Abstract
Tissue progenitor cells are an attractive target for regenerative therapy. In various organs, bone marrow cell (BMC) therapy has shown promising preliminary results, but to date no definite mechanism has been demonstrated to account for the observed benefit in organ regeneration. Tissue injury and regeneration is invariably accompanied by macrophage infiltration, but their influence upon the progenitor cells is incompletely understood, and direct signaling pathways may be obscured by the multiple roles of macrophages during organ injury. We therefore examined a model without injury; a single i.v. injection of unfractionated BMCs in healthy mice. This induced ductular reactions (DRs) in healthy mice. We demonstrate that macrophages within the unfractionated BMCs are responsible for the production of DRs, engrafting in the recipient liver and localizing to the DRs. Engrafted macrophages produce the cytokine TWEAK (TNF-like weak inducer of apoptosis) in situ. We go on to show that recombinant TWEAK activates DRs and that BMC mediated DRs are TWEAK dependent. DRs are accompanied by liver growth, occur in the absence of liver tissue injury and hepatic progenitor cells can be isolated from the livers of mice with DRs. Overall these results reveal a hitherto undescribed mechanism linking macrophage infiltration to DRs in the liver and highlight a rationale for macrophage derived cell therapy in regenerative medicine.
Keywords: liver regeneration, adult stem cell
Tissue progenitor cells show promise as a regenerative therapeutic target in a variety of organs (1, 2). Within the liver, hepatocytes regenerate the liver efficiently but following severe injury hepatocyte regeneration may fail and a second regenerative compartment becomes activated. Ductular reactions (DRs) are seen in the liver and contain the population of hepatic progenitor cells (HPCs). DRs have been used to describe the activated biliary epithelial cells and their associated inflammatory reaction. Here we will use the term DR to refer to the epithelial component alone. HPCs are bipotential adult stem-like cells that are thought to reside in the smallest branches of the biliary tree and are defined through their capacity to differentiate under clonogenic conditions in vitro into hepatocytes and biliary epithelial cells (3, 4). DRs are found in almost all major forms of human liver disease, as well as in a variety of animal models of both chronic and severe acute liver injury (5). A correlation exists between the extent of liver disease and magnitude of accompanying DRs (6, 7). The therapeutic manipulation of DRs and HPCs offers the potential to regenerate severely injured liver without recourse to liver transplantation (8). Whereas transplantation of HPCs has been successful in rodents (9), isolation of human HPCs is not a practical therapeutic option (10). Manipulation of the liver’s endogenous DRs and HPCs represents a more realistic therapeutic approach (11, 12).
There is no accepted marker specific for HPCs as the markers that have been used to isolate HPCs are also expressed upon bile duct cells, and DRs. Cytokeratins (CK) 7 and 19, EpCAM (Epithelial Cell Adhesion Molecule), Dlk1 (Delta Like Homolog), MIC1-1C3, and Sox9 [sex determining region Y (SRY) box] are all accepted, albeit unspecific, markers of DRs, and have been used to define essential mediators, including many cytokines, which control the DR response to experimental liver injury (5, 13–15). Among the most recently identified of these paracrine signals is TNF-like weak inducer of apoptosis (TWEAK), a cytokine of the TNF family, which directly stimulates proliferation of DRs in vivo and HPCs in vitro (16, 17).
During liver injury, macrophages are recruited from the bone marrow (BM) to engraft within the liver adjacent to DRs (18). Regeneration of any organ in response to injury is accompanied by macrophage infiltration, which is believed primarily to clear cellular debris. However, following tissue injury macrophages have other roles in tissue repair, which include a role in influencing stem and progenitor cell behavior (4, 19).
Related work in other organs has reported that even in the absence of their own prolonged engraftment, BM-derived cells may stimulate tissue progenitor cells, a process that is speculated to be reliant on paracrine signaling (20). Nonetheless, interpretation of a direct paracrine pathway from BM-derived cells to tissue progenitor cells is complicated during tissue injury by the association of local inflammation and tissue remodeling, processes that are affected by BM-derived cells (21, 22) and that also indirectly influence tissue progenitor cell activation (23).
The BM represents a readily available, autologous source of cells for cell therapy. Recent studies in patients with liver injury have suggested that i.v. delivery of whole bone marrow cells (BMCs) may have beneficial effects, potentially improving liver regeneration and function (2). Earlier reports that BMCs could themselves adopt an epithelial phenotype in the liver have proven to be unfounded (24). A recent study by us in a chronic liver injury model has shown infusion of BM-derived macrophages improves fibrosis and is associated with improved liver function (22). However, a direct effect of macrophages upon the DRs or HPCs themselves has not been shown. The reduction of liver fibrosis itself is associated with improved hepatic regeneration (25) and the degree of matrix remodeling correlates to the magnitude of the DR response (23). Furthermore macrophages themselves are critical for the reduction in fibrosis (21). All these factors mean that the study of a direct effect of macrophages upon DRs becomes near impossible in the setting of chronic liver injury.
Therefore, we investigated the existence of a paracrine stimulation directly by BMCs upon ductular cells in normal murine liver. Here we report that BMC transfer activates the proliferation of DRs in the absence of liver injury. This has enabled the analysis of the direct effect of macrophages upon DRs in the absence of confounding matrix changes, inflammation, and tissue damage. This DR activation is due to paracrine, macrophage-derived, TWEAK signaling. The understanding of both the signaling mechanisms mediating DR activation, and the cell type responsible for this, may enable the development of novel therapies to stimulate liver regeneration.
Results
Transfer of Syngeneic BMCs to Healthy Mice Results in Ductular Activation in Healthy Recipient Mice.
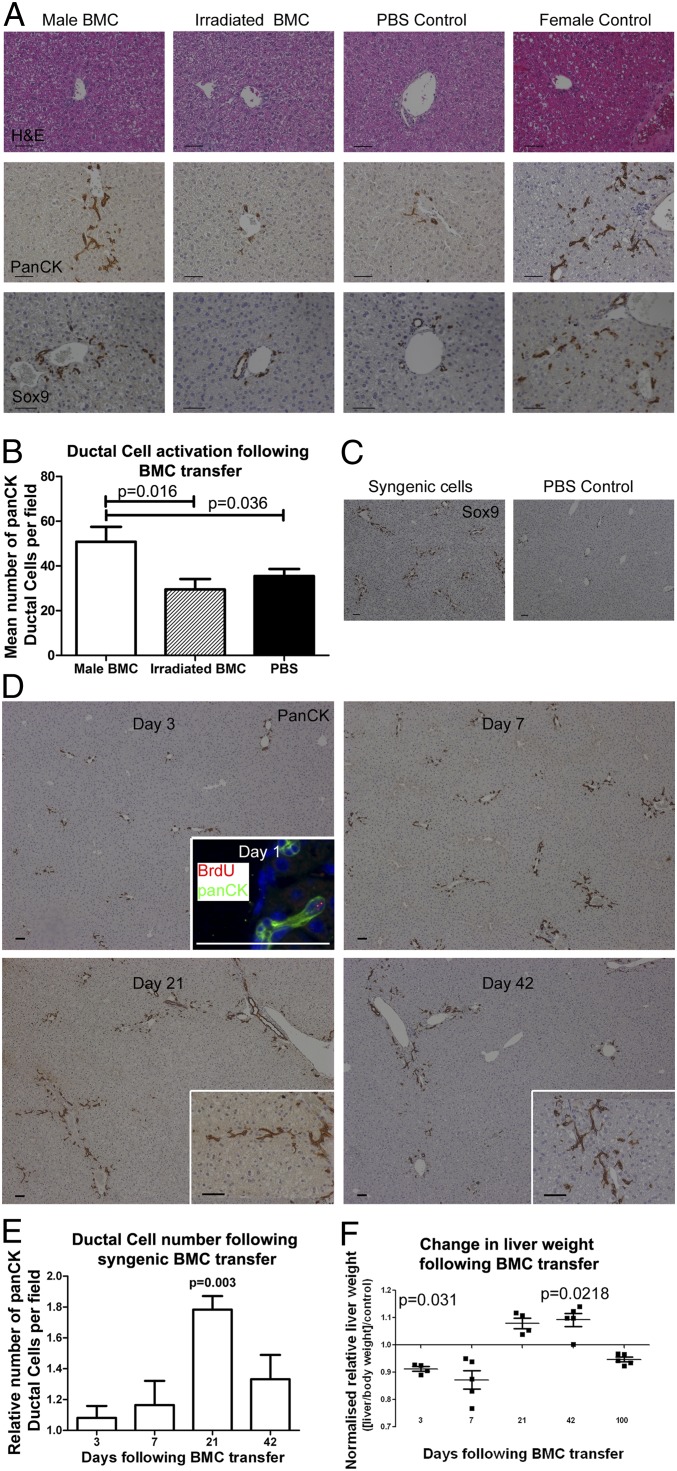
To allow tracking of donor-derived BMCs in uninjured recipient liver tissue, initial observations were made in female mice that received a single transfer of 107 unfractionated BMCs from male donors. These animals showed a consistent, periportal expansion of panCK+, Dlk1+, EpCAM+, and Sox9+ DRs, from day 7 post transfer, which lasted for up to 6 wk (Fig. 1A and Fig. S1A). No evidence of hepatocellular injury or inflammation was observed in recipient livers at any time point (Fig. 1A and Fig. S1A). Irradiation of BMCs before injection rendered them incapable of inducing ductular activation (Fig. 1 A and B and Fig. S1A), implying a requirement for functionally intact BMCs and arguing against the involvement of either a bystander reaction to debris or immunogenic antigens via a minor histocompatability reaction. Syngeneic (inbred female to female) cell transfer yielded an identical ductular activation to that observed in sex-mismatched transfer experiments (Fig. 1C). Again, ductular expansion began as early as 1 d following transfer and progressed over 3–6 wk (Fig. 1 D and E).
Fig. 1.
Ductular reactions occur following bone marrow cell (BMC) transfer. (A) Absence of injury with simultaneous expansion of panCK+/Sox9+ DRs in female mouse liver 42 d following tail vein injection of 107 BMCs, additional ductular markers (EpCAM and Dlk1 shown in Fig. S1A). Male BMC transfer experiments were repeated four times, each with n = 5 animals per group. (B) Quantification of DRs 42 d after transfer of 107 male BMCs. (C) Sox9+ (21 d) and (D) PanCK+ DRs in inbred female recipients of syngenic BMC transfer with corresponding DR quantification over time (E). Proliferation of DRs is seen as early as 1 d following cell transfer (Inset BrdU 2 h before tissue harvest), with progressive expansion over subsequent 3 wk. Data are presented as relative change in cells per 200× magnification field (experimental mean/time matched control mean) ± SEM. Mean panCK+ ductular cells 34.0 ± 2.4, 36.1 ± 4.9, 41.9 ± 2.1, and 35.6 ± 4.2 for days 3, 7, 21, and 42, respectively following BMC transfer. (F) Initial reduction in liver size is followed by increase in relative liver weight (liver weight/body weight)/(control liver weight/body weight) over the period of ductular expansion (n ≥ 4 each group). Data are presented as mean number of cells per field ± SEM. P values denote Student t test result vs. control.
Although liver weight fell initially following BMC transfer, relative liver size increased in association with ductular expansion, resulting in consistently larger livers 6 wk following BMC therapy versus PBS control (Fig. 1F). Whole liver albumin gene expression increased following BMC transfer, although later this gradually normalized (Fig. S1B). No long-term changes were seen in livers of mice followed for 400 d following BMC transfer (Fig. S1C). Identical ductular expansion was observed when BMCs from aged donors was delivered to healthy young mice (Fig. S1 D and E).
BM Transfer Results in Transient Liver Engraftment by Donor-Derived Macrophages.
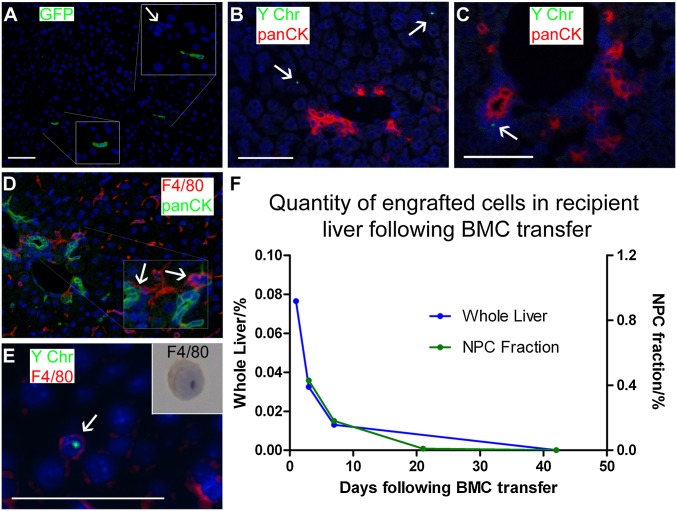
Two independent cell-tracking strategies were used to study BMC fate in recipient livers: Y chromosome detection in a sex-mismatched BMC therapy and GFP mismatch, whereby GFP+ donor BMCs were delivered to WT littermates. Both analyses found transient engraftment in the liver by donor-derived cells, with no evidence of transdifferentiation of donor BMCs into panCK+ DRs or hepatocytes (Fig. 2). Six hours and 1 d following transfer, donor cells were seen throughout all zones of the hepatic lobule with no specific localization (Fig. 2 A and B, respectively). From day 3, however, donor-derived cells were found directly adjacent to DRs and make physical contact with the panCK+ DRs (Fig. 2C), at which time F4/80+ macrophages also make contact with panCK+ DRs (Fig. 2D). To characterize the phenotype of engrafted donor-derived cells, FACS selection of GFP+ cells in WT liver was performed 3 d after BMC delivery from GFP+ donors. The resultant cell isolate was exclusively CD45+ (Fig. S2C) and by majority F4/80+ macrophages (81%); this was confirmed by combining in situ hybridization for the Y chromosome and F4/80 immunostaining (Fig. 2E). Donor cells were consistently undetectable in recipient livers by 42 d postinjection (Fig. 2F). Therefore, the expansion of panCK+ DRs occurred principally following the loss of engrafted donor-derived cells.
Fig. 2.
Transfer of BMCs leads to transient hepatic engraftment of macrophages. (A) Engraftment of GFP+ cells within the liver 6 h following tail vein injection of 107 GFP+ cells into WT mice. Portal tract highlighted by arrow. (B) One day following sex-mismatched BMC transfer, donor-derived cells remain scattered through hepatic parenchyma. Confocal analysis of recipients using panCK immunohistochemistry (red) and Y chromosome FISH (green, white arrows) with DAPI (blue). (C) Confocal analysis 3 d following sex-mismatched BMC transfer reveal association of donor-derived cells with panCK+ ductular cells. No evidence of transdifferentiation of donor cells to hepatocytes or ductular cells (A–C). Male and female controls (Fig. S2 A and B). (D) Dual immunohistochemistry for F4/80 (red) and panCK (green) confirms their association 3 d following BMC transfer. (E) Donor-derived cells (Y chromosome, green) express macrophage markers (F4/80, red) on microscopic analysis 3 d following sex-mismatched BMC transfer. Inset: representative F4/80 immunocytochemistry performed on GFP+ cells sorted by FACS from the nonparenchymal fraction of digested liver 3 d following GFP-mismatched BMC transfer. (F) Quantification of engrafted donor cells from whole liver by real-time PCR of genomic DNA in sex-mismatched BMC transfer model and in the nonparenchymal cell (NPC) fraction by FACS for GFP in GFP-mismatched BMC transfer (Fig. S2C). PCR data (blue) is presented as group mean n = 2 at each time point. FACS data (green) are presented as fraction of the NPC population; each data point represents pooled cell populations, n = 5.
Macrophages Are Themselves Capable of Activating a Ductular Reaction.
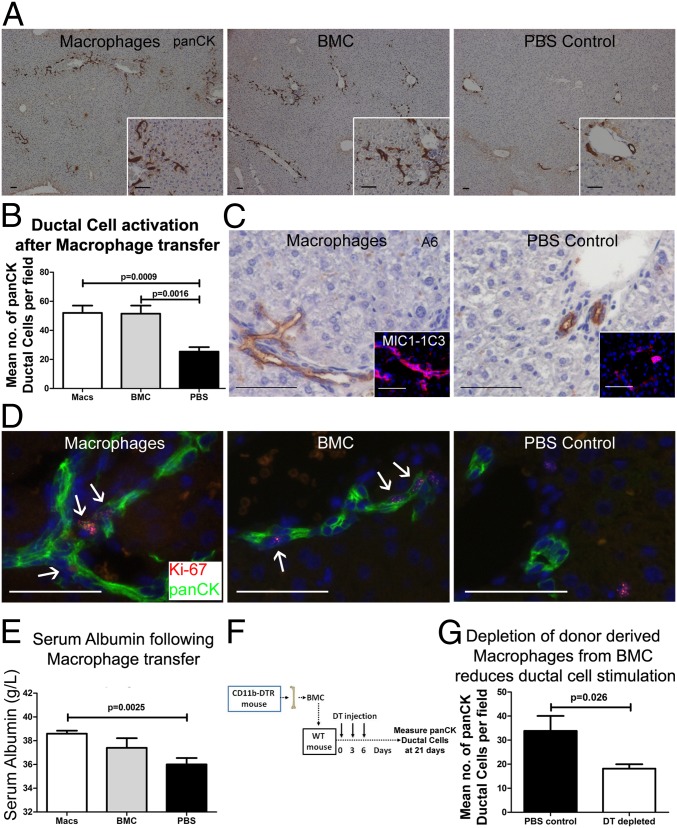
The experiments described above suggest a central role for macrophages in the activation of DRs following BMC injection. We therefore hypothesized that in vitro matured macrophages would stimulate a similar DR response to whole BMCs. Macrophage cell transfer resulted in activation of DR proliferation, analogous to that seen with BMCs (Fig. 3 A–D and Fig. S3D), once again in the absence of hepatocellular injury (Fig. S3 E and F). A raised serum alkaline phosphatase together with expression of other biliary/DR marker genes was also observed in mice receiving macrophage transfer (Fig. S3 G–L). Increased serum albumin was observed 21 d following macrophage cell transfer (Fig. 3E).
Fig. 3.
Macrophage transfer recapitulates whole BMC transfer. Twenty-one days following infusion of 107 syngenic macrophages expansion of panCK+ DRs is observed, reproducing both morphologically (A) and quantitatively (B) the expansion seen with BMC (each group n = 5). Expansion of A6+ and MIC1-1C3+ DRs is also observed (C and Inset, respectively), together with active proliferation of Ki67+/panCK+ ductular cells (D). (E) Raised serum albumin is observed 21 d following macrophage transfer. (F) Macrophage depletion was performed by delivering 107 BMCs from CD11b-DTR mice to WT littermates. DT was given on 0, 3, and 6 d post-BMC transfer to deplete donor-derived macrophages. Tissue was analyzed at 21 d (n ≥ 5 each group). Control mice received PBS injection instead of DT. (G) Following transfer of 107 BMCs from CD11b-DTR donors. Data are presented mean ± SEM, P values denote two (E) and one (B and G) tailed Student t test.
To examine the role of the macrophage subpopulation within whole BM, whole BMCs were isolated from donor CD11b-diphtheria toxin (DT) receptor (DTR) mice and transferred to healthy WT littermates. DT was then used to selectively deplete donor-derived CD11b+ macrophages from the transferred population; then the resultant DR response in recipients was measured (Fig. 3F). Sole depletion of donor CD11b+ macrophages from whole BMCs resulted in reduction of recipient panCK+ DR expansion (Fig. 3G). At 21 d, a time when donor cells were undetectable, host liver macrophage numbers significantly increased (Fig. S3M).
Functional Effects of DR Activation Following Macrophage Transfer.
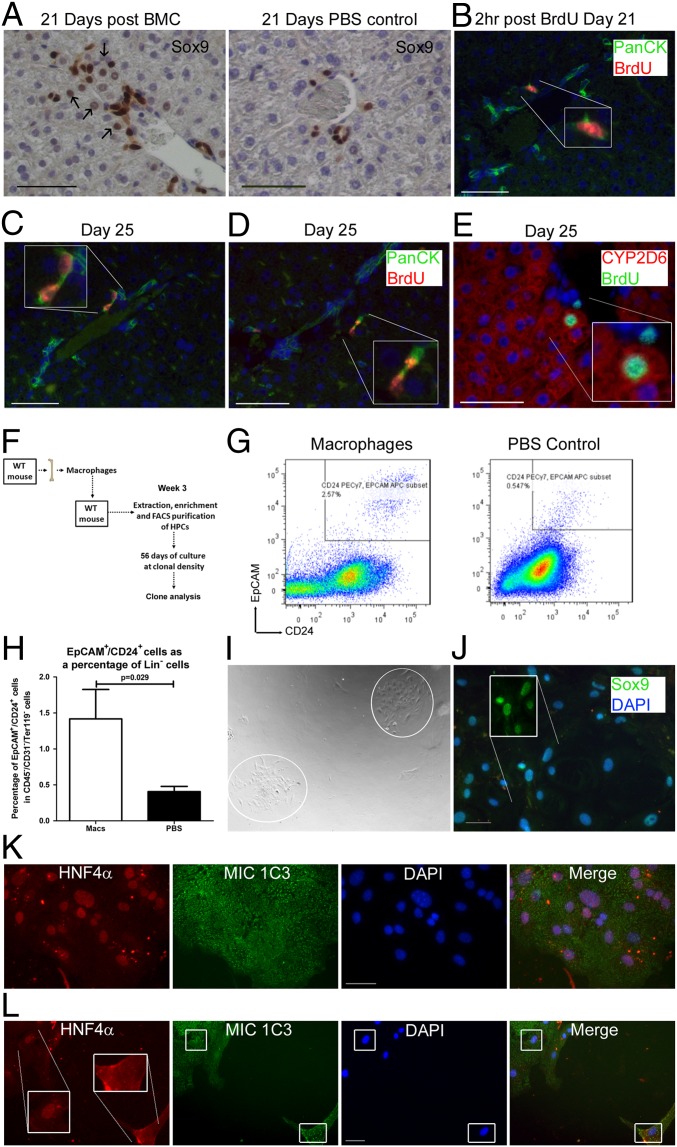
While demonstrating expansion of cells expressing DR markers in vivo, we wished to functionally observe the effect of these expanded DRs containing putative HPCs following macrophage/BMC transfer. At the time of previously observed liver enlargement following BMC transfer, Sox9 staining revealed multiple larger cells with hepatocyte-like morphology and Sox9+ nuclei (Fig. 4A) adjacent to smaller Sox9+ DR cells, implying differentiation of DRs into functional parenchyma. In agreement with the proliferation and differentiation of DRs into hepatocytes, BrdU label was taken up by DRs 21 d following macrophage transfer (Fig. 4B). A further 4 d later evidence of both retention of BrdU label within clusters of DRs was observed (Fig. 4C) together with BrdU label in both ductular cells and hepatocytes adjacent to one another (Fig. 4 D and E). A functional assessment for the presence of HPCs was performed 3 wk following macrophage cell transfer (Fig. 4F). Using FACS, Ter119−/CD31−/CD45−/CD24+/EpCAM+ cells were isolated (Fig. 4F). This population was significantly increased (approximately threefold) in healthy livers following macrophage cell transfer vs. PBS control (total pooled Ter119−/CD31−/CD45−/CD24+/EpCAM+ cells from three livers = 3,600 vs. 1,200 cells, respectively; Fig. 4G). Following macrophage injection, we found an increase in cells with HPC-defining characteristics i.e., colony formation from clonal density growth in vitro with the resultant cells of both a hepatocellular and biliary morphology (Fig. 4H) that could be maintained in the long term and passaged. A total of six viable clonal colonies were obtained after 2 mo from 3,600 sorted Ter119−/CD31−/CD45−/CD24+/EpCAM+ cells in the macrophage recipient group (from n = 3 whole livers); in the PBS recipient group there were no viable colonies formed from the total pooled-sorted Ter119−/CD31−/CD45−/CD24+/EpCAM+ cells (from n = 3 whole livers). Colonies from the macrophage injected group expressed the biliary markers Sox9 and MIC1-1C3 together with the hepatocyte marker HNF4α (Fig. 4 I and J); within colonies, nuclear Hepatocyte Nuclear Factor 4α (HNF4α) expression was heterogenous with some cells exhibiting reduced nuclear HNF4α expression (Fig. 4K).
Fig. 4.
Functional parenchymal regeneration following BMC transfer. (A) Twenty-one days following BMC transfer, periportal Sox9+ ductular expansion is observed compared with PBS control, with frequent nuclear Sox9 expression in hepatocyte-like cells adjacent to ductular cells (arrows). Twenty-one days following transfer of 107 syngenic macrophages mice were administered i.p. BrdU and killed either 2 h (B) or 4 d later (C–E), n = 3 each group. (B) Labeling of panCK+ ductular cells with BrdU 2 h following pulse. Four days later, evidence of division of BrdU+/panCK+ into two daughter BrdU+/panCK+ cells (C) and separately into hepatocyte-like cells (D) expressing cytochrome P450 (CYP) 2D6 (E). Individual channels and controls are shown in Fig. S4 A–C. (F) Diagram explaining isolation of FACS sorted viable (7AAD−), lineage negative (Ter119−/CD31−/CD45−), CD24+/EpCAM+ cells followed by their clonal analysis, performed 3 wk following injection with 107 sygenic macrophages or PBS control. (G) Representative CD24+/EpCAM+ plots, gating strategy is shown in Fig. S4. (H) CD24+/EpCAM+ cells were more frequent in the lineage negative population by FACS in animals receiving macrophages than PBS injections (here shown as a mean of n ≥ 3 each group); P value denotes one-tailed Mann–Whitney test. (I) Following up to 56 d plating at clonal density discrete heterogeneous colonies were identified from the CD24+/EpCAM+ cells in mice that had received macrophage injection. (J) Colonies expressed Sox9 (Inset Sox9 channel only) and (K) the activated biliary marker MIC1-1C3, together with hepatocellular differentiation marker HNF4α. (L) Cells at the periphery of the main colony were negative for nuclear HNF4α.
DR Activation Following BMC Transfer Is Dependent on TWEAK Signaling.
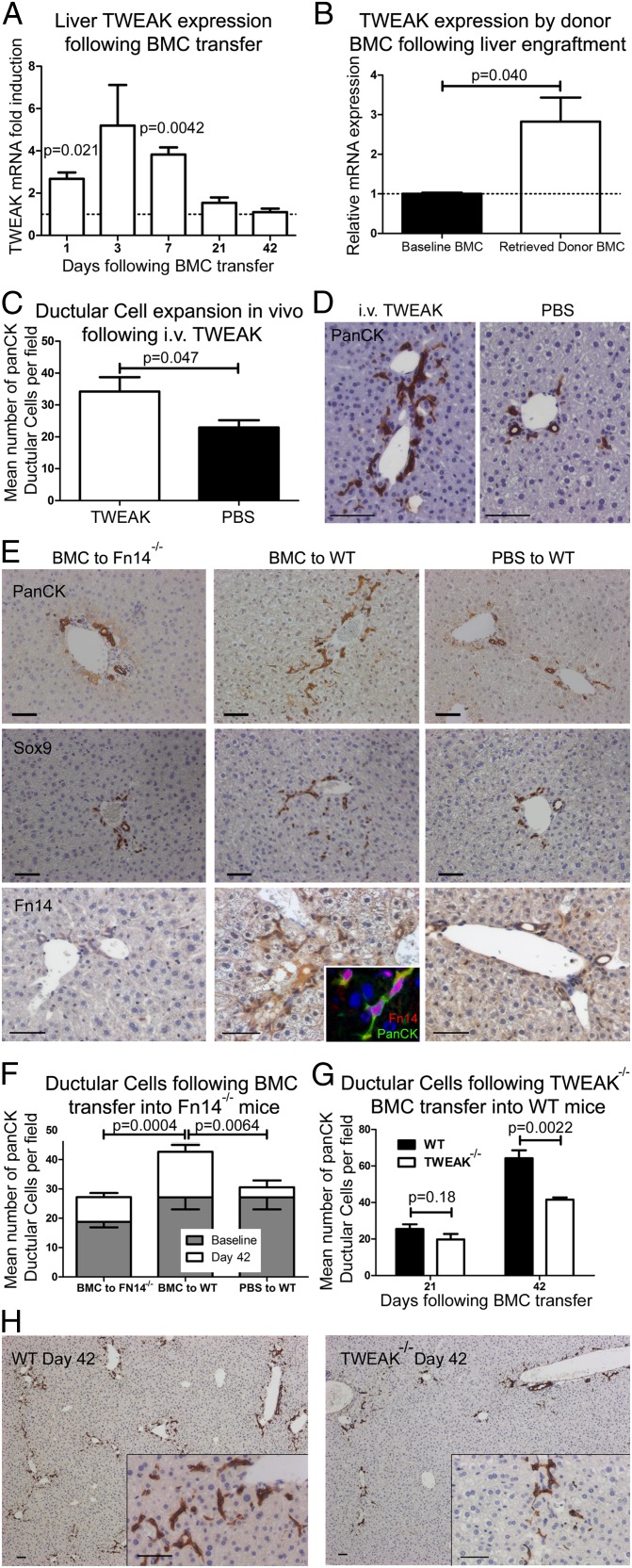
To determine the mechanism by which engrafted BM-derived macrophages stimulate the DR, we screened whole liver RNA for the presence of growth factors known to be associated with ductular proliferation, including: Hepatocyte Growth Factor (HGF), IFNγ, TNF, IL6, Lymphotoxin α (LTα), LTβ, Oncostatin (OSM), and TWEAK. Whereas most of these growth factors either were undetectable or did not change over the time course of ductular expansion (Fig. S5 A–D), TWEAK mRNA levels were strongly induced during the first week following BMC transfer and returned to normal as engrafted cells were lost from the liver (Fig. 5A). In vitro maturation of BMCs to macrophages resulted in a 20-fold up-regulation of TWEAK mRNA (Fig. S5E). To assess if donor-derived cells were the source of TWEAK message post-BMC injection, GFP+ donor-derived cells were sorted from WT recipient livers and examined for TWEAK expression. Relative to whole BM, TWEAK mRNA levels were 2.8-fold elevated in the GFP+ pullout fraction (Fig. 5B). Recombinant (r)TWEAK administered to healthy mice results in expansion of DRs (Fig. 5 C and D and Fig. S5) and the Fibroblast growth factor-inducible immediate-early response protein 14 (Fn14) receptor is also shown to be expressed in both bile ducts and DRs (Fig. 5E), but not in periportal myofibroblasts (Fig. S5K).
Fig. 5.
BMC-induced ductular expansion is dependent on in-situ TWEAK from engrafted macrophages. (A) Whole liver TWEAK mRNA following syngenic BMC transfer. Values are normalized against PBS control and expressed as mean ± SEM, n = 5 for each time point. No change was observed in control TWEAK expression over time. (B) TWEAK gene expression by GFP+ liver engrafted donor-derived cells retrieved by FACS sorting 3 d following GFP-mismatched BMC transfer. Values are normalized against whole BMCs control. (C and D) Administration of 0.4 µg i.v. rTWEAK daily for 7 d resulted in expansion of panCK ductular cells in vivo; n = 5 each group, P value denotes one-tailed Mann–Whitney test (see also Fig. S5). (E and F) Reduced BMC-mediated ductular expansion from baseline in Fn14−/− mice; n = 5 each group. BMC to Fn14−/−, 18.8 ± 1.9–27.2 ± 1.4; BMC to WT, 27.1 ± 4.0–42.7 ± 2.3; and PBS to WT, 27.1 ± 4.0−30.6 ± 2.4, baseline to day 21, respectively (mean ± SEM). Baseline Fn14−/− to WT, P = 0.098. Similar reduction in expansion of Sox9+ ductular cells is observed. Fn14 is expressed by panCK+ ductular cells in WT animals (Inset); individual channels for Fn14/PanCK are shown in Fig. S5J. A total of 107 BMCs from TWEAK−/− donors were administered to healthy WT recipients and compared with WT BMC control for panCK+ ductular expansion at 21 and 42 d (G and H); each group n = 6. All data are presented as mean ± SEM, with P values denoting two-tailed Student t test unless otherwise stated.
To directly test whether TWEAK signaling mediates DR expansion following BMC transfer, we used two methods: (i) transfer of WT BMC into recipient mice lacking the TWEAK receptor Fn14 (Fn14−/−), and (ii) transfer of BMC lacking TWEAK (TWEAK−/− donors) into WT recipients and compared the extent of DR response to appropriate WT controls. In the absence of recipient Fn14 expression, DRs were reduced (Fig. 5 E and F and Fig. S5). The engraftment of WT BMCs into Fn14−/− recipients was unchanged (Fig. S5H). Whereas a trend was observed for reduced panCK+ DRs in baseline Fn14−/− mice compared with baseline WT animals (P = 0.098), greater DR expansion (in both absolute and relative terms) is observed in WT recipients compared with Fn14−/− recipient mice following infusion of WT BMCs. To eliminate the differences between Fn14−/− and WT recipient mice in analysis of ductular expansion, transfer of BMCs from TWEAK−/− donors vs. WT donors to WT recipients was examined and revealed reduced DR expansion in the recipients of TWEAK−/− BMCs (Fig. 5 G and H). Both transgenic models of impaired TWEAK/Fn14 signaling demonstrate the necessity of this signaling pathway for the DR expansion observed in response to BMC administration.
Discussion
There is an increasing appreciation that BM-derived cells may influence the regenerative response of an injured organ. Although paracrine signaling from the BMCs to the target organ has been suggested to underlie this phenomenon, such signaling has not been well characterized. Furthermore in the damaged liver any improvement in regeneration seen following BM injection may be an indirect phenomena secondary to the effects upon the surrounding matrix (23). Here we have demonstrated that a single infusion of unfractionated BMCs results in direct activation of a DR response in the undamaged liver. We show that the macrophage population within unfractionated BMCs is both necessary and sufficient to recapitulate this DR activation. Infusion of BMCs/macrophages results in changes to both liver structure and function and includes the expansion of clonogenic HPCs. We go on to show that TWEAK/Fn14 signaling is a key component of the macrophage-stimulated DR activation in this context.
Following peripheral venous infusion of BMCs, the donor derived macrophages transiently engraft within the uninjured liver in juxtaposition to DRs, analogous to their recruitment during injury (18). Ductular proliferation and liver growth then occurs, principally following the loss of engrafted cells but with the additional accumulation of endogenous macrophages, again mirroring observations in an injury model (22). During the DR, liver weight transiently increases but without evidence of cellular enlargement or tissue oedema to account for this change. These changes are transient with restoration of normal liver architecture and weight in the following 6 wk.
There is an urgent need to develop novel therapies for chronic liver disease and manipulating the endogenous DR and HPC populations is a potential option. Previous proof of principle of pharmacological targeting of the DR has been shown in mice in the context of liver injury where DRs were inhibited (12). The precise characterization of hepatic stem cells and HPCs remains contentious with numerous, often nonspecific markers used to identify this population, although the majority consensus is that HPCs exist within the biliary tree and DRs. Various cells of the DR have been demonstrated to be capable of hepatocellular regeneration (9, 14, 15, 26). Following macrophage transfer we go on to show that accompanying the DR response identified histologically, there is an expansion of a defined Ter119−/CD31−/CD45−/CD24+/EpCAM+ cell population in the healthy livers that contains HPCs. This expanded Ter119−/CD31−/CD45−/CD24+/EpCAM+ cell population forms heterogenous colonies when grown at clonal density ex vivo, may be passaged, and throughout expresses the same markers as the expanded cells observed in vivo. The clonal colonies when formed contain both hepatocellular and biliary markers, indicating that the isolated and purified HPCs are bipotential and self-renewing. These markers include MIC1-1C3, identified by Dorrell et al. following generation of antibodies to murine DRs (27) and more recently has been shown to select an enriched population of clonogenic HPCs (15). Our findings therefore highlight a therapeutic means by which these host DRs can be directly stimulated and we propose this pathway as a possible mechanism by which autologous BMC therapy may be beneficial in human liver disease. Animal models of BMC therapy for liver disease and clinical autologous BMC therapy studies are underway with encouraging preliminary results (2, 28–30). A recent report by our group has shown that a single portal vein injection of macrophages significantly reduces fibrosis in a model of chronic liver injury. Alongside this reduction in fibrosis an improvement in liver function was seen, including an increase in DRs. The complexity of assigning a cause and effect relationship is highlighted by the fact that in the chronic injury model, the macrophages increase recruitment of inflammatory cells to the hepatic scar. Subsequent to this, a large number of matrix metalloproteinases, cytokines, and growth factors were up-regulated and matrix remodeling took place (22). The model described here is not confounded by other factors occurring during injury such as matrix remodeling, inflammation, and necrotic tissue, such that a cause–effect relationship between the injected cells and the DRs can be established. This has enabled a precise role for TWEAK to be established using the TWEAK−/− donor mice and the Fn14−/− recipient mice.
A previous report describes a transgenic mouse, which constitutively overexpresses TWEAK and in which DRs are expanded (16). We now show that exogenous TWEAK administration results in DR expansion in vivo and in addition confirm TWEAK expression by hepatic macrophages and Fn14 expression by panCK+ DRs, which have been shown to mediate ductular proliferation via NFκB activation (16, 17). Herein we demonstrate a direct link between macrophage TWEAK production and paracrine signaling to recipient DRs, resulting in their expansion using complimentary TWEAK/Fn14 KO models. Similar inhibition of ductular expansion in both KO scenarios is in keeping with the monogamous relation between this signal–receptor pair (31). Ductular expansion has previously been shown to be possible in the absence of Fn14 expression (17). Regarding the localization of macrophages to the biliary cells, previous data has described the recruitment of C-X-C chemokine receptor 4 (CXCR4+) cells to the biliary tracts via the chemokine Stromal Cell Derived Factor (SDF)-1 (32, 33). Macrophages strongly express CXCR4, and this may be a mechanism responsible for cell trafficking in this model. Interestingly the injected macrophages recruited endogenous macrophages to the liver, which may act to amplify any macrophage-derived signal.
Following tissue injury, macrophages can modulate progenitor cell behavior, suggesting a role for these cells in linking injury to regeneration (4, 19, 20, 34). Our observations both support and extend this, crucially showing that macrophages are able to modulate DRs even in the absence of injury. We also describe TWEAK as a central molecular target for future trophic/small molecule therapy. Moreover a therapeutic approach to tissue regeneration based on macrophage therapy offers several advantages: the ready availability of donor cells from an autologous source; the feasibility of the approach; and a defined mechanism of action involving paracrine signaling between donor and host liver cells, without the disadvantages associated with exogenously administered stem cells or their derivatives.
Materials and Methods
Animals were housed in a specific pathogen free environment and kept under standard conditions with a 12 hour day/night cycle and access to food and water ad libitum. All animal experiments had local ethical approval and were conducted under UK Home Office Legislation. Recipients received 1 ×107 BMCs via tail vein injection. For details of the animal models used, macrophage differentiation protocol, harvest of transferred cells, immunohistochemistry, fluorescence in situ hybridization, real time PCR, statistical analysis, microscopy and cell counting methodology please see SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Alicia Cole for help managing the Fn14−/− mouse colony, Dr. V. Factor (National Cancer Institute) for kindly supplying A6 antibody, Forbes Howie for help with serum analysis, and Biogen Idec for their generous provision of Fn14−/− and TWEAK−/− mice. T.G.B. is funded by a Wellcome Trust research training fellowship (WT081604AIA). L.B. is funded by a Medical Research Council (MRC) project grant. M.J.W. is funded by an MRC research training fellowship. S.J.F. is funded by an MRC project grant and the Sir Jules Thorn Trust. J.P.I. is supported by an MRC program grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302168110/-/DCSupplemental.
References
- 1.Bergmann A, Steller H. Apoptosis, stem cells, and tissue regeneration. Sci Signal. 2010;3(145):re8. doi: 10.1126/scisignal.3145re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houlihan DD, Newsome PN. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology. 2008;135(2):438–450. doi: 10.1053/j.gastro.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 3.Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137(2):466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulter L, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18(4):572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird TG, Lorenzini S, Forbes SJ. Activation of stem cells in hepatic diseases. Cell Tissue Res. 2008;331(1):283–300. doi: 10.1007/s00441-007-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999;154(2):537–541. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight B, et al. Liver inflammation and cytokine production, but not acute phase protein synthesis, accompany the adult liver progenitor (oval) cell response to chronic liver injury. Immunol Cell Biol. 2005;83(4):364–374. doi: 10.1111/j.1440-1711.2005.01346.x. [DOI] [PubMed] [Google Scholar]
- 8.Alison MR, Islam S, Lim SM. Cell therapy for liver disease. Curr Opin Mol Ther. 2009;11(4):364–374. [PubMed] [Google Scholar]
- 9.Wang X, et al. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmelzer E, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204(8):1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spahr L, et al. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48(1):221–229. doi: 10.1002/hep.22317. [DOI] [PubMed] [Google Scholar]
- 12. Knight B, Tirnitz-Parker JE, Olynyk JK (2008) C-kit inhibition by imatinib mesylate attenuates progenitor cell expansion and inhibits liver tumor formation in mice. Gastroenterology 135(3):969–979. [DOI] [PubMed]
- 13.Furuyama K, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43(1):34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 14.Español-Suñer R, et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143(6):1564–1575, e7. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Dorrell C, et al. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev. 2011;25(11):1193–1203. doi: 10.1101/gad.2029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakubowski A, et al. TWEAK induces liver progenitor cell proliferation. J Clin Invest. 2005;115(9):2330–2340. doi: 10.1172/JCI23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tirnitz-Parker JE, et al. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology. 2010;52(1):291–302. doi: 10.1002/hep.23663. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzini S, et al. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut. 2010;59(5):645–654. doi: 10.1136/gut.2009.182345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102(1):99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8(4):389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffield JS, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas JA, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53(6):2003–2015. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- 23.Kallis YN, et al. Remodelling of extracellular matrix is a requirement for the hepatic progenitor cell response. Gut. 2011;60(4):525–533. doi: 10.1136/gut.2010.224436. [DOI] [PubMed] [Google Scholar]
- 24.Thorgeirsson SS, Grisham JW. Hematopoietic cells as hepatocyte stem cells: A critical review of the evidence. Hepatology. 2006;43(1):2–8. doi: 10.1002/hep.21015. [DOI] [PubMed] [Google Scholar]
- 25.Issa R, et al. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 2003;17(1):47–49. doi: 10.1096/fj.02-0494fje. [DOI] [PubMed] [Google Scholar]
- 26.Endo Y, Zhang M, Yamaji S, Cang Y. Genetic abolishment of hepatocyte proliferation activates hepatic stem cells. PLoS ONE. 2012;7(2):e31846. doi: 10.1371/journal.pone.0031846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorrell C, et al. Surface markers for the murine oval cell response. Hepatology. 2008;48(4):1282–1291. doi: 10.1002/hep.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terai S, Sakaida I, Nishina H, Okita K. Lesson from the GFP/CCl4 model—translational research project: the development of cell therapy using autologous bone marrow cells in patients with liver cirrhosis. J Hepatobiliary Pancreat Surg. 2005;12(3):203–207. doi: 10.1007/s00534-005-0977-0. [DOI] [PubMed] [Google Scholar]
- 29.Yannaki E, et al. Lasting amelioration in the clinical course of decompensated alcoholic cirrhosis with boost infusions of mobilized peripheral blood stem cells. Exp Hematol. 2006;34(11):1583–1587. doi: 10.1016/j.exphem.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Gaia S, et al. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol. 2006;45(1):13–19. doi: 10.1016/j.jhep.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: Discovery, biology and therapeutic targeting. Nat Rev Drug Discov. 2008;7(5):411–425. doi: 10.1038/nrd2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kollet O, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112(2):160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terada R, et al. Stromal cell-derived factor-1 from biliary epithelial cells recruits CXCR4-positive cells: Implications for inflammatory liver diseases. Lab Invest. 2003;83(5):665–672. doi: 10.1097/01.lab.0000067498.89585.06. [DOI] [PubMed] [Google Scholar]
- 34.Seno H, et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci USA. 2009;106(1):256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.