Abstract
Studies showing reduced PKCζ expression or enzymatic activity in different types of human cancers support the clinical relevance of PKCζ as a tumor suppressor. However, the in vivo role of PKCζ and its mechanisms of action in prostate cancer remain unclear. Here we demonstrate that the genetic inactivation of PKCζ in mice results in invasive prostate carcinoma in vivo in the context of phosphatase and tensin homolog deficiency. Bioinformatic analysis of human prostate cancer gene-expression sets revealed increased c-Myc transcriptional activity in PKCζ-inactive cells, which correlated with increased cell growth, invasion, and metastasis. Interestingly, PKCζ knockdown or the overexpression of a kinase-inactive mutant resulted in enhanced cell proliferation and invasion in vitro through increased c-Myc mRNA and protein levels and decreased Ser-373 phosphorylation of c-Myc. Analysis of prostate cancer samples demonstrated increased expression and decreased phosphorylation of c-Myc at Ser-373 in PKCζ knockout tumors. In vivo xenograft studies revealed that c-Myc phosphorylation by PKCζ is a critical event in the control of metastasis. Collectively, these results establish PKCζ as an important tumor suppressor and regulator of c-Myc function in prostate cancer.
The atypical protein kinase C (aPKC) subfamily is composed of two members, PKCζ and PKCλ/ι (1). The most salient features of these kinases reside in the regulatory domain, which is substantially different from that of other members of the extended PKC family (2, 3). That is, the aPKCs have only one zinc finger, whereas the other PKCs have two (3). Through the zinc-finger domain, the aPKCs bind prostate apoptosis response-4 (Par-4), a protein that acts as a negative regulator of their enzymatic activity, whereas the classical and novel PKC isoforms bind lipids (4). Similar to the novel PKCs, the aPKCs lack the characteristic C2 domain that is present in the classical isoforms, and therefore are insensitive to Ca2+ (5). The most distinctive feature of the aPKCs’ structure is the existence of a unique type of interaction module, termed the Phox/Bem domain 1 (PB1) domain, in their regulatory region (2). This domain is also present in Par-6 and p62, two signaling adapters involved in the control of cell polarity and signaling, respectively (2).
A number of studies support the clinical relevance of PKCζ as a tumor suppressor, including reports on altered expression in different types of human cancers (1). Also, studies in patients led to the identification of a mutated form of PKCζ (S514F) with significantly impaired enzymatic activity (6). This means that tumorigenesis is associated with impaired PKCζ expression, activity, or both. The mechanisms whereby PKCζ affects tumorigenesis are unclear, but must be understood if this pathway is to be explored as a potential therapeutic target in cancer. Here we have demonstrated the role of PKCζ as a tumor suppressor in prostate cancer (PCa) as well as its mechanism of action. We found, in a relevant in vivo PCa mouse model and in human cell cultures that PKCζ restrains tumorigenesis by the inactivation of c-Myc through direct phosphorylation.
Results
Simultaneous Deficiency of PTEN and PKCζ Promotes Invasive Prostate Carcinoma.
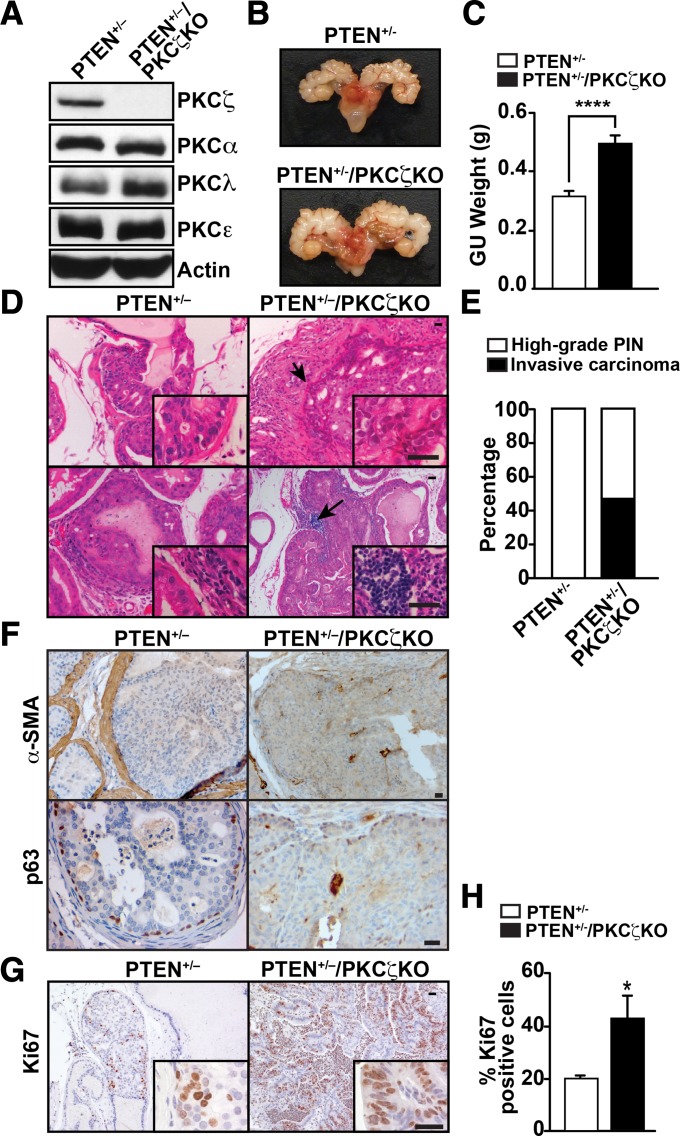
To rigorously test the in vivo role of PKCζ in PCa, we crossed PTEN+/− mice with PKCζ KO mice. Expression of different PKC isoforms was analyzed in prostate tissues of PTEN+/− and PTEN+/−/PKCζ KO mice. No changes in expression of other PKCs, including the other aPKC, PKCλ/ι, were detected in PKCζ KO samples compared with controls (Fig. 1A). Deletion of PKCζ in PTEN+/− mice resulted in larger prostate tumors (Fig. 1B) and a significant increase in genitourinary tract weight (Fig. 1C). No metastasis was identified in PTEN+/− or double-mutant mice, because these mice die earlier due to multicentric lymphoproliferative disease (7). Histological analysis of PKCζ-deficient mice in a wild-type PTEN background showed normal development of the prostate, whereas PTEN+/− mice in a PKCζ wild-type background had a high incidence of prostate hyperplasia and regions of low- and high-grade prostatic intraepithelial neoplasia (HGPIN) (Fig. 1 D, Upper and E). HGPIN lesions are characterized by an intraglandular proliferation of crowding cells with atypia, enlarged nuclei, and prominent nucleoli and have an onset of 8 mo of age in PTEN+/− mice, but they do not progress to invasive carcinoma, as previously published (7, 8). In contrast, prostates of PTEN+/−/PKCζ KO mice revealed not only regions of HGPIN with earlier onset (6 mo) than those of PTEN+/− mice, but also developed foci of invasive adenocarcinoma, predominantly in the dorsolateral prostate with a penetrance of 50% (Fig. 1 D, Upper and E). Consistent with the invasive phenotype, we found increased infiltration of immune cells, with enhanced recruitment of T and B cells, in prostates of PTEN+/−/PKCζ KO mice compared with PTEN+/− (Fig. 1D, Lower), and a profound impact on the stroma with a marked loss of smooth muscle actin (α-SMA) and disruption of the basal membrane, as shown by p63 staining (Fig. 1F). The proliferative index, measured by Ki67 staining, was also increased in the double-mutant prostates (Fig. 1 G and H). Collectively, these results demonstrate that PKCζ deficiency cooperates with PTEN heterozygosity to promote prostate carcinogenesis and unveil an unanticipated role for PKCζ as a critical tumor suppressor in PCa.
Fig. 1.
Loss of PKCζ cooperates with PTEN+/− to promote invasive prostate carcinoma. (A) PKC levels were analyzed by immunoblot in prostates of PTEN+/− and PTEN+/−/PKCζ KO mice. (B) Representative prostate glands of PTEN+/− and PTEN+/−/PKCζ KO mice at 8 mo of age. (C) Genitourinary (GU) tract weight of mice at 10 mo of age and of both genotypes: PTEN+/− (n = 23) and PTEN+/−/PKCζ KO (n = 9) mice, ****P < 0.0001. (D) H&E staining of prostates of the indicated genotypes. (E) Quantification of the incidence of invasive carcinoma in PTEN+/− (n = 7) and PTEN+/−/PKCζ KO mice (n = 15). (F) α-SMA and p63 staining of prostate sections of 9-mo-old mice of the indicated genotypes (n = 5). (G) Ki67 staining of prostate sections from 9-mo-old mice of the indicated genotypes. (H) Quantification of Ki67-positive cells. Results are the mean ± SD of 10 different fields per mouse sample (n = 5; *P < 0.05). (Scale bars, 20 μm in all panels.)
PKCζ Is a Tumor Suppressor in Human PCa in the Context of PTEN Deficiency.
To further examine the role of PKCζ as a tumor suppressor in PCa, we analyzed PKCζ protein levels in an invasive PCa model that is also mediated by PTEN cooperation. In this model, deficiencies in the tumor suppressors Par-4 and PTEN cooperate to promote invasive adenocarcinoma (7). Interestingly, PKCζ protein levels were completely lost in prostates from PTEN+/−/Par-4 KO mice, as measured by both Western blot analysis and immunostaining (Fig. S1 A and B). As a control for specificity of the PKCζ antibody, prostates from PTEN+/−/PKCζ KO mice showed no staining (Fig. S1B). Surprisingly, in contrast to the protein expression data, mRNA levels measured in parallel samples were highly up-regulated in the double-mutant prostates with invasive adenocarcinoma (Fig. S1C). Similar inconsistencies between PKCζ mRNA and protein levels were observed when these two parameters were compared in a panel of human epithelial prostate and PCa cell lines (Fig. S1 D and E). That is, PKCζ protein levels were down-regulated in metastatic PCa cell lines (DU145 and PC3M) compared with normal prostate epithelial cells (RWPE-1), in keeping with a tumor-suppressor role of PKCζ in PCa, whereas PKCζ mRNA levels were increased in the same samples (Fig. S1 D and E). This suggests that, although PKCζ mRNA levels could be highly up-regulated upon PCa disease progression, as previously reported (9), its protein levels are down-regulated. This is inconsistent with previous data that propose PKCζ as a predictive biomarker for survival in PCa (10). However, the antibody used for that study recognizes both isoforms, PKCζ and PKCλ/ι, which precludes any conclusion on the specific role of PKCζ in those tumors. It should be noted that PKCλ/ι has been reported to be a prooncogenic kinase, with up-regulation observed in a variety of tumor types (1, 11, 12).
Therefore, to more definitively evaluate the role of PKCζ in human PCa, we analyzed tissue microarrays (TMAs) with the PKCζ antibody used above, and validated with PKCζ KO samples (Fig. S1B). Because PKCζ cooperates with PTEN as a tumor suppressor, we examined the expression of PKCζ in the context of PTEN deficiency. For that, we stained human PCa TMAs to detect PTEN and PKCζ with a total of 15 normal samples and 146 PCa tumors (Fig. S2A). Of note, we found a significant positive correlation between PKCζ and PTEN levels, consistent with both being tumor suppressors (Fig. S2B). To further assess the PTEN-conditional association between PKCζ expression levels and metastatic PCa, we also analyzed a public domain gene expression dataset of 164 samples of normal prostate tissues, primary PCas, and metastatic PCas (13). As expected, PTEN expression levels were strongly associated with tumor progression, with expression being lowest in metastatic tumor samples (Fig. S2C). Then, we systematically examined the differences in PKCζ levels between primary and metastatic tumors for samples with different PTEN expression levels. For this analysis, PTEN expression levels in tumor samples were sorted in increasing order (Fig. S2D). Using each PTEN expression level as a cutoff, we performed a comparison between PKCζ levels in metastatic and primary tumors for samples in which PTEN expression is below the cutoff. Interestingly, there was a statistically significant reduction in PKCζ levels in metastatic versus primary tumors for tumors with PTEN levels below the cutoff, whereas no significant differences were observed in samples with PTEN levels above the cutoff (Fig S2E). These results reinforce the notion that PKCζ is a tumor suppressor in PCa and unveil its dependency on PTEN.
Cellular Mechanisms of Tumor Suppression by PKCζ in Prostate Carcinogenesis.
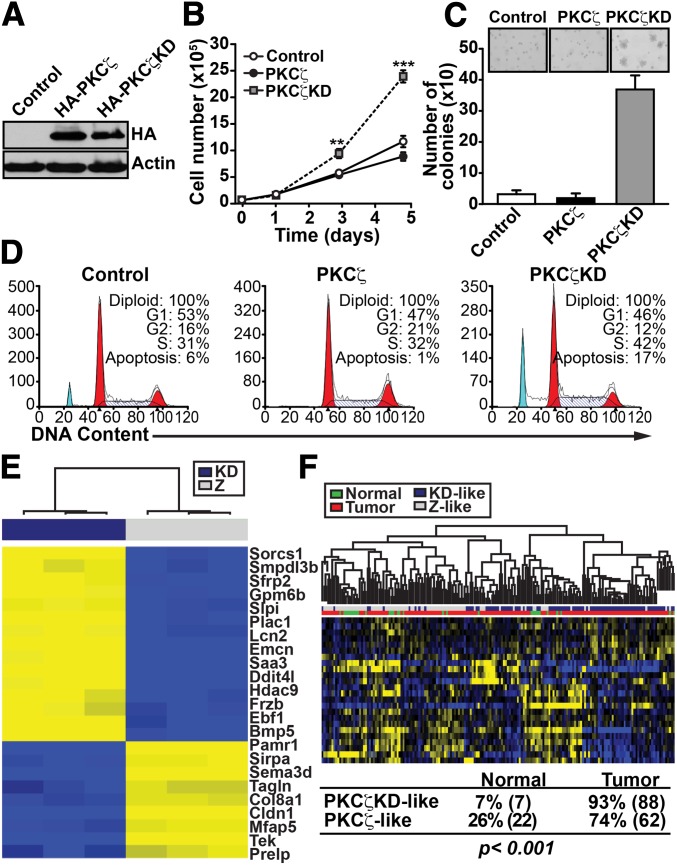
To investigate the molecular mechanisms by which PKCζ restrains PCa in the context of PTEN haploinsufficiency, and to determine whether PKCζ’s kinase activity is required for this function, we set up a physiologically relevant in vitro system. We reconstituted murine PTEN-P2 prostate epithelial cells by retroviral infection with exogenous PKCζ, either wild type (PKCζ-WT) or a kinase-dead mutant (PKCζ-KD). The two cell lines expressed similar levels of PKCζ construct (Fig. 2A). Interestingly, PKCζ-KD cells, but not PKCζ-WT cells, showed a robust increase in cell proliferation (Fig. 2B) and in their ability to form colonies in soft agar compared with control cells (Fig. 2C). In addition, the basal apoptosis in these cells was inhibited by expression of wild-type PKCζ and enhanced by the expression of the kinase-dead form of PKCζ (Fig. 2D). These results are consistent with the notion that the in vivo effects of PKCζ deficiency are actually cell autonomous and related to PKCζ’s enzymatic activity.
Fig. 2.
Role of PKCζ in tumorigenesis of PCa cells. (A) PTEN-P2 prostate epithelial cells were infected with retrovirus expressing HA-tagged PKCζ (HA-PKCζ) or kinase-dead mutant PKCζ (HA-PKCζ-KD) and cell lysates were analyzed by immunoblotting with the indicated antibodies. (B) Cell proliferation of PKCζ-WT– or PKCζ-KD–expressing cells in the presence of 0.1% FCS. Cell number was determined by trypan blue exclusion assay. Values are the mean ± SEM of triplicate counts from three different experiments, **P < 0.01, ***P < 0.001. (C) Colony formation in soft agar by PKCζ-WT– or PKCζ-KD–expressing cells. The total number of colonies per plate was scored by counting and is represented as the mean ± SD of six plates from two independent experiments. Insets are representative pictures showing colony size differences. (D) Cell-cycle analysis of PKCζ- or PKCζ-KD–expressing cells. (E) Genes differentially expressed in PKCζ-KD vs. PKCζ-WT cells (FDR <0.01 and fold change higher than three) with human homolog in the GSE21034 dataset. Yellow color indicates high expression levels and blue indicates low expression levels. (F) Unsupervised patient clustering analysis of the human PCa dataset GSE21034 using mouse genes with significantly altered expression, with FDR <0.01 and fold change higher than three when comparing PKCζ-KD vs. PKCζ-WT. Patient signatures were classified as PKCζ-KD–like or PKCζ-like based on whether the expression levels of signature genes were similar to PKCζ-KD or PKCζ-WT samples. If the expression levels of signature genes in a patient sample correlated better with PKCζ-KD samples, the sample was classified as PKCζ-KD–like, and vice versa. The association between such classification and the disease status of the sample (normal vs. tumor) was statistically significant (Fisher’s exact test P < 0.001).
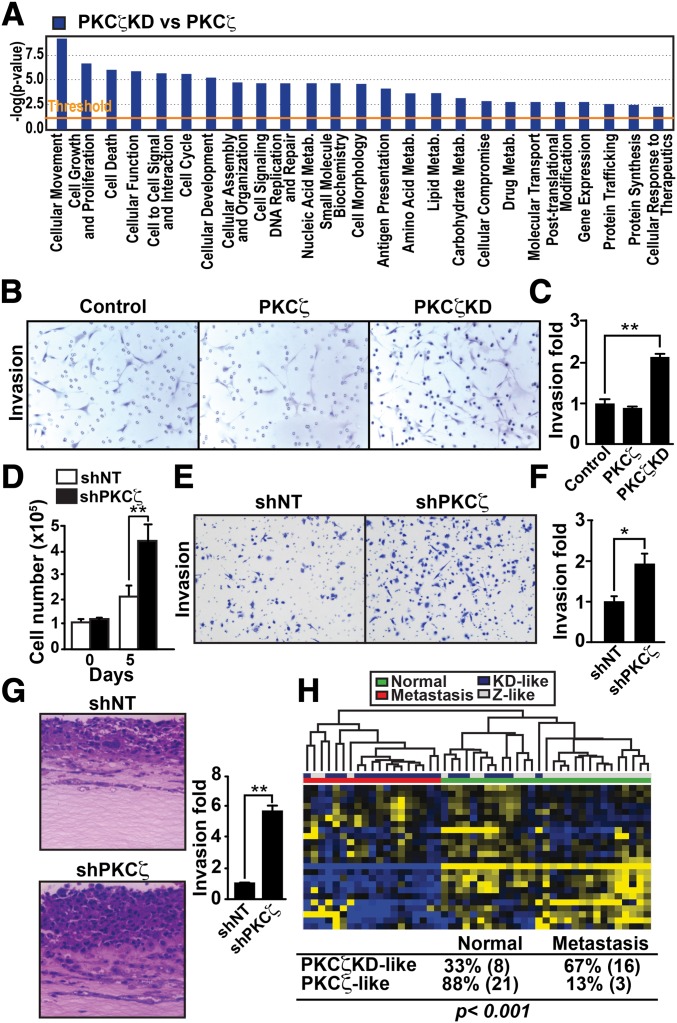
To gain insight into the kinase-dependent pathways that PKCζ might influence, we performed a genome-wide transcriptome analysis of PKCζ-KD cells vs. PKCζ-WT cells followed by unsupervised patient-clustering analysis. We interrogated the Gene Expression Omnibus code GSE21034 human PCa dataset, which contains 179 PCa samples, among which 19 are metastatic and 29 are normal, adjacent, benign prostate tissue (14). By clustering patients using the PKCζ-KD vs. PKCζ-WT gene signature (Fig. 2E), we found a significant association of the PKCζ-KD–like phenotype with tumors compared with normal tissue (P < 0.001; Fig. 2F). This is consistent with the concept that the loss of PKCζ results in increased tumorigenesis in PCa cells. To better understand the biological processes that underlie what appears to be a prometastatic phenotype of PKCζ-KD cells, we next used ingenuity pathway analysis (IPA) and found that the genes that were most significantly enriched were classified in the functional categories of “cellular movement” and “cell growth and proliferation” (Fig. 3A). Therefore, we tested the ability of PKCζ-KD cells to promote migration and invasion. Consistent with the IPA analysis, PKCζ-KD cells showed increased proliferation (Fig. 2B) as well as enhanced invasion in a modified Boyden chamber assay (Fig. 3 B and C). Another functional category with enriched gene expression was “cell death” (Fig. 3A), consistent with the increased apoptosis observed in PKCζ-KD cells (Fig. 2D). These results indicate that PKCζ inactivation influences cell proliferation, cell survival, and invasion to modulate prostate carcinogenesis. These results are in good agreement with previous data showing that overexpression of PKCζ in a rat cell-culture model of PCa can suppress invasion and metastasis (15). We next used the human PCa cell line DU145 to extend our analysis to other in vitro cell systems that are more relevant to human cancer. Of note, lentiviral knockdown of PKCζ (shPKCζ) in these cells resulted in significantly enhanced cell proliferation (Fig. 3D), and invasion in a modified Boyden chamber assay (Fig. 3 E and F). Similar results were also obtained using three different PKCζ shRNA constructs (Fig. S3A). In addition, reexpression of an shRNA-resistant PKCζ cDNA rescued the effect of PKCζ knockdown by two different shRNAs (Fig. S3B). A similar invasive phenotype was also observed in mouse embryo fibroblasts derived from PKCζ KO mice (Fig. S3C).
Fig. 3.
PKCζ controls cell movement and invasion. (A) Ingenuity knowledge-based molecular and cellular functions of the PKCζ-KD transcriptome shows significant enrichment of cell-movement genes (rank 1, P = 1.36E-14). (B and C) Invasion determined by modified Boyden chamber assay for control PTEN-P2 cells or cells expressing PKCζ or PKCζ-KD. Results are shown as mean ± SEM n = 3. **P < 0.01. (D) Cell proliferation of shNT and shPKCζ-infected DU145 cells determined by trypan blue exclusion assay. Values are mean ± SEM of triplicate counts of three different experiments. **P < 0.01. (E and F) Invasion in shNT- and shPKCζ-infected DU145 cells. Results are shown as mean ± SEM n = 3. *P < 0.05. (G) H&E-stained sections of shNT- and shPKCζ-infected DU145 cells cultured in an organotypic system. Quantification of invasion fold (Right). Results are shown as mean ± SEM n = 3. **P < 0.01. (H) Unsupervised patient clustering analysis of human PCa dataset GSE21034 using genes significantly altered in PKCζ-KD vs. PKCζ-WT mice with FDR <0.01 and fold change higher than three. Patient signatures were classified as in Fig. 2F. The association between such classification and the disease status of the sample (normal vs. metastasis) was statistically significant (Fisher’s exact test P < 0.001).
Currently, the organotypic culture model is the most physiologically relevant in vitro quantitative assay for studying tumor cell invasion. In this 3D system, tumor cells are grown at an air/liquid interface on collagen/Matrigel matrices and can be cultured with other cells, such as stromal cells, to mediate the invasion process. Furthermore, the pattern of invasion produced in this assay is more similar to invasion patterns observed in human tissues in vivo. Therefore, we used this organotypic model to test whether PKCζ-deficient cells were able to influence tumor invasion. Interestingly, shPKCζ cells showed a significant increase in the invasion index, compared with non-targeting shRNA (shNT) cells (Fig. 3G). Collectively, these results suggest that PKCζ’s ability to modify tumor-cell invasion, and thus influence metastasis, could be a critical driver in its role as a tumor suppressor in PCa. Interestingly, the clustering of patients based on the PKCζ-KD vs. PKCζ-WT gene signature, as above, revealed a significant association of the PKCζ-KD–like phenotype with metastasis, compared with normal tissue (P < 0.001; Fig. 3H), and with metastasis compared with primary tumors (P < 0.01; Fig. S4).
Myc Regulation by PKCζ in the Control of Tumor Cell Invasion.
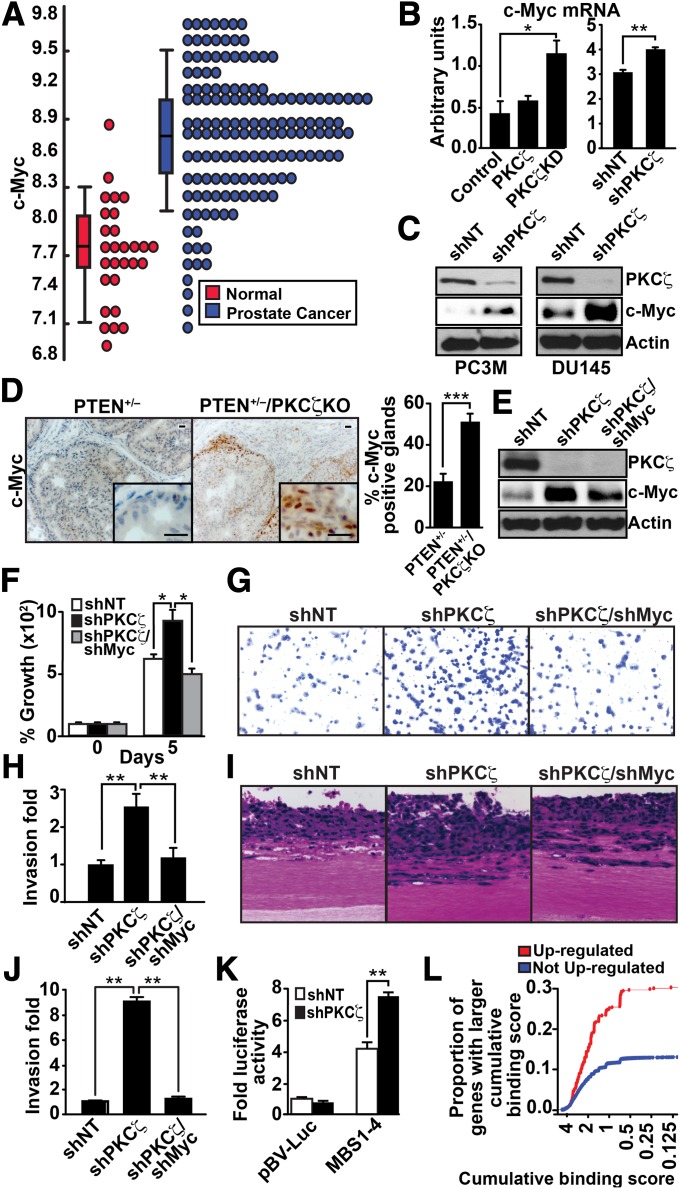
In an effort to identify molecular target(s) downstream of PKCζ, we examined the P2 PKCζ-KD gene signature by using human orthologs of the PKCζ-KD vs. PKCζ-WT gene signature identified above to develop a predictive model of human PCa progression. This analysis led us to the identification of c-Myc as a potentially relevant target of PKCζ in this system (Fig. 4A). This is a key finding as c-Myc has been shown to be one of the most prominently overexpressed genes in human PCa (16). Interestingly, we found that c-Myc mRNA levels were increased in P2 PKCζ-KD cells, as well as in DU145 human PCa cells with knockdown levels of PKCζ (Fig. 4B). Consistent with this, c-Myc protein levels were also dramatically increased in DU145 cells and in PC3M cells, which both express knockdown PKCζ levels, compared with their respective PKCζ-proficient controls (Fig. 4C). This is very interesting because Myc activation has been implicated in increased proliferation and invasion of cancer cells and in enhanced apoptosis under conditions of growth factor or nutrient stress (17). It is worth noting that all of the phenotypic alterations observed upon Myc activation resembled the phenotype of PKCζ knockdown cells or those expressing a PKCζ kinase-dead mutant, as shown in Figs. 2 and 3.
Fig. 4.
PKCζ regulates c-Myc levels. (A) Dot plot view of the distributions of c-Myc expression data in normal and PCa samples. (B) Quantification by Q-PCR of c-Myc mRNA levels in control, PKCζ, and PKCζ-KD P2 cells (Left), and in shNT and shPKCζ DU145 cells (Right). Results are shown as mean ± SEM; n = 3. *P < 0.05; **P < 0.01. (C) Immunoblot analysis of c-Myc and PKCζ levels in shNT- and shPKCζ-expressing PC3M and DU145 cells. (D) Immunostaining of c-Myc in prostate sections and quantification of staining (Right). Results are the mean ± SEM (n = 5 mice; ***P < 0.001). (Scale bar, 20 μm.) (E) Immunoblotting for c-Myc, PKCζ, and actin levels of DU145 cells infected with the indicated shRNA lentiviral vectors. (F) Cell proliferation was determined by trypan blue exclusion assay in DU145 cells infected with the indicated lentiviral knockdown vectors. Values are mean ± SEM of triplicate counts of three different experiments. *P < 0.05. (G–J) Invasion by the modified Boyden chamber assay (G and H) or by organotypic cultures (I and J) for DU145 cells infected with the indicated shRNA lentiviral vectors. Results are shown as mean ± SD n = 3. **P < 0.01. (K) shNT or shPKCζ DU145 cells were transfected with c-Myc–luciferase reporters. Luciferase activity was normalized to Renilla activity. Values are mean ± SEM of triplicate counts of two different experiments. **P < 0.01. (L) Comparisons of the distributions of Myc cumulative binding scores for the 519 genes up-regulated (FDR < 0.05) in PKCζ-KD samples (red) and the remaining 20,693 genes (blue). The proportion of up-regulated genes with high cumulative binding score (CBS) is significantly higher (Kolmogorov–Smirnov test P value = 4.3 × 10−13) than the proportion of genes with high CBS that were not up-regulated.
As further validation of the physiological relevance of these observations, we found that c-Myc expression was also highly increased in vivo in prostates from PTEN+/−/PKCζ KO double-mutant mice compared with prostates from PTEN+/− mice (Fig. 4D). Interestingly, increased c-Myc staining was detected in the leading edges of glands and in areas where foci were beginning to invade the surrounding stroma, suggesting that c-Myc up-regulation in PKCζ-deficient PCa cells could be an important factor in invasion. To rigorously test the functional relevance of these findings and the contribution of c-Myc to the tumor-suppressor mechanism of PKCζ, we next knocked down c-Myc in shPKCζ cells, and determined the impact on proliferation and invasion of PKCζ-deficient DU145 cells. Results in Fig. 4E demonstrate that infecting shPKCζ cells with shMyc vector restored c-Myc levels to those of shNT cells. Interestingly, upon restoration of c-Myc levels to those of shNT cells, the proliferation of shPKCζ cells was reduced to match the levels of shNT cells (Fig. 4F). We also found that c-Myc levels were important for mediating the invasive phenotype. That is, restoration of c-Myc levels rescued the increased invasion index of PKCζ-deficient cells in both the Boyden chamber assay (Fig. 4 G and H) and in organotypic cultures (Fig. 4 I and J). Similar results were obtained in PC3M cells (Fig. S5A). Collectively, these data demonstrate that the loss of PKCζ results in an accumulation of c-Myc in PCa cells, which is a key contributor to the more aggressive phenotype associated with PKCζ deficiency. In keeping with this notion, the activity of a Myc-driven luciferase reporter was significantly increased in PKCζ-deficient DU145 cells compared with their PKCζ-proficient controls (Fig. 4K). Furthermore, ChIP-seq experiments designed to assess the genome-wide Myc DNA-binding patterns in mouse embryonic stem cells (18) allowed us to calculate the Myc cumulative binding score (CBS) for each gene identified (19). We then examined genes that were differentially expressed in cells expressing kinase-dead PKCζ, as opposed to wild-type PKCζ, and specifically assessed the enrichment of genes with high Myc CBSs by using the Kolmogorov–Smirnov (KS) test (20). Interestingly, genes with high Myc CBSs were enriched among those that were up-regulated (false discovery rate, FDR <0.05) in PKCζ-KD samples (KS P value = 4.3 × 10−13) (Fig. 4L). However, there was no statistically significant enrichment among down-regulated genes (KS P value = 0.999). Collectively, these results establish that PKCζ is a negative regulator of c-Myc transcriptional function.
c-Myc Phosphorylation by PKCζ.
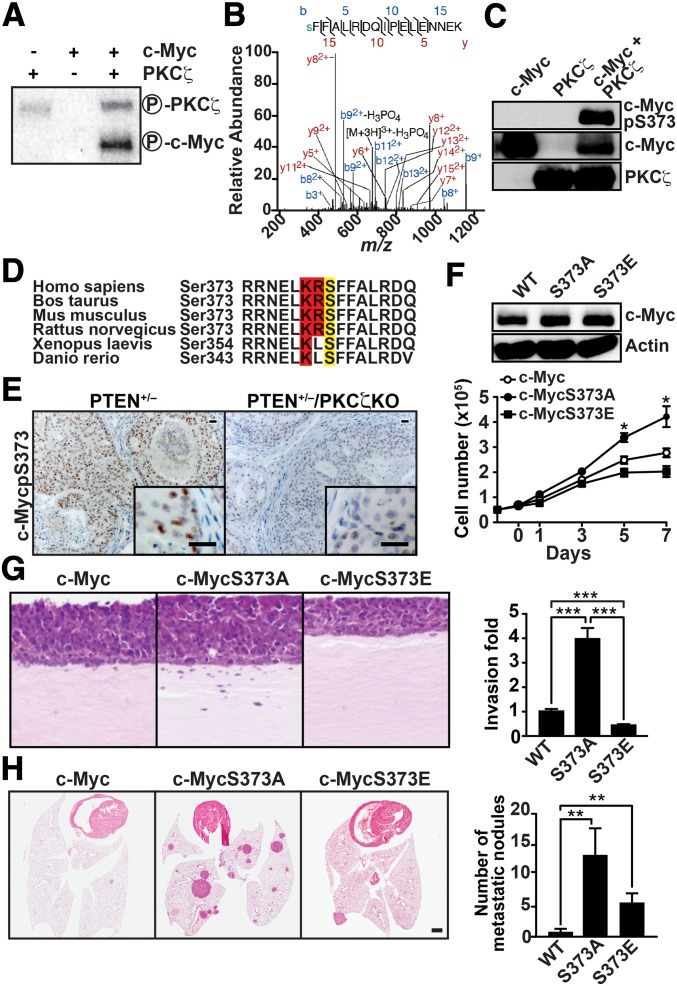
Abundant literature has documented that c-Myc is not only regulated at a gene-transcription level, but also by phosphorylation, which mediates its stability and function (17). As PKCζ is a kinase and its enzymatic activity is critical to the control of PCa cell proliferation, survival, and invasion (Figs. 2 and 3), we hypothesized that, in addition to mediating transcriptional repression of c-Myc, PKCζ might also control c-Myc function by direct phosphorylation. To test this hypothesis, we carried out an in vitro phosphorylation assay using recombinant bacullovirus-expressed PKCζ and recombinant bacterially expressed c-Myc as a substrate. Interestingly, recombinant PKCζ efficiently phosphorylated c-Myc, establishing c-Myc as a direct target of PKCζ (Fig. 5A). To investigate the functional relevance of this phosphorylation event, we next determined by mass spectrometry the Myc phosphorylation sites targeted by PKCζ. These analyses allowed us to identify c-Myc’s Ser-373 as the major PKCζ phosphorylation residue (Fig. 5B). To confirm this finding, recombinant c-Myc was phosphorylated in vitro by PKCζ using unlabeled ATP, after which the reaction was fractionated by SDS/PAGE and analyzed by immunoblotting with an anti–phospho-Ser373 c-Myc antibody. Results of Fig. 5C show that PKCζ-phosphorylated, but not unphosphorylated, c-Myc was readily recognized by the anti–phospho-Ser373 c-Myc antibody. This site was also identified with high stringency (using the Web-based bioinformatics tool Motif Scan) as a PKCζ phosphorylation consensus site (Scansite, Massachusetts Institute of Technology, Cambridge, MA), which was conserved across species (Fig. 5D). This suggests that c-Myc is a bona fide substrate of PKCζ. In agreement with these results, the immunohistochemical analysis of prostate tumors from mice that were either haploinsufficient for PTEN or doubly mutant for PTEN and PKCζ revealed that the loss of PKCζ dramatically inhibited the phospho-Ser373 staining of PTEN mutant prostates (Fig. 5E).
Fig. 5.
Functional role of c-Myc phosphorylation by PKCζ. (A) PKCζ phosphorylates c-Myc in vitro. (B) MS/MS spectra of phosphopeptides containing the pS373 site of c-Myc. Fragment ions are shown, as is the sequence coverage due to identified fragment ions. Mass-to-charge ratio, m/z. (C) Immunoblotting analysis of c-Myc phosphorylated by PKCζ in vitro. (D) The Ser-373 phosphorylation site is highly conserved among different species. (E) Immunostaining of phospho-S373–c-Myc in prostate sections of the indicated genotypes (n = 5). (Scale bar, 20 μm.) (F) DU145 cells were infected with retroviral vectors expressing c-Myc, c-Myc–S373A, or c-Myc–S373E mutants, and cell proliferation was determined by trypan blue exclusion. Values are mean ± SEM of triplicate counts of three different experiments. Cell lysates were analyzed by immunoblotting for c-Myc and actin (Upper). (G) Invasion was determined in organotypic cultures of DU145 cells expressing c-Myc, c-Myc–S373A, or c-Myc–S373E. Results (Right) are shown as mean ± SD (n = 3 ***P < 0.001). (H) H&E staining of lung sections of mice i.v. inoculated with DU145 cells expressing c-Myc or c-Myc mutants. Total numbers of lung metastatic nodules in individual mice injected with the indicated cells (Right) are shown as mean ± SEM **P < 0.01. n = 6–7 mice per group.
To determine whether Ser-373 phosphorylation is actually relevant to c-Myc’s function in the enhanced proliferative and invasive phenotype of PKCζ-deficient cells, Ser-373 of c-Myc was mutated to alanine to abolish its phosphorylation by PKCζ or to glutamate to partially mimic the negative charge of the phosphorylated serine. These mutant constructs, or their wild-type controls, were expressed in DU145 cells and the effect on cell proliferation and invasion was determined. We found that the c-Myc Ala-373 mutant enhanced, whereas the Glu-373 mutant reduced the proliferative (Fig. 5F) and invasive (Fig. 5G) properties of these cells compared with those expressing wild-type c-Myc. This is consistent with phosphorylation of Ser-373 accounting for the negative regulation of c-Myc function by PKCζ. To test the in vivo role of c-Myc’s Ser-373 phosphorylation, we transfected either wild-type c-Myc, or its Ala-373 or Glu-373 mutant variants into DU145 cells and determined their ability to produce lung metastasis using a well-established in vivo protocol. Consistent with our hypothesis, expression of the Ala-373 nonphosphorylatable mutant revealed the metastatic potential of c-Myc, whereas the phosphorylation mimetic Glu-373 did not (Fig. 5H). Interestingly, expression of the Glu-373 mutant in shPKCζ cells clearly abolished their enhanced invasiveness compared with shNT cells (Fig. S5B), indicating that PKCζ-induced phosphorylation of c-Myc’s Ser-373 is essential for its tumor-suppressor activity.
Discussion
Previous studies have discussed correlative data from different types of human cancers, suggesting that PKCζ is likely to have clinical relevance as a tumor suppressor (6, 21–23). However, the role of PKCζ in human PCa was controversial (9, 10, 15), and the in vivo molecular and cellular mechanisms whereby PKCζ affects tumorigenesis in this type of cancer were not known. Here we show that the genetic inactivation of PKCζ in mice resulted in enhanced prostate tumorigenesis in vivo. Furthermore, PKCζ knockdown or the overexpression of a kinase-inactive mutant induced increased proliferation and invasion in vitro, and in an organotypic culture model that faithfully mimicked the in vivo situation. Interestingly, we show that the inactivation of PKCζ results in increased levels of c-Myc mRNA and protein, which are required for the tumor-suppressor activity of PKCζ. We know this because the reduction of c-Myc levels in PKCζ-deficient cells to match the levels of normal cells resulted in the complete rescue of the protumorigenic phenotype, both in vitro and in organotypic cultures. PCa is the most common malignancy among men in Western countries. Our observations that PKCζ is a tumor suppressor in this type of neoplasia, and that it acts by repressing c-Myc expression and function, are likely to be highly relevant in the design of new therapeutic approaches, which are sorely needed.
This study also reveals further details about the relationship between c-Myc and PKCζ by showing that Ser-373 on c-Myc is a direct target of PKCζ. Previous results suggested that this site is a target of the stress kinase Pak2 (24, 25). p21-activated kinase-2 (Pak2) phosphorylates c-Myc at two other residues, Thr-358 and Thr-400. Whereas phosphorylation of Thr-358 inhibited the interaction of Myc with DNA, phosphorylations at Ser-373 and Thr-400 reduced the ability of Myc to interact with Max, which is an important mechanism by which Myc acts as a transcriptional regulator of a collection of genes involved in proliferation, cell transformation, and apoptosis (17). However, in contrast to Pak2, which is important for the regulation of Myc under conditions of stress in vitro, the activity of PKCζ is required for the phosphorylation of c-Myc at Ser-373 under basal conditions, both in transformed cells in vitro and in mouse-derived PCa samples in vivo. Also, in contrast to Pak2, PKCζ targets only Ser-373 and not Thr-350 or Thr-358. Nevertheless, Ser-373 phosphorylation is necessary and sufficient to inhibit cell proliferation and invasion, not only in vitro but also in an in vivo model of lung metastasis. The fact that the knockdown of PKCζ does not rescue the inhibitory effect of the c-Myc–S373E mutant on the proliferation of PCa cells demonstrates that this is a critical event in the tumor-suppressor activity of PKCζ.
Materials and Methods
PKCζ KO and PTEN+/− mice were described previously (7). Both mouse strains were in C57BL/6 background. All mice were born and maintained under pathogen-free conditions. Animal handling and experimental procedures conformed to institutional guidelines (Sanford-Burnham Medical Research Institute Institutional Animal Care and Use Committee). All genotyping was done by PCR. Information on histology, cellular assays, in vitro kinase assays, bioinformatics analysis, and statistical analysis are included in SI Material and Methods.
Supplementary Material
Acknowledgments
We thank Maryellen Daston for editing this manuscript; Diantha LaVine for the artwork; and Tom Hudson, Jessica Leung, and Nahid Hamidy for their technical assistance. This work was funded by National Institutes of Health Grants R01CA134530 (to M.T.D.-M.); R01CA132847, R01AI072581, and R01DK088107 (to J.M.); a Department of Defense Grant PC080441 (to M.T.D.-M.); and a CCSPG Pilot Project Grant (to M.T.D.-M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221799110/-/DCSupplemental.
References
- 1.Moscat J, Diaz-Meco MT, Wooten MW. Of the atypical PKCs, Par-4 and p62: Recent understandings of the biology and pathology of a PB1-dominated complex. Cell Death Differ. 2009;16(11):1426–1437. doi: 10.1038/cdd.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137(6):1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moscat J, Diaz-Meco MT, Albert A, Campuzano S. Cell signaling and function organized by PB1 domain interactions. Mol Cell. 2006;23(5):631–640. doi: 10.1016/j.molcel.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Díaz-Meco MT, et al. The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell. 1996;86(5):777–786. doi: 10.1016/s0092-8674(00)80152-x. [DOI] [PubMed] [Google Scholar]
- 5.Moscat J, Diaz-Meco MT. The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep. 2000;1(5):399–403. doi: 10.1093/embo-reports/kvd098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galvez AS, et al. Protein kinase Czeta represses the interleukin-6 promoter and impairs tumorigenesis in vivo. Mol Cell Biol. 2009;29(1):104–115. doi: 10.1128/MCB.01294-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Marcos PJ, et al. Simultaneous inactivation of Par-4 and PTEN in vivo leads to synergistic NF-kappaB activation and invasive prostate carcinoma. Proc Natl Acad Sci USA. 2009;106(31):12962–12967. doi: 10.1073/pnas.0813055106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trotman LC, et al. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441(7092):523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes DR, et al. Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9(2):166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao S, et al. PRKC-ζ expression promotes the aggressive phenotype of human prostate cancer cells and is a novel target for therapeutic intervention. Genes Cancer. 2010;1(5):444–464. doi: 10.1177/1947601910376079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishiguro H, et al. aPKClambda/iota promotes growth of prostate cancer cells in an autocrine manner through transcriptional activation of interleukin-6. Proc Natl Acad Sci USA. 2009;106(38):16369–16374. doi: 10.1073/pnas.0907044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray NR, Kalari KR, Fields AP. Protein kinase Cι expression and oncogenic signaling mechanisms in cancer. J Cell Physiol. 2011;226(4):879–887. doi: 10.1002/jcp.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu YP, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22(14):2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 14.Taylor BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell CT, et al. Overexpression of protein kinase C-zeta (PKC-zeta) inhibits invasive and metastatic abilities of Dunning R-3327 MAT-LyLu rat prostate cancer cells. Cancer Res. 1996;56(18):4137–4141. [PubMed] [Google Scholar]
- 16.Koh CM, et al. MYC and prostate cancer. Genes Cancer. 2010;1(6):617–628. doi: 10.1177/1947601910379132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dang CV. MYC on the path to cancer. Cell. 2012;149(1):22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133(6):1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang Z, Zhou Q, Wong WH. ChIP-Seq of transcription factors predicts absolute and differential gene expression in embryonic stem cells. Proc Natl Acad Sci USA. 2009;106(51):21521–21526. doi: 10.1073/pnas.0904863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mootha VK, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 21.Diouf B, et al. Somatic deletions of genes regulating MSH2 protein stability cause DNA mismatch repair deficiency and drug resistance in human leukemia cells. Nat Med. 2011;17(10):1298–1303. doi: 10.1038/nm.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pu YS, et al. Down-regulation of PKCζ in renal cell carcinoma and its clinicopathological implications. J Biomed Sci. 2012;19:39. doi: 10.1186/1423-0127-19-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma L, et al. Control of nutrient stress-induced metabolic reprogramming by PKCζ in tumorigenesis. Cell. 2013;152(3):599–611. doi: 10.1016/j.cell.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Z. Stress signaling and Myc downregulation: Implications for cancer. Cell Cycle. 2004;3(5):593–596. [PubMed] [Google Scholar]
- 25.Huang Z, Traugh JA, Bishop JM. Negative control of the Myc protein by the stress-responsive kinase Pak2. Mol Cell Biol. 2004;24(4):1582–1594. doi: 10.1128/MCB.24.4.1582-1594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.