Abstract
Although we now have thousands of studies focused on the nano-, micro-, and whole-animal mechanics of gecko adhesion on clean, dry substrates, we know relatively little about the effects of water on gecko adhesion. For many gecko species, however, rainfall frequently wets the natural surfaces they navigate. In an effort to begin closing this gap, we tested the adhesion of geckos on submerged substrates that vary in their wettability. When tested on a wet hydrophilic surface, geckos produced a significantly lower shear adhesive force (5.4 ± 1.33 N) compared with a dry hydrophilic surface (17.1 ± 3.93 N). In tests on an intermediate wetting surface and a hydrophobic surface, we found no difference in shear adhesion between dry and wet contact. Finally, in tests on polytetrafluoroethylene (PTFE), we found that geckos clung significantly better to wet PTFE (8.0 ± 1.09 N) than dry PTFE (1.6 ± 0.66 N). To help explain our results, we developed models based on thermodynamic theory of adhesion for contacting surfaces in different media and found that we can predict the ratio of shear adhesion in water to that in air. Our findings provide insight into how geckos may function in wet environments and also have significant implications for the development of a synthetic gecko mimic that retains adhesion in water.
Keywords: contact angle, superhydrophobicity, van der Waals, friction, bioinspired adhesive
Over the past decade, researchers have made extraordinary progress in understanding how the gecko adhesive system works (1–8). Indeed, many laboratories have tested hundreds of synthetic mimics for potential use in robotics, medicine, space, and everyday life (9–21). Although the range and performance of synthetic “gecko-tapes” are impressive, important gaps remain in our knowledge of the system and its capabilities in natural environments. Geckos are extremely diverse, constituting more than 1,400 species worldwide (22, 23). However, knowledge of the natural substrates and conditions geckos use is very limited. For example, it is likely that many species move across leaves and other plant structures that are not perfectly smooth and have variable surface chemistries (24, 25). In principle, the interaction of gecko feet with such surfaces may have a significant effect on adhesion, yet gecko research has only just begun to tackle such questions (26–28). Additionally, natural surfaces are likely to become wet (especially in the tropics) and dirty, potentially reducing adhesion. Although research on the ability of geckos to remove dirt from their toes has received some attention (29, 30), studies on wetting and the effect of water are limited, despite the well-known antiwetting properties of the toes, which are superhydrophobic and have a low–contact-angle hysteresis (31, 32).
Somewhat surprisingly, geckos cannot stick to hydrophilic glass when it is covered with a layer of water (33). Anecdotally, this effect has been long and widely appreciated; nevertheless, the effect of water on gecko adhesion is complex. For example, a thin water layer on a hydrophilic sapphire substrate can be expelled at the adhesive interface between a gecko toe and the substrate (34), likely because of the gecko’s superhydrophobic toe pads. Conversely, a thick water layer (∼0.5 cm deep) on a hydrophilic glass surface cannot similarly be expelled. Moreover, a large drop in adhesive strength occurs when the adhesive system is submerged in water (33). At face value, this is perplexing: many geckos live in tropical habitats where surface wetting from rain and humidity is expected to be common. However, arboreal geckos likely use plant surfaces more than other substrates, such as dirt, sand, or manmade glass and plastics, and many plant surfaces are hydrophobic (24). This begs the obvious question: Can geckos stick to wet hydrophobic surfaces? Unfortunately, data necessary to answer this question are very limited. Early experiments by Hiller (35) tested for the effect of surface wettability on adhesion using surfaces whose water contact angle ranged from 62.6° to 92.7°. Although gecko adhesion was inversely related to water contact angle, adhesion when these surfaces were wetted with water was not tested. More recent work demonstrated that a submerged atomic force microscopy (AFM) probe contacting the tips (spatula) of a gecko’s adhesive hairs showed a significant decrease in normal pull-off force compared with a dry environment (36). Similarly, Pesika et al. (37) tested adhesion between a small patch of the gecko’s adhesive hairs (setae) and a silica surface in water under different loads and also found a significant drop in adhesive force. Taken together, however, these studies do not predict how the gecko adhesive system behaves under conditions that likely are quite common in their native environment.
In this study, we tested the effect of water on the gecko adhesive system using a range of surfaces with different wettability defined by water contact angle (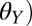 . Because geckos probably encounter hydrophilic surfaces (such as some flowers and roots) as well as hydrophobic surfaces (most plant leaves) (24, 38), we tested surfaces that are hydrophilic (
. Because geckos probably encounter hydrophilic surfaces (such as some flowers and roots) as well as hydrophobic surfaces (most plant leaves) (24, 38), we tested surfaces that are hydrophilic (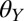 = 50 ± 1.4°), intermediately wetting (
= 50 ± 1.4°), intermediately wetting (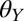 = 85 ± 0.5°), and hydrophobic (
= 85 ± 0.5°), and hydrophobic (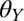 = 94 ± 0.5°). We also tested the effect of water on adhesion to polytetrafluoroethylene (PTFE), a synthetic substrate to which geckos cannot adhere in dry conditions (35, 39).
= 94 ± 0.5°). We also tested the effect of water on adhesion to polytetrafluoroethylene (PTFE), a synthetic substrate to which geckos cannot adhere in dry conditions (35, 39).
To explain our experimental results, we developed a model in which we take into account adhesion between a “gecko hair-like” surface and surfaces with different wettability in different media (in air and in water). We used a classic thermodynamic approach to calculate the work of adhesion between two separating surfaces: a gecko hair-like surface and each of the four surfaces we used in whole-animal experiments with different 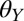 . Further, we expected that the gecko’s setal morphology would have a significant effect on adhesion, because the small adhesive hairs form a multicontact interface rather than a flat, uniform contact. To take into account this geometric effect, we calculated the thermodynamic work of adhesion between a structured gecko hair-like surface and the substrates with varying
. Further, we expected that the gecko’s setal morphology would have a significant effect on adhesion, because the small adhesive hairs form a multicontact interface rather than a flat, uniform contact. To take into account this geometric effect, we calculated the thermodynamic work of adhesion between a structured gecko hair-like surface and the substrates with varying 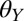 . Our model allows us to predict adhesive forces across a range of surface wettability and environments that either the gecko or a synthetic mimic may encounter and, thus, highlights not only a very important aspect of the gecko adhesive system but also crucial design parameters for mimetic systems.
. Our model allows us to predict adhesive forces across a range of surface wettability and environments that either the gecko or a synthetic mimic may encounter and, thus, highlights not only a very important aspect of the gecko adhesive system but also crucial design parameters for mimetic systems.
Materials and Methods
Whole-Animal Adhesion.
Six tokay geckos (Gekko gecko) with an average weight of 99.3 ± 2.25 g were used during experimental trials. Detailed husbandry procedures are outlined by Niewiarowski et al. (40). Before each experiment, the geckos were allowed 30 min to acclimate to the testing environment, which was maintained at 26 ± 0.1 °C and 33 ± 0.3% relative humidity through all experimental trials. Procedures involving live animals followed guidelines published by the Society for the Study of Amphibians and Reptiles (SSAR 2004) and were approved by University of Akron Institutional Animal Care and Use Committee Protocol 07–4G.
Four substrates—glass, polymethylmethacrylate (PMMA), PTFE, and an octadecyltrichlorosilane self-assembled monolayer (OTS-SAM) formed on the surface of glass (SI Text, section 1)—were used in this experiment. Surfaces were mounted securely with Velcro to the bottom of a Rubbermaid container, which was used to hold water during trials. Each gecko was fitted with two harnesses, which were attached to a force sensor positioned horizontally on a motorized track, similar to the rig described by Niewiarowski et al. (40) and Stark et al. (33). Maximum shear adhesive force was defined as the point at which all four feet began to slip along the surface. In the wet surface condition, the substrate was fully submerged in water (24 ± 0.2 °C) so that ∼1 cm of standing water covered the surface, completely submerging the gecko’s feet. Geckos were tested randomly on all surfaces except the OTS-SAM–coated surface, which was tested last. Each gecko was tested on all four surfaces and under both surface conditions (wet and dry) three times. Only the highest maximum shear force value collected from the three trials per individual was used in statistical analysis. The effect of surface type (glass, PMMA, OTS-SAM–coated glass, or PTFE) on surface treatment (wet or dry) was tested using a repeated-measures multivariate ANOVA. A matched-pairs analysis was used to compare specific treatments of interest. All sample means are reported as mean ±1 SEM.
Adhesion Model.
To explain adhesion between a gecko foot and the four different surfaces used in whole-animal adhesion experiments, a classical thermodynamic model of adhesion was used to predict the adhesive interaction between the two contacting surfaces in either air or water.
There are three main assumptions for the model calculations: First, the surface of the gecko foot at the contact interface is assumed to have surface properties similar to those of lipid-like n-hexadecane. This assumption is based on our previous experiments indicating that gecko setae have phospholipids on their surface (34). Second, the model assumes a normal direction of adhesion, whereas the experimental trials were carried out in the shear direction. We know that typically normal and shear adhesions are proportional for hard surfaces (41), whereas for soft surfaces, normal adhesion is only lower by the one-half power (42). Considering the proportionality between normal and shear force, our assumption is reasonable. In addition, we compared only relative adhesion energies, rather than exact numbers, by calculating the ratio of the work of normal adhesion in water (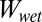 ) to the work of normal adhesion in air (
) to the work of normal adhesion in air (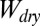 ) for each surface. Finally, our third assumption is that the contact interface is flat and contact between the two surfaces is chemically and structurally homogeneous. This includes the assumption that contact between the surfaces is dry, and no intervening layer of water is present between the two surfaces during contact.
) for each surface. Finally, our third assumption is that the contact interface is flat and contact between the two surfaces is chemically and structurally homogeneous. This includes the assumption that contact between the surfaces is dry, and no intervening layer of water is present between the two surfaces during contact.
Smooth Surface.
We used the Young–Dupré equation to calculate the work of adhesion between each of our four surfaces (glass, PMMA, OTS-SAM–coated glass, and PTFE) and the gecko hair-like n-hexadecane surface (43) (SI Text, section 2.1).
In the case of wet adhesion, i.e., where water is the medium of contact, the work of adhesion (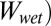 is calculated using the following equation (derived in SI Text, section 2.2):
is calculated using the following equation (derived in SI Text, section 2.2):
Here, 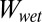 is the work of adhesion between two surfaces contacting underwater, Ac is the area of contact,
is the work of adhesion between two surfaces contacting underwater, Ac is the area of contact, 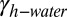 is the gecko hair-like n-hexadecane–water interfacial energy,
is the gecko hair-like n-hexadecane–water interfacial energy, 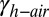 is the gecko hair-like n-hexadecane–air interfacial energy, and
is the gecko hair-like n-hexadecane–air interfacial energy, and 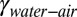 is the interfacial energy at the water–air interface. To obtain the interfacial energies, we measured the contact angle of n-hexadecane (θ1) and water (θ2) on all four surfaces used in the experimental trials (Table 1; see SI Text, section 3 for procedure).
is the interfacial energy at the water–air interface. To obtain the interfacial energies, we measured the contact angle of n-hexadecane (θ1) and water (θ2) on all four surfaces used in the experimental trials (Table 1; see SI Text, section 3 for procedure).
Table 1.
Contact angles 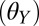 of water and n-hexadecane on all four surfaces used in whole-animal experiments and modeling
of water and n-hexadecane on all four surfaces used in whole-animal experiments and modeling
| Surface | Water, ° | n-Hexadecane, ° |
| Glass | 50 ± 1.4 | 13 ± 2.0 |
| PMMA | 85 ± 0.5 | 9 ± 2.3 |
| OTS-SAM | 94 ± 0.5 | 29 ± 2.9 |
| PTFE | 97 ± 0.3 | 30 ± 1.6 |
Errors are means ±1 SEM.
Patterned Surface.
To take into account the surface morphology of gecko toes, we used a model unit cell. The unit cell consists of a tetrad pattern, which is representative of the setal pattern on the surface of the tokay gecko (G. gecko) toe (Fig. 1 A and B). The tetrad unit cell was made up of four square pillars, each with a square side width of 4 μm and height of 60 μm. The distance between any two adjacent pillars in one tetrad was 1 μm, and the separation between two adjacent tetrads was 2 μm (Fig. 1C).
Fig. 1.
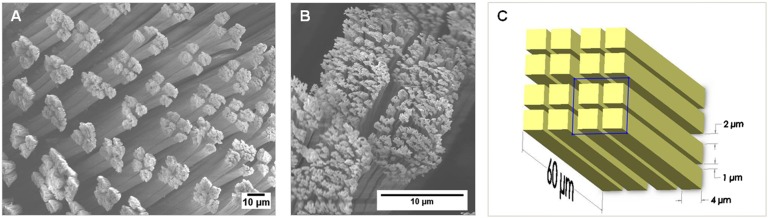
Scanning electron microscope images of (A) the tetrad patterning of the tokay gecko (G. gecko) setal mats and (B) the four setae that are grouped together to form a single tetrad. To represent the tetrad morphology of the toe pad, a patterned surface was used to model the gecko setal mats (C) using dimensions similar to those of the gecko setal mat. Each column, representing one seta, was 60 μm high and 4 μm wide. The separation distance between columns (setae) was 1 μm, and separation between tetrad unit cells was 2 μm. The model unit cell used for calculations is boxed in C. Scale bars are 10 μm.
When the tetrad structure of gecko hair-like n-hexadecane is in contact with any of the four flat surfaces (glass, PMMA, OTS-SAM–coated glass, or PTFE), 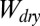 is estimated using the Young–Dupré equation (SI Text, section 4.1). For
is estimated using the Young–Dupré equation (SI Text, section 4.1). For 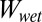 of a patterned surface, there are four different cases possible of “separated” and “in-contact” states that are associated with air pockets between the square columns before or after contact with the flat test surface (see Table 2 for schematics of each case). In all cases, the water surrounding the unit cell also is accounted for in
of a patterned surface, there are four different cases possible of “separated” and “in-contact” states that are associated with air pockets between the square columns before or after contact with the flat test surface (see Table 2 for schematics of each case). In all cases, the water surrounding the unit cell also is accounted for in 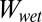 , whereas the adhesive interface is always assumed to be dry. In case 1, both the in-contact and the separated states maintain air pockets in the space separating the square columns.
, whereas the adhesive interface is always assumed to be dry. In case 1, both the in-contact and the separated states maintain air pockets in the space separating the square columns. 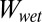 for case 1 may be calculated using the following equation (Eq. 2), where A1 and A2 correspond to total surface areas of the gecko hair-like n-hexadecane tetrad-patterned unit cell and the surface with which it is in contact (glass, PMMA, OTS-SAM–coated glass, or PTFE), respectively:
for case 1 may be calculated using the following equation (Eq. 2), where A1 and A2 correspond to total surface areas of the gecko hair-like n-hexadecane tetrad-patterned unit cell and the surface with which it is in contact (glass, PMMA, OTS-SAM–coated glass, or PTFE), respectively:
Table 2.
Ratios of wet to dry adhesion on all four surfaces used in whole-animal experiments and modeling
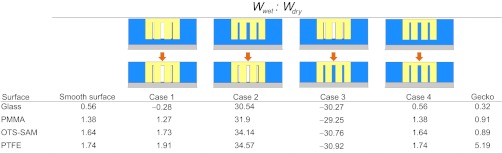 |
The 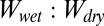 ratio in the smooth and patterned surface models is calculated as the work of normal adhesion of two surfaces coming into contact in water
ratio in the smooth and patterned surface models is calculated as the work of normal adhesion of two surfaces coming into contact in water 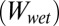 and the work of normal adhesion of two surfaces coming into contact in air
and the work of normal adhesion of two surfaces coming into contact in air 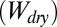 . Models were calculated using a chemically similar gecko hair-like surface (n-hexadecane), represented as the yellow surface, and each of the four surfaces used for whole-animal experiments (glass, PMMA, OTS-SAM–coated glass, and PTFE), represented as the gray surface. Ratios for normal adhesion of a patterned surface may be separated into four cases of precontact (separated) and in-contact (shown by the arrow) with the gecko hair-like surface and the four experimental surfaces. When submerged in water (blue), the space between the patterned unit cell pillars is filled with either air or water. These are shown schematically, where case 1 shows a consistently dry interpillar region and case 4 shows one that is consistently wet. Case 2 represents the scenario in which the interpillar region first is wet and then becomes dry on contact, and case 3 represents the opposite, in which the interpillar region first is dry and then becomes wet after contact. The wet-to-dry ratios for whole-animal shear adhesion on each of the four surfaces are shown in the last column.
. Models were calculated using a chemically similar gecko hair-like surface (n-hexadecane), represented as the yellow surface, and each of the four surfaces used for whole-animal experiments (glass, PMMA, OTS-SAM–coated glass, and PTFE), represented as the gray surface. Ratios for normal adhesion of a patterned surface may be separated into four cases of precontact (separated) and in-contact (shown by the arrow) with the gecko hair-like surface and the four experimental surfaces. When submerged in water (blue), the space between the patterned unit cell pillars is filled with either air or water. These are shown schematically, where case 1 shows a consistently dry interpillar region and case 4 shows one that is consistently wet. Case 2 represents the scenario in which the interpillar region first is wet and then becomes dry on contact, and case 3 represents the opposite, in which the interpillar region first is dry and then becomes wet after contact. The wet-to-dry ratios for whole-animal shear adhesion on each of the four surfaces are shown in the last column.
In the separated state of case 2, water penetrates completely inside the tetrad asperities in such a way that the entire surface area of the tetrad unit cell is in contact with water. However, the in-contact state expels all the water, and the asperities occupied with water initially are replaced by air pockets. The equation to calculate 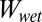 for case 2 is
for case 2 is
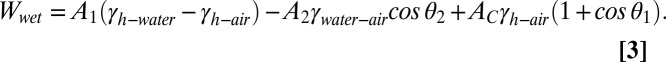 |
Cases 3 and 4 represent the possibility that the gaps between the pillars in a tetrad are completely filled with water in the in-contact state; however, in the separated state, the gaps are either air pockets or filled with water for cases 3 and 4, respectively. Eq. 4 is used to calculate 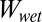 for case 3, and Eq. 1 calculates
for case 3, and Eq. 1 calculates 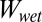 for case 4 (because it is only AC that is different for the patterned surface compared with the flat surface).
for case 4 (because it is only AC that is different for the patterned surface compared with the flat surface).
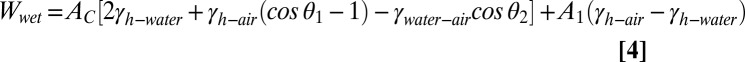 |
Derivations for each case are included in Supporting Information (SI Text, section 4.2), as are derivations for a non–tetrad-patterned unit cell model (SI Text, section 5, Fig. S1, and Table S1).
Results
Whole-Animal Adhesion.
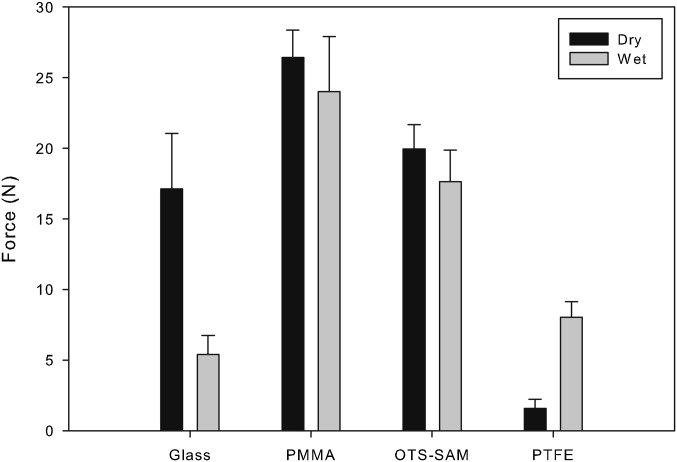
Gecko adhesion in the shear direction (frictional adhesion) was tested on four surfaces with different wetting properties: glass, PMMA, OTS-SAM–coated glass, and PTFE. Water contact angles ranged from ∼50° to 100° (Table 1). Whole-animal shear adhesion was measured on all four surfaces when submerged in water and when dry to test for a difference in adhesion when either air or water was the medium of contact. When comparing surfaces of different wettability (glass, PMMA, OTS-SAM, and PTFE) and the medium of contact (wet or dry), we found a significant interaction between surface wettability and medium of contact (F3,8 = 8.82, P = 0.0064; Table S2). As reported previously (33), gecko adhesion on the glass surface was significantly higher (t = −3.35, df = 5, P = 0.0204) when the surface was dry (17.1 ± 3.93 N) than when the glass substrate was submerged in water (5.4 ± 1.33 N). When tested on the PMMA and the OTS-SAM–coated surfaces, geckos did not show a significant difference (t = −0.61, df = 5, P = 0.5691 for PMMA and t = −0.80, df =5, P = 0.4615 for OTS-SAM) in wet (24.0 ± 3.92 N, PMMA; 17.6 ± 2.22 N, OTS-SAM) and dry surface conditions (26.4 ± 1.94 N, PMMA; 20.0 ± 1.71 N, OTS-SAM). Conversely, when tested on PTFE, geckos produced a significantly higher (t = 6.61, df = 5, P = 0.0012) adhesive force underwater (8.0 ± 1.09 N) than on dry PTFE (1.6 ± 0.66 N), unlike the results on all other surfaces (Fig. 2).
Fig. 2.
Whole-animal shear (frictional) adhesion values from tokay geckos (G. gecko) tested on four surfaces in dry or wet contact. Each gecko (n = 6) was tested three times on each surface (glass, PMMA, OTS-SAM–coated glass, and PTFE), and the highest of the three tests was used for data analysis. Surfaces were tested either without water (dry) or fully submerged in water (wet). Error bars are means ±1 SEM.
Similar to our previous report, geckos could not support their body weight (1 N of force to support a 100-g gecko) on a wet glass surface (33). These experiments, however, were very controlled as we allowed the gecko to take steps only one foot at a time. In the results reported in this study, we allowed each gecko to place all four of its feet naturally before beginning the experiment and we measured only one force reading per experimental trial. Consequently, our cling force values for geckos on a glass surface submerged in water were higher (5.4 ± 1.33 N) than those reported previously [0.4 ± 0.04N (33)]. Because the geckos were allowed to position all their feet at one time, we noticed that five of the six geckos were successful in achieving sufficient contact with the glass surface and one of their feet. This occurred for these five geckos only once in three trials, or about 28% of the total experiment time. When one foot was planted successfully on the hydrophilic glass surface, it was clear by visual inspection that this foot was the only foot contributing to overall whole-animal adhesion, and these events all produced over 1 N of force. If these data were removed from analysis, our results on the submerged glass surface would be 0.7 ± 0.07 N, similar to our previous findings. Using this value to calculate the wet-to-dry ratio of adhesion on a glass surface, the ratio would be even lower (0.04) than that reported (0.32; Table 2).
Smooth Surface Adhesion Model.
We tested the hypothesis that the ratio of shear adhesion values from the whole-animal experiments on wet and dry surfaces could be predicted from a classical thermodynamic model, based on the work of normal adhesion (W) between two flat surfaces in air and in water. Using the Young–Dupré equation and Eq. 1, we calculated the 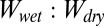 ratio for our model and compared this to the ratio of wet-to-dry shear adhesion values obtained experimentally from whole-animal adhesion measurements on each of the four surfaces (Table 2). The model calculations show that relative normal adhesion on any given test surface when tested in air and underwater is a function of surface wettability. As the hydrophobicity of the surface increases (water contact angle increases), the value of
ratio for our model and compared this to the ratio of wet-to-dry shear adhesion values obtained experimentally from whole-animal adhesion measurements on each of the four surfaces (Table 2). The model calculations show that relative normal adhesion on any given test surface when tested in air and underwater is a function of surface wettability. As the hydrophobicity of the surface increases (water contact angle increases), the value of 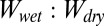 increases. The experimental values from whole-animal adhesion experiments also show a similar trend (Table 2), supporting our hypothesis that surface hydrophobicity may have a significant thermodynamic impact on gecko adhesion when tested underwater.
increases. The experimental values from whole-animal adhesion experiments also show a similar trend (Table 2), supporting our hypothesis that surface hydrophobicity may have a significant thermodynamic impact on gecko adhesion when tested underwater.
Patterned Surface Adhesion Model.
To consider the effect of patterning of the setal mats, we took into account the gecko toe surface morphology by modeling a tetrad-patterned gecko hair-like surface. Using our calculations of the 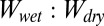 ratios of each surface in various conditions, we qualitatively compared our model with experimental results. The
ratios of each surface in various conditions, we qualitatively compared our model with experimental results. The 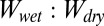 ratios for smooth surfaces and ratios for case 4 of the patterned surface are the same (Table 2); this is because the common multiplication factor corresponding to the total surface area for
ratios for smooth surfaces and ratios for case 4 of the patterned surface are the same (Table 2); this is because the common multiplication factor corresponding to the total surface area for 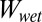 and
and 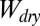 cancels out as the ratio of the two is taken. Similar to the smooth surface model, hydrophobicity of the surface has a significant impact on the
cancels out as the ratio of the two is taken. Similar to the smooth surface model, hydrophobicity of the surface has a significant impact on the 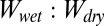 ratio; however, each case presents different results for a given surface, suggesting that the presence of water between or around the hairs also plays an important role in adhesion underwater. Based on our results, we clearly can rule out cases 2 and 3 as possible conditions of pre- and postcontact between the patterned gecko hair-like surface and the flat test surfaces, as ratio values are either extremely high, favoring wet adhesion over dry (case 2), or extremely low, favoring dry adhesion over wet (case 3), neither of which occurs in the whole-animal system.
ratio; however, each case presents different results for a given surface, suggesting that the presence of water between or around the hairs also plays an important role in adhesion underwater. Based on our results, we clearly can rule out cases 2 and 3 as possible conditions of pre- and postcontact between the patterned gecko hair-like surface and the flat test surfaces, as ratio values are either extremely high, favoring wet adhesion over dry (case 2), or extremely low, favoring dry adhesion over wet (case 3), neither of which occurs in the whole-animal system. 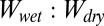 ratios for cases 1 and 4 are most similar to those from whole-animal experiments, in which glass is the least favorable in wet conditions and ratios on the hydrophobic surfaces are near 1, supporting equal adhesion in wet and dry conditions (Table 2). Modeled ratios involving PTFE do not explain our experimental results (the experimental ratio is much higher than all modeled ratios) and are discussed later.
ratios for cases 1 and 4 are most similar to those from whole-animal experiments, in which glass is the least favorable in wet conditions and ratios on the hydrophobic surfaces are near 1, supporting equal adhesion in wet and dry conditions (Table 2). Modeled ratios involving PTFE do not explain our experimental results (the experimental ratio is much higher than all modeled ratios) and are discussed later.
To further investigate cases 1 and 4, we imaged the gecko foot contacting glass and the OTS-SAM–coated surfaces underwater after the gecko took four steps, similar to experimental trials (Fig. 3). The hydrophobic OTS-SAM–coated surface (Fig. 3B) shows typical characteristics of a dry contact (Fig. 3A), such as a white rather than a gray appearance of the adhesive toe pads and their tendency to remain dry when taken out of the water. This suggests that our results are most similar to case 1, a constantly dry contact underwater with air pockets between the surface asperities in both the separated and in-contact states. On the hydrophilic glass surface, however, we typically see one of three scenarios taking place: a consistently dry contact (case 1), an entrapped air bubble between the toe and the glass surface (shown as a silvery layer on the toe; Fig. 3C), or a scenario in which water wets the toes (case 3) and the wet toes appear gray when removed from the test surface.
Fig. 3.
Images of a gecko foot (A) in dry contact with glass, (B) in wet contact with a hydrophobic surface (OTS-SAM–coated glass), and (C) in wet contact with a hydrophilic surface (glass). Geckos were allowed to take four natural steps to ensure adhesive contact with the surface. Areas where the toe is in dry contact with the surface appear white, similar to the dry contact in A, and areas where the toe appears gray have been wetted with water. Silvery air bubbles may be seen between wet lamellae in B and on most of the toe pad in C. Scale bars are 0.5 cm.
Discussion
Despite substantial interest in the field, little work has been done to investigate the performance of the gecko adhesive system under conditions similar to the gecko’s native environment. Although gecko species are widely distributed, many are native to tropical environments where surfaces are likely to be dirty or wet. In this study, we took into account the effect of surface wettability on adhesion to wet and dry surfaces by testing geckos on four different surfaces ranging from hydrophilic (glass), intermediately wetting (PMMA), hydrophobic (OTS-SAM–coated glass), and finally PTFE, a hydrophobic surface to which geckos fail to cling in dry conditions. To explain our findings from whole-animal experiments, we modeled adhesion between two surfaces, a gecko hair-like surface (n-hexadecane) and each of the four surfaces tested in whole-animal experiments, using both air and water as the media of contact. Results from whole-animal experiments and surface modeling suggest that gecko adhesion is highly dependent on surface wettability and the presence of water or air between the toe pad and the contact surface.
Similar to our previous report (33), shear adhesion to the hydrophilic glass surface was much lower in water than in air experimentally (Fig. 2) and was consistently lower in our model calculations compared with the other three surfaces (Table 2). The anomalous behavior in the case of the glass surface is not surprising given that the surface is hydrophilic and thus shows higher affinity to water compared with other test surfaces. For example, three scenarios may occur when two surfaces contact underwater (44). First, a layer of water between the surfaces acts as a barrier to the establishment of contact between the two. Second, roughness of the contact surface may change the contact area and also may leave small pools of water behind. Third, water may be fully excluded from the two surfaces and completely dry contact occurs. Depending on the gecko’s foot placement, we found that either a dry contact formed by squeezing water out completely (case 1) or an air bubble formed (Fig. 3C) that acted as a lubricating layer, causing the gecko to slip. There is another scenario that may occur in whole-animal gecko adhesion on wet surfaces in which a wetting transition occurs and the toe wets completely, causing a substantial drop in adhesion experimentally (33) and theoretically (case 3). These observations suggest that natural placement of the foot on the wet surface is important and adhesion may be highly variable when the surface is hydrophilic, potentially limiting a gecko’s movement on wet hydrophilic surfaces. Interestingly, not all feet, and possibly not all toes, show the same scenario in any single trial; rather, it is always a combination of two or more behaviors happening at the same time that complicates the use of models, like ours, that assume homologous contact.
Our results on PMMA and OTS-SAM–coated glass are consistent with our hypothesis that adhesion will not be affected by water on surfaces that are more hydrophobic in nature than glass. This suggests that gecko adhesion is not impaired by surface wetting in geckos’ natural environments, assuming their native substrates are at least moderately hydrophobic (perhaps 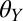 ≥ 85°). Some of the main surfaces we expect geckos to be walking on are plant surfaces, such as leaves, which are primarily hydrophobic because of their waxy cuticle (24). By imaging the foot in contact with the hydrophobic OTS-SAM–coated glass, it is clear that water is excluded from most of the adhesive toe pad and the contact made underwater is dry (Fig. 3B). These results are similar to the contact made by a terrestrial beetle underwater, where trapped air bubbles actually allow dry contact to occur on hydrophobic surfaces and traction force of the beetle walking underwater does not differ from forces collected when the beetle was walking in air (45). Our experimental results also are supported by our model, and ratios of wet to dry adhesion are near 1 (wet adhesion is not different from dry adhesion) in case 1, in which the gecko hair-like surface remains dry before and during contact. The maintenance of dry contact is interesting because when two surfaces become separated, water likely will penetrate the separation crack forming between the two surfaces and even propagate further growth (46). This may be what occurs when testing normal and frictional (shear) adhesion of a patch of setae, where normal adhesion in water is much lower, as the result of water penetration, than frictional adhesion in water under high loads that presumably keep water from separating the two surfaces (37).
≥ 85°). Some of the main surfaces we expect geckos to be walking on are plant surfaces, such as leaves, which are primarily hydrophobic because of their waxy cuticle (24). By imaging the foot in contact with the hydrophobic OTS-SAM–coated glass, it is clear that water is excluded from most of the adhesive toe pad and the contact made underwater is dry (Fig. 3B). These results are similar to the contact made by a terrestrial beetle underwater, where trapped air bubbles actually allow dry contact to occur on hydrophobic surfaces and traction force of the beetle walking underwater does not differ from forces collected when the beetle was walking in air (45). Our experimental results also are supported by our model, and ratios of wet to dry adhesion are near 1 (wet adhesion is not different from dry adhesion) in case 1, in which the gecko hair-like surface remains dry before and during contact. The maintenance of dry contact is interesting because when two surfaces become separated, water likely will penetrate the separation crack forming between the two surfaces and even propagate further growth (46). This may be what occurs when testing normal and frictional (shear) adhesion of a patch of setae, where normal adhesion in water is much lower, as the result of water penetration, than frictional adhesion in water under high loads that presumably keep water from separating the two surfaces (37).
The dominant mechanism behind the gecko’s ability to stick is van der Waals forces, which in dry contact should be relatively insensitive to surface chemistry (2). Of the three surfaces with similar Hamaker constants (see derivations in SI Text, section 6 and Tables S3 and S4)—glass (6.5 × 10−20 J), PMMA (6.2 × 10−20 J) and a glass plate coated with OTS-SAM (6.5 × 10−20 J, assuming the OTS coating has no effect)—dry adhesion on the PMMA surface was almost significantly greater than both the dry glass (t = 2.33, df = 5, P = 0.0672) and the dry glass coated with OTS-SAM (t = −2.52, df = 5, P = 0.0535). The PMMA surface had a contact angle of 85 ± 0.5° and a slightly lower Hamaker constant. Although it is not entirely clear why dry PMMA was marginally better than the other two surfaces in our whole-animal experiments, it is interesting to consider an optimal surface for the gecko adhesive system and how such a surface may correlate to the natural surfaces of the gecko’s environment.
In contrast, the Hamaker constants calculated in water are lower than in air for all the substrates studied here (SI Text, section 6, and Table S4). This is expected based on van der Waals interactions and may indicate that the adhesion forces to separate nonpolar surfaces in water should be lower in water. However, this is misleading because Hamaker constants do not accurately predict the interfacial energies of nonpolar materials in water and require the addition of hydrogen bonding (table 11.4 in ref. 47). A simpler explanation for higher shear adhesion forces for nonpolar surfaces in water is the higher interfacial energies of nonpolar materials in water than in air, as calculated by our model (42).
Our final surface, PTFE, provided surprising results. We quantitatively confirmed previous observations (35, 39) that geckos do not stick well to dry PTFE (1.6 ± 0.66 N). When tested on PTFE submerged in water, however, the geckos clung to the fully wetted surface significantly better than on the dry (8.0 ± 1.09 N), contrary to our findings on all other tested surfaces. Unlike our previous results on each of the other surfaces, our model does not predict the whole-animal experimental results. Our experimental ratio is fivefold higher than our theoretical ratio, and this discrepancy might be a result of the low adhesion of geckos to dry PTFE or the comparably high adhesion geckos have on wet PTFE. Additionally, the experimentally measured shear adhesion to dry PTFE is 10–15 times lower than shear adhesion to a surface with a similar water contact angle (OTS-SAM–coated glass). This low adhesion on PTFE cannot be explained by the small difference in the Hamaker constants (4.6 × 10−20 J for PTFE compared with 6.5 × 10−20 J for OTS-SAM–coated glass). We also do not believe that static charging is playing a role here, because the adhesion values for PTFE are lower, rather than higher, than those predicted by contact angles or Hamaker constants. In addition, surface charges will be neutralized underwater and cannot influence the shear adhesion values measured in water. One reason the experimental values of dry shear adhesion on PTFE are lower than the expected values from our theoretical models, or compared with a surface with similar water contact angle, may be related to the abnormally low coefficient of friction of PTFE. Interestingly, underwater shear adhesion values for PTFE are vastly improved and closer to our expected values for hydrophobic surfaces. We believe that the adhesion in air is anomalous for PTFE, resulting in much larger ratios for wet vs. dry shear adhesion forces. Additionally, we hypothesize that the roughness of PTFE also may play an important role. Although the roughness of the PTFE surface does not change when wet or dry, when PTFE is submerged in water, the roughness may be less important to adhesion because water can penetrate between the rough surface asperities. However, when dry, the roughness of PTFE may cause air gaps and a reduced contact area, lowering adhesion values. It is clear that further work is necessary to clarify the effect of roughness on adhesion to wet and dry surfaces. Interestingly, synthetic gecko-like PTFE pillars tested underwater with a silica probe also were successful in achieving adhesion; however, adhesion values underwater were not five times higher than dry as measured in our experiments (48).
Our main goal was to answer a puzzling question: Can geckos living in tropical environments maintain adhesion on wet hydrophobic surfaces? Using our whole-animal adhesion results, we found that wet surfaces that are even weakly hydrophobic allow the gecko adhesive system to remain functional for clinging and likely locomotion as well. Our findings suggest a level of versatility in the gecko adhesive system that previously was not accounted for and calls into question interesting evolutionary, ecological, and behavioral predictions. For example, maintenance of the superhydrophobic toe pads likely is critical for geckos living in the tropics. The ability of the toes to shed water droplets relies on the wettability of the toe pad, and, as such, a surface chemistry conducive to water shedding should be conserved in species native to wet environments. Our recent finding of lipid-like molecules at the contact interface (34) may help maintain or prolong this antiwetting property; however, further experiments are needed to confirm this. Evolutionarily, geckos are an interesting group in that their adhesive toe pads have evolved multiple times (23) and, in at least one group, digital toe pads appear to be strongly correlated with ecological factors such as substrate utilization (49). Although our experiment focuses on one tropical species of gecko (G. gecko), it is not unreasonable to consider potential variation in surface chemistry, toe pad roughness, or other antiwetting mechanisms that are dictated by environment. Although this study highlights yet another remarkable property of the gecko adhesive system, it is important to remember that the system remains limited on hydrophilic surfaces and loses functionality when the toes become wet (33). It is unclear how these limitations affect adhesive performance in natural conditions; however, the answer to this might be related to compensatory behaviors or ecological constraints that have yet to be evaluated. Our findings highlight the importance of considering the natural environments in which geckos use their adhesive system, as well as how the chemical composition and patterning of their adhesive structures may play a role in the success of the system in challenging environments, such as those that frequently become wet. Our study also provides important information for the design of synthetic mimics that can attach equally well in water and in air or, in the case of PTFE, better in water than air, similar to what the gecko can achieve.
Supplementary Material
Acknowledgments
The authors thank Jocelyn Ohlemacher for help with experimental trials, Edward A. Ramirez for the photographs in Fig. 3, and Ethan Knapp for help with the schematics in Fig. 1, Fig. S1, and Table 2. Financial support was provided by National Science Foundation Grant DMR-1105370 (to A.D.) and Goodyear Tire and Rubber Company (I.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219317110/-/DCSupplemental.
References
- 1.Autumn K, et al. Adhesive force of a single gecko foot-hair. Nature. 2000;405(6787):681–685. doi: 10.1038/35015073. [DOI] [PubMed] [Google Scholar]
- 2.Autumn K, et al. Evidence for van der Waals adhesion in gecko setae. Proc Natl Acad Sci USA. 2002;99(19):12252–12256. doi: 10.1073/pnas.192252799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autumn K, Peattie AM. Mechanisms of adhesion in geckos. Integr Comp Biol. 2002;42(6):1081–1090. doi: 10.1093/icb/42.6.1081. [DOI] [PubMed] [Google Scholar]
- 4.Tian Y, et al. Adhesion and friction in gecko toe attachment and detachment. Proc Natl Acad Sci USA. 2006;103(51):19320–19325. doi: 10.1073/pnas.0608841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autumn K, Dittmore A, Santos D, Spenko M, Cutkosky M. Frictional adhesion: A new angle on gecko attachment. J Exp Biol. 2006;209(Pt 18):3569–3579. doi: 10.1242/jeb.02486. [DOI] [PubMed] [Google Scholar]
- 6.Puthoff JB, Prowse MS, Wilkinson M, Autumn K. Changes in materials properties explain the effects of humidity on gecko adhesion. J Exp Biol. 2010;213(Pt 21):3699–3704. doi: 10.1242/jeb.047654. [DOI] [PubMed] [Google Scholar]
- 7.Gravish N, et al. Rate-dependent frictional adhesion in natural and synthetic gecko setae. J R Soc Interface. 2010;7(43):259–269. doi: 10.1098/rsif.2009.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravish N, Wilkinson M, Autumn K. Frictional and elastic energy in gecko adhesive detachment. J R Soc Interface. 2008;5(20):339–348. doi: 10.1098/rsif.2007.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yurdumakan B, Raravikar NR, Ajayan PM, Dhinojwala A. Synthetic gecko foot-hairs from multiwalled carbon nanotubes. Chem Commun (Camb) 2005;(30):3799–3801. doi: 10.1039/b506047h. [DOI] [PubMed] [Google Scholar]
- 10.Ge L, Sethi S, Ci L, Ajayan PM, Dhinojwala A. Carbon nanotube-based synthetic gecko tapes. Proc Natl Acad Sci USA. 2007;104(26):10792–10795. doi: 10.1073/pnas.0703505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett MD, et al. Looking beyond fibrillar features to scale gecko-like adhesion. Adv Mater (Deerfield Beach Fla) 2012;24(8):1078–1083. doi: 10.1002/adma.201104191. [DOI] [PubMed] [Google Scholar]
- 12.Lee H, Lee BP, Messersmith PB. A reversible wet/dry adhesive inspired by mussels and geckos. Nature. 2007;448(7151):338–341. doi: 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- 13.Menon C, Murphy M, Sitti M. 2004. Gecko inspired surface climbing robots. IEEE International Conference on Robotics and Biomimetics, 2004. ROBIO 2004, pp 431–436.
- 14.Murphy MP, Kim S, Sitti M. Enhanced adhesion by gecko-inspired hierarchical fibrillar adhesives. ACS Appl Mater Interfaces. 2009;1(4):849–855. doi: 10.1021/am8002439. [DOI] [PubMed] [Google Scholar]
- 15.Murphy MP, Aksak B, Sitti M. Gecko-inspired directional and controllable adhesion. Small. 2009;5(2):170–175. doi: 10.1002/smll.200801161. [DOI] [PubMed] [Google Scholar]
- 16.Aksak B, Murphy MP, Sitti M. 2008. Gecko inspired micro-fibrillar adhesives for wall climbing robots on micro/nanoscale rough surfaces. International Conference on Robotics and Automation—ICRA, pp 3058–3063.
- 17.Northen MT, Greiner C, Arzt E, Turner KL. A Gecko-inspired reversible adhesive. Adv Mater (Deerfield Beach Fla) 2008;20(20):3905–3909. [Google Scholar]
- 18.Parness A, et al. A microfabricated wedge-shaped adhesive array displaying gecko-like dynamic adhesion, directionality and long lifetime. J R Soc Interface. 2009;6(41):1223–1232. doi: 10.1098/rsif.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TI, Jeong HE, Suh KY, Lee HH. Stooped nanohairs: Geometry-controllable, unidirectional, reversible, and robust gecko-like dry adhesive. Adv Mater (Deerfield Beach Fla) 2009;21(22):2276–2281. [Google Scholar]
- 20.Jeong HE, Lee JK, Kim HN, Moon SH, Suh KY. A nontransferring dry adhesive with hierarchical polymer nanohairs. Proc Natl Acad Sci USA. 2009;106(14):5639–5644. doi: 10.1073/pnas.0900323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahdavi A, et al. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc Natl Acad Sci USA. 2008;105(7):2307–2312. doi: 10.1073/pnas.0712117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han D, Zhou K, Bauer AM. Phylogenetic relationships among gekkotan lizards inferred from C-mos nuclear DNA sequences and a new classification of the Gekkota. Biol J Linn Soc Lond. 2004;83(3):353–368. [Google Scholar]
- 23.Gamble T, Greenbaum E, Jackman TR, Russell AP, Bauer AM. Repeated origin and loss of adhesive toepads in geckos. PLoS ONE. 2012;7(6):e39429. doi: 10.1371/journal.pone.0039429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch K, Bhushan B, Barthlott W. Diversity of structure, morphology and wetting of plant surfaces. Soft Matter. 2008;4(10):1943–1963. [Google Scholar]
- 25.Jetter R, Schäffer S. Chemical composition of the Prunus laurocerasus leaf surface. Dynamic changes of the epicuticular wax film during leaf development. Plant Physiol. 2001;126(4):1725–1737. doi: 10.1104/pp.126.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niewiarowski PH, Stark A, McClung B, Chambers B, Sullivan T. Faster but not stickier: Invasive house geckos can out-sprint resident mournful geckos in Moorea, French Polynesia. J Herpetol. 2012;46(2):194–197. [Google Scholar]
- 27.Russell AP, Johnson MK. Real-world challenges to, and capabilities of, the gekkotan adhesive system: Contrasting the rough and the smooth. Can J Zool. 2007;85(12):1228–1238. [Google Scholar]
- 28.Huber G, Gorb SN, Hosoda N, Spolenak R, Arzt E. Influence of surface roughness on gecko adhesion. Acta Biomater. 2007;3(4):607–610. doi: 10.1016/j.actbio.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Hansen WR, Autumn K. Evidence for self-cleaning in gecko setae. Proc Natl Acad Sci USA. 2005;102(2):385–389. doi: 10.1073/pnas.0408304102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu S, Lopez S, Niewiarowski PH, Xia Z. Dynamic self-cleaning in gecko setae via digital hyperextension. J R Soc Interface. 2012;9(76):2781–2790. doi: 10.1098/rsif.2012.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Autumn K, Hansen W. Ultrahydrophobicity indicates a non-adhesive default state in gecko setae. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192(11):1205–1212. doi: 10.1007/s00359-006-0149-y. [DOI] [PubMed] [Google Scholar]
- 32.Liu KS, Du JX, Wu JT, Jiang L. Superhydrophobic gecko feet with high adhesive forces towards water and their bio-inspired materials. Nanoscale. 2012;4(3):768–772. doi: 10.1039/c1nr11369k. [DOI] [PubMed] [Google Scholar]
- 33.Stark AY, Sullivan TW, Niewiarowski PH. The effect of surface water and wetting on gecko adhesion. J Exp Biol. 2012;215(Pt 17):3080–3086. doi: 10.1242/jeb.070912. [DOI] [PubMed] [Google Scholar]
- 34.Hsu PY, et al. Direct evidence of phospholipids in gecko footprints and spatula-substrate contact interface detected using surface-sensitive spectroscopy. J R Soc Interface. 2012;9(69):657–664. doi: 10.1098/rsif.2011.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiller U. Comparative studies on the functional morphology of 2 Gekkonid lizards. J Bombay Nat Hist Soc. 1976;73(2):278–282. [Google Scholar]
- 36.Huber G, et al. Evidence for capillarity contributions to gecko adhesion from single spatula nanomechanical measurements. Proc Natl Acad Sci USA. 2005;102(45):16293–16296. doi: 10.1073/pnas.0506328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pesika NS, et al. Gecko adhesion pad: A smart surface? J Phys Condens Matter. 2009;21(46):464132. doi: 10.1088/0953-8984/21/46/464132. [DOI] [PubMed] [Google Scholar]
- 38.Feng L, Zhang Y, Cao Y, Ye X, Jiang L. The effect of surface microstructures and surface compositions on the wettabilities of flower petals. Soft Matter. 2011;7(6):2977–2980. [Google Scholar]
- 39.Hiller U. Untersuchungen zum Feinbau und zur Funktion der Haftborsten von Reptilien (Studies on fine structure and function of the digital setae of lizards) Zoomorphology. 1968;62(4):307–362. [Google Scholar]
- 40.Niewiarowski PH, Lopez S, Ge L, Hagan E, Dhinojwala A. Sticky gecko feet: The role of temperature and humidity. PLoS ONE. 2008;3(5):e2192. doi: 10.1371/journal.pone.0002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpick RW, Agrait N, Ogletree DF, Salmeron M. Variation of the interfacial shear strength and adhesion of a nanometer-sized contact. Langmuir. 1996;12(13):3334–3340. [Google Scholar]
- 42.Chaudhury MK, Chung JY. Studying friction and shear fracture in thin confined films using a rotational shear apparatus. Langmuir. 2007;23(15):8061–8066. doi: 10.1021/la700501m. [DOI] [PubMed] [Google Scholar]
- 43.Chaudhury MK. Interfacial interaction between low-energy surfaces. Mater Sci Eng Rep. 1996;16(3):97–159. [Google Scholar]
- 44.Chaudhury MK, Whitesides GM. Direct measurement of interfacial interactions between semispherical lenses and flat sheets of poly(dimethylsiloxane) and their chemical derivatives. Langmuir. 1991;7(5):1013–1025. [Google Scholar]
- 45.Hosoda N, Gorb SN. Underwater locomotion in a terrestrial beetle: combination of surface de-wetting and capillary forces. Proc Biol Sci. 2012;279(1745):4236–4242. doi: 10.1098/rspb.2012.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haidara H, Chaudhury MK, Owen MJ. A direct method of studying adsorption of a surfactant at solid-liquid interfaces. J Phys Chem-Us. 1995;99(21):8681–8683. [Google Scholar]
- 47.Israelachvili J. Intermolecular and Surface Forces. London: Academic; 1991. [Google Scholar]
- 48.Izadi H, Zhao B, Han Y, McManus N, Penlidis A. Teflon hierarchical nanopillars with dry and wet adhesive properties. J Polym Sci B Polym Phys. 2012;50(12):846–851. [Google Scholar]
- 49.Lamb T, Bauer AM. Footprints in the sand: Independent reduction of subdigital lamellae in the Namib-Kalahari burrowing geckos. Proc Biol Sci. 2006;273(1588):855–864. doi: 10.1098/rspb.2005.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.