Abstract
We have generated a transgenic rat model using RNAi and used it to study the role of the membrane protein Nogo-A in synaptic plasticity and cognition. The membrane protein Nogo-A is expressed in CNS oligodendrocytes and subpopulations of neurons, and it is known to suppress neurite growth and regeneration. The constitutively expressed polymerase II-driven transgene was composed of a microRNA-targeting Nogo-A placed into an intron preceding the coding sequence for EGFP, thus quantitatively labeling cells according to intracellular microRNA expression. The transgenic microRNA in vivo efficiently reduced the concentration of Nogo-A mRNA and protein preferentially in neurons. The resulting significant increase in long-term potentiation in both hippocampus and motor cortex indicates a repressor function of Nogo-A in synaptic plasticity. The transgenic rats exhibited prominent schizophrenia-like behavioral phenotypes, such as perseveration, disrupted prepulse inhibition, and strong withdrawal from social interactions. This fast and efficient microRNA-mediated knockdown provides a way to silence gene expression in vivo in transgenic rats and shows a role of Nogo-A in regulating higher cognitive brain functions.
Keywords: animal model, Rtn4, learning, memory
Gene knockout (KO) technology has spurred the analysis of gene functions in mice during the past two decades (1) and has recently been expanded to other species using new genome modification technologies (2). Although germ-line gene ablation is a very powerful tool for investigating gene function in vivo, its most important drawback is that the complete loss of gene function often leads to molecular compensation, obscuring the role of the deleted gene. Tissue- or cell-specific KOs are more specific but are currently confined to mice as a model system. RNA interference (RNAi) is a viable alternative to the KO approach and represents a fast and powerful tool for manipulating gene expression (3). RNAi technology not only allows keeping the endogenous genomic locus intact, but it also enables the knockdown of multiple genes at the same time or the selective depletion of a specific isoform of mRNA transcripts (4). Another advantage is offered by the possibility of creating hypomorphic alleles instead of complete KOs, which can avoid embryonic lethality and better mirrors many human diseases and therapeutic interventions.
Elucidating gene functions in transgenic rats has several important advantages over using mice (5). Their larger size simplifies interventions, such as microsurgery and multiple site in vivo electrophysiological recordings (6). Furthermore, higher-order cognitive functions are more developed in this social rodent species than in the more solitarily living mice (7, 8). Hence, many behavioral tests are more advanced or validated for the rat species, especially regarding the behavioral assessment of complex neuropsychiatric disease phenotypes, such as negative symptoms in schizophrenia. For the rat, only polymerase (Pol) III-controlled shRNA RNAi models have been created, but this methodology does not easily allow for tissue-specific or simultaneous expression of translated mRNAs (9). MicroRNAs (miRNAs) (10) can be efficiently expressed by Pol II promoters in eukaryotic cells and a tissue-specific manner (11). Similar to shRNAs, miRNAs bind a defined target site in an endogenous mRNA, leading to efficient knockdown of target genes (12). In mice, transgenic RNAi expression not only led to phenotypes that closely resembeled those observed in KO mice (13, 14) but also enabled the generation of hypomorphic animal models (14).
In this study, we present and investigate the potential of Pol II-driven knockdown in a rat model that quantitatively monitors the transgenic miRNA expression using EGFP as a reporter. Such cellular visualization facilitates selection and analysis of transgenic animals and targeted cell populations, opening avenues for additional cellular analysis in vivo. As an experimental example, we created Nogo-A (Rtn4) knockdown rats using this technology. In these rats, we found enhancement of long-term potentiation (LTP) in not only hippocampus but also cortex, and we observed behavioral phenotypes related to schizophrenia, extending those phenotypes described in the Nogo-A KO mouse (15). These findings validate the miRNA-based knockdown technology and show an important role of Nogo-A repressing neuronal plasticity. Moreover, unlike Nogo-A KO mice, which show a significant Nogo-B up-regulation, there is no such compensatory mechanism apparent in our transgenic model.
Results
Design of the miRNA Expression Unit and Generation of Nogo-A Knockdown Rats.
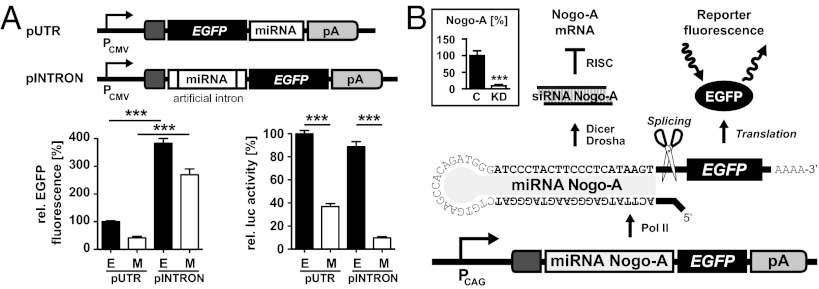
To determine the most efficient expression design of Pol II-driven miRNAs, we first tested two constructs, in which the miRNA was located in either the 3′ UTR of EGFP (pUTR) or an intronic sequence located 5′ of EGFP (pINTRON) (11). The intronic design enables about fourfold higher knockdown efficacy compared with 3′ design coupled with high expression of miRNA/EGFP (Fig. 1A). Different synthetic siRNAs selectively targeting Nogo-A mRNA were tested for knockdown efficiencies by transient transfections of oligonucleotides into 3T3 fibroblasts, which express Nogo-A endogenously. The most efficient RNAi sequence was chosen for the design of a miRNA targeting Nogo-A mRNA, which was then cloned into the intronic sequence of the vector pINTRON (Fig. 1A). Placing the miRNA sequences in an intron ensures effective labeling of the miRNA-expressing cells with EGFP, while at the same time, enhances and stabilizes transgenic expression (16). For strong transgenic expression of the miRNA targeting Nogo-A, we replaced the human cytomegalovirus (CMV) promoter of pINTRON with a hybrid construct comprising the CMV early enhancer element and the chicken β-actin (CAG) promoter (17). The miRNA is transcribed as bifunctional pre-mRNA coding for EGFP, which is processed into functional miRNA and equimolar amounts of EGFP mRNA. To test the knockdown efficiency of the final construct, we transfected 3T3 fibroblasts and quantified endogenous Nogo-A mRNA 3 d posttransfection; 91% of the Nogo-A mRNA expression was abolished compared with control conditions (Fig. 1B, Inset).
Fig. 1.
Design and testing of the intronic miRNA/EGFP expression system. (A) Expression vectors pUTR and pINTRON containing a rationally designed miRNA targeting firefly luciferase (M) or no miRNA as controls (E). In pUTR, the miRNA is integrated between the 3′ end of EGFP and the polyadenylation signal (pA). In pINTRON, the miRNA is embedded within an artificial intron located 5′ of EGFP. Transient transfection experiments using miRNA/EGFP together with luciferase expression vectors show that relative EGFP activities and luciferase knockdown efficiencies are much higher for pINTRON than pUTR. Means (n = 9) and SEM are shown. (B) Schematic drawing of the Nogo-A miRNA insert-containing construct used for the generation of transgenic rats. Inset displays results of Nogo-A qPCR quantification relative to housekeeping genes from Nogo-A–expressing 3T3 cells transfected with either pCAG-INTRON-(miRNA Nogo-A)-EGFP (KD) or control (C) vector. ***P < 0.001.
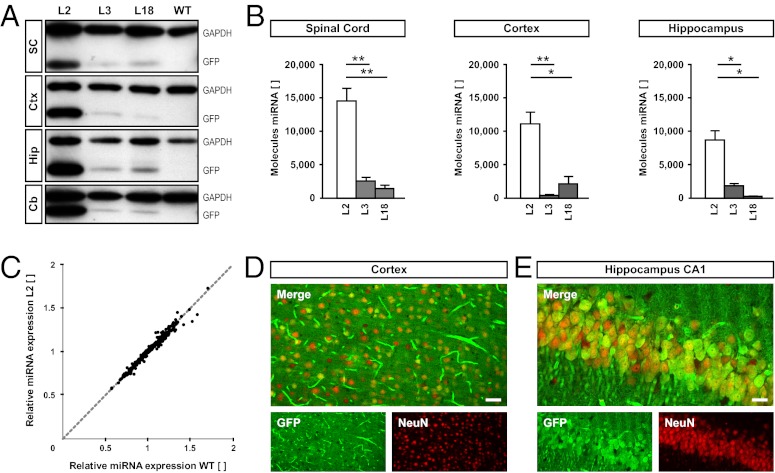
For the production of transgenic rats, the pCAG-INTRON-(miRNA Nogo-A)-EGFP DNA construct was microinjected into fertilized Sprague–Dawley rat oocytes, resulting in the generation of eight individual founders. Primary fibroblast cultures were established from ear punches of these animals (18) and tested for EGFP fluorescence. The four founders with strongest EGFP expression in fibroblasts were mated with WT Sprague–Dawley animals to establish the respective lines L2, L3, L18, and L33. From those four lines, L2, L3, and L18 transmitted the transgene in Mendelian fashion and therefore were chosen for detailed analysis. To validate CAG promoter activity in the CNS, we analyzed EGFP expression in brain lysates and on histological brain sections. Western blot analyses showed expression of EGFP in all brain regions tested; the expression was strongest in line L2 (Fig. 2A). To assess functional processing of the transgenic miRNA targeting Nogo-A, we quantified the absolute number of Nogo-A miRNA copies per cell in spinal cord, cortex, and hippocampus RNA preparations by quantitative PCR (qPCR) (Fig. 2B). As expected, RNA preparations from L2 rats contained the highest concentration of transgenic miRNA, being at least fivefold above the content of all other lines in the examined regions. To ensure that overexpression of the transgenic miRNA had no influence on the overall miRNA-processing machinery (19), we compared the expression profiles of 359 abundantly expressed miRNA sequences in L2 and WT rats (Fig. 2C). The results show that the transgenic miRNA did not influence endogenous miRNA expression and thus, did not interfere with the overall miRNA-processing machinery. Histological sections of the brains of L2 rats revealed that, in the cortex, 48.8% (±7.8%, n = 5) and in the hippocampus, 72.8% (±11.2%, n = 6) of neuronal nuclei (NeuN)-positive neurons expressed EGFP (Fig. 2 D and E). Expression was also detectable in blood vessels (Fig. 2D) but absent in the vast majority of oligodendrocytes and interneurons (Fig. S1).
Fig. 2.
Rat line L2 expresses transgenic EGFP and miRNA at the highest level without affecting the miRNA-processing machinery. (A) Western blot results of EGFP and GAPDH for different CNS regions derived from the transgenic rat lines L2, L3, and L18 as well as WT rats. Cb, cerebellum; Ctx, cortex; Hip, hippocampus; SC, spinal cord. (B) Absolute qPCR quantification (expressed as molecules per cell) of transgenic Nogo-A miRNA expression levels in different rat lines and CNS regions. (C) Scatter plot of qPCR expression levels of 359 abundantly expressed miRNAs from L2 and WT rats. Nonregulated miRNA levels of L2 vs. WT are identical and converge on the bisector of the angle. (D and E) Immunofluorescence of EGFP (green) and the neuronal nuclear marker neuronal nuclei (NeuN) (red) in the cortex (D) and hippocampus (E) of L2 shows a largely neuronal expression of the transgene. (Scale bar: D, 50 µm; E, 20 µm.) *P < 0.05; **P < 0.01.
Nogo-A Levels Are Significantly Diminished in L2 Animals.
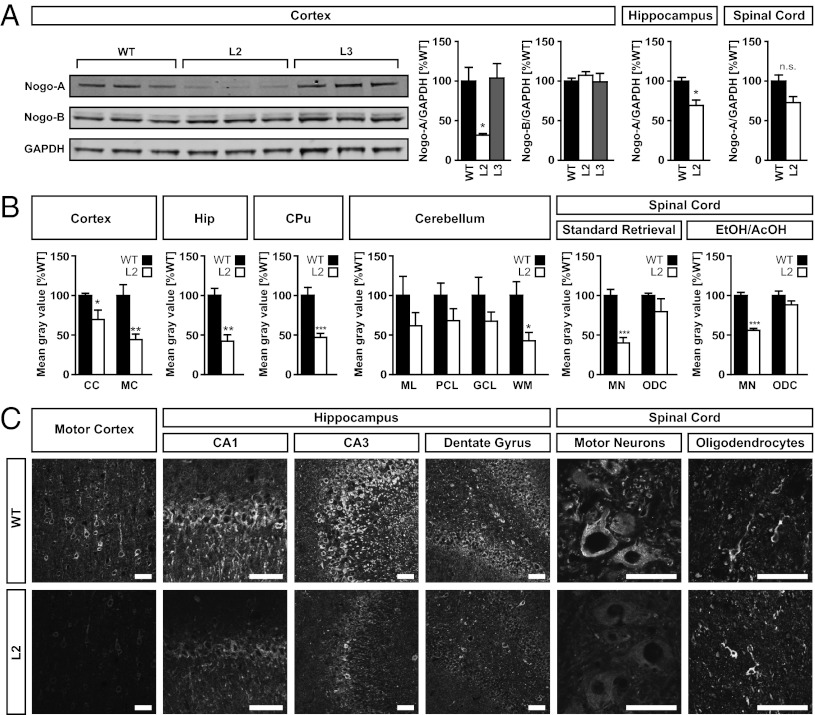
qPCR revealed significant reduction of Nogo-A mRNA in several CNS regions in L2 and L3 rats to about 50% and 75% of WT levels, respectively (Fig. S2). Nogo-A protein expression was assessed by Western blotting (for determination of dynamic range, see Fig. S3) and immunofluorescence analysis in different regions of the CNS of adult L2 and L3 transgenic rats. Western blots revealed a reduction of Nogo-A expression in the cortex of L2 rats to about 30% of WT mean but no detectable decrease in the cortex of L3 rats. Importantly, Nogo-B expression remained unchanged in both transgenic lines (Fig. 3A). Hippocampal Nogo-A expression was reduced by 30% in L2 rats (Fig. 3A). More detailed results were obtained from densitometric measurements of L2 histological brain sections, where Nogo-A expression was quantified by immunofluorescence. Here, the cingulate cortex of L2 rats showed a decrease of Nogo-A expression to 70%, and the motor cortex to 40% of WT levels (Fig. 3 B and C). The densitometric measurements revealed a major loss of Nogo-A expression in neurons (Fig. 3 B and C). Quantified Nogo-A levels were 40% of WT in the hippocampus, 45% of WT in the caudate-putamen, and in total, about 60% of WT in the cerebellum. In the spinal cord, Nogo-A expression decreased to 40–50% in motor neurons, whereas expression was not significantly changed in white matter oligodendrocytes of L2 animals (Fig. 3 B and C). Treatment of spinal cord slices with ethanol and acetic acid known to specifically visualize Nogo-A expression in myelin (20) confirmed retention of Nogo-A expression in oligodendrocytes of L2 rats (Fig. 3 B and C).
Fig. 3.
Expression of Nogo-A is reduced in L2 rats. (A) Western blot analysis of Nogo-A (∼200 kDa) and Nogo-B (∼50 kDa) expression compared with GAPDH (∼40 kDa) in different CNS tissues. Whereas Nogo-A is significantly down-regulated in the cortex of L2 rats (P = 0.0173), no cortical knockdown can be seen in animals of L3. In addition, Nogo-B remains unchanged in both L2 and L3. Nogo-A knockdown can also be observed in the hippocampus (P = 0.0195) of L2 rats, whereas only a trend is visible in whole spinal cord (P = 0.0659). Averaged Nogo-A/GAPDH ratios from three animals per transgenic line or WT are reported as a percentage of WT mean ± SEM. (B) Densitometric quantification of Nogo-A immunoreactivity as determined by epifluorescence microscopy in a Zeiss Axiophot microscope. Down-regulation of Nogo-A is evident in the cingulate (CC; P = 0.0499) and motor cortex (MC; P = 0.0041) of L2 rats. In addition, Nogo-A immunoreactivity is decreased in the cell layers of the hippocampus (Hip; P = 0.0080) and the caudate putamen (CPu; P = 0.0008). Down-regulation can also be observed in the cerebellar white matter (WM; P = 0.0166), whereas only a trend is seen in the molecular layer (ML; P = 0.2155), Purkinje cell layer (PCL; P = 0.1675), and granular cell layer (GCL; P = 0.2310). In the spinal cord, whereas a marked reduction of Nogo-A immunoreactivity is detected in motoneurons (MNs; P < 0.0001), no significant change can be observed in spinal oligodendrocytes (ODCs; P = 0.1135). Similar results were obtained after ethanol/acetic acid treatment (EtOH/AcOH) of the spinal cord sections (MN, P = 0.0002; ODC, P = 0.2449). (C) Representative confocal images show the decrease of Nogo-A immunoreactivity in L2 rats in different CNS regions. Images of the spinal cord show sections after EtOH/AcOH treatment. (Scale bars: 50 μm.) Data are presented as mean + SEM. Asterisks represent P values obtained by comparing L2 and WT rats with unpaired t test: *P < 0.05; **P < 0.01; ***P < 0.001.
Overall, although regional variations were noted, transgenic rat line L2 with the highest miRNA expression showed about 50% reduction of Nogo-A protein expression in CNS neurons. Copy number quantification of transgenic insertions into the genome of L2 showed six copies per cell (Fig. S4). We selected L2 for additional behavioral and electrophysiological experiments.
Nogo-A–Deficient Rats Exhibit Distinct Behaviors Resembling Neuropsychiatric Phenotypes.
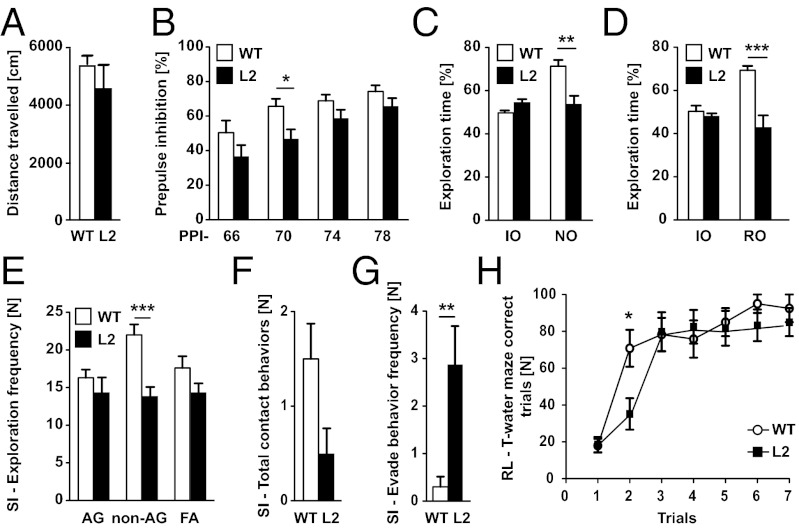
Cohorts of L2 and WT animals (n = 10 each) were used to investigate the consequences of reduced Nogo-A expression on behavior. We focused on the analysis of distinct neuropsychiatric intermediate phenotypes, some of which have been associated with Nogo-A function in a KO mouse model (15). Baseline behavioral parameters like locomotor activity were similar in L2 and WT rats (Fig. 4A). The two groups were indistinguishable in terms of anxiety-related behavior, deduced from the time spent in the center of the open field and the elevated plus maze task (Fig. S5 A and B). However, when animals were tested for their sensorimotoric gating, a marked deficit in prepulse inhibition (PPI) could be observed in L2 rats compared with WT controls [FGenotype(1.54) = 4.97; P = 0.039] (Fig. 4B). Cognitive functions of the animals were evaluated using the novel object recognition and the object relocation paradigms. In these tasks, the animal had to distinguish a novel or relocated object from familiar objects that it had memorized. L2 animals displayed significantly decreased short-term memory capabilities compared with WT controls in discriminating both a novel object (Fig. 4C) and a relocated object (Fig. 4D) from a familiar one. No significant differences were observed between the animals of both genotypes regarding object exploration time during the training phase of the tasks. Behavioral differences between L2 rats and controls were also observed during testing of social interaction. A significant decrease in total social interaction was observed in L2 rats compared with WT (Fig. S5C). This difference originated only from a highly significant decrease in nonanogenital exploration, because no significant differences between the groups were detected for anogenital exploration and following/approach during interaction with the unfamiliar social partner (Fig. 4E). L2 rats showed a strong tendency for lower social contact behaviors compared with WT animals (P = 0.054) (Fig. 4F). Finally, L2 rats were found to withdraw significantly more often from social contact if initiated by the social partner (social evade) (Fig. 4G). No significant differences between the groups were detected for self-grooming behavior (Fig. S5D). Spatial learning and behavioral flexibility were assessed in a reversal learning paradigm using a water T maze. Performance during the initial learning of the task was indistinguishable between rats of both genotypes (Fig. S5E). However, L2 rats showed perseveration in reversal learning, requiring one additional trial for the reversal of the escape strategy compared with WT rats (Fig. 4H and Fig. S5 F and G).
Fig. 4.
L2 rats exhibit schizophrenia-like phenotypes. (A) Locomotor activity in the open field arena. No difference on distance traveled between WT and Nogo-A knockdown rats (L2). (B) PPI. Two-way ANOVA revealed a significant reduction in PPI in L2 rats compared with WT rats [FGenotype (1,54) = 4.97; P = 0.039]. However, Bonferroni posthoc testing revealed that, only at a PPI of 70 dB (PPI-70), startle amplitudes of L2 rats were significantly lower than those startle amplitudes of WT rats. (C) Effects of Nogo-A knockdown on novel object recognition memory in the novel object recognition task. L2 and WT rats showed no significant differences in percent of time spent in exploration of the identical objects (IOs) during the training phase of the test. In contrast, L2 rats have a significant impairment in discriminating between the novel and the familiar object during testing (NO). (D) Novel object relocation task. L2 and WT rats showed no significant differences in exploration of the IOs during the training phase of the test. During the test phase, L2 rats had a significant impairment in discriminating the relocated object (RO). (E–G) Behavioral performance during social interaction with an unknown social partner (social interaction). (E) Significant differences between WT and L2 rats were found for nonanogenital exploration (non-AG), whereas no differences were observed for anogenital exploration (AG) and following/approach (FA). (F) A strong trend was found for a decrease in the number of social contact behaviors (grooming/crawling). (G) L2 rats show significantly more social withdrawal behavior than WT littermates. (H) Reversal learning in the water T maze. Two-way ANOVA revealed that there was no significant difference between animals of the two genotypes in the reversal of their escape strategy in the water T maze, which was seen from their percentage of correct trails within the task [L, FGenotype(1.108) = 1.22; P = 0.284]. In contrast, Bonferroni posthoc testing revealed that, in trial 2, L2 rats have a significant impairment in finding the escape platform. All data are mean values ± SEM. Asterisks represent P values obtained by comparing L2 and WT rats with either unpaired t or Bonferroni posthoc test after two-way ANOVA of repeated measures: *P < 0.05; **P < 0.01; ***P < 0.001.
Reduced Nogo-A Expression Results in Increased Synaptic Plasticity in the Hippocampus and Motor Cortex.
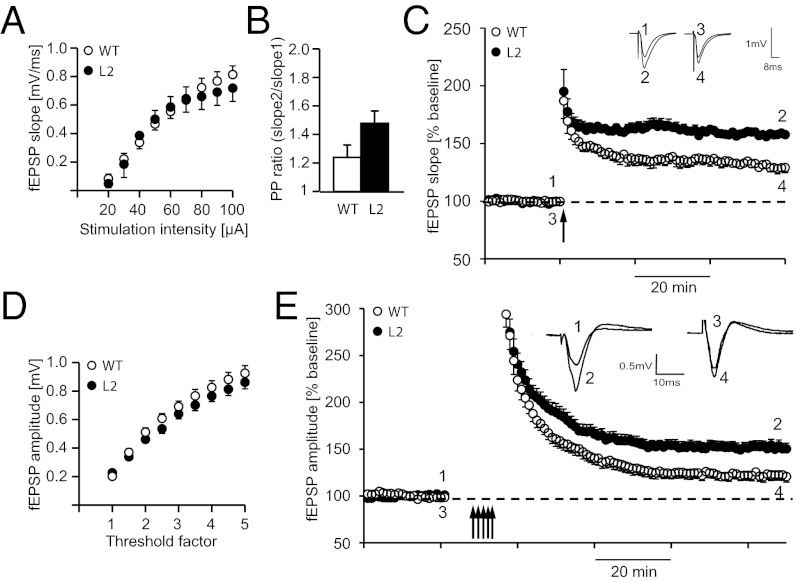
Because Nogo-A expression is high in hippocampus and primary motor cortex (M1) neurons (20) (Fig. 3), we used our transgenic rat model to investigate the role of Nogo-A in regulating synaptic transmission and plasticity in these areas. To assess baseline synaptic transmission at the hippocampal CA3-CA1 Schaffer collateral pathway, input–output curves were generated by measuring evoked field excitatory postsynaptic potential (fEPSP) slopes at increasing stimulation intensities (20–100 μA). No difference was seen between the L2 and WT rats (Fig. 5A). To analyze the influence of Nogo-A on short-term plasticity, we measured paired pulse facilitation by applying two stimuli separated by a temporal interstimulus interval of 40 ms and recorded fEPSPs. We obtained slightly higher paired pulse ratios for the L2 line (Fig. 5B), which were, however, not significantly different from the ratios of control littermates (L2: 1.47 ± 0.07 SEM; WT: 1.24 ± 0.08; P = 0.13). To investigate the role of Nogo-A in regulating hippocampal synaptic plasticity, we induced LTP using a theta-burst stimulation (TBS) protocol after 20 min of baseline recordings. We observed a significant increase in LTP in L2 rats (157.5 ± 0.76%, n = 4) compared with littermate controls (132.2 ± 1.9%, n = 4; P < 0.05) (Fig. 5C). To determine whether the presence of Nogo-A in M1, a structure involved in motor skill acquisition (21), is relevant for synaptic transmission and/or synaptic plasticity, we measured evoked fEPSPs in layer II/III horizontal connections in acute brain slices containing the M1 forelimb area. Baseline synaptic transmission as determined by input–output relationships using multiples of threshold intensity was not affected in Nogo-A–deficient rats (Fig. 5D). fEPSP amplitudes were not significantly different in L2 vs. WT rats (3× threshold intensity: L2: 0.64 ± 0.035 mV; WT: 0.69 ± 0.037 mV; P = 0.35; n = 15).
Fig. 5.
Hippocampal as well as cortical LTP are increased in Nogo-A knockdown rats. (A) Input–output relationships (I/O curves) from WT (n = 6) and L2 Nogo-A knockdown rats (n = 6) recorded in the hippocampal CA3-CA1 Schaffer collateral pathway. I/O curves indicate no significant difference in synaptic strength across stimulation intensities. (B) Paired pulsed ratio measured at different interstimulus intervals did not significantly differ between WT and L2 (n = 6 each). (C) LTP was induced by TBS (arrow) in L2 and WT rats. At 60 min after TBS, a significant difference between L2 and WT rats could be observed. Inset shows original traces from representative individual experiments; numbers correspond to the time point when traces were taken. (D) I/O curves from WT (n = 15) and L2 Nogo-A knockdown rats (n = 15) recorded in layer II/III horizontal connections in the M1 forelimb area of brain slices. I/O curves indicate no significant difference in synaptic strength across stimulation intensities. (E) Maximum synaptic strength (LTP saturation) was determined by repeated induction of LTP (multiple arrows). Peak amplitudes were significantly larger in L2 (n = 16) compared with WT rats (n = 15). Each field potential trace represents an average of 10 individual responses at times indicated by numbers.
The maximum potential for synaptic plasticity was determined by multiple attempts of LTP. After 20 min of baseline recordings at 50% maximum response amplitude, LTP was induced by TBS preceded by local transient application of the GABAA receptor antagonist bicuculline (22). This stimulation protocol was repeated until responses did not increase. Maximum synaptic strength (LTP saturation) was significantly increased in L2 rats (152 ± 5.9%; n = 16) compared with WT rats (122 ± 5.5%; n = 15; P = 0.001) (Fig. 5E), suggesting that Nogo-A is a repressor of synaptic plasticity in the motor cortex as well.
Discussion
Nogo-A is an important neurite growth inhibitory protein that stabilizes the adult CNS wiring, restricts regeneration, and also negatively regulates hippocampal plasticity (23). Recent evidence suggests that aberrant Nogo-A signaling poses an increased risk for schizophrenia (detailed review in ref. 24). We applied a Pol II-driven miRNA expression strategy for RNAi-mediated mRNA knockdown in transgenic rats, resulting in an ∼50% reduction of neuronal Nogo-A protein in different regions of the CNS. The rat model that we present here extended the range of schizophrenia-like phenotypes previously reported for conventional Nogo-A KO mice and allowed for a more detailed investigation of learning and memory-related phenotypes. RNAi-mediated genetic depletion of Nogo-A significantly increased synaptic plasticity in not only the hippocampus but also the motor cortex.
Pol II-Driven Expression of Intronic Synthetic miRNA Induces Down-Regulation of the CNS Protein Nogo-A.
Traditionally, rats have been the preferred experimental animal model system in biomedical research (25), particularly for the analysis of complex neurological disorders and behavioral psychology, where they are superior to mice in many respects (26). Pol II-based miRNA expression systems are a reverse genetic strategy for overexpression of miRNAs. In our experiments, placing an miRNA sequence into an intron 5′ of a coding gene significantly enhances expression compared with 3′ insertions and at the same time, enables simultaneous expression of Pol II-controlled genes. EGFP coexpression with a similar construct in mice failed so far (14), and in rats, only shRNAs driven by a Pol III human H1 promoter have been successfully applied (9, 27). The latter method does not allow quantitative coexpression of an interfering RNA together with a protein coding gene or tissue-specific gene expression. We inserted the Nogo-A–targeting miRNA in an intronic sequence preceding the ORF of EGFP in the vector pCAG-INTRON-EGFP (11). The CAG promoter has been shown to be well-suited for transgenic expression in rats, particularly for neurons (28–30). This construct enables labeling of miRNA-expressing cells and a quantitative measure of the amount of miRNA produced (Fig. S6), because the miRNA is spliced from the EGFP mRNA. An overall reduction of Nogo-A mRNA and protein levels by 50% in several regions of the CNS of adult rats was achieved in line L2.
Reduced Nogo-A Expression Leads to Defects in Cognition, Sensorimotor Gating, and Social Behavior.
Rat transgenic line L2 with high neuronal and not detectable oligodendrocytic EGFP reporter expression was chosen for in-depth analyses. Immunohistochemistry detecting Nogo-A confirmed the significant Nogo-A depletion in neurons. Behaviorally, these rats showed disruptions in sensorimotor gating and selective attention, and an increased perseverative behavior in reversal learning was observed. These changes are hallmarks for schizophrenia (31). Indeed, similar phenotypes were recently observed in conventional Nogo-A KO mice (15). Compared with the mouse model, unique phenotypic traits could be identified in our transgenic Nogo-A knockdown rats: they showed significantly lower exploration and reduced social contact behavior as well as much higher withdrawal from social interaction initiated by the social partner compared with their WT littermates. The presence of these negative symptoms in the rat model is of particular significance. In free social interactions, L2 rats showed normal exploratory behavior but a marked attenuation and avoidance of social contact. Although such social withdrawal behavior might be related to increased anxiety (32), we consider it unlikely, because in the open field test and the elevated plus maze task L2 rats showed no signs of anxiety and Nogo-A KO mice do not differ in anxiety-related behaviors from their WT controls (33). Social withdrawal and isolation are among the key components of negative symptoms in schizophrenia (34), and thus, social withdrawal observed in L2 rats supports a schizophrenia-like phenotype.
Current pharmacological treatments are focusing on the positive symptoms of schizophrenia in man, because genetic mouse models were quite successful in modeling these symptoms (35). In contrast, only very few mouse models exhibit the negative symptoms associated with this disease (36, 37). The Nogo-A knockdown rat with both positive and negative schizophrenia-like symptoms may provide a tool for testing compounds selective for negative symptoms, the severity of which is most predictive for poor therapeutic outcome in patients (38).
Nogo-A Is a Negative Regulator of Synaptic Plasticity in the Hippocampus and Motor Cortex.
Depletion of Nogo-A might affect the synaptic equilibrium, resulting in the observed behavioral dysfunctions. In schizophrenic patients, increased hippocampal activity at baseline and during auditory hallucinations is often observed (39, 40). We have recently found significantly increased LTP in mouse hippocampal slices treated with Nogo-A blocking antibodies and a trend for increased hippocampal LTP in Nogo-A KO mice (41). Here, we show that rats with reduced neuronal Nogo-A expression exhibit a significant increase in hippocampal LTP compared with WT littermates. To determine whether synaptic plasticity was generally changed in these animals, we measured cortical LTP induction after saturated TBS in the primary motor cortex, a region critically important for motor learning, which correlates with increased LTP (21). After TBS of horizontal connections, LTP was doubled in Nogo-A knockdown rats compared with WT littermates. These results show that neuronal Nogo-A mediates repression of synaptic plasticity in the hippocampus and the motor cortex, probably by regulating synapse maturation, size, and/or numbers (42, 43).
Inhibitory constraints on synaptic plasticity and learning and memory were shown in Aplysia, Drosophila, mice, and humans (44–46); removal of such molecular constraints can result in increased synaptic plasticity and improved learning and memory (47–49). Although generally increased hippocampal LTP correlates with improved learning and memory, pathological and prolonged increase in LTP may also lead to cognitive defects (50–54). The perseveration that we observed could indicate an effect of Nogo-A knockdown on memory, which is currently being analyzed in more detail. However, the LTP results would also be consistent with detrimental effects on cognitive functions. Nogo-A knockdown rats, KO mice, and in vivo neutralization experiments with antibodies can now be used to further analyze the roles of neuronal Nogo-A for learning, memory and cognitive functions, and neuropsychiatric diseases, particularly schizophrenia.
Specific Advantages of miRNA Knockdown Transgenic Rats.
Compensation and lethality are frequent undesirable side effects of conventional KO mice. In Nogo-A KO mice (55–57), significant up-regulation of the small splice-isoform Nogo-B was found (58), whereas Nogo-B was not affected in our transgenic Nogo-A knockdown rats. One reason might be that the miRNA-based approach targets splice form-specific mRNAs and leaves the endogenous genetic locus intact. Reducing the concentration of a target protein rather than completely removing it may also help to prevent compensation and particularly, also lethality. Importantly, partial knockdowns also mirror some human pathologic conditions more accurately, especially conditions caused by hypomorphic mutations, epigenetic silencing events, or altered RNAi expression (59, 60) [e.g., autoimmune and neurological diseases (61)]. miRNA-based knockdown models seem ideally suited to study the disease implications of allelic (e.g., single nucleotide) mutations in disease susceptibility genes. Partial knockdown of protein function also resembles pharmacological blockade more closely than complete ablation of a given protein. Many KO phenotypes in mice are known to be strictly strain-specific because of background genes and/or compensation (58); using outbred rat strains, such as Sprague–Dawley, avoids this problem and guarantees a much more bias-free model. In addition, endogenous miRNAs can easily be studied with this technology. Finally, using polycistronic constructs for targeting multiple transcripts, replacing the CAG promoter by cell type-specific promoters, or combining the Cre/loxP or Tet system can further enlarge the spectrum of applications of this promising technology.
Materials and Methods
Detailed descriptions of experimental procedures can be found in SI Materials and Methods. A brief summary of materials and methods is give here.
Generation and Molecular Characterization of Transgenic Rats.
siRNAs targeting Nogo-A–specific exon 3 of Rtn4 were designed and cloned into the Pol II-driven vector pCAG-INTRON-EGFP. The construct pCAG-INTRON-(miRNA Nogo-A)-EGFP was tested by transfections of 3T3 cells using fluorescent microscopy and Nogo-A mRNA measurements with real-time qPCR. The linearized vector was used for the generation of transgenic Sprague–Dawley rats denominated SD-Tg(CAG-RNAi:Nogo-A,EGFP)#ZI, where # stands for the number of the transgenic line (in this work, it is abbreviated as L#). Quantification of processed miRNA and analyses of the endogenous mRNA expression levels in transgenic and WT animals were determined by qPCR. Protein levels of Nogo-A, Nogo-B, EGFP, and housekeeping genes were measured qualitatively and quantitatively by near-fluorescent Western blotting and immunohistochemistry using epifluorescence and confocal microscopy. Copy number quantification of transgenes per cell was done by genomic qPCR.
Behavioral Analysis.
For the behavioral assessment, 6-mo-old male L2 (n = 10) or WT littermate (n = 10) rats were used. The following behaviors were analyzed: basal locomotor activity, object recognition and relocation memory, reversal learning, PPI of the acoustic startle response, and social interaction (contact behavior, social exploration, and approach/following; social evade; and self-grooming behavior).
Cortical and Hippocampal LTP.
Differences in LTP were measured in hippocampal (n: L2 = 4; WT = 4) and primary motor cortical slices (n: L2 = 16; WT = 15) of L2 rats and WT littermates, respectively.
Statistical Analysis.
Statistical significance was calculated by means of unpaired, two-tailed Student t tests if not indicated otherwise.
Supplementary Material
Acknowledgments
We thank Dr. Roman Willi for discussions; Dr. Anita Buchli for management; Ariana Frömmig, Tien Nguyen, Brigitte Pesold, and Lena Wendler for technical assistance; and our colleagues Dr. Linard Filli, Miriam Gullo, Dr. Andreas Luft, Dr. Michelle Starkey, and Dr. Björn Zörner for experimental support. This work was funded by German Ministry for Education and Research (BMBF) Grant 01GQ1003B, a National Bernstein Network for Computational Neuroscience (http://www.gesundheitsforschung-bmbf.de/en/2478.php#Heidelberg) grant, a HEALTH-F2-2007-201714 DEVANX (http://devanx.vitamib.com) grant, and Swiss National Science Foundation Grant 31-122527/1 (to M.E.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217665110/-/DCSupplemental.
References
- 1.Glaser S, Anastassiadis K, Stewart AF. Current issues in mouse genome engineering. Nat Genet. 2005;37(11):1187–1193. doi: 10.1038/ng1668. [DOI] [PubMed] [Google Scholar]
- 2.Tong C, et al. Rapid and cost-effective gene targeting in rat embryonic stem cells by TALENs. J Genet Genomics. 2012;39(6):275–280. doi: 10.1016/j.jgg.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemann MT, et al. An epi-allelic series of p53 hypomorphs created by stable RNAi produces distinct tumor phenotypes in vivo. Nat Genet. 2003;33(3):396–400. doi: 10.1038/ng1091. [DOI] [PubMed] [Google Scholar]
- 4.Volpicelli-Daley LA, Li Y, Zhang CJ, Kahn RA. Isoform-selective effects of the depletion of ADP-ribosylation factors 1-5 on membrane traffic. Mol Biol Cell. 2005;16(10):4495–4508. doi: 10.1091/mbc.E04-12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aitman TJ, et al. Progress and prospects in rat genetics: A community view. Nat Genet. 2008;40(5):516–522. doi: 10.1038/ng.147. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, et al. Genetic enhancement of memory and long-term potentiation but not CA1 long-term depression in NR2B transgenic rats. PLoS One. 2009;4(10):e7486. doi: 10.1371/journal.pone.0007486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buehr M, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135(7):1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Grant E, Mackintosh H. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21(3/4):246–259. [Google Scholar]
- 9.Kotnik K, et al. Inducible transgenic rat model for diabetes mellitus based on shRNA-mediated gene knockdown. PLoS One. 2009;4(4):e5124. doi: 10.1371/journal.pone.0005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wienholds E, Plasterk RH. MicroRNA function in animal development. FEBS Lett. 2005;579(26):5911–5922. doi: 10.1016/j.febslet.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 11.Berger SM, et al. Quantitative analysis of conditional gene inactivation using rationally designed, tetracycline-controlled miRNAs. Nucleic Acids Res. 2010;38(17):e168. doi: 10.1093/nar/gkq616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karagiannis TC, El-Osta A. RNA interference and potential therapeutic applications of short interfering RNAs. Cancer Gene Ther. 2005;12(10):787–795. doi: 10.1038/sj.cgt.7700857. [DOI] [PubMed] [Google Scholar]
- 13.Kunath T, et al. Transgenic RNA interference in ES cell-derived embryos recapitulates a genetic null phenotype. Nat Biotechnol. 2003;21(5):559–561. doi: 10.1038/nbt813. [DOI] [PubMed] [Google Scholar]
- 14.Xia XG, Zhou H, Samper E, Melov S, Xu Z. Pol II-expressed shRNA knocks down Sod2 gene expression and causes phenotypes of the gene knockout in mice. PLoS Genet. 2006;2(1):e10. doi: 10.1371/journal.pgen.0020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willi R, et al. Constitutive genetic deletion of the growth regulator Nogo-A induces schizophrenia-related endophenotypes. J Neurosci. 2010;30(2):556–567. doi: 10.1523/JNEUROSCI.4393-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinster RL, Allen JM, Behringer RR, Gelinas RE, Palmiter RD. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci USA. 1988;85(3):836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108(2):193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 18.Schönig K, Bujard H. Generating conditional mouse mutants via tetracycline-controlled gene expression. Methods Mol Biol. 2003;209:69–104. doi: 10.1385/1-59259-340-2:69. [DOI] [PubMed] [Google Scholar]
- 19.Grimm D, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441(7092):537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 20.Huber AB, Weinmann O, Brösamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22(9):3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290(5491):533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 22.Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75(5):1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- 23.Schwab ME. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 2010;11(12):799–811. doi: 10.1038/nrn2936. [DOI] [PubMed] [Google Scholar]
- 24.Willi R, Schwab ME. Nogo and Nogo receptor: Relevance to schizophrenia? Neurobiol Dis. 2013 doi: 10.1016/j.nbd.2013.01.011. 10.1016/j.nbd.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Cozzi J, Fraichard A, Thiam K. Use of genetically modified rat models for translational medicine. Drug Discov Today. 2008;13(11–12):488–494. doi: 10.1016/j.drudis.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Gill TJ, 3rd, Smith GJ, Wissler RW, Kunz HW. The rat as an experimental animal. Science. 1989;245(4915):269–276. doi: 10.1126/science.2665079. [DOI] [PubMed] [Google Scholar]
- 27.Herold MJ, van den Brandt J, Seibler J, Reichardt HM. Inducible and reversible gene silencing by stable integration of an shRNA-encoding lentivirus in transgenic rats. Proc Natl Acad Sci USA. 2008;105(47):18507–18512. doi: 10.1073/pnas.0806213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hakamata Y, et al. Green fluorescent protein-transgenic rat: A tool for organ transplantation research. Biochem Biophys Res Commun. 2001;286(4):779–785. doi: 10.1006/bbrc.2001.5452. [DOI] [PubMed] [Google Scholar]
- 29.Michalkiewicz M, et al. Efficient transgenic rat production by a lentiviral vector. Am J Physiol Heart Circ Physiol. 2007;293(1):H881–H894. doi: 10.1152/ajpheart.00060.2007. [DOI] [PubMed] [Google Scholar]
- 30.Weber T, Schönig K, Tews B, Bartsch D. Inducible gene manipulations in brain serotonergic neurons of transgenic rats. PLoS One. 2011;6(11):e28283. doi: 10.1371/journal.pone.0028283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geyer MA, Moghaddam B. Animal models relevant to schizophrenia disorders. In: Davis KL, Charney DS, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 689–701. [Google Scholar]
- 32.File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62(1):19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willi R, Aloy EM, Yee BK, Feldon J, Schwab ME. Behavioral characterization of mice lacking the neurite outgrowth inhibitor Nogo-A. Genes Brain Behav. 2009;8(2):181–192. doi: 10.1111/j.1601-183X.2008.00460.x. [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 35.Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 2009;32(6):347–358. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drew MR, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27(29):7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward RD, Simpson EH, Kandel ER, Balsam PD. Modeling motivational deficits in mouse models of schizophrenia: Behavior analysis as a guide for neuroscience. Behav Processes. 2011;87(1):149–156. doi: 10.1016/j.beproc.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho BC, Nopoulos P, Flaum M, Arndt S, Andreasen NC. Two-year outcome in first-episode schizophrenia: Predictive value of symptoms for quality of life. Am J Psychiatry. 1998;155(9):1196–1201. doi: 10.1176/ajp.155.9.1196. [DOI] [PubMed] [Google Scholar]
- 39.Silbersweig DA, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378(6553):176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- 40.Dierks T, et al. Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22(3):615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 41.Delekate A, Zagrebelsky M, Kramer S, Schwab ME, Korte M. NogoA restricts synaptic plasticity in the adult hippocampus on a fast time scale. Proc Natl Acad Sci USA. 2011;108(6):2569–2574. doi: 10.1073/pnas.1013322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aloy EM, et al. Synaptic destabilization by neuronal Nogo-A. Brain Cell Biol. 2006;35(2–3):137–156. doi: 10.1007/s11068-007-9014-3. [DOI] [PubMed] [Google Scholar]
- 43.Petrinovic MM, et al. Neuronal Nogo-A negatively regulates dendritic morphology and synaptic transmission in the cerebellum. Proc Natl Acad Sci USA. 2013;110(3):1083–1088. doi: 10.1073/pnas.1214255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abel T, Martin KC, Bartsch D, Kandel ER. Memory suppressor genes: Inhibitory constraints on the storage of long-term memory. Science. 1998;279(5349):338–341. doi: 10.1126/science.279.5349.338. [DOI] [PubMed] [Google Scholar]
- 45.Bartsch D, et al. Aplysia CREB2 represses long-term facilitation: Relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83(6):979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 46.Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108(5):689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- 47.Malleret G, et al. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001;104(5):675–686. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 48.Chen A, et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39(4):655–669. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- 49.Abel T, et al. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88(5):615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 50.Gerlai R, Henderson JT, Roder JC, Jia Z. Multiple behavioral anomalies in GluR2 mutant mice exhibiting enhanced LTP. Behav Brain Res. 1998;95(1):37–45. doi: 10.1016/s0166-4328(98)00002-3. [DOI] [PubMed] [Google Scholar]
- 51.Kaksonen M, et al. Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Mol Cell Neurosci. 2002;21(1):158–172. doi: 10.1006/mcne.2002.1167. [DOI] [PubMed] [Google Scholar]
- 52.Kim MH, et al. Enhanced NMDA receptor-mediated synaptic transmission, enhanced long-term potentiation, and impaired learning and memory in mice lacking IRSp53. J Neurosci. 2009;29(5):1586–1595. doi: 10.1523/JNEUROSCI.4306-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rutten K, et al. Enhanced long-term potentiation and impaired learning in phosphodiesterase 4D-knockout (PDE4D) mice. Eur J Neurosci. 2008;28(3):625–632. doi: 10.1111/j.1460-9568.2008.06349.x. [DOI] [PubMed] [Google Scholar]
- 54.Uetani N, et al. Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. EMBO J. 2000;19(12):2775–2785. doi: 10.1093/emboj/19.12.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JE, Li S, GrandPré T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38(2):187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 56.Simonen M, et al. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38(2):201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 57.Zheng B, et al. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38(2):213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 58.Dimou L, et al. Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J Neurosci. 2006;26(21):5591–5603. doi: 10.1523/JNEUROSCI.1103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller BH, Wahlestedt C. MicroRNA dysregulation in psychiatric disease. Brain Res. 2010;1338:89–99. doi: 10.1016/j.brainres.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 61.Buckingham SD, Esmaeili B, Wood M, Sattelle DB. RNA interference: From model organisms towards therapy for neural and neuromuscular disorders. Hum Mol Genet. 2004;13(Spec No 2):R275–R288. doi: 10.1093/hmg/ddh224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.