtle indices of non-cystic injury are still very common. Conventional MRI scans show qualitative structural abnormalities in white matter, including thinning of the corpus callosum (Maalouf et al., 1999) and diffuse regions of high signal echodensity (Maalouf et al., 2001).
DTI is a relatively new MRI technique that enables quantitative analysis of the white matter of the brain. DTI capitalizes on the differential patterns of water diffusion in different brain compartments: isotropic diffusion (equal in all directions) occurs in cerebral spinal fluid and cell bodies and anisotropic diffusion (greater in one direction than the other directions) occurs in axons sheathed in myelin. Fractional anisotropy (FA) is a DTI measure that reflects the directional coherence of axons. High FA values (closer to 1) indicate greater directionality and coherence, dense axonal packing, and large diameter axons. Elevated FA has been associated with maturity (Asato, Terwilliger, Woo, & Luna, 2010; Paus et al., 2001; Schmithorst, Wilke, Dardzinski, & Holland, 2002). Reduced FA values have been found in clinical conditions, such as multiple sclerosis as evidence of injury (Ge et al., 2004). However, the interpretation of FA is not straightforward. Elevated FA has been found among individuals in clinical populations which impaired connectivity was assumed, including Williams syndrome (Arlinghaus, Thornton-Wells, Dykens, & Anderson, 2011; Hoeft et al., 2007) and in Attention-Deficit/Hyperactivity disorder (Davenport, Karatekin, White, & Lim, 2010).
Compared to controls, children born preterm have been found to have relatively low FA in many different brain regions, including the corpus callosum, superior longitudinal fasciculus, and inferior frontal-occipital fasciculus. The reduction in FA can be documented through adolescence (Mullen et al., 2011; Nagy et al., 2003; Vangberg et al., 2006) and into adulthood (Kontis et al., 2009). However, not all studies of preterm and full-term participants find group differences in FA (Allin et al., 2011; Counsell et al., 2006; Feldman et al., 2012; Frye et al., 2010). In the sample we are reporting on here, we found that FA was not lower in the preterm than full-term group.
Even in the absence of cystic white matter lesions, measures of white matter, such as FA, have been shown to correlate with neurodevelopmental outcomes in children born preterm, including motor skills (Adams et al., 2010; Berman et al., 2005), attention (Nagy et al., 2003; Skranes et al., 2007), and executive function (Frye et al., 2009; Skranes et al., 2009). The present study examines language and reading skills in relation to white matter characteristics in a sample of children and adolescents born preterm compared to children born at term.
1.1.1. Associations of language skills after prematurity and white matter characteristics
Studies of language in children born preterm have documented associations between measures of language performance and properties of white matter. A qualitative clinical measure of the extent of white matter injury on MRI scans that were obtained near term age (Woodward, Anderson, Austin, Howard, & Inder, 2006) was associated with language outcomes at age 4 years (Foster-Cohen et al., 2010). Correlations between right temporal white matter volume and verbal memory skills were found in a combined sample of preterm and full-term children at age 12 years (Fraello et al., 2011). Positive correlations between verbal IQ and verbal fluency scores and two measures of the corpus callosum—mid-sagittal size and mid-posterier surface area—were found in a sample of 15-year olds born preterm and full-term, but only among the males (Nosarti et al., 2004). Conversely, in another study, the size of the corpus callosum was associated with performance IQ and memory but not with verbal IQ or other language measures (Caldu et al., 2006).
Associations have also been found between language measures and white matter characteristics using DTI. Verbal IQ and vocabulary were associated with FA values within the left and right anterior uncinate fasciculus in preterm children at age 12 (Constable et al., 2008). In another study, compared to full-term participants, preterm subjects had lower fractional anisotropy (FA) values in multiple regions including bilateral uncinate fasciculi, bilateral external capsules, the splenium of the corpus callosum, and white matter serving the inferior frontal gyrus bilaterally (Mullen et al., 2011). FA values in both the left and right uncinate fasciculi correlated with receptive vocabulary scores.
1.1.2. Associations of reading skills after prematurity and white matter characteristics
Reading abilities in children born preterm have also been associated with measures from DTI. In a group of preterm and full-term children and adolescents 9–16 years of age, performance on single word reading was significantly correlated with FA in the genu and body of the corpus callosum, and performance on reading comprehension was associated bilaterally with the temporal–parietal junction and the corpus callosum (Andrews et al., 2010). In a group of 15-year old preterm and full-term adolescents, FA and volume of the left superior longitudinal fasciculus were associated with letter-word identification and phoneme reversal (Frye et al., 2010). Another study of 16-year olds found that FA values in the left and right arcuate fasciculi correlated with a phonological task in the preterm subjects only (Mullen et al., 2011). Based on the findings of bilateral dorsal correlations for the preterm group, the authors of that paper concluded that prematurely-born subjects rely more heavily on the right hemisphere than do typically developing full-term peers in performing phonological language or reading tasks (Mullen et al., 2011). A similar alteration in the expected laterality of spelling functions was also found in an adolescent preterm sample (Scott et al., 2011). Consistent with the structural findings of altered connectivity, Gozzo and colleagues (2009) reported that functional connectivity in the language network was abnormal in a sample of children born preterm with low language skills; they had stronger connections between Wernicke's area and the right inferior frontal gyrus, and between bilateral superior marginal gyri than did the full-term controls.
1.2. Associations of language and white matter properties in full-term healthy children
Much of what we know about the neural basis of language and reading in the typically developing child stems from functional activation studies of gray matter regions during relevant tasks. Such studies generally find bilateral activation during auditory language processing and left hemisphere activation for expressive language and reading (Cabeza & Nyberg, 2000). Increasingly, interest has focused on the neural systems or circuits that link the different cortical areas activated during language tasks, particularly in the left hemisphere. White matter fascicles comprise these circuits.
Two systems have been proposed for the language network (Hickok & Poeppel, 2004). The ventral stream projects from auditory cortex in a ventral and lateral direction, projecting into the cortex of the temporal lobe. The ventral stream is generally conceptualized to allow linkage of speech sound information with conceptual knowledge. White matter tracts that could carry signals along the ventral stream include the inferior fronto-occipital, inferior longitudinal, and uncinate fasciculi. The inferior fronto-occipital fasiculus actually begins in the occipital lobe and courses to the frontal lobe, allowing visual information to integrate with speech sound information and conceptual knowledge. The dorsal stream projects from posterior superior temporal lobe or the temporal–parietal junction in the dorsal direction, initially posteriorly toward the parietal lobe and ultimately anteriorly to the frontal lobes. The nature of processing along this stream is conceptualized variably, but may link auditory representations of speech to motor representations (Hickok & Poeppel, 2004). White matter tracts that could carry signals along the dorsal stream include the superior longitudinal and arcuate fasciculi.
Many studies have emphasized the role of the dorsal stream, particularly in the left hemisphere, in normal language function (Catani, Jones, & ffytche, 2005; Glasser & Rilling, 2008). However, recent evidence suggests that the ventral route may be important in children. Brauer and colleagues (Brauer, Anwander, & Friederici, 2011) correlated functional MRI (fMRI) with DTI tractography results. In adults, they found functional activation in the pars opercularis of Broca's area that corresponded to the termination of the arcuate and superior longitudinal fasciculi. In children, they found functional activation in the pars opercularis and pars triangularis, beyond the termination of the arcuate and superior longitudinal fasciculi. Based on this wide area of activation, the authors inferred that connections from the temporal to frontal lobes in children also travel along the ventral pathway. These findings suggest that in full-term children and adolescents, measures of language ability may correlate with measures of both the dorsal and ventral pathways, particularly in the left hemisphere.
1.3. Associations of reading skills and white matter in full-term individuals
Klingberg and colleagues compared DTI scans of adults who were good and poor readers and detected bilateral differences in the white matter of a region at the temporal–parietal junction (Klingberg et al., 2000). Similar findings have since been replicated in children; lower FA in this region of the left hemisphere is associated with lower reading and rapid naming scores (Ben-Shachar et al., 2007; Deutsch et al., 2005). Though initially the regions were assumed to be on the arcuate fasciculus, some studies found the fibers in that area to be oriented in the inferior– superior direction, suggesting that the associated tracts may be part of the corona radiata (Niogi & McCandliss, 2006,Odegard, Farris, Ring, McColl, & Black, 2009). Interestingly, a recent DTI study of children 7–11 years of age using sophisticated analytic methods found that measurements of diffusivity in the left arcuate were negatively correlated with phonological awareness (Yeatman et al., 2011). In addition, FA in the right hemisphere has been associated with reading; decoding and single word reading were associated with right inferior fronto-occipital and inferior longitudinal fasciculus as well as the right posterior limb of the internal capsule and right superior (Odegard, 2009). FA in the right superior longitudinal fasciculus, including arcuate fasciculus, significantly predicted future reading gains in adolescents with poor reading skills (Hoeft et al., 2011).
Associations of phonological awareness and properties of the temporal connections of the corpus callosum have also been found. In this case, the FA of good readers has been consistently lower than that of the poor readers (Dougherty et al., 2007; Frye et al., 2008; Odegard et al., 2009). Various interpretations of this finding is that good readers may have fewer but larger axons connecting the temporal lobes, their axon membranes may be more permeable than the membranes of poor readers, or good readers may use the bilateral connections less actively than do poor readers.
1.4. Laterality of white matter tracts
Language and reading abilities are lateralized in terms of function and also in terms of gray matter structure. Recent studies have also demonstrated laterality of white matter. In adults, significant rightward asymmetry in parallel diffusion was found in the parietal and frontal lobes and significant leftward asymmetry in the temporal and occipital lobes (Iwabuchi et al., 2011). In children, lateralization of white matter tracts, including the arcuate fasciculus, begins early in life (Bonekamp et al., 2007; Dubois et al., 2009; Iwabuchi et al., 2011). Left lateralization of white matter properties has been found to be weakly associated with receptive vocabulary and with phonological processing; left lateralization was associated with higher performance (Lebel & Beaulieu, 2009).
1.5. Hypotheses and study contributions
Based on this review of the literature, we anticipated that in this sample of preterm and full-term children and adolescents, we would find a widely distributed white matter network associated with clinical measures of language. In the preterm group, we specifically hypothesized we would find positive associations between measures of verbal IQ (Constable et al., 2008), verbal memory (Fraello et al., 2011), and receptive vocabulary (Constable et al., 2008; Mullen et al., 2011) and white matter properties in the ventral pathways of both hemispheres. In addition, we hypothesized that we would find similar associations between white matter and other language measures, such as syntactic comprehension and linguistic processing speed, functions not previously assessed in relation to DTI. By contrast, in the full-term group, we hypothesized that we would find that these language measures, especially syntactic comprehension, would be associated with dorsal and ventral tracts of the left hemisphere (Brauer et al., 2011).
We further anticipated that in this sample of preterm and full-term children and adolescents, we would find positive associations between measures of single word reading and FA in multiple areas of white matter. Based on the findings in preterm participants, in the preterm group, we hypothesized that we would find associations between single word reading and white matter properties in the dorsal pathways of the left (Richard E. Frye et al., 2010) and right hemisphere (Mullen et al., 2011) and the corpus callosum (Andrews et al., 2010). By contrast, in the full-term group, we hypothesized that we would find single word reading to be positively associated with left hemisphere dorsal tracts and negatively associated with regions of the corpus callosum (Dougherty et al., 2007; Odegard et al., 2009). We anticipated a broadly distributed network associated with reading comprehension, another function not previously explored in relation to DTI. Finally, we assessed the degree of white matter laterality to assess whether group differences in brain-behavior associations could be due attributed to differences in laterality in the preterm and full-term groups.
This study contributes to the previous literature in several ways. First, we used a large battery of measures in order to assess different subdomains of both language and reading in relation to white matter (Lee et al., 2011b). The use of multiple measures allowed us to determine whether specific areas on specific tracts were associated with a single outcome or with multiple outcomes. Second, we used a different but established method to analyze white matter from DTI. Tract-based spatial statistics identifies the core of white matter tracts throughout the brain. Its advantage is that it creates a core white matter skeleton that is anatomically equivalent across participants; it thereby minimizes differences across groups based on the differences in the ratio of gray and white matter within tracts that can arise in other whole-brain analysis methods. It also sets a high threshold for significance because it looks for associations throughout the white matter skeleton and corrects for multiple comparisons. Third, when multiple tracts were associated with an outcome measure, we conducted a regression analysis that allowed us to determine whether the different tracts each made an independent contribution to the outcome of interest. Finally, we considered whether structural laterality differences in the white matter between the preterm and full-term groups could explain any differences in the associations of language or reading and FA.
2. Methods
2.1. Participants
Participants were 9–16 years old and enrolled in the Palo Alto CA site of a larger multi-site study of prematurity outcomes (N=100) (Lee et al., 2011b; Loe, Lee, Luna, & Feldman, 2011; Loe, Lee, Luna, & Feldman, 2012). This study reports on participants who underwent MRI scanning at Stanford University. The Stanford University institutional review board approved this study. A parent provided informed consent; participants provided assent.
Preterm subjects were born at <36 weeks gestation with birth weight <2500 g. Controls were born ≥37 weeks. Exclusion criteria for all participants included seizure disorder; hydrocephalus; receptive vocabulary score <70; sensorineural hearing loss; and non-English speaker. Controls were excluded for identified language, learning, or psychiatric disorders on a screening interview because these conditions have been associated with abnormalities on brain imaging (Casey, Nigg, & Durston, 2007; Freitas-Ferrari et al., 2010; Hoeft et al., 2007). Three preterm subjects were excluded from analysis because of extremely enlarged ventricles that interfered with the analysis of white matter tracts.
Demographic data for 23 preterm and 19 control subjects in the sample are presented in Table 1. Age (preterm mean=12.5 years, S.D.=2.0; full-term mean=13.1 years, S.D.=2.1) and gender (pre-term % males 47.8, full-term % males 42.1) were not statistically different. Maternal education, the index for socioeconomic status (SES), was dichotomized: “low” was defined as less than a college degree and “high” as at least a college degree. The preterm and full-term groups did not differ significantly in level of maternal education. By design there was a group difference in gestational age, p<.001, and birth weight, p<.001. One control and two preterms were left-handed, and two controls and two preterms were ambidextrous.
Table 1.
Demographics and mean scores (standard deviation) of outcome measures (N=42).
| Measures | Preterm (n=23) | Control (n=19) | χ2 or t |
|---|---|---|---|
| Boys—n (%) | 11 (47.8) | 8 (42.1) | .14 |
| Low maternal education—n (%) | 3 (13.0) | 5 (26.3) | 1.19 |
| Mean age in years | 12.5 (2.0) | 13.1 (2.1) | .90 |
| Mean gestational age in weeks | 28.7 (2.5) | 39.3 (1.0) | 18.41*** |
| Mean birth weight in grams | 1184 (431) | 3178 (413) | 15.20*** |
| Performance IQ, WASIa, SSb | 107.0 (14.7) | 109.4 (11.8) | .58 |
| Verbal IQ, WASI, SS | 108.7 (16.6) | 115.3 (16.3) | 1.31 |
| Receptive vocabulary, PPVT-IIIc, SS | 111.3 (18.5) | 110.2 (12.8) | −.21 |
| Verbal memory, CELF-4d Language Memory Index, SS | 104.6 (15.8) | 107.8 (9.8) | .82 |
| Linguistic processing speed, TROG-Re reaction time, milliseconds | 2136 (374) | 1956 (327) | −1.64 |
| Syntactic comprehension, sentence comprehension, error z-score | .47 (1.45) | .14 (.90) | −.86 |
| Decoding, WJ-IIIf Basic Reading Skills Cluster, SS | 103.4 (13.2) | 108.0 (9.3) | 1.26 |
| Reading comprehension, WJ-III Passage Comprehension, SS | 102.8 (12.9) | 109.8 (14.2) | 1.67 |
p<.001.
WASI=Wechsler Abbreviated Scale of Intelligence.
SS=standard score.
PPVT-III=Peabody Picture Vocabulary Test - 3rd Edition.
CELF-4=Clinical Evaluation of Language Fundamentals—4th Edition.
TROG-R=Test for Reception of Grammar—Revised.
WJ-III=Woodcock—Johnson Tests of Achievement—3rd Edition.
Medical complications at birth in the preterm group were: 4 with abnormal findings on head ultrasounds or MRIs (≥grade 2 intraventricular hemorrhage, echodensities, or cystic lesions), two with mildly abnormal findings (grade 1 hemorrhage or choroid plexus cyst); 13 had respiratory distress syndrome, five developed bronchopulmonary dysplasia (BPD) or chronic lung disease; none had necrotizing enterocolitis; and two were small for gestational age (≤3rd percentile birth weight for gestational age).
Of the 23 preterm subjects, 9 had mildly abnormal findings on T1 images, including mildly enlarged ventricles (n=8), minimal to mild ventricular asymmetry (n=8, of whom 6 had a larger left ventricle), irregular ventricular margins (n=4), signs of cerebellar abnormality (n=2), cavum septum vergae (n=2), and a thin corpus callosum (n=1). Of the 19 control subjects, two had minimal ventricular asymmetry (one had a larger left ventricle, and the other had a larger right ventricle), and one of these two had mildly enlarged ventricles.
2.2. Behavioral measures
Subjects underwent two testing sessions to assess language and reading skills. Assessments and standard scores were based on birth date.
2.2.1. Verbal IQ
The Wechsler Abbreviated Scale of Intelligence (WASI) is a widely used, nationally standardized test of general intellectual ability (Horn, 1995; Kaufman, 1994; Wechsler, 1999; Richard W. Woodcock, 1990). We used the Verbal IQ, which is composed of Vocabulary and Similarities subtests and assesses verbal intelligence. Reliability coefficients on the test range from .90 to .98 for Vocabulary, .84 to .96 for Similarities. The reliability coefficients for the scale scores range from .92 to .98 for Verbal IQ. The four-subtest version of the WASI correlates highly (.92) with full-scale IQ tests.
2.2.2. Receptive and expressive language skills
The Comprehensive Evaluation of Language Fundamentals— Fourth Edition (CELF-4) is a norm-referenced test for the identification, diagnosis, and follow-up evaluation of language and communication disorders in students 5–21 years old (Semel, Wiig, & Secord, 2003). The Receptive Language Index (RLI) and Expressive Language Index (ELI) from the CELF-4 were generated. The RLI is a measure of listening and auditory comprehension. For ages 9–12 years, the RLI is composed of Word Classes 2-Receptive and Concepts and Following Directions; for ages 13–21 years, the RLI is composed of Word Classes 2-Receptive, Semantic Relationships, and Understanding Spoken Paragraphs. For all age groups, the ELI is a measure of language production and is composed of Word Classes 2-Expressive, Formulated Sentences, and Recalling Sentences. The stability coefficients range from .71 to .86 for subtests and .88 to .92 for the composite scores. The test has high sensitivity and specificity in detecting language disorders.
2.2.3. Receptive vocabulary
Peabody Picture Vocabulary Test—Third Edition (PPVT-III) is a widely used test of receptive vocabulary that generates a standard score (Dunn & Dunn, 1997). Each item consists of four black-and-white drawings on a page. Subjects are asked to identify which of the four illustrations best represents the stimulus word presented orally by the examiner. Reliability varies by age group, falls between .92 and .98 and has strongly positive correlations with tests of verbal intelligence.
2.2.4. Syntactic comprehension
Two tests of on-line sentence comprehension were combined to generate a score for syntactic comprehension. First, the Test for Reception of Grammar—Version Two (TROG-2) is a computerized measure that assesses syntactic comprehension by presenting sentences in the auditory mode and using a four picture multiple choice format with lexical and grammatical foils (Bishop, 2003). We chose this test because it is a pure measure of syntax, and it generates both an error score and a processing speed. The vocabulary is simple and familiar to school-aged children. Subjects can press a button to hear the sentence as many times as necessary. The test, consisting of 80 items, organized into 20 four-sentence blocks of increasing syntactic difficulty, is designed to tap grammatical skills of school-aged children and adolescents. The error score is the total number of errors out of 80 items. Standardization of the paper form of the test was carried out in Britain; split half reliability was .77 for children age 7–8 years old. Second, we also created sentence-picture verification tasks that consist of the oral presentation of the same sentences from the TROG-2 followed by the presentation of a single colored picture. The vocabulary is very simple so that the emphasis is on the interpretation of syntax. Subjects decide whether the picture matches the sentence by pressing a yes or no button. We used both measures to increase the variance in syntactic comprehension. Because syntactic comprehension errors correlated with age, an age-adjusted z-score was calculated for each subject. In pilot testing, we found that the accuracy and reaction time on the sentence verification tests were highly associated with age and with group as indicators of validity.
2.2.5. Linguistic processing speed
The TROG-2 results were used for this measure. The reaction time for each item is the time it took for subjects to respond to the sentence by selecting the matching picture. The mean reaction time was calculated from correct trials only. We also excluded trials in which the subject repeated the sentence presentation in order to eliminate from analysis distractions and re-checked responses of very high-functioning children.
2.2.6. Verbal memory
The Language Memory Index (LMI) from the CELF-4 provides a measure of the ability to apply working memory to linguistic content and structure. It is composed of the following subtests: Formulated Sentences, Recalling Sentences, as well as Concepts and Following Directions for ages 9–12 and Semantic Relationships for ages 13–21.
2.2.7. Single word reading or decoding
The Woodcock–Johnson III Tests of Achievement (WJ-III) assesses reading abilities (Woodcock, McGrew, & Mather, 2001). The Basic Reading Skills Cluster assesses decoding skills, the ability to translate written letters into speech sounds and recognize written words, and is a composite score of two subtests: Letter-Word Identification (single word reading) and Word Attack (pseudoword reading). Reliability of Letter-Word Identification is .94 and Word Attack is .87. The cluster score has higher reliability than the subtest scores.
2.2.8. Reading comprehension
The WJ-III Passage Comprehension subtest (Woodcock et al., 2001) assesses reading comprehension skills. Reliability of Passage Comprehension is .88.
2.3. MRI protocol
MRI data were acquired on a 3T Signa Excite (GE Medical Systems, Milwaukee, WI) at Stanford University. Two high resolution IR-prep 3D FSPGR scans (FOV=24 × 18 cm, matrix size= 260 × 192, .9 mm slices, TI=300 ms, flip angle = 15°, 1 NEX) and one IR-prep 3D FSPGR scan (FOV=24 × 15.6 cm, matrix size= 256 × 192, 1.2 mm slices, TI=300ms, flip angle=15°, 1 NEX) were collected. The three T1 images were averaged. They were coregistered based on a mutual information algorithm (SPM5, http://www.fil.ion.ucl.ac.uk/spm/). A trained experimenter manually identified the anterior and posterior commissures and mid-sagittal plane, and these points were used to put the image in a canonical orientation.
For DTI, a diffusion-weighted, single-shot, spin-echo, echo-planar imaging sequence (TE=80 ms, TR=6500 ms, FOV=240 mm, matrix size=128 × 128) was used to acquire 60 slices, 2 mm thick, in 30 different diffusion directions (b=900). The sequence was repeated 4 times, and 10 non-diffusion weighted (b=0) volumes were collected.
2.4. DTI processing
We used the voxelwise “Tract Based Spatial Statistics” (TBSS) method from the Oxford Center for Functional MRI of the Brain (FMRIB) Diffusion Toolbox (FDT) (Smith et al., 2004, 2006). This method identifies a core white matter “skeleton” that is anatomically equivalent across subjects. It allows for comparison of diffusion parameters across clinical populations and healthy controls. TBSS avoids spatial smoothing (averaging voxels). By only analyzing voxels of core white matter, it minimizes partial volume effects that can occur when more than one tract goes through a voxel, thereby leading to decreases in anatomical specificity (Smith et al., 2006).
The DTI parameter analyzed in this study was fractional anisotropy (FA), a ratio from 0 to 1 that indexes the magnitude of diffusion in one direction compared to other directions. FA values are assumed to reflect the directional coherence of axons within a white matter tract. High FA values (closer to 1) are considered evidence of healthy and mature microstructure.
DTI images were corrected for eddy current distortions by affine registration to a non-diffusion weighted volume. A tensor model was fit to each voxel (Basser, Mattiello, & LeBihan, 1994), and FA images were calculated. Because we were analyzing child subjects (Smith et al., 2006), the FA image of the most representative subject in the study (target) was chosen based on the minimum necessary warping, with FMRIB Software Library's (FSL) non-linear registration tool FNIRT (Andersson, Jenkinson, & Smith, 2007a, 2007b). The target image was sampled to 1 mm3 resolution and aligned by affine transformation to the Montreal Neurological Institute template (MNI152), allowing us to use standard space coordinates for describing regions. Then, each subject's image was aligned to the target. We found no differences between preterm and control groups in the degree of nonlinear warping using independent samples t-test. This finding strongly suggests that the results of subsequent analyses are not related to biases in warping across groups in the creation of the FA-skeleton.
Using a threshold of FA ≥ .2 to restrict analyses to white matter, a mean FA image was generated and thinned to create the mean FA-skeleton, a representation of the centers or core of all major white matter tracts common to the group. Finally, each subject's maximum local FA was orthogonally projected onto the FA-skeleton. The resulting data was fed into voxelwise cross-subject statistics.
2.5. Laterality analysis
In order to determine whether the preterm group showed a different pattern of laterality from the full-term group, we used TBSS results to test for asymmetries in FA as described in FSL and used in other studies (Takao, Hayashi, & Ohtomo, 2011). A symmetric mean FA-skeleton was generated using the script “tbss_sym”. The symmetric mean FA-skeleton was restricted to only those parts of the original FA-skeleton that were sufficiently close to being symmetric in terms of the general tract structure between the left and right hemispheres of the brain. Each subject's aligned FA data were then projected onto this symmetric skeleton. The left was subtracted from the right. These difference values were tested for statistical significance to find areas of the FA-skeleton that had greater FA on the left than on the right that were greater in one group than in the other group. The inverse of the difference values were used to find areas of the FA-skeleton that had greater FA on the right than on the left in one group over the other group.
2.6. Statistical analysis
Chi-square tests (for categorical variables) and independent t-tests (for continuous variables) were used to evaluate between-group differences on demographic variables and outcome measures.
Analysis of the FA values of the FA-skeleton at the voxel level was conducted using a permutation-based approach (Nichols & Holmes, 2002). These analyses controlled for age and set significance at p<.05 after correction for multiple comparisons. Statistical maps were obtained for each voxelwise test in both the positive and negative direction.
First, we analyzed group differences in FA across the entire FA-skeleton.
Second, we analyzed associations of scores on the language and reading measures with FA using two-tailed tests of significance. We generated a map of the regions on the FA-skeleton in which there were significant associations with one or more language or reading functions. Significant clusters of voxels on the FA-skeleton were visually inspected with reference to John Hopkins University (JHU) DTI-based white matter atlases (Hua et al., 2008; Mori, Wakana, van Zijl, & Nagae-Poetscher, 2005; Wakana et al. 2007; Setsu Wakana, Jiang, Nagae-Poetscher, van Zijl, & Mori, 2004) to determine their tract assignments. We used the following criteria to select the clusters of substantial size and specificity in locations along tracts:
The clusters were identifiable on the JHU atlas as predominantly a single tract.
The clusters have a minimum of 30 voxels.
The clusters are contiguous or near contiguous (no more than 12 voxels apart).
The mean FA of the cluster is greater than .30.
The mean FA values of these clusters were described in terms of the standard space coordinates (MNI152) for the voxel with the highest average association across the outcomes.
We then used these clusters for region-of-interest (ROI) analyses. Partial correlations, controlling for age, were used to evaluate the degree of association between the mean FA of these regions and each of the outcomes. One-tailed significance tests were used because the direction of association between FA and outcome was already established from the whole FA-skeleton analyses.
To determine if one or more than one tract contributed to the variance in a given language and reading outcome, we performed exploratory multiple regressions (Field, 2009). We chose one cluster from the composite map to represent each of the tracts that correlated with the outcomes. For tracts with multiple significant clusters, for each regression model, we selected the cluster with the highest r-value in relation to that outcome. Using this method, for each outcome, the regression model included the cluster with the highest r-value in the univariate analyses. Cluster-FAs were entered into a regression model, and age was included because white matter properties change with age (Asato et al., 2010; Schmithorst et al., 2002). The final model for each outcome was automatically determined based on mathematical criteria for the stepwise method of regression. This method is preferable to other regression methods when there is no a priori hypothesis about the selection or order of potential predictors (Field, 2009).
3. Results
3.1. Behavioral results and overall DTI results
There were no statistically significant group differences in the language and reading scores in this sample of children by independent t-tests (Table 1).
3.2. Associations between the FA-skeleton and language and reading scores
In the preterm group only, voxelwise analysis of the entire FA-skeleton with scores from language and reading tasks showed positive associations with FA for four measures: verbal IQ, linguistic processing speed, syntactic comprehension, and decoding. The regions of significant positive correlation for the different measures overlapped. There were no statistically significant associations negative correlations between scores and FA in the preterm group and no significant positive or negative correlations in the control group with any of the measures.
In order to examine the degree of correlation more closely, we created a single composite map of all voxels that were associated with scores from any of the four measures. This composite map can be conceptualized as the language and reading network for the preterm group. Fig. 1 shows that this network includes 15 tracts distributed across hemispheres: corpus callosum, forceps major, forceps minor, bilateral anterior thalamic radiation, bilateral corticospinal tract, bilateral inferior fronto-occipital fasciculus, bilateral inferior longitudinal fasciculus, bilateral superior longitudinal fasciculus, and bilateral uncinate fasciculus.
Fig. 1.
White matter language and reading network. White matter regions (red) on the FA-skeleton (green), overlaid on axial slices of mean FA image (grayscale), associated with at least one behavioral measure, p < .05, in the preterm group (n=23). Regions located on 15 tracts: corpus callosum (z=4–34), forceps major (z=4–16), forceps minor (z=7–22), bilateral anterior thalamic radiation (z=4–19), bilateral corticospinal tract (z=4–37), bilateral inferior fronto-occipital fasciculus (z=4–22), bilateral inferior longitudinal fasciculus (z=4–7), bilateral superior longitudinal fasciculus (z=16–37), and bilateral uncinate fasciculus (z= − 25 to − 7). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Region-of-interest (ROI) analyses
We then identified 22 clusters from the 15 major tracts on the composite map for ROI analyses. Table 2 shows the partial correlations, controlling for age, between the behavioral measures in the preterm group and the mean FA values for the 22 ROIs. Higher language and reading performance on all measures was positively correlated with FA in several white matter tracts in both hemispheres. Note that syntactic comprehension was measured by a z-score of the errors made in sentence comprehension tasks and linguistic processing speed was measured by a z-score of the reaction time. For both of these measures, a negative correlation coefficient indicates that lower FA is associated with poor performance. Syntactic comprehension and decoding scores were correlated with FA in all 15 tracts. All language and reading scores showed a positive correlation with FA in the corpus callosum, forceps minor, bilateral inferior longitudinal fasciculus, right anterior thalamic radiation, right corticospinal tract, and right inferior fronto-occipital fasciculus. All language and reading scores except reading comprehension showed a positive correlation with the FA from the left inferior fronto-occipital fasciculus and left superior longitudinal fasciculus.
Table 2.
Partial correlations, controlling for age, between FA values of 22 distinct regions of interest on the White Matter Language and Reading Network and behavioral measures: verbal IQ, receptive vocabulary, verbal memory, syntactic comprehension, linguistic processing speed, decoding, and reading comprehension (n=23). Locations of the clusters are reported in MNI coordinate system for the voxel in that cluster with the highest average association across outcomes.
| White matter tract (MNI coordin ate) # of voxels | VIQ | Vocab | Verbal memory | Syntactic comp (z)a | Processing speed (z)b | Decoding | Reading comp |
|---|---|---|---|---|---|---|---|
| Corpus callosum | |||||||
| Body (15, 19, 22) 1590 | .31 | .31 | .33 | −.35 | −.43* | .39* | .12 |
| Genu (17, 26, 20) 493 | .57** | .52** | .58** | −.57** | −.52** | .67*** | .39* |
| Splenium (18, −37, 30) 467 | .18 | .18 | .20 | −.39* | −.31 | .20 | − .01 |
| Forceps major | |||||||
| Left (30, −61, 17) 120 | .27 | .32 | .37* | −.43* | −.34 | .26 | .32 |
| Right (− 25, −69, 12)222 | .28 | .30 | .29 | −.38* | −.27 | .44* | .20 |
| Forceps minor | |||||||
| (18, 26, 21) 165 | .54** | .56** | .61** | − .48* | −.54** | .48* | .46* |
| Left anterior thalamic radiation | |||||||
| (− 4, −8, 5) 193 | .49* | .41* | .41* | − .46* | − .03 | .58** | .32 |
| Left corticospinal tract | |||||||
| (− 22, −23, 1) 119 | .44* | .38* | .26 | −.53** | −.29 | .43* | .35 |
| (− 22, −23, 36) 129 | .19 | .25 | .24 | −.16 | −.05 | .53** | .23 |
| Left inferior fronto — occipital fasciculus | |||||||
| (−31, −51, 14)578 | .38* | .40* | .44* | − .50** | −.46* | .47* | .27 |
| Left inferior longitudinal fasciculus | |||||||
| (−37, —37, 3) 245 | .41* | .41* | .49* | −.47* | −.49* | .57* | .44* |
| Left superior longitudinal fasciculus | |||||||
| (−36, —40, 32) 356 | .41* | .37* | .43* | −.59** | −.45* | .42* | .21 |
| Left uncinate fasciculus | |||||||
| (−36, —6, —21) 77 | .49* | .33 | .43* | −.38* | −.36 | .53** | .50** |
| Right anterior thalamic radiation | |||||||
| (24, 14, 16) 228 | .56** | .54** | .55** | −.47* | −.33 | .45* | .38* |
| (11, —5, —7) 39 | .43* | .47* | .39* | −.45* | −.51** | .31 | .30 |
| Right corticospinal tract | |||||||
| (26, —16, 13) 855 | .53** | .58** | .50** | − .59** | −.40* | .52** | .48* |
| Right inferior fronto —occipital fasciculus | |||||||
| (26, 21, 14) 353 | .70*** | .56** | .59** | .67*** | .49* | .47* | .50** |
| (31, —58, 17) 50 | .31 | .31 | .35 | −.57** | −.12 | .37* | .25 |
| (39, —16, —12) 429 | .44* | .42* | .49* | 57*** | −.35 | .32 | .23 |
| Right inferior longitudinal fasciculus | |||||||
| (40, —4, —22) 314 | .54** | .46* | .51** | − .64** | −.40* | .51** | .39* |
| Right superior longitudinal fasciculus | |||||||
| (31, 0, 17) 1114 | .40* | .36 | .46* | −.48* | −.25 | .49* | .25 |
| Right uncinate fasciculus | |||||||
| (36, —1, —24) 74 | .53** | .47* | .31 | − .59** | −.22 | .43* | .37* |
All significance tests are one-tailed.
p<.05.
p<.01.
p<.001.
Syntactic comprehension is measured by a z-score of the errors made in sentence comprehension tasks. A negative correlation coefficient indicates that lower FA is associated with more errors.
Linguistic processing speed is measured by a z-score of the reaction time in sentence comprehension task. A negative correlation coefficient indicates that lower FA is associated with longer reaction time.
We also examined the degree of correlation between FA and outcome measure within the full-term group. Any associations that are statistically significant in the ROI analysis of the full-term group are considered weak because they did not rise to the level of statistical significance in the voxelwise analysis that corrects for multiple comparisons. Because no direction for the correlation was established in the voxelwise analysis, we used a 2-tailed test of significance. We found four positive associations of outcomes and an ROI: verbal memory with the ROI on the right inferior fronto-occipital fasciculus (r = .48 p < .05), vocabulary with the ROI at the genu of the corpus callosum (r=.50, p < .05), and linguistic processing speed with the right anterior thalamic radiation (r=.51, p < .05) and with the right uncinate (r=.50, p<.05). We also found six negative associations of outcomes and an ROI: verbal IQ and the right uncinate (r = −.51, p<.05), vocabulary and the right inferior fronto-occipital fasciculus (r = −.49, p < .05), verbal memory and the right uncinate (r = −.48, p < .05), decoding and the right anterior thalamic radiation (r= − .47, p < .05), and reading comprehension and the right inferior longitudinal fasciculus (r = − .48, p < .05) and right uncinate (r = −.58, p < .05).
3.4. Regression analysis in the preterm group
Since several tracts correlated with each measure, we performed an exploratory multiple regression analysis for each outcome to determine the tract or tracts that would be the most important independent contributors to that outcome. We found only one significant predictor in each of the final regression models and that predictor was the cluster with the highest correlation in the univariate analyses:
Right inferior fronto-occipital fasciculus for verbal IQ, β = .66, p < .01, R2 = .44, and syntactic comprehension (errors), β= −.65, p <.01, R2 = .42;
Forceps minor for receptive vocabulary, β = .56, p<.01, R2=31, and verbal memory, β=.61, p < .01, R2=.38;
Right anterior thalamic radiation for linguistic processing speed (reaction time), β=−.53, p<.01, R2=.28;
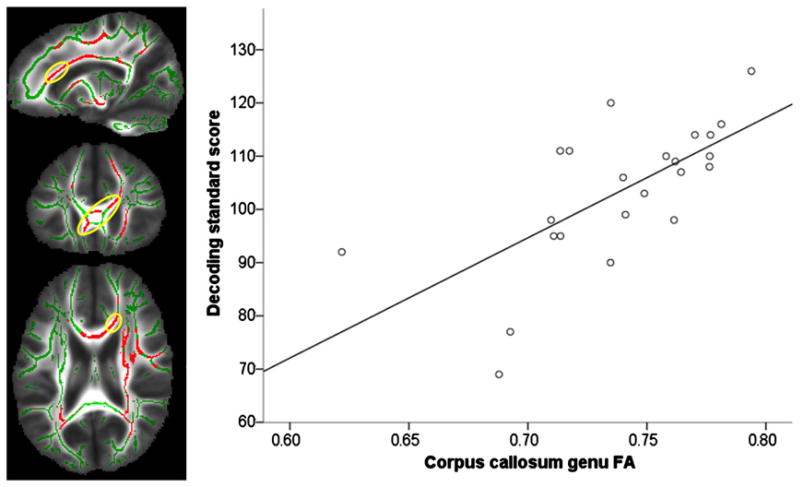
Genu of the corpus callosum for deoding, β=.67, p<.001, R2=.45;
Left uncinate fasciculus for reading comprehension, β = .52, p < .05, R2=.27 (Figs. 2-6).
Fig. 2.
A region of interest (left panel) on the right inferior fronto-occipital fasciculus (red circled in yellow) shown on sagittal (x=26), coronal (y=21), and axial (z=14) slices of FA-skeleton (green) overlaid on the mean FA image (grayscale). Though the FA of 14 tracts correlated with verbal IQ, the FA of this region was the only significant predictor in the regression model predicting verbal IQ, β=.66, p<.01, R2=.44, (top right panel); and though the FA of 15 tracts correlated with syntactic comprehension, the FA of this region was the only significant predictor in the regression model predicting syntactic comprehension, β=− .65, p<.01, R2=.42, (bottom right panel) in children born preterm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
A region of interest (left panel) on the left uncinate fasciculus (red circled in yellow) shown on sagittal (x = −36), coronal (y = −6), and axial (z= −21) slices of FA-skeleton (green) overlaid on the mean FA image (grayscale). Though the FA of 9 tracts correlated with reading comprehension, the FA of this region was the only significant predictor in the regression model predicting reading comprehension, β=.52, p < .05, R2=.27 (right panel) in children born preterm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Laterality analysis
Using the laterality analysis on TBSS, we found only two small regions (88 and 43 voxels) (Supplemental Figure) on the inferior fronto-occipital fasciculus showed greater left laterality in the controls than in the preterm group. These results indicate that the positive difference between FA in these regions in the left versus right hemisphere was significantly greater in the control group than it was in the preterm group only in these two small regions. We found no other regions in which the controls had greater laterality in the left hemisphere. There were no other laterality differences between groups in either direction in the right hemisphere. Given that the language and reading network included 15 tracts, we do not think that the main associations relate to differences in laterality.
4. Discussion
4.1. Summary of results
Four language and reading measures—verbal IQ, linguistic processing speed, syntactic comprehension, and decoding—were associated with areas of the FA-skeleton in the preterm group in the voxelwise analysis. Contrary to the hypotheses, we found that language and reading measures were not associated with FA in the full-term group in the voxelwise analysis. A bilateral and widely distributed network of tracts was associated with the measures in the preterm group. The network included 22 clusters on 15 bilateral tracts. Multiple language and reading scores were correlated with FA in multiple tracts in the preterm group. Consistent with the hypotheses, language measures were correlated with FA in left and right hemisphere ventral tracts. However, in addition to the predicted associations, we found positive correlations with FA in dorsal tracts. Decoding and reading comprehension were also correlated with FA in bilateral dorsal and ventral tracts. We also found associations beyond the dorsal and ventral pathways, in the corticospinal tract and the anterior thalamic radiation, both running in the inferior–superior direction.
In the regression models, only one tract contributed to the variance in each of the behavioral scores. These results suggest that the FA of different tracts was correlated such that only a single region accounted for the variance in scores. The regression model identified the right inferior fronto-occipital fasciculus (a ventral tract) as making the strongest contribution to the variance in both verbal IQ and syntactic comprehension and the right anterior thalamic radiation as making the strongest contribution to the variance in linguistic processing speed. The bilateral forceps minor made the strongest contribution to the variance in receptive vocabulary and verbal memory. A midline structure, the genu of the corpus callosum, made the strongest contribution to the variance in decoding skills. The left uncinate fasciculus (a ventral tract) made the strongest contribution to the variance in reading comprehension.
4.2. Language, reading, and white matter in children born full-term
We did not find significant associations between language or reading measures and FA within the full-term group in the voxelwise analysis. When we restricted analyses to the ROIs that were significantly associated with measures in the preterm group, we found weak associations in both the positive and negative directions. We recognize that the sample size is modest and the language and reading performance of the participants above the mean with a small standard deviation. We may not have had sufficient sample size or variation among scores to detect significant associations. However, other studies of children find that favorable behavioral outcomes are negatively associated with FA in healthy and typically developing children (Dougherty et al., 2007; Odegard et al., 2009; Yeatman et al., 2011). Explanations for the relatively low FA in association with relatively high performance has been attributed to decreased dendritic branching or crossing fibers, and fewer but larger axons, factors that could contribute to relative increases in FA. Moreover, it is possible many factors in addition to white matter characteristics may contribute to individual differences in language and reading abilities in the full-term group, thereby diluting any associations of FA and language or reading scores. Mullen and colleagues (2011) also did not find associations between language or reading and FA in the full-term group in their study.
4.3. White matter and language and reading in children born preterm
The network associated with language and reading included bilateral long-range fasciculi and the corpus callosum. In this next section we will discuss the network in terms of the dorsal and ventral pathways proposed to serve language and reading functions.
4.3.1. Dorsal pathways
The left superior longitudinal fasciculus and segments of the arcuate fasciculus have long been considered important components of the language network because they connect Wernicke's and Broca's area as the dorsal stream (Friederici & Friederici, 2009). The dorsal stream bilaterally appears to be important in individual differences in language processing in children born preterm based on the positive associations of language measures with FA in the superior longitudinal fasciculus. All measures except reading comprehension were associated with the left tract and four of seven measures were associated with the right tract, but neither side accounted for the variance in the multiple regression analyses. Interestingly, previous studies of preterm groups using other DTI analytic approaches have not found significant correlations between language functions and FA of the superior longitudinal fasciculus (Constable et al., 2008; Mullen et al., 2011). The superior longitudinal fasciculus shows wide variation in size and shape among individuals (Yeatman et al., 2011) reducing the likelihood of finding associations unless tractography in native space is used. In addition, in several studies regions associated with reading appear to be part of the inferior– superior axis, including the corona radiate, rather than are part of the posterior–anterior axis or the superior longitudinal fasciculus. Future analyses using tractography may clarify which tracts are associated with the language and reading skills in this sample.
4.3.2. Ventral pathways
The ventral pathways associated with language measures included the inferior fronto-occipital fasciculus that courses from the occipital to frontal lobe, the inferior longitudinal fasciculus that connects posterior regions to the anterior temporal lobe, and the uncinate fasciculus that courses between the anterior temporal lobe and frontal lobe. In the regression analysis, FA of the right inferior fronto-occipital fasciculus accounted for most of the variance in verbal IQ. These findings are consistent with previous findings that children and adolescents born preterm show significant associations of right-sided ventral pathways and language measures. We were initially surprised to find that the right inferior fronto-occipital fasciculus was also the tract most highly associated with syntactic comprehension because of the long history of neuropsychology research implicating the left hemisphere in syntactic processing (Friederici & Friederici, 2009). The tasks used in this study required the participant to interpret a sentence and determine whether a subsequently presented picture depicted the meaning of the sentence. This complex task required not only analysis of grammatical features but also sentence-level comprehension and other processes to come to accurate interpretation. The processing is a complex combination of various kinds of computations that likely requires the collective activity of a distributed network involving multiple systems (Hickok & Poeppel, 2004). Associations of language functions with right-sided tracts are consistent with the findings within the preterm group in other studies (Mullen et al., 2011).
The pathway that explained most of the variance in reading comprehension in the preterm group was the left uncinate fasciculus. Other studies have found that the uncinate fasciculus bilaterally is associated with verbal IQ (Constable et al., 2008) and receptive vocabulary (Constable et al., 2008; Mullen et al., 2011). The uncinate fasiculus courses from the temporal lobe to the frontal lobe. Therefore, it may be important for carrying semantic and conceptual information to the frontal lobes for integration and processing. We are not certain why the left uncinate fasciculus is more associated with reading comprehension than the right uncinate fasciculus, particularly given the findings of right ventral pathway associations for other functions. Decoding skills were associated with the corpus callosum, suggesting that both hemispheres may be collaborating in reading processes.
4.3.3. Corpus callosum and bilateral forceps
The corpus callosum and its extensions to the forceps major posteriorly and forceps minor anteriorly were important parts of the network and the main tracts associated with verbal memory and receptive vocabulary. These tracts provide inter-hemispheric connections. Functional imaging studies find that aspects of language, such as syntactic processing, generally activate the left hemisphere, and different aspects of language, such as metaphor interpretation, activate the right hemisphere. One intriguing explanation for the importance of midline and bilateral structure in this study is that children born preterm draw on multiple concurrent strategies to remember long sentences and to identify vocabulary items. They must integrate information from multiple semi-specialized brain regions to accomplish their tasks. Because of the requirement for integration, FA of the corpus callosum might be associated with language function.
The corpus callosum accounted for most of the variance in decoding in this study. The corpus callosum has been implicated in reading functions in studies of otherwise healthy good and poor readers (Dougherty et al., 2007; Odegard et al., 2009) However, in these studies the direction of association is negative; the good readers have lower FA than the poor readers. Yeatman et al. (2011) also found negative associations of phonological awareness and FA of the arcuate fasciculus. Our confidence in our findings of positive correlations in the corpus callosum is increased because Andrews et al., 2010 similarly found that the genu of the corpus callosum was the midline structure most highly associated with decoding using a different analytic methodology on a different sample of children.
We are not certain how to explain the differences in the direction of association between groups. Dougherty and colleagues (2007) hypothesized that the good readers in their study may have a higher proportion of large axons and therefore a lower density of cell membranes in the perpendicular direction, accounting for higher radial diffusivity in the good readers. They offer a less likely alternative, that the membranes and myelin sheaths in healthy good readers are more permeable to diffusing water. We found that within the ROIs in which there was a consistent positive association of language and reading measures within the preterm group, there were ROIs with weak negative associations between language and reading in the full-term group. The meaning of high FA may differ in different clinical populations. FA is higher within selected tracts among individuals in clinical populations than within the same tracts in healthy participants in William syndrome (Arlinghaus et al., 2011; Hoeft et al., 2007) and attention-deficit/hyperactivity disorder (Davenport, Karatekin, White, & Lim, 2010), both conditions associated with relatively weak skills. Future imaging studies using methods sensitive to the amount of myelin will be important for understanding the factors associated with FA in healthy and clinical populations and direction of association between FA and behavioral outcomes, such as reading.
4.3.4. Additional tracts
The right anterior thalamic radiation accounted for the variance in linguistic processing speed. This tract in the medial portion of the internal capsule connects the anterior and medial thalamic nuclei to the frontal lobe. Using voxel-based lesion-symptom mapping, this region has been associated with processing speed in a large sample of adults with cerebral small vessel disease (Duering et al., 2011). Anisotropy of the right anterior thalamic radiation has also been associated with executive function and working memory abilities in individuals with schizophrenia (Mamah et al., 2010).
Other tracts that were found as part of the network included the corticospinal tracts and the forceps major. We suspect that these tracts have associations with language and reading measures because of the complex tasks that were used in this study that require the integration of multiple different processes in terms of understanding and also in producing required responses. The splenium of the corpus callosum has been implicated in reading tasks (Dougherty et al., 2007; Frye et al., 2008; Odegard et al., 2009), possibly because the need in reading to link visual processing to language processing.
4.4. Laterality analysis
Though the visual appearance of the T1 weighted images of the preterm group suggested that there might be more injury on the left than the right, the laterality analysis showed no major changes in white matter laterality in the preterm group with the exception of two small regions of the inferior frontal-occipital fasciculus. These findings indicate that throughout most of the white matter network, laterality in the two groups is comparable using the TBSS technique. These findings indicate that the relative FA of either hemisphere does not account for the level of the correlations.
4.5. Limitations
The study utilized a convenience sample that may not be representative of all children born preterm. The sample had a range of gestation ages at birth and ages at scanning, which may introduce variability to the results. However, increased variation may also facilitate finding associations, particularly between structure and function. The sample size was modest and included high-functioning preterm children. The DTI analysis method of TBSS is conservative in that it focuses on the centers of white matter tracts and may not detect peripheral damage in the tracts. Tractography may allow for more sensitive analyses of FA within white matter tracts in relation to language and reading.
4.6. Conclusions and future directions
In this DTI study using TBSS, we found that FA was positively associated with performance on several language and reading measures in a relatively high-functioning sample of preterm children and adolescents. Multiple regions from several tracts were associated with the measures. However, only a single tract explained most of the variance in these outcomes, suggesting that white matter microstructural properties are highly correlated throughout the brain in the preterm group. We plan to confirm these results in this sample using tractography in native space in order to increase confidence in the results obtained with TBSS. In the future analyses of this sample, we will also add measures of white matter volume because of the significant associations of volume and outcomes in full-term (Yeatman et al., 2011) and preterm children (Frye et al., 2010). In the future, longitudinal analyses of language and reading in relation DTI and other neuroimaging methods from young ages through adolescence may be able to increase our understanding of the long-term impact of prematurity on brain structure while simultaneously providing insights into the contribution of development and plasticity on outcomes (Ment et al., 2009).
Supplementary Material
Fig. 3.
A region of interest (left panel) on the forceps minor (red circled in yellow) shown on sagittal (x=18), coronal (y=26), and axial (z=21) slices of FA-skeleton (green) overlaid on the mean FA image (grayscale). Though the FA of 13 tracts correlated with verbal memory, the FA of this region was the only significant predictor in the regression model predicting verbal memory, β=.61, p <.01, R2=.38, (top right panel); and though the FA of 12 tracts correlated with receptive vocabulary, the FA of this region was the only significant predictor in the regression model predicting receptive vocabulary, β= −.56, p < .01, R2 =.31 (bottom right panel) in children born preterm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
A region of interest (left panel) on the right anterior thalamic radiation (red circled in yellow) shown on sagittal (x=11), coronal (y = −5), and axial (z = − 7) slices of FA-skeleton (green) overlaid on the mean FA image (grayscale). Though the FA of 9 tracts correlated with linguistic processing speed, the FA of this region was the only significant predictor in the regression model predicting linguistic processing speed, β= − .53, p <.01, R2=.28 (right panel) in children born preterm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
A region of interest (left panel) on the genu of the corpus callosum (red circled in yellow) shown on sagittal (x=17), coronal (y=26), and axial (z=20) slices of FA-skeleton (green) overlaid on the mean FA image (gray scale). Though the FA of 15 tracts correlated with decoding, the FA of this region was the only significant predictor in the regression model predicting decoding, β=.67, p<.001, R2=.45 (right panel) in children born preterm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Acknowledgments
This work was supported by a grant from the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, RO1 HD046500 to Heidi M. Feldman; and by the Clinical and Translational Science Award 1UL1 RR025744 for the Stanford Center for Clinical and Translational Education and Research (Spectrum) from the National Center for Research Resources, National Institutes of Health. We thank the children and families who participated in our study. We thank Lynne C. Huffman MD, Irene M. Loe, Laura H.F.Barde, and Michal Ben-Shachar for helpful comments on earlier versions.
Footnotes
Appendix A. Supplementary material: Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.neuropsychologia. 2012.10.014.
References
- Adams E, Chau V, Poskitt KJ, Grunau RE, Synnes A, Miller SP. Tractography-based quantitation of corticospinal tract development in premature newborns. Journal of Pediatrics. 2010;156(6):882–888 888.e881. doi: 10.1016/j.jpeds.2009.12.030. Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MC. Neurodevelopmental outcomes of preterm infants. Current Opinion in Neurology. 2008;21(2):123–128. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- Allin MPG, Kontis D, Walshe M, Wyatt J, Barker GJ, Kanaan RAA, et al. White matter and cognition in adults who were born preterm. PLoS One. 2011;6(10):e24525. doi: 10.1371/journal.pone.0024525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Doyle LW, Callanan C, Carse E, Casalaz D, Charlton MP, et al. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA: Journal of the American Medical Association. 2003;289(24):3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation FMRIB Analysis Group Technical Reports. TR07JA1. Oxford: University of Oxford; 2007a. [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation FMRIB Analysis Group Technical Reports. TR07JA2. Oxford: University of Oxford; 2007b. [Google Scholar]
- Andrews JS, Ben-Shachar M, Yeatman JD, Flom LL, Luna B, Feldman HM. Reading performance correlates with white-matter properties in preterm and term children. Developmental Medicine & Child Neurology. 2010;52(6):e94–100. doi: 10.1111/j.1469-8749.2009.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlinghaus LR, Thornton-Wells TA, Dykens EM, Anderson AW. Alterations in diffusion properties of white matter in Williams syndrome. Magnetic Resonance Imaging. 2011;29(9):1165–1174. doi: 10.1016/j.mri.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato M, Terwilliger R, Woo J, Luna B. White matter development in adolescence: A DTI study. Cerebral Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward GP. Cognitive and neuropsychological outcomes: More than IQ scores. Mental Retardation & Developmental Disabilities Research Reviews. 2002;8(4):234–240. doi: 10.1002/mrdd.10043. [DOI] [PubMed] [Google Scholar]
- Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38(2 Suppl.):724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: A meta-analysis. Journal of Pediatrics. 2011;158(5):766–774 and 761. doi: 10.1016/j.jpeds.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Wandell BA. White matter pathways in reading. Current Opinion in Neurobiology. 2007;17(2):258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, et al. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27(4):862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Test for Reception of Grammar (TROG-2) Oxford, United Kingdom: Pearson Assessment; 2003. [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WMA, Barker PB, et al. Diffusion tensor imaging in children and adolescents: Reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34(2):733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Friederici AD. Neuroanatomical prerequisites for language functions in the maturing brain. Cerebral Cortex. 2011;21(2):459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caldu X, Narberhaus A, Junque C, Gimenez M, Vendrell P, Bargallo N, et al. Corpus callosum size and neuropsychologic impairment in adolescents who were born preterm. Journal of Child Neurology. 2006;21(5):406–410. doi: 10.1177/08830738060210050801. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Nigg JT, Durston S. New potential leads in the biology and treatment of attention deficit-hyperactivity disorder. Current Opinion in Neurology. 2007;20(2):119–124. doi: 10.1097/WCO.0b013e3280a02f78. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chyi LJ, Lee HC, Hintz SR, Gould JB, Sutcliffe TL, Chyi LJ, et al. School outcomes of late preterm infants: Special needs and challenges for infants born at 32 to 36 weeks gestation. Journal of Pediatrics. 2008;153(1):25–31. doi: 10.1016/j.jpeds.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, et al. Prematurely born children demonstrate white matter micro-structural differences at 12 years of age, relative to term control subjects: An investigation of group and gender effects. Pediatrics. 2008;121(2):306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Shen Y, Boardman JP, Larkman DJ, Kapellou O, Ward P, et al. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics. 2006;117(2):376–386. doi: 10.1542/peds.2005-0820. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Karatekin C, White T, Lim KO. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Research. 2010;181(3):193–198. doi: 10.1016/j.pscychresns.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JDE, Wandell B. Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41(3):354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(20):8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cerebral Cortex. 2009;19(2):414–423. doi: 10.1093/cercor/bhn097. [DOI] [PubMed] [Google Scholar]
- Duering M, Zieren N, Herve D, Jouvent E, Reyes S, Peters N, et al. Strategic role of frontal white matter tracts in vascular cognitive impairment: A voxel-based lesion-symptom mapping study in CADASIL. Brain. 2011;134(Part 8):2366–2375. doi: 10.1093/brain/awr169. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody picture vocabulary test-III. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Feldman HM, Lee ES, Loe IM, Yeom KW, Grill-Spector K, Luna B. White matter microstructure Q5 on diffusion tensor imaging is associated with conventional MRI findings and cognitive function in adolescents born preterm. Developmental Medicine & Child Neurology. 2012;54(9):809–814. doi: 10.1111/j.1469-8749.2012.04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. Discovering statistics using SPSS. 3rd. London, UK: SAGE Publications Ltd; 2009. [Google Scholar]
- Fletcher JM, Bohan TP, Brandt ME, Brookshire BL, Beaver SR, Francis DJ, et al. Cererbral white matter and cognition in hydrocephalic children. Archives of Neurology. 1992;49(8):818–824. doi: 10.1001/archneur.1992.00530320042010. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Landry SH, Bohan TP, Davidson KC, Brookshire BL, Lachar D, et al. Effects of intraventricular hemorrhage and hydrocephalus on the long-term neurobehavioral development of preterm very-low-birthweight infants. Developmental Medicine & Child Neurology. 1997;39(9):596–606. doi: 10.1111/j.1469-8749.1997.tb07495.x. [DOI] [PubMed] [Google Scholar]
- Foster-Cohen SH, Friesen MD, Champion PR, Woodward LJ. High prevalence/low severity language delay in preschool children born very preterm. Journal of Developmental & Behavioral Pediatrics. 2010;31(8):658–667. doi: 10.1097/DBP.0b013e3181e5ab7e. [DOI] [PubMed] [Google Scholar]
- Fraello D, Maller-Kesselman J, Vohr B, Katz KH, Kesler S, Schneider K, et al. Consequence of preterm birth in early adolescence: The role of language on auditory short-term memory. Journal of Child Neurology. 2011;26(6):738–742. doi: 10.1177/0883073810391904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Ferrari MC, Hallak JE, Trzesniak C, Filho AS, Machado-de-Sousa JP, Chagas MH, et al. Neuroimaging in social anxiety disorder: A systematic review of the literature. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(4):565–580. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Friederici AD. Pathways to language: Fiber tracts in the human brain. Trends in Cognitive Sciences. 2009;13(4):175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Frye RE, Hasan K, Malmberg B, Desouza L, Smith K, Landry S. Superior longitudinal fasciculus and cognitive dysfunction in adolescents born preterm and at term. Developmental Medicine & Child Neurology. 2010;2010(52):760–766. doi: 10.1111/j.1469-8749.2010.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Hasan K, Malmberg B, Desouza L, Swank P, Smith K, et al. Superior longitudinal fasciculus and cognitive dysfunction in adolescents born preterm and at term. Developmental Medicine & Child Neurology. 2010;52(8):760–766. doi: 10.1111/j.1469-8749.2010.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Hasan K, Xue L, Strickland D, Malmberg B, Liederman J, et al. Splenium microstructure is related to two dimensions of reading skill. Neuroreport. 2008;19(16):1627–1631. doi: 10.1097/WNR.0b013e328314b8ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RE, Landry SH, Swank PR, Smith KE, Frye RE, Landry SH. Executive dysfunction in poor readers born prematurely at high risk. Developmental Neuropsychology. 2009;34(3):254–271. doi: 10.1080/87565640902805727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Law M, Johnson G, Herbert J, Babb JS, Mannon LJ, et al. Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. Journal of Magnetic Resonance Imaging. 2004;20(1):1–7. doi: 10.1002/jmri.20083. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK. DTI tractography of the human brain's language pathways. Cerebral Cortex. 2008;18(11):2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J, et al. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009;484(2):458–463. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Whitfield MF, Davis C. Pattern of learning disabilities in children with extremely low birth weight and broadly average intelligence. Archives of Pediatrics & Adolescent Medicine. 2002;156(6):615–620. doi: 10.1001/archpedi.156.6.615. [DOI] [PubMed] [Google Scholar]
- Guarini A, Sansavini A, Fabbri C, Alessandroni R, Faldella G, Karmiloff-Smith A, et al. Reconsidering the impact of preterm birth on language outcome. Early Human Development. 2009;85(10):639–645. doi: 10.1016/j.earlhumdev.2009.08.061. [DOI] [PubMed] [Google Scholar]
- Hack M. Young adult outcomes of very-low-birth-weight children. Seminars In Fetal & Neonatal Medicine. 2006;11(2):127–137. doi: 10.1016/j.siny.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Minich N. School-age outcomes in children with birth weights under 750 g. New England Journal of Medicine. 1994;331(12):753–759. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hille ET, den Ouden AL, Bauer L, van den Oudenrijn C, Brand R, Verloove-Vanhorick SP. School performance at nine years of age in very premature and very low birth weight infants: Perinatal risk factors and predictors at five years of age. Collaborative Project on Preterm and Small for Gestational Age (POPS) infants in The Netherlands. Journal of Pediatrics. 1994;125(3):426–434. doi: 10.1016/s0022-3476(05)83290-1. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Barnea-Goraly N, Haas BW, Golarai G, Ng D, Mills D, et al. More is not always better: Increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. Journal of Neuroscience. 2007;27(44):11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, et al. Neural systems predicting long-term outcome in dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(1):361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(10):4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JL. Theory of fluid and crystallized intelligence. In: Sternberg RJ, editor. Encyclopedia of human intelligence. New York, NY: Simon & Schuster/Macmillan; 1995. pp. 443–451. [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi SJ, Haberling IS, Badzakova-Trajkov G, Patston LLM, Waldie KE, Tippett LJ, et al. Regional differences in cerebral asymmetries of human cortical white matter. Neuropsychologia. 2011;49(13):3599–3604. doi: 10.1016/j.neuropsychologia.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Kaufman AS. Intelligent testing with the WISC-III. New York, NY: Wiley; 1994. [Google Scholar]
- Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: A review. Seminars in Perinatology. 2006;30(2):81–88. doi: 10.1053/j.semperi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, et al. Microstructure of temporo-parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25(2):493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Kontis Db, Catani Ma, Cuddy Mb, Walshe Mb, Nosarti Cb, Jones Dd, et al. Diffusion tensor MRI of the corpus callosum and cognitive function in adults born preterm. Neuroreport. 2009;20(4):424–428. doi: 10.1097/WNR.0b013e328325a8f9. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Human Brain Mapping. 2009;30(11):3563–3573. doi: 10.1002/hbm.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Yeatman JD, Luna B, Feldman HM. Specific language and reading skills in school-aged children and adolescents are associated with prematurity after controlling for IQ. Neuropsychologia. 2011a;49:906–913. doi: 10.1016/j.neuropsychologia.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Yeatman JD, Luna B, Feldman HM. Specific language and reading skills in school-aged children and adolescents are associated with prematurity after controlling for IQ. Neuropsychologia. 2011b;49(5):906–913. doi: 10.1016/j.neuropsychologia.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe I, Lee ES, Luna B, Feldman HM. Executive function skills in children born prematurely are associated with behavior. Reading, and Overall Function. 2012 doi: 10.1016/j.earlhumdev.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe IM, Lee ES, Luna B, Feldman HM. Behavior problems of 9–16 year old preterm children: Biological, sociodemographic, and intellectual contributions. Early Human Development. 2011;87(4):247–252. doi: 10.1016/j.earlhumdev.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M. Cognitive development in children born preterm: Implications for theories of brain plasticity following early injury. Development and Psychopathology. 2003;15(04):1017–1047. doi: 10.1017/s095457940300049x. [DOI] [PubMed] [Google Scholar]
- Maalouf EF, Duggan PJ, Counsell SJ, Rutherford MA, Cowan F, Azzopardi D, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001;107(4):719–727. doi: 10.1542/peds.107.4.719. [DOI] [PubMed] [Google Scholar]
- Maalouf EF, Duggan PJ, Rutherford MA, Counsell SJ, Fletcher AM, Battin M, et al. Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. Journal of Pediatrics. 1999;135(3):351–357. doi: 10.1016/s0022-3476(99)70133-2. [DOI] [PubMed] [Google Scholar]
- Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, et al. Anterior thalamic radiation integrity in schizophrenia: A diffusion-tensor imaging study. Psychiatry Research. 2010;183(2):144–150. doi: 10.1016/j.pscychresns.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Hirtz D, Huppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurology. 2009;8(11):1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- Mikkola K, Ritari N, Tommiska V, Salokorpi T, Lehtonen L, Tammela O, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996–1997. Pediatrics. 2005;116(6):1391–1400. doi: 10.1542/peds.2005-0171. http://dx.doi.org/10.1542/peds.2005-0171. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, van Zijl PC, Nagae-Poetscher LM. MRI atlas of human white matter. Amsterdam, Netherlands: Elsevier; 2005. [Google Scholar]
- Msall ME, Tremont MR. Measuring functional outcomes after prematurity: Developmental impact of very low birth weight and extremely low birth weight status on childhood disability. Mental Retardation & Developmental Disabilities Research Reviews. 2002;8(4):258–272. doi: 10.1002/mrdd.10046. [DOI] [PubMed] [Google Scholar]
- Mullen KM, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M, et al. Preterm birth results in alterations in neural connectivity at age 16 years. NeuroImage. 2011;54(4):2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Skare S, Andersson JL, Lilja A, Flodmark O, et al. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatric Research. 2003;54(5):672–679. doi: 10.1203/01.PDR.0000084083.71422.16. [DOI] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44(11):2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human Brain Mapping. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Rushe TM, Woodruff PW, Stewart AL, Rifkin L, Murray RM. Corpus callosum size and very preterm birth: Relationship to neuropsychological outcome. Brain. 2004;127(Part 9):2080–2089. doi: 10.1093/brain/awh230. [DOI] [PubMed] [Google Scholar]
- O'Callaghan MJ, Burns YR, Gray PH, Harvey JM, Mohay H, Rogers YM, et al. School performance of ELBW children: A controlled study. Developmental Medicine & Child Neurology. 1996;38(10):917–926. doi: 10.1111/j.1469-8749.1996.tb15048.x. [DOI] [PubMed] [Google Scholar]
- Odegard TN, Farris EA, Ring J, McColl R, Black J. Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 2009;47(8–9):1972–1977. doi: 10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Ornstein M, Ohlsson A, Edmonds J, Asztalos E. Neonatal follow-up of very low birthweight/extremely low birthweight infants to school age: A critical overview. Acta Paediatrica Scandinavica. 1991;80(8–9):741–748. doi: 10.1111/j.1651-2227.1991.tb11943.x. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Research Bulletin. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Peterson BS. Brain imaging studies of the anatomical and functional consequences of preterm birth for human brain development. Annals of the New York Academy of Sciences. 2003;1008:219–237. doi: 10.1196/annals.1301.023. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF. Memory and processing speed in preterm children at eleven years: A comparison with full-terms. Child Development. 1996;67(5):2005–2021. [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ, Rose SA, Feldman JF, Jankowski JJ. A cognitive approach to the development of early language. Child Development. 2009;80(1):134–150. doi: 10.1111/j.1467-8624.2008.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: A cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222(1):212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott FE, Mechelli A, Allin MP, Walshe M, Rifkin L, Murray RM, et al. Very preterm adolescents show gender-dependent alteration of the structural brain correlates of spelling abilities. Neuropsychologia. 2011;49(9):2685–2693. doi: 10.1016/j.neuropsychologia.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals. 4th. Toronto, Canada: The Psychological Corporation/A brand of Harcourt Assessment, Inc; 2003. [Google Scholar]
- Skranes J, Lohaugen GC, Martinussen M, Indredavik MS, Dale AM, Haraldseth O, et al. White matter abnormalities and executive function in children with very low birth weight. Neuroreport. 2009;20(3):263–266. doi: 10.1097/wnr.0b013e32832027fe. [DOI] [PubMed] [Google Scholar]
- Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130(Part 3):654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:1. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Soria-Pastor S, Gimenez M, Narberhaus A, Falcon C, Botet F, Bargallo N, et al. Patterns of cerebral white matter damage and cognitive impairment in adolescents born very preterm. International Journal of Developmental Neuroscience. 2008;26(7):647–654. doi: 10.1016/j.ijdevneu.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Strang-Karlsson S, Andersson S, Paile-Hyvarinen M, Darby D, Hovi P, Raikko-nen K, et al. Slower reaction times and impaired learning in young adults with birth weight < 1500 g. Pediatrics. 2010;125(1):e74–82. doi: 10.1542/peds.2009-1297. [DOI] [PubMed] [Google Scholar]
- Takao H, Hayashi N, Ohtomo K. White matter asymmetry in healthy individuals: A diffusion tensor imaging study using tract-based spatial statistics. Neuroscience. 2011;193:291–299. doi: 10.1016/j.neuroscience.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Van Lierde KM, Roeyers H, Boerjan S, De Groote I. Expressive and receptive language characteristics in three-year-old preterm children with extremely low birth weight. Folia Phoniatrica et Logopedica. 2009;61(5):296–299. doi: 10.1159/000238401. [DOI] [PubMed] [Google Scholar]
- van Noort-van der Spek IL, Franken MCJP, Weisglas-Kuperus N. Language functions in preterm-born children: A systematic review and meta-analysis. Pediatrics. 2012;129(4):745–754. doi: 10.1542/peds.2011-1728. [DOI] [PubMed] [Google Scholar]
- Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk AM, Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. NeuroImage. 2006;32(4):1538–1548. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurology. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. http://dx.doi.org/10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation/A brand of Harcourt Assessment, Inc; 1999. [Google Scholar]
- Woodcock RW. Theoretical foundations of the WJ-R measures of cognitive ability. Journal of Psychoeducational Assessment. 1990;8:231–258. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock–Johnson III tests of achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. New England Journal of Medicine. 2006;355(7):685–694. doi: 10.1056/NEJMoa053792. see comment. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Rykhlevskaia E, Sherbondy AJ, Deutsch GK, Wandell BA, et al. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. Journal of Cognitive Neuroscience. 2011;23(11):3304–3317. doi: 10.1162/jocn_a_00061. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung A, Poon G, Qiu DQ, Chu J, Lam B, Leung C, et al. White matter volume and anisotropy in preterm children: A pilot study of neurocognitive correlates. Pediatric Research. 2007;61(6):732–736. doi: 10.1203/pdr.0b013e31805365db. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.