Abstract
Most Forkhead box P3+ (Foxp3+) CD4 regulatory T cell (Treg) precursors are newly formed thymocytes that acquire Foxp3 expression on antigen encounter in the thymus. Differentiation of Treg, however, can also occur in the periphery. What limits this second layer of self- and nonself-reactive Treg production in physiological conditions remains to be understood. In this work, we tested the hypothesis that, similarly to thymic Treg, the precursors of peripheral Treg are immature T cells. We show that CD4+CD8−Foxp3− thymocytes and recent thymic emigrants (RTEs), contrarily to peripheral naïve mature cells, efficiently differentiate into Treg on transfer into lymphopenic mice. By varying donor and recipient mice and conducting ex vivo assays, we document that the preferential conversion of newly formed T cells does not require intrathymic preactivation, is cell-intrinsic, and correlates with low and high sensitivity to natural inhibitors and inducers of Foxp3 expression, such as IL-6, T-cell receptor triggering, and TGF-β. Finally, ex vivo analysis of human thymocytes and peripheral blood T cells revealed that human RTE and newly developed T cells share an increased potential to acquire a FOXP3brightCD25high Treg phenotype. Our findings indicating that RTEs are the precursors of Tregs differentiated in the periphery should guide the design of Treg-based therapies.
Keywords: tolerance, development, lymphopoiesis, polarization
Forkhead box P3 (Foxp3) -expressing CD4 regulatory T cells (Tregs) are antigen-specific suppressor cells that prevent autoimmunity and allergy, dampen protective responses, and restrict associated immunopathologies (1). Although never directly shown, it is predicted that tight regulation of Treg production and expansion is required to prevent immune paralysis while ensuring tolerance to tissue antigens. Treg were first shown to differentiate in the thymus from newly developed thymocytes on T-cell receptor (TCR) engagement by antigen expressed and/or presented locally (2). Time and space constraints defined by intrathymic development would suffice to explain the restricted domain of specificity and physiological competence of Treg reaching the periphery. Hence, the thymic Treg (tTreg) reactive repertoire is necessarily restricted to self-antigens, including peripheral tissue-specific gene products expressed promiscuously by thymic epithelial cells (3), and peripheral antigens accessing the thymus (4, 5). It is now well-documented, however, that Treg can also differentiate in the periphery (pTreg) in both therapeutic and physiologic settings. Antigen-specific pTregs were reported on s.c. administration of peptides (6) or exposure to intestinal food (7) or microbiota (8) antigens. Peripheral de novo differentiation of Treg was also documented during pregnancy (9) and in conditions of lymphopenia (10). Hence, pTreg differentiation may complement tTreg self-reactive repertoire by including reactivities against those self-antigens not expressed in the thymus, but it may also recruit clones that are reactive to fully foreign antigens. It follows that the mechanisms limiting extrathymic Treg differentiation in physiological conditions are essential to ensure tolerance while maintaining protective immune responses. Such mechanisms remain to be elucidated, and it can be anticipated that interfering with these processes would help to improve the development of therapies that aim at enhancing or inhibiting de novo Treg differentiation, such as in autoimmune diseases and transplantation or cancer, respectively.
In the last few years, several studies have addressed differences between tTreg and pTreg both phenotypically and functionally as well as concerning the signals required for their differentiation. This body of work, however, does not provide a consensual picture. Thus, expression of various membrane and nuclear molecules tentatively subdivided tTreg and pTreg in some but not other experimental settings (8, 11, 12). Epigenetic marks, notably CG methylation at the Foxp3 regulatory sequences, were shown to dissociate tTreg from in vitro-induced Treg (iTreg) but not pTreg (13). Controversies also arose as to whether TGF-β is required for Foxp3 induction solely in peripheral cells, notably through engagement of the smad3 motif in the CNS1 regulatory sequence 5′ of the coding region (14–17). Overall, tTreg and pTreg share many features, among which is a similar two-step process for their differentiation from TCR to IL-2 signaling (18–20). Because these signals are abundant in the periphery, physiological limitation of pTreg production should rely on other features.
In this work we hypothesized that the precursors of pTreg are limiting and similarly to tTreg, that they are recently developed T cells. Adoptive transfer experiments in lymphopenic mice indicated that thymocytes as well as recent thymic emigrants (RTEs) readily acquire a Treg phenotype in the periphery. In contrast, very few peripheral mature resident naïve cells, if purged of RTEs by either thymectomy or cell sorting, ever converted to Foxp3+ Treg in this assay. T-cell maturation stage also conditioned in vitro-induced Foxp3 expression in both mice and humans. The decreased susceptibility of mature cells to acquire a Treg phenotype was further correlated with their increased sensitivity to inhibitors, such as IL-6, and decreased sensitivity to facilitators, such as TGF-β. Together, our results show that T-cell maturation stage conditions de novo Foxp3 expression and that RTEs are the preferential or exclusive precursors of Tregs differentiated in the periphery. These findings provide a rationale for limited pTreg in physiology and raise specific concerns for the success of pTreg-based therapies in all situations of limited thymopoiesis.
Results
Newly Developed CD4 T Cells Are Enriched in Precursors of Tregs.
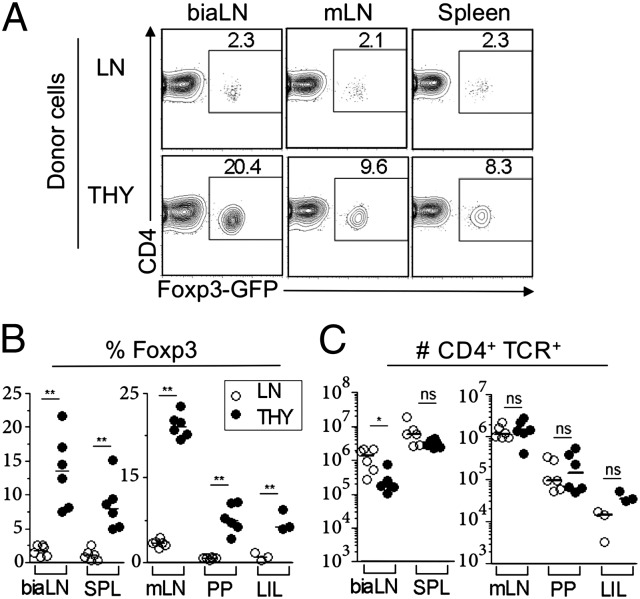
To directly test whether maturation stage correlates with CD4 T-cell ability to acquire Foxp3 expression in vivo, we first compared newly formed single-positive (SP) CD4 thymocytes with peripheral CD4 cells isolated from lymph nodes (LNs) in assays of lymphopenia-induced Treg differentiation (8). The two cell subsets were isolated from Foxp3-gfp reporter mice (21), purified as GFP−, and separately injected i.v. into T cell-deficient animals (TCRβ−/−). Because donor and recipient mice were raised in strict specific pathogen free conditions, adoptive transfer did not result in noticeable pathology for at least 8 wk. Cellular analysis was performed at 4 wk posttransfer, and donor cells were identified as CD4+ TCRβ+ (Fig. 1). As described earlier (8), a small but readily detectable fraction of LN cells acquired Foxp3 expression, never exceeding 2.5% of the recovered cells. Strikingly, this frequency was 4- to 10-fold higher in recipients of CD4 SP thymocytes in all lymphoid organs analyzed, including those LNs draining the intestine (Fig. 1 A and B). Skin-draining LNs, such as brachial, inguinal, and axillary, analyzed either pooled or separately appeared as preferential sites for Foxp3+ cell differentiation and/or accumulation. Importantly, thymocytes expanded less than CD4 LN cells in these organs but equally well in other sites (Fig. 1C); however, thymocytes invariably gave rise to more Foxp3+ cells than LN cells (Fig. S1A shows the summary of six independent experiments). We ascertained that our results did not owe to trivial cell-sorting artifacts by performing voluntary contamination experiments (Fig. S1B). We also tested and evidenced that resident lymphocytes in WT recipient mice rendered mildly lymphopenic the day before injection do not interfere with the preferential capacity of thymocytes to acquire Foxp3 expression (Fig. S1C). Together, these results establish that thymocytes are selectively prone to undergo true de novo differentiation into a Foxp3+ phenotype after reaching the periphery.
Fig. 1.
Thymocytes are enriched in precursors of Tregs that differentiate in the periphery on lymphopenia. TCRβ−/− mice received i.v. 3 × 105 CD4+CD8−Foxp3− cells purified from either thymi (THY; black symbols) or pooled lymph nodes (LNs; white symbols) isolated from Foxp3-gfp reporter mice and analyzed 4 wk later by FACS. (A) Foxp3 expression in gated CD4+TCR+ lymphocytes from the indicated organs in recipient mice. (B and C) Frequency of Foxp3+ cells within CD4+TCR+ lymphocytes (B) and number of CD4+TCR+ cells (C) recovered in one experiment (representative of seven repeats) (Fig. S1). Each dot represents one mouse. biaLN, pooled branchial, inguinal, and axillary LN; LIL, large intestine lamina propria and intraepithelial lymphocytes; mLN, pooled mesenteric LN; PP, pooled payer patches; spl, spleen. Note the different scales in Left and Right. *P < 0.05; **P < 0.01, Mann–Whitney test. LIL data in B: P value of 0.0059, t test.
Peripheral Tregs Derived from Thymocytes and LN Cells Are Functionally and Phenotypically Indistinguishable.
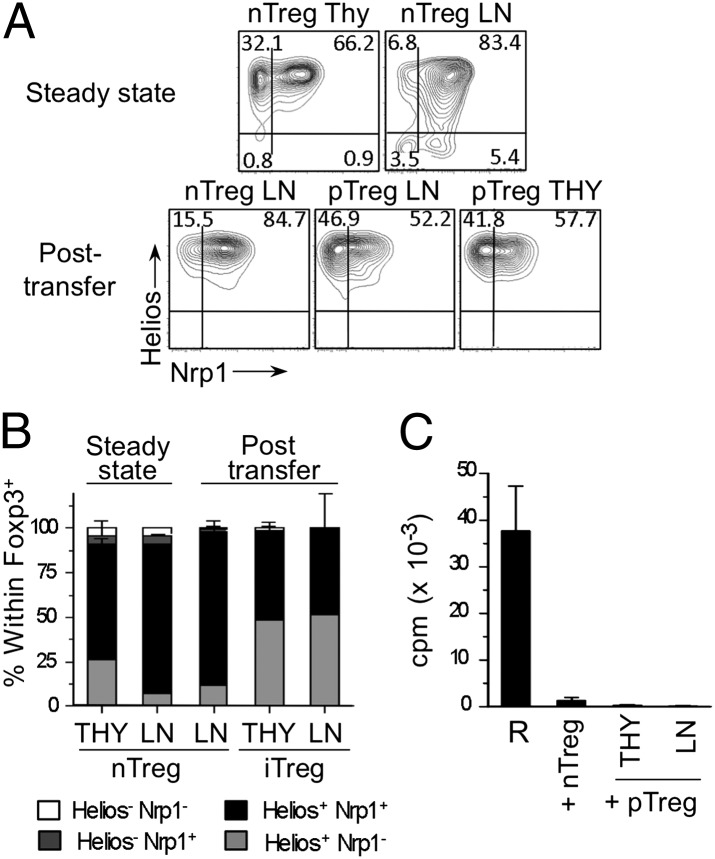
Several surface markers have been proposed to discriminate Tregs that were generated in the thymus or induced in the periphery. We tested whether Foxp3+ cells originated from thymocytes or LN cells in the experiments above either differ or share phenotypes. Thymic and peripheral cells from unmanipulated WT mice served as references. An additional control consisted of in vivo expanded Tregs obtained from TCRβ−/− mice that had received a mixture of Thy1.2 Foxp3+ and Thy1.1 Foxp3− cells isolated from LNs of unmanipulated WT mice 4 wk earlier. Pairwise analysis of the surface markers CD103 and killer cell lectin-like receptor subfamily G member 1 (KLRG1) or glucocorticoid-induced TNFR family related gene (GITR) and CD25 revealed no differences between Foxp3+ cells that differentiated from either LN cells or thymocytes in conditions of lymphopenia (Fig. S2). These two populations shared a phenotype resembling the previously described induced Treg [that is, both were enriched in CD103+KLRG1+ (11) and GITR+CD25+ (12) cells], thus clearly distinguishable from tTreg and pTreg at steady state but strikingly similar to in vivo expanded Treg. Analysis of Helios and Nrp1 expression also did not discriminate LN- or thymocyte-derived Treg in our adoptive transfers (Fig. 2 A and B). However, both cell subsets displayed a phenotype similar to thymic Treg, namely Helios+, with a clear Nrp1− subpopulation (12) that was only residual in Tregs found in the periphery, whether at steady state or expanded in vivo. Finally, a classical proliferation assay confirmed that both thymocyte- and LN-derived Foxp3+ cells are suppressors (Fig. 2C). Together, these analyses indicate that Foxp3+ cells developed from thymocyte or LN cells in lymphopenic hosts are remarkably similar to bona fide Treg.
Fig. 2.
Thymocytes and LN cells converted into Treg on lymphopenia are functionally and phenotypically indistinguishable. (A and B) FACS analysis for Helios and Nrp1 expression in gated Foxp3+CD4+TCR+ cells (pTreg) from the spleens of animals treated as in Fig. 1A (A) and distribution of each cell subset (B). Three populations of Foxp3+ natural Tregs (nTregs) served as control: thymic (THY) and peripheral (LN) cells from unmanipulated mice (steady state) and Thy1.2 peripheral cells from TCRβ−/−–recipient mice that received a mixture of Thy1.1 Foxp3− and Thy1.2 Foxp3+ LN cells at a 9:1 ratio (nTregLN; posttransfer) 4 wk earlier. (C) Standard suppression assay. Naïve Foxp3− cells (R) were mixed at a 2:1 ratio with either LN nTreg or pTreg differentiated in vivo as in Fig. 1.
Enhanced Susceptibility of Thymocytes to Differentiate into Treg Is a Cell-Intrinsic Property.
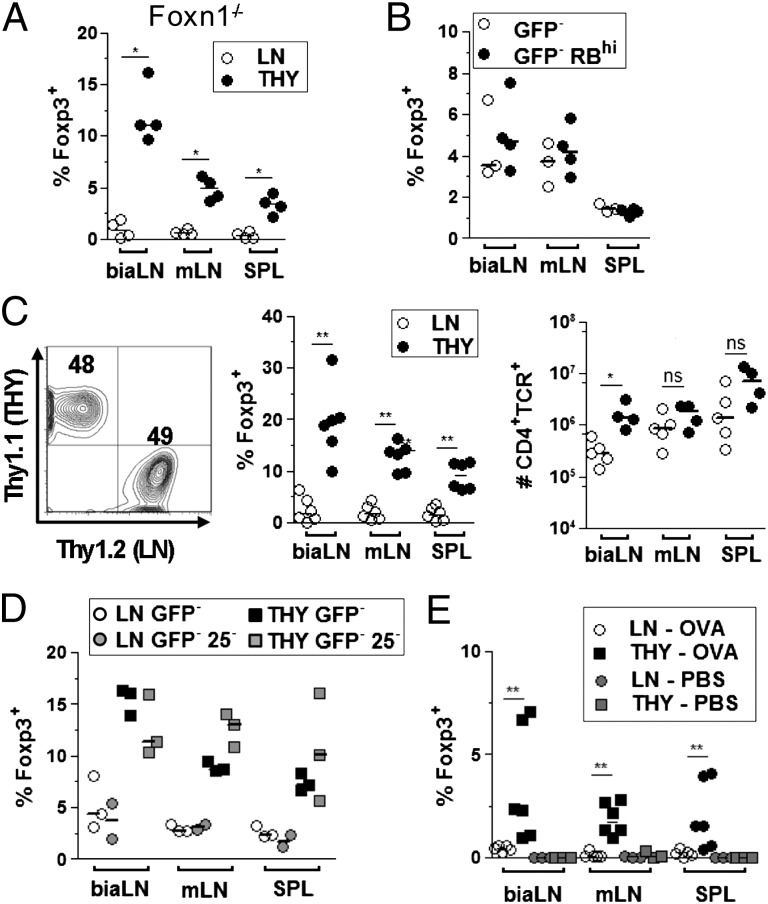
We next tested whether the differential capacity of thymocyte and peripheral cells to acquire Foxp3 expression was controlled by environmental factors. We first addressed whether Treg differentiation relied on donor cell recirculation to the thymus, a process for which thymocytes might be more competent. Transfer of thymocytes or LN cells into athymic nude Foxn1−/− animals reproduced the results obtained in TCRβ−/− recipients, excluding a contribution of the thymus (Fig. 3A). Secretion of proinflammatory cytokines by activated T cells has been proposed to limit naïve T-cell conversion into Treg in other systems (22). It was conceivable that LN preparations, enriched in naturally activated cells compared with thymocytes, would produce more of these inhibitory factors on lymphopenia-induced proliferation. However, adoptive transfers of peripheral CD4+Foxp3− cells, either unfractionated or purged of activated cells (CD45RBhi), resulted in similar low frequencies of Foxp3+ cells (Fig. 3B and Fig. S3A). Moreover, in mice recipient of both thymocytes and LN cells, each prepared from Thy1 congenic donors and mixed at a 1:1 ratio, thymocytes again gave rise to 5- to 10-fold higher frequency of Treg compared with LN cells (Fig. 3C). These results exclude specific modifications of environmental factors on lymphopenia as an explanation for thymocyte and peripheral cell differential conversion to Treg.
Fig. 3.
Enhanced susceptibility of thymocytes to differentiate into Treg in vivo is a cell-intrinsic property. Adoptive transfers as in Fig.1 with the following alterations. (A) Recipients were athymic nude Foxn1−/− mice. (B) Donor cells were from LN purged (GFP−RBhi) or not (GFP−) of activated cells by selecting CD45RBhigh cells (Fig. S3). (C) LN and thymocyte donor cells were isolated from Thy1.1 and Thy1.2 Foxp3-gfp mice, respectively, and coinjected at a 1:1 ratio (Left). Analyses were gated on Thy1.1+ (LN) or Thy1.2+ (THY). (D) Donor cells were either total (GFP−) or purged of potential precommitted CD25+ cells (GFP−25−) as illustrated in Fig. S3. (E) Donor cells were isolated from OTII Foxp3-gfp Rag2−/− animals. TCRβ−/−-recipient mice received 0.1 mg OVA or PBS i.v. the day before and 5 d after adoptive transfer. Analysis was performed at day 8. Shown is one of at least two experiments for each condition. *P < 0.05; **P < 0.01.
Because the thymus is the site of natural Treg differentiation, it was plausible that our thymocyte preparations were enriched in precommitted Foxp3− Treg. Expression of CD25 by Foxp3− cells has been proposed to indicate an early step along the Treg differentiation pathway resulting from TCR triggering (18–20). Depleting CD25+ cells from thymocyte and LN cell preparations before adoptive transfer (Fig. S3B) did not modify the frequency of recovered Treg from either donor population (Fig. 3D). Incidentally, these latter experiments provided the experimental design to exclude that our findings would be because of the fusion Foxp3-gfp allele, recently shown to be an occasional hypomorph, of potentially unstable expression (23, 24). Exclusion of Foxp3+ cells from WT lymphocytes by selecting CD45RBhighCD25− LN cells and CD25− thymocytes before adoptive transfer fully reproduced the differential phenotype (Fig. S3C). To further exclude antigen-triggered precommitted Treg in our thymocyte preparations, we performed adoptive transfer of monoclonal TCR transgenic cells specific for the foreign antigen ovalbumin (OVA) never exposed to their nominal antigen. Recipient TCRβ−/− mice were injected i.v. with either PBS or endotoxin free OVA the day before and 5 d after cell infusion. Strikingly, antigen-dependent induction of Foxp3 expression was efficient in mice that received thymocytes but barely detectable in animal recipients of LN cells (Fig. 3E). These results indicated that neither intrathymic preselection nor specific repertoire features sufficed to explain the increased susceptibility of thymocytes to differentiate into Treg. From this set of experiments, we conclude that cell-intrinsic properties account for the differential susceptibility of thymocytes and LN cells to acquire Foxp3 expression.
Recent Thymic Emigrants Are the Precursors of Tregs That Differentiate in Conditions of Lymphopenia.
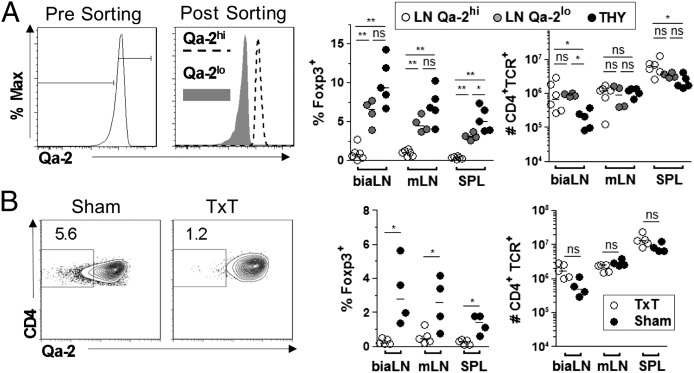
The results above show that thymocytes are selectively prone to undergo de novo differentiation into Treg after exposed to the periphery. We next tested whether naturally exported thymocytes (i.e., RTEs) share similar features (Fig. 4). CD4+SP cells naturally exit the thymus before full maturation and complete their differentiation in the periphery (25). Consistent with their intermediate maturation stage, recent thymic emigrants (RTEs) can be identified through their intermediate levels of expression for CD24 and Qa-2 (25) compared with thymocytes and peripheral mature cells (Fig. S4A). On transfer into TCRβ−/− mice, recent thymic emigrants, isolated as Foxp3− Qa-2lo cells from LN preparation, gave rise to similar frequencies and numbers of Foxp3+ cells as thymocytes. In contrast, peripheral mature cells purged of RTEs (Foxp3− Qa-2hi) showed only a residual capacity to differentiate into Treg, which was indicated by less than 1% Foxp3+ cells in the recovered CD4 T lymphocytes (Fig. 4A).
Fig. 4.
Recent thymic emigrants are the main precursors of peripherally differentiated Tregs. Adoptive transfers as in Fig.1 with the following alterations. (A) Donor LN cells were either purged or enriched for Qa-2lo RTEs (Left) before adoptive transfer (Right). (B) Donor mice were TxT or sham 2–3 mo before LN CD4+Foxp3− cells were purified (Left) and transferred to TCRβ−/−-recipient mice (Right). *P < 0.05; **P < 0.01.
To confirm that most or all Treg progenitors in the periphery are encompassed in the RTE subset, we tested LN cells prepared from mice naturally purged of RTE by prior thymectomy (Fig. 4B and Fig. S4B). Donor mice were thymectomized (TxT) or as a control, submitted to the surgery procedure except for thymus removal (sham) and left to rest for at least 2 mo. At this time point, preexisting RTEs in TxT mice had incorporated the mature T-cell pool as indicated by the absence of Qa2lo cells in the peripheral organs In support of our hypotheses, de novo differentiated Foxp3+ cells were not at all or only barely detectable in mice that had received cells from thymectomized donors, whereas as expected, cells from sham-operated donors converted into Treg at low but readily detectable levels. Taken together, these results confirm that maturation stage defines T-cell susceptibility to acquire Foxp3 expression and show that RTEs are the main, if not the exclusive, precursors of Treg differentiating in the periphery under conditions of lymphopenia.
Peripheral T-Cell Maturation Associates with Altered Sensitivity to Signals Modulating Treg Differentiation in Vitro and in Vivo.
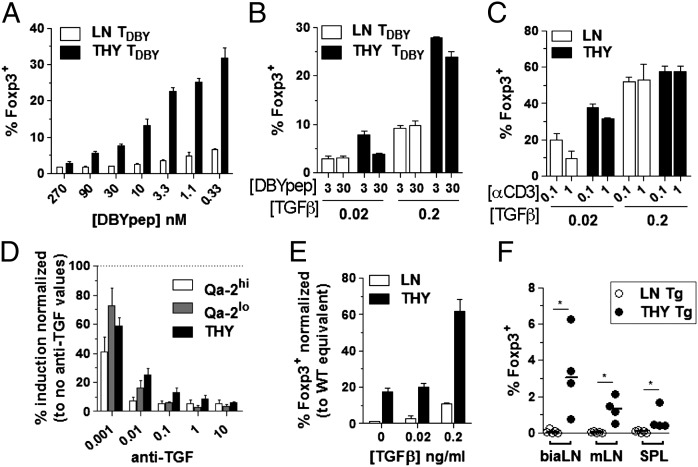
Our results above indicating that cell-intrinsic features restrict Foxp3 induction in mature cells suggested that peripheral maturation alters T-cell sensitivity to signals either favoring or inhibiting Foxp3 induction. To test these possibilities, we first assessed the sensitivity of each T-cell subset to bona fide inducers of Foxp3 in vitro, namely TCR triggering and TGF-β. Monitoring the response of anti-minor histocompatibility Y antigen (HY) TCR transgenic (tg) cells to broad-range titration of their nominal peptide (Fig. 5A) confirmed that the lower the TCR triggering, the larger the frequency of cells acquiring Foxp3 expression (26, 27). More importantly, the slope of the dose–response obtained in thymocyte cultures was much steeper than the slope in peripheral cells, indicating differential sensitivity to TCR triggering. Decreased sensitivity of mature cells to TGF-β was evidenced in three independent in vitro assays. First, it was evidenced by monitoring cultures of monoclonal or polyclonal cells stimulated by the nominal peptide or anti-CD3 Ab in the presence of varying concentrations of exogenous TGF-β (Fig. 5 B and C). Second, it was evidenced by performing titration of TGF-β–neutralizing antibodies to reach suboptimal concentrations of TGF-β (Fig. 5D). Third, it was evidenced by testing cells prepared from donor mice expressing a dominant negative form of the TGF-β receptor II (28) that are partially impaired in signaling (Fig. 5E). Finally, analysis of recipient mice that received thymocytes or LN cells from the same dnTGF-βRII donors confirmed that TGF-β signaling promotes Treg induction during lymphopenia (Fig. 5F).
Fig. 5.
Enhanced sensitivity of immature T cells to TGF-β–dependent induction of Foxp3 expression in vitro. (A and B) CD8− CD4+ thymocytes and LN cells isolated from female Rag−/−Foxp3-gfp anti-HY TCR tg mice were set in cultures containing T cell-depleted splenocytes (spAPCs), IL-2, and TGF-β at 0.2 ng/mL (A) or as indicated (B) and the nominal peptide at the indicated concentration (nM). (C) Polyclonal CD8−CD4+Foxp3− cells stimulated as in B except for anti-CD3Ab (μg/mL) instead of peptide. (D) Polyclonal LN cells fractionated as mature resident cells (Qa-2hi) or RTEs (Qa-2lo) were cultured in the presence of spAPCs, 0.1 µg/mL anti-CD3 Ab, IL-2, and the indicated concentration (μg/mL) of neutralizing anti–TGF-β Abs. Results are expressed as percentage of response in the absence of neutralizing Abs. (E and F) Peripheral (LN) and thymic (THY) CD8−CD4+Foxp3− cells were purified from Foxp3-gfp TGF-βRIIdn tg double mutant mice and cultured (E) as in C or transferred (F) to TCRβ−/− mice as in Fig.1. *P < 0.05.
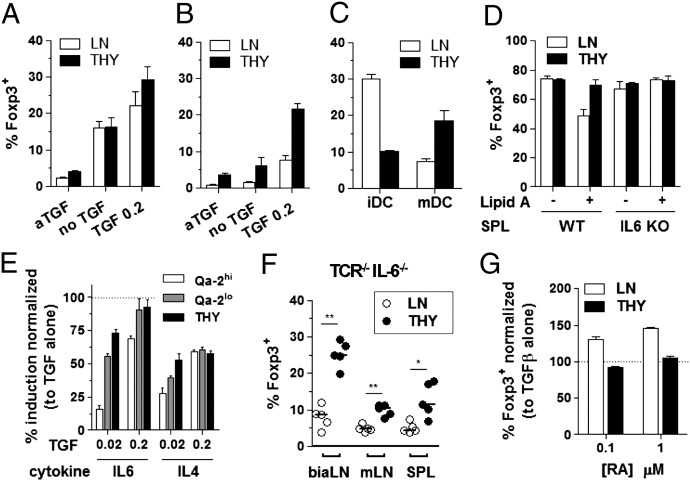
We next assessed the sensitivity of each T-cell subset to inflammatory signals known to inhibit Foxp3 induction (29, 30) (Fig. 6). The acquisition of Foxp3 expression by LN cells but not thymocytes was reduced in cultures containing preactivated instead of immature antigen-presenting cells (APCs) (Fig. 6 A–D). This effect was detectable in cultures supplemented or not with IL-2 (Fig. 6 A and B vs. Fig. 6C). In the latter conditions, mature cells converted better than thymocytes when stimulated with immature dendritic cells (iDC), a result likely related to the reduced ability of immature T cells to produce IL-2 on TCR engagement (25). In agreement with this proposition, activation of APCs enhanced thymocytes conversion, presumably by increasing their IL-2 production. However, mature T-cell conversion was inhibited by APC activation. This inhibitory effect was mediated by IL-6, because it was lost in cultures seeded with APC from IL-6−/− mutants (Fig. 6D). We further tested and confirmed that Qa-2lo RTEs, similarly to newly formed thymocytes (5), are less sensitive than mature T cells to IL-6 in an APC-free system, where IL-6 could be titrated (Fig. 6E). In agreement with these results, the frequency of peripheral cells that converted on adoptive transfer into TCRβ−/−IL-6−/− double mutant mice was increased compared with IL-6–sufficient recipients. However, irrespective of IL-6 deficiency, thymocytes performed better than LN cells at acquiring Foxp3 expression in vivo (Fig. 6F and Fig. S5 A and B), confirming that other features limit mature cell conversion. Among other cytokines that could affect Foxp3 induction, only IL-4 and to a lower extent, TNFα showed a noticeable inhibitory effect that affected mature more than immature T cells, possibly explained by the lower level of surface receptor expression (Fig. 6E and Fig. S5 C and D). We next tested the effect of retinoic acid, known to enhance Treg differentiation, both in vitro and in vivo (31) and revealed that it did not affect thymocytes (Fig. 6G). From this set of experiments, we conclude that T-cell maturation associates with several features impairing their susceptibility to acquire Foxp3 expression.
Fig. 6.
Enhanced sensitivity of mature T cells to modulators of Foxp3 induction. CD8−CD4+Foxp3− cells purified as in Fig. 1 were set in cultures containing anti-CD3 Ab at 0.1 µg/mL and supplemented with varying APCs and/or compounds. (A and B) APCs were either immature (A) or activated (B) bone marrow-derived dendritic cells (DCs), and media were supplemented with IL-2 and either neutralizing anti–TGF-β Ab or TGF-β. (C) The same as A and B, except that media did not contain IL-2 and TGF-β was fixed at 0.2 ng/mL. (D) The same as A and B, except that spAPCs were from either WT or IL-6−/− mice and media contained IL-2 and TGF-β (0.2 ng/mL). (E) LN cells were fractionated as mature resident cells (Qa-2hi) and RTEs (Qa-2lo), plates were coated with anti-CD3 Abs, and media were supplemented with IL-2 as well as the indicated cytokines. Shown is normalized frequency (%) of Foxp3+ cells in TGF-β and IL-6 or IL-4 conditions relative to TGF-β alone. (F) Adoptive transfers as in Fig. 1, except that recipient mice were TCRβ−/− IL-6−/− double mutants. (G) APCs were naïve spAPCs, and media contained IL-2, 0.2 ng/mL TGF-β, and the indicated concentration of retinoic acid (RA). *P < 0.05; **P < 0.01.
Human RTEs Are More Susceptible than Mature Cells to Differentiate into Treg.
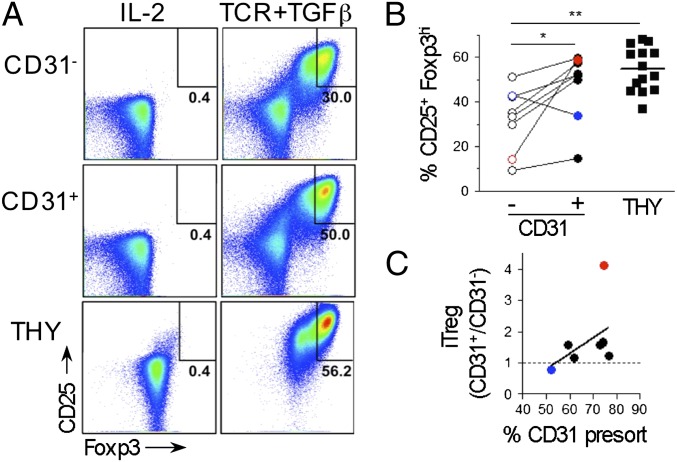
We next tested whether our findings, indicating that peripheral maturation limits T-cell susceptibility to differentiate into Treg, can be extended to humans. Human naïve CD4+CD25−CD127hi lymphocytes are devoid of Foxp3-expressing cells and can acquire Foxp3 expression and suppressive functions in vitro on stimulation with anti-CD3 in the presence of TGF-β (Fig. 7 and Fig. S6). Among these cells, FOXP3brightCD25high are bona fide Treg (32). Human RTEs are enriched in the CD31+ cell subset (33) that represents 50–80% of the naïve cells in peripheral blood of healthy donors (Fig. S6), a variation previously associated with age and thymic involution (34). We tested CD4SP CD25−CD127hi thymocytes isolated from patients undergoing heart surgery in parallel with CD31+ and CD31− naïve cells both isolated from blood of the same healthy donor for their capacity to acquire a Treg phenotype in vitro (Fig. 7A). A pairwise comparison revealed that seven of eight donors analyzed showed more efficient conversion when CD31+ instead of CD31− cells were tested (Fig. 7B). Noticeably, the percentage of CD31+ in total naïve cells presorting correlated with the increased efficiency of this cell subset to acquire Foxp3 expression, a result that we interpret as reflecting proportional enrichment in RTEs (Fig. 7C). Finally, analysis of thymocytes compared with peripheral blood cells also supported the notion that, in human as in mice, peripheral maturation affects T-cell susceptibility to differentiate into Treg.
Fig. 7.
Preferential differentiation of human thymocytes and RTEs into Treg ex vivo. Human thymocytes and peripheral blood mononuclear cells sorted as CD8−CD4+CD25−CD127hi, and for peripheral blood mononuclear cells, according to CD31 expression, they were cultured for 5 d in media containing IL-2 alone or supplemented with plate-bound anti-CD3 mAbs, soluble anti-CD28 mAbs, and TGF-β (TCR+TGF). (A) FACS analysis within live lymphocytes after culture. (B) Frequency of induced FOXP3brightCD25high. Each pair of CD31−/CD31+ represents one blood donor (paired t test). Thymocytes were from another cohort (n = 14). (C) Correlation between the frequencies of CD31+ cells among naïve cells before sorting and the fold increase in iTreg frequency comparing CD31+ with CD31− cell cultures. Colored symbols highlight the highest and lowest responders in B and C. Spearman coefficient test: r and P were 0.5238 and 0.0983, respectively. *P < 0.05; **P < 0.01.
Discussion
There is increasing hope that inducing de novo differentiation of Treg would offer a potential therapeutic solution for autoimmunity and allergy. However, preventing de novo Treg development is needed to improve tumor immunotherapies and vaccine efficacy. Our findings that extrathymic maturation of CD4 T cells limits their capacity to differentiate into Tregs in mice and humans have several consequences for our understanding of immune regulation and should be relevant for the rational development of therapeutic approaches.
We tested the hypothesis that T-cell maturation stage limits peripheral Treg differentiation by monitoring induction of Foxp3 expression under lymphopenic conditions. The lymphopenia-induced Foxp3 expression assay presents the advantage of testing polyclonal cells developed in a WT host for their spontaneous differentiation into Tregs. It also mimics the severe peripheral lymphopenia that takes place in various pathophysiological situations, such as on infection or in clinical settings (for instance, on high-dose corticosteroid administration). Moreover, the physiological relevance of pTreg generated during lymphopenia has been previously shown in a model of colitis (8). In addition, induction of Treg on lymphopenia is readily detectable, which allows for reliable quantitative measurements.
Our findings indicating that cell maturation restricts T-lymphocyte susceptibility to acquire a Treg phenotype are consistent with other studies concerned with thymic Treg differentiation. Hence, thymocytes from newborns were more readily differentiated into Tregs than thymocytes from adults (35). Moreover, thymic APCs were shown to be more potent at sustaining thymocyte than peripheral naïve cell conversion (36). Although these studies did not provide a molecular mechanism to these differences, our present work indicates that they relate to specific sensitivity to factors promoting (TCR and TGF-β) and inhibiting (IL-6) Treg differentiation. Others have already argued how unlikely it is that a single molecule or pathway would explain the functional differences between immature and mature T cells (37). Importantly, our findings are also compatible with the notion that mucosa, a microenvironment rich in TGF-β and retinoic acid where inflammation is maintained at check, efficiently supports the differentiation of Tregs (31, 38). The concept brought about by our analysis is that systemic peripheral conversion (i.e., in situations of limited retinoic acid and TGF-β and/or in inflammatory context) is restricted to RTEs.
Earlier works supported directly or indirectly the notion that RTEs preferentially mediate peripheral dominant tolerance [for instance, to mismatched graft (39) and myelin basic protein on oral administration in a preventive setting for encephalomyelitis (40)]. In contrast, the more recent literature on Foxp3+ cells seems to assume that any naïve cell can be a precursor of pTreg provided that they express the proper TCR specificity and are presented antigen in the right context. Intriguingly, only a small fraction of cells has been successfully induced to expressed Foxp3 in vivo in various assays, including in monoclonal TCR transgenic mice infused for long periods with the nominal peptide (6). Our present finding that RTEs, a limited cell subset in the periphery, are the preferential or exclusive precursors of pTreg explains this limitation. In turn, a limited pool of pTreg precursors should guarantee efficient immunity again pathogens. Our findings also provide a rationale to older notions linking ontogeny and immune tolerance. Thus, as shown already in the work by Billingham et al. (41) in 1953, immune tolerance prevails early in life. Moreover, after the development of a functional thymus, the peripheral pool of T cells is first formed by output from the thymus with little peripheral expansion (42). Hence, during perinatal development, peripheral T cells are RTEs, and as we show here, they are prone to differentiate into Treg, ensuring robust tolerance.
Finally, our work reveals a third mechanism by which the adult thymus contributes to peripheral tolerance. As first evidenced in the 1990s, selection of natural Treg takes place in the thymus on TCR triggering by self-antigens. As shown later, these self-antigens include the products of tissue-specific genes expressed by thymic epithelial cells (2). Moreover, recirculation of peripheral antigens to the thymus also shapes Treg repertoire (5). Lastly, as we show here, recently differentiated T cells have a limited developmental time window before full maturation in the periphery to readily acquire Foxp3, placing RTEs as the precursors of pTregs. Because the thymus involutes with age and is depleted in the course of infections and on administration of various drugs, these properties call for specific attention to thymic activities in the rational development of therapies to enhance or prevent immune tolerance.
Materials and Methods
SI Materials and Methods contains detailed description of (i) human donors and samples preparation, (ii) animals and methods of thymectomy, cell adoptive transfer, and antigen administration, and (iii) reagents and methods for cell purification, in vitro assays, FACS, and statistical analysis. Table S1 details antibodies used for FACS analysis and cell culture. Human and animal studies were approved by the Ethical Board of the Faculty of Medicine, Lisbon, the Institutional Ethical Committee, and the Portuguese Veterinary General Division.
Supplementary Material
Acknowledgments
We thank Drs. Miguel Abecasis and Rui Anjos from Hospital de Santa Cruz, Lisbon, Portugal, for human thymus sample collection. We also thank Ana Regalado and Catarina Martins for assistance with antibody production and mouse colonies maintenance; Rui Gardner and Manuel Rebelo for managing the FACS and animal house facilities; and Thiago Carvalho and António Coutinho for discussions and critical reading of the manuscript. This work was funded by the Portuguese Research Council (FCT) under Grant PTDC/SAU-IMU/113541/2009 (to I.C.), fellowships (to R.S.P., A.C.L., and M.-L.B.), and a contract (to I.C.) and the European Union’s 7th Framework Programme (FP7/2007-2013) under Grant 241447 [Natural Immunomodulators as Novel Immunotherapies for Type 1 Diabetes (NAIMIT); to J.D.].
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221955110/-/DCSupplemental.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2(4):301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 3.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2(11):1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009;206(3):607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelenay S, et al. Cutting edge: Intrathymic differentiation of adaptive Foxp3+ regulatory T cells upon peripheral proinflammatory immunization. J Immunol. 2010;185(7):3829–3833. doi: 10.4049/jimmunol.1001281. [DOI] [PubMed] [Google Scholar]
- 6.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199(10):1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mucida D, et al. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115(7):1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haribhai D, et al. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182(6):3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150(1):29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202(10):1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feuerer M, et al. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci USA. 2010;107(13):5919–5924. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haribhai D, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35(1):109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polansky JK, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38(6):1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9(6):632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 15.Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9(2):194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463(7282):808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med. 2012;209(9):1529–1535. doi: 10.1084/jem.20112646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28(1):100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burchill MA, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28(1):112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schallenberg S, Tsai PY, Riewaldt J, Kretschmer K. Identification of an immediate Foxp3(-) precursor to Foxp3(+) regulatory T cells in peripheral lymphoid organs of nonmanipulated mice. J Exp Med. 2010;207(7):1393–1407. doi: 10.1084/jem.20100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Hill JA, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29(5):758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bettini ML, et al. Loss of epigenetic modification driven by the Foxp3 transcription factor leads to regulatory T cell insufficiency. Immunity. 2012;36(5):717–730. doi: 10.1016/j.immuni.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darce J, et al. An N-terminal mutation of the Foxp3 transcription factor alleviates arthritis but exacerbates diabetes. Immunity. 2012;36(5):731–741. doi: 10.1016/j.immuni.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5(4):418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira VG, Caridade M, Paiva RS, Demengeot J, Graca L. Sub-optimal CD4+ T-cell activation triggers autonomous TGF-β-dependent conversion to Foxp3+ regulatory T cells. Eur J Immunol. 2011;41(5):1249–1255. doi: 10.1002/eji.201040896. [DOI] [PubMed] [Google Scholar]
- 27.Gabryšová L, et al. Integrated T-cell receptor and costimulatory signals determine TGF-β-dependent differentiation and maintenance of Foxp3+ regulatory T cells. Eur J Immunol. 2011;41(5):1242–1248. doi: 10.1002/eji.201041073. [DOI] [PubMed] [Google Scholar]
- 28.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12(2):171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 29.Korn T, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105(47):18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei J, et al. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci USA. 2007;104(46):18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 32.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 33.den Braber I, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36(2):288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Kimmig S, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195(6):789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G, et al. “Default” generation of neonatal regulatory T cells. J Immunol. 2010;185(1):71–78. doi: 10.4049/jimmunol.0903806. [DOI] [PubMed] [Google Scholar]
- 36.Wirnsberger G, Mair F, Klein L. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proc Natl Acad Sci USA. 2009;106(25):10278–10283. doi: 10.1073/pnas.0901877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fink PJ, Hendricks DW. Post-thymic maturation: Young T cells assert their individuality. Nat Rev Immunol. 2011;11(8):544–549. doi: 10.1038/nri3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nolting J, et al. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009;206(10):2131–2139. doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Modigliani Y, et al. Establishment of tissue-specific tolerance is driven by regulatory T cells selected by thymic epithelium. Eur J Immunol. 1996;26(8):1807–1815. doi: 10.1002/eji.1830260822. [DOI] [PubMed] [Google Scholar]
- 40.Song F, et al. The thymus plays a role in oral tolerance in experimental autoimmune encephalomyelitis. J Immunol. 2006;177(3):1500–1509. doi: 10.4049/jimmunol.177.3.1500. [DOI] [PubMed] [Google Scholar]
- 41.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 42.Modigliani Y, Coutinho G, Burlen-Defranoux O, Coutinho A, Bandeira A. Differential contribution of thymic outputs and peripheral expansion in the development of peripheral T cell pools. Eur J Immunol. 1994;24(5):1223–1227. doi: 10.1002/eji.1830240533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.