Abstract
Declarative memory is thought to rely on two processes: recollection and familiarity. Recollection involves remembering specific details about the episode in which an item was encountered, and familiarity involves simply knowing that an item was presented even when no information can be recalled about the episode itself. There has been debate whether the hippocampus supports only recollection or whether it supports both processes. We approached this issue in a relatively theory-neutral way by fitting two prominent models that have been used to describe recognition memory: dual process signal detection and unequal variance signal detection. Both models yield two parameters of interest when fit to recognition memory data. The dual process signal detection model yields estimates of recollection (r) and familiarity (d′). The unequal variance signal detection model yields estimates of the ratio of the variance of target and foil memory strength distributions (σtarget/σfoil) and the difference in the means of the two distributions (d). We asked how the two parameters of each model were affected by hippocampal damage. We tested five patients with well-characterized bilateral lesions thought to be limited to the hippocampus and age-matched controls. The patients exhibited a broad memory deficit that markedly reduced the value of both parameters in both models. In addition, the pattern of results exhibited by the patients was recapitulated in healthy controls as the delay between learning and testing was extended. Thus, hippocampal damage impairs both component processes of recognition memory.
Keywords: amnesia, medial temporal lobe
The formation of declarative memory depends on the integrity of the hippocampus and related medial temporal lobe (MTL) structures (1). A widely studied example of declarative memory is recognition memory, the ability to correctly judge that an item was encountered previously. Recognition memory is thought to consist of two component processes, recollection and familiarity (ref. 2; for review, see ref. 3). Recollection involves recalling specific details about the episode in which an item was encountered. Familiarity involves simply knowing that an item was presented without remembering anything about the episode itself. Whereas the hippocampus and other MTL structures are important for recognition memory (4), their relative importance for recollection and familiarity is unclear. One view is that the hippocampus is important for recollection but is entirely uninvolved in familiarity (for review, see ref. 5). A second view is that the hippocampus contributes to both processes (for review, see ref. 6).
We focus here on two models that have been used to characterize the memory impairment associated with hippocampal lesions: the dual process signal detection (DPSD) model (7, 8) and the unequal variance signal detection (UVSD) model (9, 10). These models are typically fit to experimental data from recognition memory tests in which participants use a confidence rating scale to discriminate targets that appeared on a prior study list from foils that did not. Both models yield two parameters of interest. For the DPSD model, the two parameters consist of the proportion of targets that theoretically achieve a qualitatively distinct state of memory such that they are recognized with high confidence and high accuracy; and d′, the quantitative difference between the average memory strength of targets and the average memory strength of foils, divided by the SD of the two distributions (which is assumed to be identical). These two parameters have been termed recollection (r) and familiarity (d′) because the parameter values are assumed to correspond directly to the strength of these two processes. For the UVSD model, the two parameters consist of σtarget/σfoil, the ratio of the SDs of memory strengths associated with targets and foils, and d, the quantitative difference between the average memory strength of targets and the average memory strength of foils, divided by the SD of the foil distribution. In the UVSD model, these two parameters capture distinct quantitative properties of the memory signal but are neutral with respect to the constructs of recollection and familiarity.
Although the DPSD and UVSD models do not provide the same theoretical interpretation of recognition memory performance, the two parameters in each model may nevertheless capture similar trends in the data. Thus, it is of interest to know whether hippocampal lesions affect one parameter of each model (consistent with a selective memory impairment) or both parameters of both models (consistent with a broad memory impairment). Previous research using a model-based approach to understanding the effect of hippocampal lesions has yielded inconsistent results. The present study sought to clarify the role of the hippocampus in recognition memory using a relatively theory-neutral approach to determine (according to each model) whether only one parameter or both parameters were affected. We also address methodological issues that may have contributed to the conflicting findings in earlier studies.
Results
Experiment 1.
Experiment 1 tested the recognition performance of patients with damage limited to the hippocampus and a matched group of healthy volunteers using 50-item word lists and a 3- to 5-min retention interval. Analysis was performed at the individual subject level.
One control was eliminated because both his DPSD recollection and UVSD σtarget/σfoil estimates were greater than 3 SDs below the means of the other estimates for these parameters. The remaining 11 controls performed better than the patients [83% vs. 65% correct; t(14) = 4.5, P < 0.01]. Both groups performed well above chance (P < 0.01; see Table 1 for hit and false alarm rates). In addition, the two groups adopted a similar response criterion (bias) (for patients β= 1.06; for controls β = 1.00).
Table 1.
Patient and control performance in experiment 1
| Performance | Patients | Controls |
| False alarm rate | 0.34 | 0.17 |
| Hit rate | 0.61 | 0.84 |
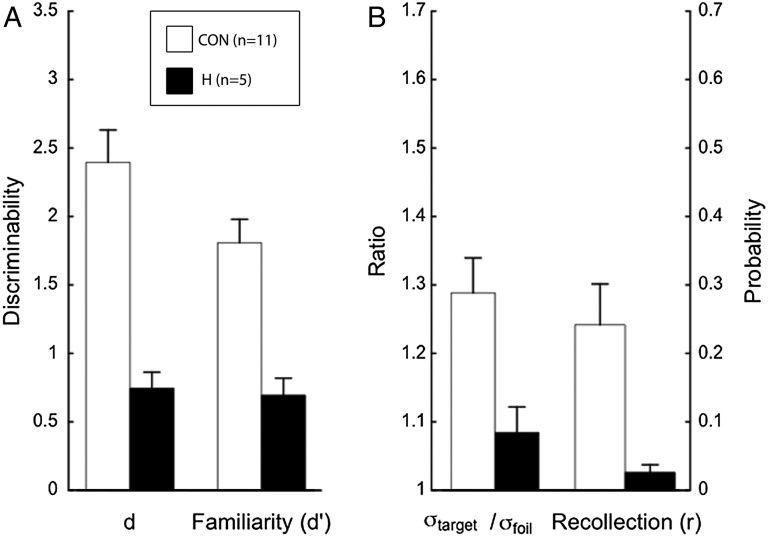
For the DPSD model, both parameter estimates of interest were lower for the patients than for the controls (Fig. 1). Estimates of familiarity were 0.70 and 1.78, respectively [t(14) = 4.27, P < 0.01]. Estimates of recollection were 0.03 and 0.22, respectively [t(10.4) = 3.04, P = 0.01; unequal variance t test]. The two parameters associated with the UVSD model were also reduced (Fig. 1). Estimates of d were 0.75 and 2.35 for patients and controls, respectively [t(14) = 4.90, P < 0.01). Estimates of σtarget/σfoil were 1.09 and 1.23, respectively [t(13.3) = 2.31, P = 0.04; unequal variance t test).
Fig. 1.
Parameter estimates for recognition memory performance of controls (CON) and patients with hippocampal lesions (H) based on two prominent models. Both models yield two parameters of interest. The DPSD model yields estimates of familiarity and recollection. Familiarity is a discriminability estimate, d′ (A), and recollection is a probability estimate, r (B). The UVSD model yields d, a discriminability estimate (A) and the ratio of the SD of the target distribution to the SD of the foil distribution, σtarget/σfoil (B). All estimates were lower for the patients than controls. Error bars show SEM.
The parameter estimates from the two models indicated that declarative memory was broadly impaired in the patients. To test whether the parameters of the two models capture the same empirical trends in the data, we computed correlations between the corresponding parameters across participants. The familiarity estimate from the DPSD model and the d estimate from the UVSD model correspond to each other in the sense that they both determine the degree to which the curvilinear Receiver Operating Characteristic bows away from the diagonal line (7). These parameters were strongly correlated for both the patients and the controls [r(3) = 0.97 and r(9) = 0.77, respectively; P < 0.01]. The recollection and σtarget/σfoil parameters were also correlated in the patient group but not in the control group [r(3) = 0.93 and r(9) = 0.11, respectively; P = 0.02 and 0.74, respectively].
Finally, the goodness of fit of the two models to the data was assessed for each participant using a χ2 test. Thus, the frequency of responses at each confidence level (levels 1–6) predicted by the two models was compared with the frequency of responses that was observed. The UVSD model outperformed the DPSD model for 7 of the 11 controls and for four of the five patients.
Experiment 2.
Experiments 2 and 3 were designed to assess whether the pattern of performance exhibited by the patient group in experiment 1 would be recapitulated in controls when their performance matched patient performance. Experiment 2 characterized memory in controls as a function of increasing retention interval. This procedure identified retention intervals (1 d, 7 d) at which control performance approximated the performance of the patients. In experiment 3, sufficient data were collected at these two retention intervals for analysis at the individual subject level. In this way, it was possible to compare directly the data collected from patients in experiment 1 with data from controls with matched memory performance.
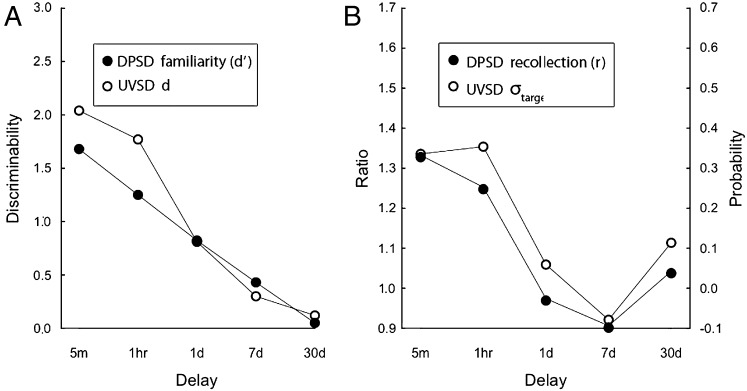
Accuracy, hit rate, and false alarm rate data are presented in Table 2. For the DPSD model, estimates of both familiarity and recollection decreased monotonically with delay (Fig. 2, filled symbols). Note that the recollection estimate (Fig. 2B) decreased more rapidly than the familiarity estimate, reaching a score of zero after only 1 d. For the UVSD model, estimates of both d and σtarget/σfoil also decreased monotonically (Fig. 2, open symbols). The σtarget/σfoil estimate decreased more rapidly than the d estimate, approaching the minimum value of 1.0 after 1 d.
Table 2.
Control performance at variable delay intervals in experiment 2
| Delay |
|||||
| Performance | 5 min | 1 h | 1 d | 7 d | 30 d |
| False alarm rate | 0.15 | 0.18 | 0.31 | 0.46 | 0.40 |
| Hit rate | 0.84 | 0.77 | 0.66 | 0.60 | 0.47 |
Fig. 2.
Parameter estimates for recognition performance of controls (CON, n = 9) as a function of retention delay. The DPSD model yields estimates of familiarity, d′ (A) and a probability estimate labeled recollection, r (B). The UVSD model yields d, a discriminability estimate (A) and the ratio of the SD of the target distribution to the SD of the foil distribution, σtarget/σfoil (B). In both models, the two parameters decrease as time passes after learning.
Based on group χ2 values, the UVSD model fit the data better than the DPSD model at all five delays.
Experiment 3.
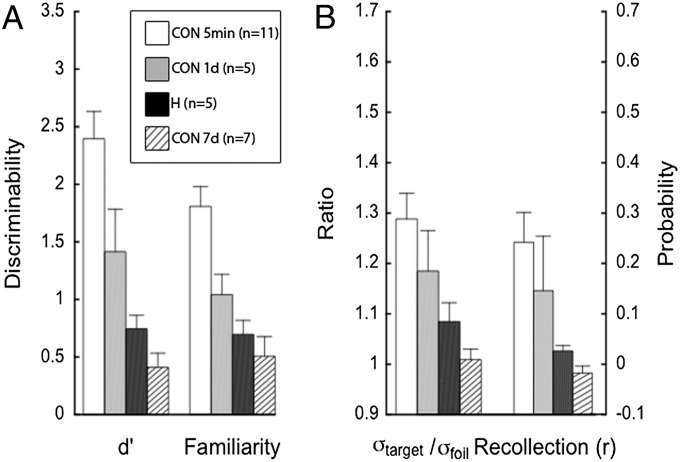
Controls tested after a 1-d delay performed similarly to, albeit a little better than, the patients in experiment 1, who were tested after a 3- to 5-min delay (Fig. 3). Controls tested after a 7-d delay also performed similarly to, but a little worse than, the patients in experiment 1. The 1-d controls scored 71% correct, the patients in experiment 1 scored 65% correct, and the 7-d controls scored 58% correct. Hit and false alarm rate data for all groups are presented in Table 3. There were no significant differences between the model parameter estimates for the patients and either group of controls (1-d and 7-d; P > 0.05).
Fig. 3.
Parameter estimates for recognition performance of controls tested 1 or 7 d after learning (CON 1 d, n = 5; CON 7 d, n = 7) based on two models. Corresponding estimates from Fig. 1 for controls (CON, n = 11) and patients with hippocampal lesions (H, n = 5) tested 3 min after learning are shown for comparison. At 1 d after learning, control performance was numerically better than performance of the patients tested 3 min after learning whereas, at 7 d after learning, control performance was numerically better than that of patients. The DPSD model yields estimates of familiarity, d′ (A) and a probability estimate labeled recollection, r (B). The UVSD model yields d, a discriminability estimate (A) and the ratio of the SD of the target distribution to the SD of the foil distribution, σtarget/σfoil (B). All parameter estimates were higher for controls tested at 1 d after learning than for patients tested after 3 min. This pattern was reversed when controls were tested after 7 d. Error bars show SEM.
Table 3.
Patient and control performance in experiments 1 and 3
| Performance | Controls (1 d) | Patients | Controls (7 d) |
| Accuracy | 0.71 | 0.65 | 0.58 |
| False alarm rate | 0.22 | 0.34 | 0.37 |
| Hit rate | 0.66 | 0.61 | 0.53 |
Accuracy, hit rate, and false alarm rate values for patients tested at a 3–5 min delay and controls tested at a 1-d or 7-d delay.
Correlation analyses between corresponding parameters of the DPSD and UVSD models across individual participants again indicated that the two models described similar trends in the data. Discriminability estimates of the two models were significantly correlated in the 7-d group [familiarity and d; r(5) = 0.93, P < 0.01] and marginally correlated in the 1-d group [r(3) = 0.85, P = 0.07]. Estimates of σtarget/σfoil and recollection fell short of significance in both the 1-d [r(3) = 0.83, P = 0.08] and 7-d [r(5) = 0.65, P = 0.11] conditions.
Based on individual χ2 values, the UVSD model provided a better fit to the data than did the DPSD model for three of the five controls in the 1-d group and six of the seven controls in the 7-d group.
Discussion
Patients with bilateral damage to the hippocampus exhibited a broad deficit in recognition memory, as indicated by a reduction in the two key parameter estimates of two prominent models, DPSD and UVSD (experiment 1). In addition, the parameter estimates of both models were reduced for healthy volunteers as memory became weaker during normal forgetting (experiment 2). Finally, according to both models, the performance of the patients was similar to the performance of healthy volunteers, when the memory of the volunteers was made weaker by extending the retention interval (experiment 3).
Taken together, the results indicate that the performance of patients differed quantitatively, but not qualitatively, from that of controls. Thus, to the extent that the two parameters of the DPSD and UVSD models are differentially sensitive to the processes of recollection and familiarity (an explicit assumption of the DPSD model), the results suggest that damage limited to the hippocampus impairs both recollection and familiarity.
It is of interest to know whether one parameter of either model was affected by hippocampal damage more than the other parameter of the same model. However, it is difficult to make this determination when comparing a probability estimate, on the one hand, and a discriminability estimate on the other. According to the DPSD model, the recollection parameter decreased by 86%, and the familiarity parameter decreased by 61%. According to the UVSD model, the corresponding decreases were 68% and 61%. Our main point is that both parameters of both models were affected by hippocampal lesions, a finding that counts against the view that familiarity is preserved after hippocampal lesions (5).
Three studies have used the DPSD model, or both models, to characterize the memory impairment of patients with damage thought to be limited to the hippocampus (11–13). Yonelinas et al. (11) reported that the performance of patients reflected a selective decrease in the recollection parameter of the DPSD model. Aggleton et al. (13) reached a similar conclusion for patient KN. By contrast, Wais et al. (12) found that hippocampal damage affected both the recollection and familiarity parameters of the DPSD model as well as both parameters of the UVSD model.
The study by Wais et al. (12) differed from the two other studies in two important respects. First, the analyses that were based on the DPSD and UVSD models were applied only to group data and not to individual subject data. Second, short study lists were used. When group data are analyzed, averaging artifacts can yield parameter estimates that are not representative of individual performance (14, 15). In addition, it has been suggested that, with short lists, patients might rely on working memory to maintain and then recollect words from the study list (16). If so, patient performance should not be taken as evidence for successful retrieval from long-term memory. The current study shows that these factors were not responsible for the broad memory impairment reported earlier (12). First, in the present study, the critical analyses were performed at the level of the individual participant and did not depend on group data. Second, long study lists were used in all conditions.
We next consider the two studies that reported a selective impairment in recollection after hippocampal damage (11, 13). In the first study (11), the DPSD model was fit to data from four patients thought to have damage limited to the hippocampus based on the fact that their amnesia resulted from a period of hypoxia after cardiac arrest. Magnetic resonance (MR) images were not available. Compared with the parameter estimates from a matched control group, the recollection estimate derived for the patients was significantly reduced. The familiarity estimate was also reduced, but not significantly. However, in the analysis, as reported, data from deep and shallow encoding conditions were combined and then analyzed as though the data had been drawn from a single memory strength condition. When items from different strength conditions are intermixed, the result is a non-Gaussian mixture distribution. Under these conditions, the use of Gaussian-based signal detection models (such as the DPSD and UVSD models) are not appropriate (17). Thus, no conclusions can be drawn based on a fit of the DPSD model to these data.
In the second study (13), patient KN was described as having a selective recollection deficit. However, KN had intact recognition memory scores as measured by both percent correct (KN = 72%, controls = 73%) and da (KN = 1.29, controls = 1.35). Furthermore, according to the DPSD model, neither KN’s recollection z-score (−1.14) nor his familiarity z-score (+0.34) differed by more than 1.2 SDs from the mean of the controls. It was proposed that patient KN’s memory impairment was obscured by the unusually poor performance of one control, whose recollection z-score was more than 3 SDs below the control mean. When that outlier was excluded, the DPSD recollection z-score for patient KN became −2.16 (suggesting an impairment). However, the corresponding DPSD familiarity score without the outlier was not reported, so that one does not know whether KN’s memory impairment was selective for recollection.
The question naturally arises whether differences in results between patient groups might reflect differences in the locus and extent of damage. For example, it has been proposed that two of the patients whom we have studied likely have damage outside the hippocampus because their amnesia resulted from hypoxia secondary to heroin abuse. Yonelinas et al. (18, p. 395) wrote that “heroin overdose…produce(s) neurotoxic effects beyond those typically related to hypoxia.” However, the relevant citation (19) actually made the opposite statement: “permanent brain damage seems more likely to be caused by recurrent episodes of hypoxia during severe reactions to narcotics than to be related to direct neurotoxic effects of heroin.” Whereas there is no reason to suppose that the two relevant patients in our study (GW and RS) have damage beyond the hippocampus, we reexamined the present data without GW and RS and found the same results as with the full group.
Yonelinas et al. (18) also drew attention to the severity of memory impairment in our patients, which suggested to these authors the possibility of damage beyond the hippocampus. However, the severity of memory impairment in our patients is similar to the severity of impairment reported for other patient groups studied elsewhere who are described as having limited hippocampal damage (20, 21; here, we compared our patients only to patients in these other studies with reported hippocampal lesions and not to patients that had large lesions of the medial temporal lobe). Furthermore, volumetric measurements of the lateral temporal, frontal, and parietal lobe revealed no reductions in our patient group. The impression expressed by Yonelinas et al. (18) that our patients are severely impaired may have originated from the unusually mild memory impairment in their own patients. Those patients (11) were selected based on a history of hypoxia associated with cardiac arrest, not on the basis of MR data (which was not available) and not on the basis of their memory impairment. Indeed, many of the patients in this large group of 55 patients appeared to perform normally and to have no memory impairment (see individual data for the 55 patients in ref. 22).
Two other studies (23, 24) used the DPSD model to characterize the recollection and familiarity deficits associated with damage to structures other than the hippocampus. The first study, involving patients with mammillary body lesions (23), obtained familiarity estimates by the unusual step of converting d′ estimates from the DPSD model to probabilities (d′ is the distance between the means of two equal-variance Gaussian distributions and cannot be reasonably expressed as a probability). With this procedure, the model’s familiarity parameter was calculated to be intact, and the recollection parameter was calculated to be differentially reduced. However, it is difficult to interpret the finding for the familiarity estimate, given the unusual method of calculating it. In the second study (24), a selective recollection deficit was reported for a single patient with damage to the anterior medial thalamus. Our own findings apply to patients with bilateral hippocampal lesions and showed that both parameters of the DPSD model (as well as both parameters of the UVSD model) were markedly reduced.
It is also worth mentioning that the UVSD model described our data far more accurately than did the DPSD model. This finding is consistent with many earlier studies of word list learning that have reached this same conclusion (25–28). In one instance involving memory for travel scenes taken from the Internet, the DPSD model performed better (27). It seems reasonable to use the better-fitting model to interpret the data. Accordingly, in terms of the better-fitting UVSD model, our findings suggest that hippocampal lesions reduce both the mean and the variance of the memory signal that is associated with the target items. This same result was obtained as memory weakened during the course of normal forgetting. Thus, the performance of patients with hippocampal lesions on memory tests reflects a broad impairment that is characteristic of weak memory.
Materials and Methods
Experiment 1.
Participants.
Five memory-impaired patients participated (Table 4), all with bilateral lesions thought to be limited to the hippocampus (CA fields, dentate gyrus, and subicular complex). KE became amnesic in 2004 after an episode of ischemia associated with kidney failure and toxic shock syndrome. LJ (the only female) became amnesic in 1988 during a 6-mo period with no known precipitating event. Her memory impairment has been stable since that time. Patients GW and RS became amnesic in 2001 and 1998, respectively, following drug overdose and associated respiratory failure. JRW became amnesic in 1990 following an anoxic episode associated with cardiac arrest.
Table 4.
Characteristics of memory-impaired patients
| Patient | Age, y | Education, y | WAIS-III IQ | WMS-R |
||||
| Attention | Verbal | Visual | General | Delay | ||||
| KE | 70 | 13.5 | 108 | 114 | 64 | 84 | 72 | 55 |
| LJ | 74 | 12 | 101 | 105 | 83 | 60 | 69 | <50 |
| RS | 55 | 12 | 99 | 99 | 85 | 81 | 82 | <50 |
| GW | 52 | 12 | 108 | 105 | 67 | 86 | 70 | <50 |
| JRW | 48 | 12 | 90 | 87 | 65 | 95 | 70 | <50 |
The Wechsler Adult Intelligence Scale-III (WAIS-III) and the Wechsler Memory Scale-Revised (WMS-R) yield mean scores of 100 in the normal population with a SD of 15. The WMS-R does not provide numerical scores for individuals who score below 50. IQ scores for RS and JRW are from the Wechsler Adult Intelligence Scale-Revised.
Estimates of medial temporal lobe damage were based on quantitative analysis of MR images from 19 healthy males for the four male patients and 11 healthy females for patient LJ (29). GW, KE, LJ, RS, and JRW have an average reduction in hippocampal volume of 48%, 49%, 46%, 33%, and 44%, respectively (all values >3 SDs from the control mean). On the basis of two patients (LM and WH) with similar bilateral volume loss, for whom detailed postmortem neurohistological information was obtained, the degree of volume loss in the present patient group likely reflects nearly complete loss of hippocampal neurons (30). The volume of the parahippocampal gyrus is reduced by of 10%, 11%, −17%, −5%, and 12% for GW, KE, LJ, RS, and JRW, respectively (all values within 2 SDs of the control mean). These values differ slightly from the volumes reported previously for these patients and are based on newly published, more detailed guidelines for identifying the caudal border of the gyrus (31; for eight coronal MR images from each patient, see Fig. S1).
Additional measurements, based on four controls for each patient, were performed for the frontal lobes, lateral temporal lobes, parietal lobes, occipital lobes, insular cortex, and fusiform gyrus (32). The only volume reduction in these regions >1.3 SDs of the control mean was the parietal lobe of patient RS.
A control group of twelve healthy volunteers also participated (three females; mean age, 62.7 y; mean education, 14.3 y). All procedures were approved by the Institutional Review Board at the University of California at San Diego, and participants gave written informed consent before participation.
Materials and procedure.
Six hundred common English words (4–9 letters) served as stimuli (33). The words were used to construct six tests, each with 50 study words and 100 test words (50 targets from the study list plus 50 foils that were not previously studied). For testing, individual words served equally often as targets and foils, and the words were presented in a mixed order for each participant. The order of presentation of the six tests was also mixed across participants.
Controls were tested three times in a single session. To obtain robust data, patients were tested six times in two sessions separated by an average of 5 mo. The results were similar in the two sessions. After a 250-ms fixation cross, each word was presented on a computer screen for 2.5 s and rated as pleasant or unpleasant on the keyboard. After 3–5 min of conversation to prevent rehearsal, the 50 target words were presented one at a time, intermixed with 50 foil words, and participants decided on a six-point confidence scale whether each word had been presented before [1 (sure new) to 6 (sure old)]. The end points of the confidence scale were labeled “1 = definitely new” and “6 = definitely old” in the first session for the patients. The test was self-paced.
Data analysis.
As discussed above, the DPSD model yields two parameters of interest: (i) the probability that a target will achieve a qualitatively distinct state of memory such that it is recognized with high confidence—a quantitative property of the memory signal that in this model is termed recollection (r); and (ii) the distance between the average memory strength of targets and the average memory strength of foils, divided by the SD of the two distributions (which is assumed to be identical). In the DPSD model, this value is termed familiarity (d′). The UVSD model also yields two parameters of interest: (i) the ratio of the SD of the target distribution to the SD of the foil distribution (σtarget/σfoil), and (ii) the distance between the average memory strength of targets and the average memory strength of foils, divided by the SD of the foil distribution. This value is termed d. Maximum likelihood parameter estimates for the DPSD and UVSD models were obtained for each participant by separately fitting both models to each participant’s confidence ratings using standard methods (34). For both models, seven parameters were estimated (the two memory-relevant parameters discussed above plus five criteria specified by the confidence ratings).
Experiment 2.
Participants.
Nine healthy volunteers participated (two females; mean age, 60.2 y; mean education, 14.5 y).
Materials and procedure.
Five hundred common English words (4–9 letters) served as stimuli (33). The 500 words (different from the words in experiment 1) were used to construct five tests, each with 50 study words and 100 test words (50 study words plus 50 foils). For testing, individual words served equally often as targets and foils, and the words were presented in a mixed order for each participant. The order of presentation of the five tests was also mixed across participants.
Memory was tested using five separate recognition tests. Each participant was tested once each at study-test delays of 5 min, 1 h, 1 d, 7 d, and 30 d. The order of the delays was mixed across participants. As in experiment 1, maximum likelihood parameter estimates were obtained by fitting both models to the confidence data. Group data were analyzed because there were too few observations to fit the data from each participant individually.
Experiment 3.
Participants.
Five healthy volunteers (two female; mean age, 60.6 y; mean education, 14 y) were tested on three separate occasions with a study-test delay of 1 d. In addition, seven healthy volunteers (one female; mean age, 56.2 y; mean education, 14.4 y) were tested on three separate occasions with a study-test delay of 7 d.
Materials and procedure.
Three hundred common English words (4–9 letters) served as stimuli (19). The 300 words (different from the words in experiments 1 and 2) were used to construct three tests, each with 50 study words and 100 test words (50 study words plus 50 foils). For testing, individual words served equally often as targets and foils, and the words were presented in a mixed order for each participant. The order of presentation of the three tests was also mixed across participants. Data were analyzed as in experiment 1, and parameter estimates for both models were calculated individually for each participant.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304739110/-/DCSupplemental.
References
- 1.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 2.Mandler G. Recognizing: The judgment of previous occurrence. Psychol Rev. 1980;87(3):252–271. [Google Scholar]
- 3.Diana RA, Reder LM, Arndt J, Park H. Models of recognition: A review of arguments in favor of a dual-process account. Psychon Bull Rev. 2006;13(1):1–21. doi: 10.3758/bf03193807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav Neurosci. 1997;111(4):667–675. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- 5.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends Cogn Sci. 2011;15(5):210–217. doi: 10.1016/j.tics.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yonelinas AP. Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. J Exp Psychol Learn Mem Cogn. 1994;20(6):1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- 8.Yonelinas AP. The contribution of recollection and familiarity to recognition and source-memory judgments: A formal dual-process model and an analysis of receiver operating characteristics. J Exp Psychol Learn Mem Cogn. 1999;25(6):1415–1434. doi: 10.1037//0278-7393.25.6.1415. [DOI] [PubMed] [Google Scholar]
- 9.Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychol Rev. 2007;114(1):152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- 10.Wixted JT, Stretch V. In defense of the signal detection interpretation of remember/know judgments. Psychon Bull Rev. 2004;11(4):616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- 11.Yonelinas AP, et al. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5(11):1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- 12.Wais PE, Wixted JT, Hopkins RO, Squire LR. The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron. 2006;49(3):459–466. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggleton JP, et al. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43(12):1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: a review. Psychol Bull. 2007;133(5):800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]
- 15.Macmillan NA, Kaplan HL. Detection theory analysis of group data: Estimating sensitivity from average hit and false-alarm rates. Psychol Bull. 1985;98(1):185–199. [PubMed] [Google Scholar]
- 16.Yonelinas AP, Aly M, Wang WC, Koen JD. Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus. 2010;20(11):1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang Y, Mickes L, Wixted JT. Three tests and three corrections: Comment on Koen and Yonelinas (2010) J Exp Psychol Learn Mem Cogn. 2012;38(2):513–523. doi: 10.1037/a0025880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yonelinas AP, et al. Mild hypoxia disrupts recollection, not familiarity. Cogn Affect Behav Neurosci. 2004;4(3):393–400, discussion 401–406. doi: 10.3758/cabn.4.3.393. [DOI] [PubMed] [Google Scholar]
- 19.Pearson J, Baden MB, Richter RW. Neuronal depletion in the globus pallidus of heroin addicts. Drug Alcohol Depend. 1976;1(5):349–356. doi: 10.1016/0376-8716(76)90037-5. [DOI] [PubMed] [Google Scholar]
- 20.Race E, Keane MM, Verfaellie M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci. 2011;31(28):10262–10269. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barense MD, et al. Intact memory for irrelevant information impairs perception in amnesia. Neuron. 2012;75(1):157–167. doi: 10.1016/j.neuron.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wixted JT, Squire LR. Recall and recognition are equally impaired in patients with selective hippocampal damage. Cogn Affect Behav Neurosci. 2004;4(1):58–66. doi: 10.3758/cabn.4.1.58. [DOI] [PubMed] [Google Scholar]
- 23.Vann SD, et al. Impaired recollection but spared familiarity in patients with extended hippocampal system damage revealed by 3 convergent methods. Proc Natl Acad Sci USA. 2009;106(13):5442–5447. doi: 10.1073/pnas.0812097106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlesimo GA, et al. Bilateral damage to the mammillo-thalamic tract impairs recollection but not familiarity in the recognition process: a single case investigation. Neuropsychologia. 2007;45(11):2467–2479. doi: 10.1016/j.neuropsychologia.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Heathcote A. Item recognition memory and the receiver operating characteristic. J Exp Psychol Learn Mem Cogn. 2003;29(6):1210–1230. doi: 10.1037/0278-7393.29.6.1210. [DOI] [PubMed] [Google Scholar]
- 26.Jang Y, Wixted JT, Huber DE. Testing signal-detection models of yes/no and two-alternative forced-choice recognition memory. J Exp Psychol Gen. 2009;138(2):291–306. doi: 10.1037/a0015525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onyper SV, Zhang YX, Howard MW. Some-or-none recollection: Evidence from item and source memory. J Exp Psychol Gen. 2010;139(2):341–364. doi: 10.1037/a0018926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starns JJ, Ratcliff R. Two dimensions are not better than one: STREAK and the univariate signal detection model of remember/know performance. J Mem Lang. 2008;59(2):169–182. doi: 10.1016/j.jml.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15(1):79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. RempelClower NL Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16(16):5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frankó E, Insausti AM, Artacho-Pérula E, Insausti R, Chavoix C. Identification of the human medial temporal lobe regions on magnetic resonance images. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayley PJ, Hopkins RO, Squire LR. The fate of old memories after medial temporal lobe damage. J Neurosci. 2006;26(51):13311–13317. doi: 10.1523/JNEUROSCI.4262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson M. MRC psycholinguistic database: Machine-usable dictionary, version 2.00. Behav Res Methods Instrum Comput. 1988;20(1):6–10. [Google Scholar]
- 34.Macmillan NA, Creelman CD. Detection Theory, a User's Guide. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.