Abstract
B lymphocyte-induced maturation protein-1 (Blimp-1) is a transcriptional repressor important for the differentiation and function of several types of immune cells. Because skin serves as a physical barrier and acts as an immune sentinel, we investigated whether Blimp-1 is involved in epidermal immune function. We show that Blimp-1 expression is reduced in skin lesions of some human eczema samples and in stimulated primary keratinocytes. Epidermal-specific deletion of PR domain containing 1, with ZNF domain (Prdm1), the gene encoding Blimp-1, in adult mice caused spontaneously inflamed skin characterized by massive dermal infiltration of neutrophils/macrophages and development of chronic inflammation associated with higher levels of cytokines/chemokines, including granulocyte colony-stimulating factor (G-CSF), and enhanced myelopoiesis in bone marrow. Deletion of Prdm1 in the epidermis of adult mice also led to stronger inflammatory reactions in a tape-stripping test and in a disease model of contact dermatitis. The elevated G-CSF produced by keratinocytes after deletion of Prdm1 in vitro was mediated by the transcriptional activation of FBJ osteosarcoma oncogene (Fos) and fos-like antigen 1 (Fosl1). Systemic increases in G-CSF contributed to the inflammatory responses, because deletion of the G-CSF gene [colony stimulating factor 3, (Csf3)] prevented neutrophilia and partially ameliorated the inflamed skin in Prdm1-deficient mice. Our findings indicate a previously unreported function for Blimp-1 in restraining steady-state epidermal barrier immunity.
Skin of higher organisms is composed mainly of keratinocytes and provides a physical barrier between the body and environment. Many chronic inflammatory diseases of the skin are associated with dysregulation of immune responses (1), but the precise underlying molecular mechanisms are poorly understood. In vivo, epidermal keratinocytes produce basal levels of IL-1α sufficient to initiate cutaneous inflammation (2). Accumulating evidence indicates that in both the basal and diseased states, keratinocytes produce many other cytokines/chemokines, including IL-6, IL-10, and TNF-α (1), which recruit immune cells to the epidermis/dermis and initiate inflammation. In line with their ability to secrete cytokines/chemokines, epidermal keratinocytes express several Toll-like receptors (1), arguing for an autonomous role for keratinocytes in sensing danger signals to initiate inflammatory responses. In humans, keratinocytes in allergic skin appear to be activated before immune cells (eg, T cells) enter the skin (3), again supporting the notion that skin plays an active role in controlling immune responses. How transcriptional regulation orchestrates the expression of inflammatory genes in the epidermis is unclear, however.
B lymphocyte-induced maturation protein-1 (Blimp-1) is an important transcriptional repressor involved in the regulation of differentiation and function of many types of immune cells (4). Blimp-1 negatively regulates several cytokine/chemokine genes, including Il6, C-C chemokine ligand 2 (Ccl2), Ccl3, Ccl4, and Il2, in immune cell lineages (5–7). Of note, Blimp-1 is also expressed in various mouse epithelial tissues, including the epidermis (8, 9). Lineage-specific deletion of PR domain containing 1, with ZNF domain (Prdm1), the gene encoding Blimp-1, in the epithelium during mouse development causes defects in the terminal differentiation of epidermal keratinocytes and delayed barrier formation (9). Newborn mice deficient in Prdm1 in epithelial tissues display abnormal cornified layers and expanded granular layers, possibly resulting from increased cell proliferation (9). Several genes, including FBJ osteosarcoma oncogene (Fos), nuclear factor of activated T-cells 5 (Nfat5), and Prdm1, are directly repressed by Blimp-1 in keratinocytes (9). Blimp-1 is also expressed in the unipotent stem cells residing in sebaceous glands. These cells also belong to the epidermal lineage and are required for sebaceous gland maintenance (10). Whether Blimp-1 has an immunologic role in adult epidermis is unknown, however. In the present study, adult mice carrying floxed Prdm1 alleles were subjected to inducible and conditional deletion in the epidermis and used to study the immunologic role of Blimp-1 in the epidermis.
Results
Blimp-1 Expression in Normal and Diseased Human Skin.
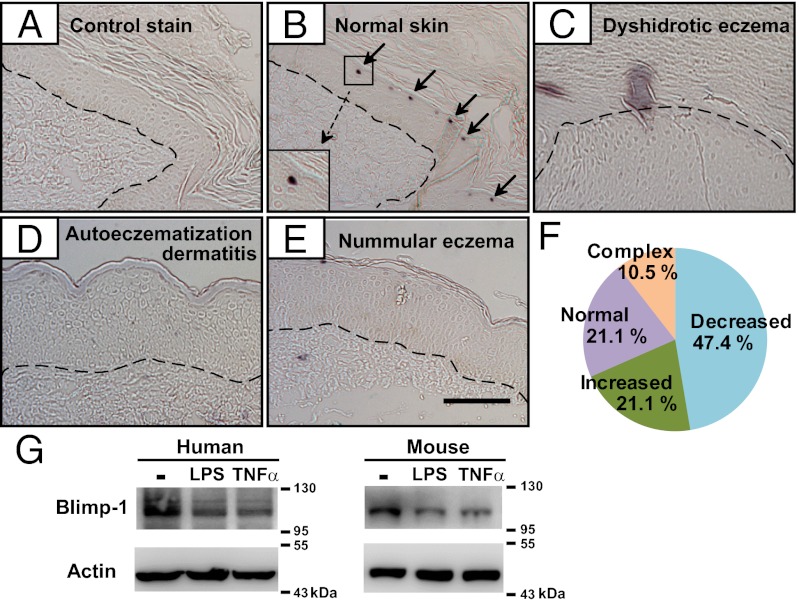
To examine whether Blimp-1 is involved in immunologic reactions in adult skin, we assessed Blimp-1 expression with immunohistochemistry (IHC) staining of normal and diseased human skin sections. Consistent with a previous report (9), compared with skin stained with the isotype control antibody (Fig. 1A), normal skin showed intense Blimp-1–specific staining in granular layer cells (Fig. 1B). Interestingly, Blimp-1 expression was reduced in skin sections with dyshidrotic eczema (Fig. 1C), autoeczematization dermatitis (Fig. 1D), and nummular eczema (Fig. 1E). Approximately 47% of the unclassifiable eczema skin samples showed decreased Blimp-1 expression (Fig. 1F). Furthermore, Blimp-1 protein levels (Fig. 1G), but not mRNA levels (Fig. S1A), were reduced in human and mouse primary keratinocytes in response to LPS or TNF-α stimulation. This altered expression of Blimp-1 in human eczematous skin prompted us to examine the physiological role of Blimp-1 in adult mouse keratinocytes.
Fig. 1.
Expression of Blimp-1 in human epidermis and stimulated keratinocytes. (A–E) Immunohistochemical staining of human skin sections with isotype control antibody, mouse IgG (A) or with anti–Blimp-1 (B–E) or from individuals with normal skin (A and B), dyshidrotic eczema (C), autoeczematization dermatitis (D), or nummular eczema (E). Each dashed line denotes the epidermis–dermis boundary. Arrows indicate Blimp-1+ cells. (Scale bar: 100 μm.) (F) Relative expression of Blimp-1 from unclassified eczema skin samples (n = 19) compared with normal skin. Eczema skin sections that contained areas of both increased and decreased Blimp-1 were categorized as complex. (G) Immunoblots showing reduced Blimp-1 protein in human (Left) and mouse (Right) primary keratinocytes treated with LPS (5 μg/mL) or TNF-α (50 ng/mL), or untreated (−), for 24 h; results are representative of three experiments.
Inducible Deletion of Prdm1 in Adult Mouse Keratinocytes.
To examine whether Blimp-1 has an immunoregulatory function in the adult epidermis in vivo, we generated mice with floxed Prdm1 alleles (Prdm1f/f) that expressed Cre recombinase fused with the mutated ligand-binding domain of the estrogen receptor (CreER) under control of the bovine keratin 5 promoter (K5-CreER). Conditional knockout (CKO) of Prdm1 in adult mouse epidermis is achieved in the presence of 4-hydroxytamoxifen (4OHT), which induces the nuclear translocation of CreER that is specifically expressed in basal layer keratinocytes. Quantitative PCR (qPCR) using genomic DNA from isolated keratinocytes from the tail skin of 4OHT-injected Prdm1f/f, K5-CreER− [control (Ctrl)] mice, and Prdm1f/f, K5-CreER+ (CKO) mice showed that ∼80% of Prdm1 alleles were effectively deleted in CKO keratinocytes (Fig. S1B, Left). Deletion of Prdm1 appears to be specific to keratinocytes, given that Prdm1 alleles remained intact in bone marrow (BM) macrophages/neutrophils and splenic T and B cells of CKO mice (Fig. S1C). Quantitative RT-PCR (qRT-PCR) using mRNA from tail skin keratinocytes of Ctrl and CKO mice demonstrated that less than 20% of Blimp-1 mRNA remained in CKO keratinocytes (Fig. S1B, Right).
We next measured Blimp-1 protein levels by immunoblot analysis using lysates of cultured primary keratinocytes from the tail skin of Ctrl and CKO mice. As expected, Blimp-1 protein was barely detectable in CKO keratinocytes (Fig. 2A). IHC staining confirmed that Blimp-1 signals were detected in the granular layers in Ctrl skin, but not in CKO skin (Fig. S1D). These data demonstrate that Blimp-1 was efficiently depleted in CKO adult epidermis.
Fig. 2.
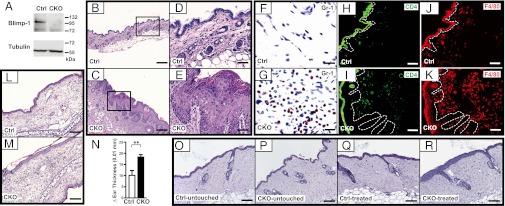
Deletion of epidermal Prdm1 in adult mice leads to spontaneous development of skin inflammation and sensitized skin. (A) Deletion of Prdm1 alleles at 2 mo after i.p. injection of 4OHT followed by immunoblotting with anti–Blimp-1 of cultured keratinocytes derived from tail skin of Ctrl or CKO mice. (B–K) At 6 mo after 4OHT injection, H&E staining of neck skin sections from CKO mice (C and E) showed inflamed skin compared with control (Ctrl) mice (B and D). Immunohistochemical (F and G) or immunofluorescence (H–K) staining of neck skin from Ctrl (F, H, and J) and CKO (G, I, and K) mice using antibodies specific for Gr-1 (F and G), CD4 (H and I), and F4/80 (J and K). (L–N) At 2 mo after 4OHT injection, Ctrl and CKO mice were used for contact hypersensitivity tests. H&E staining shows ear sections of Ctrl (L) and CKO (M) mice after rechallenging with DNFB for 24 h. The extent of ear swelling was determined by comparing changes in ear thickness before and after challenges (N) (Ctrl, n = 8; CKO, n = 9). (O–R) H&E staining of back skin sections from Ctrl and CKO mice at 2 d after receiving 10 tape-stripping treatments (treated); control skin areas are designated as “untouched.” Results are representative of three independent experiments. **P < 0.01. Images in D and E are enlarged fields of the boxed areas of B and C, respectively. The dotted line in H–K denotes the epidermis–dermis boundary. (Scale bars: 200 μm in B and C; 100 μm in L, M, and O–R; 50 μm in D, E, and H–K; 15 μm in F and G.)
CKO Mice Exhibit Alopecia, Ulceration, and Inflammation in Neck Skin.
Starting at 3 mo after 4OHT injection, CKO mice spontaneously lost hair in the neck area (Fig. S1E). At 4 mo postinjection, the neck skin of CKO mice became scaly and erythematous, accompanied by alopecia; at 6–7 mo postinjection, the neck skin developed lesions and ulcers (Fig. S1E). In addition, CKO mice had enlarged spleens and cervical lymph nodes compared with Ctrl mice, but the other lymph nodes were slightly larger than or indistinguishable from those of Ctrl mice (Fig. S1F).
Histological examination with H&E staining of skin biopsy sections revealed that, compared with Ctrl mice (Fig. 2 B and D), CKO mice had a thickened epidermis characterized by hyperplasia in the basal layer, spinous layer, and granular layer keratinocytes (Fig. 2 C and E). Moreover, the normal basket-weave feature of the stratum corneum was disrupted and replaced by hyperkeratosis in CKO mice (Fig. 2E). The enhanced skin thickness was not due to perturbation of epidermal differentiation in CKO mice, because IHC staining showed a similar distribution of epidermal markers of various stages of differentiation, including keratin (K) 5, K1, and loricrin, in Ctrl and CKO neck skin (Fig. S2A). The extent of apoptosis, as determined by TUNEL staining, was also comparable in Ctrl and CKO skin (Fig. S2B); however, CKO epidermis exhibited slightly increased expression of Ki67, a marker of cell proliferation (Fig. S2C). Of note, the thickened dermis of CKO mice exhibited dilated vessels and numerous inflammatory cells penetrating the dermis, which were mainly granulocytes and lymphocytes (Fig. S3 A and B). In some cases, nucleated corneocytes (parakeratosis) and increased sebaceous gland size were seen in CKO neck skin sections (Fig. S3 C and D). IHC staining showed markedly enhanced signals for Gr-1, a neutrophil marker, in the CKO dermis (Fig. 2G) compared with Ctrl dermis (Fig. 2F). In parallel, immunofluorescence staining for CD4 and the macrophage marker F4/80 demonstrated a slight increase in the number of CD4+ T cells (Fig. 2I), but a significantly greater number of inflammatory macrophages in the CKO dermis (Fig. 2K), compared with Ctrl dermis (Fig. 2 H and J). Increased numbers of granulocytes, macrophages, and CD4+ T cells in CKO dermis were further confirmed by quantification (Fig. S3E).
CKO Mice Develop a More Severe Skin Inflammatory Response Caused by 2,4-Dinitrofluorobenzene and Tape Stripping.
Most mouse models of skin inflammatory diseases exhibit globally abnormal skin, but some models show effects predominantly in the neck and facial areas because the skin in these areas may be subjected to more frequent stimulatory stress, such as scratching, which can exacerbate symptoms. Neck skin was predominantly affected in the CKO mice, prompting us to examine whether other skin areas of the CKO mice were also predisposed to inflammatory reactions or mechanical stress. After challenging the presensitized Ctrl and CKO mice with 2,4-dinitrofluorobenzene (DNFB) to induce contact hypersensitivity that mimics human contact dermatitis, we found that, compared with ear sections of Ctrl mice (Fig. 2L), H&E staining of the ears of CKO mice showed enhanced dermal infiltration of inflammatory cells (Fig. 2M); CKO mice also displayed increased ear thickness (Fig. 2N). Similarly, enhanced epidermal thickness and exacerbated inflammation were found on CKO back skin after tape stripping (Fig. 2R) compared with untouched back skin from Ctrl (Fig. 2O) and CKO (Fig. 2P) mice and back skin from Ctrl mice after tape stripping (Fig. 2Q). Thus, CKO skin had a higher propensity to develop an immune reaction on epicutaneous antigen sensitization and stress, and the symptoms were not restricted to the neck area.
Keratinocyte-Specific Prdm1-Deficient Mice Exhibit Aberrant Immune Responses.
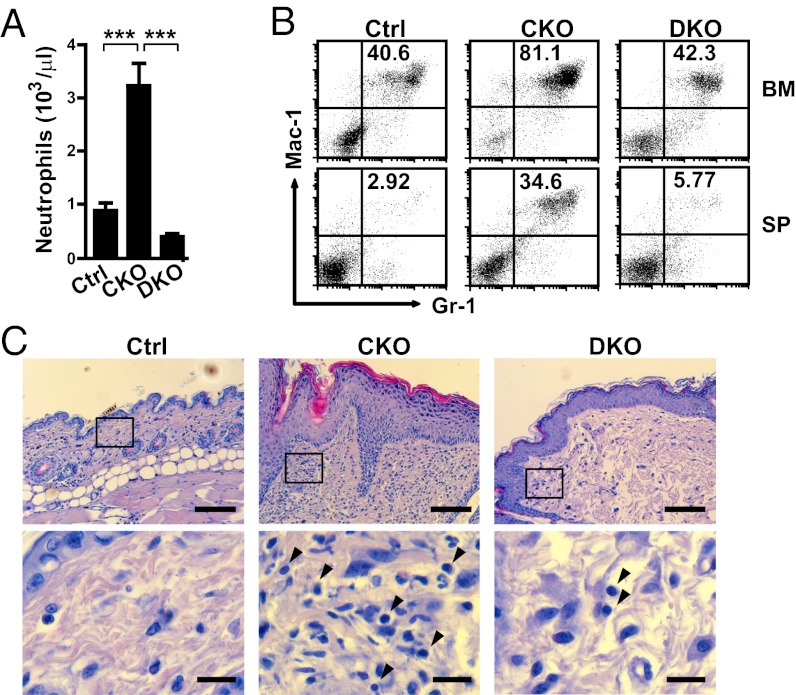
Given our finding of neutrophil- and macrophage-dominated inflammatory infiltrates in the skin of CKO mice, we next examined whether these responses were linked with the abnormal immune responses. In peripheral blood, the WBC count was similar in Ctrl and CKO mice, but the CKO mice had significantly higher numbers of neutrophils and monocytes (Fig. 3A). Similarly, the frequency of Mac-1+Gr-1+ neutrophils was also increased in CKO spleen at the time when the skin symptoms became apparent (Fig. 3B). More strikingly, and unexpectedly, beginning with the onset of skin disease, a significant expansion of neutrophils was detected in the BM of CKO mice compared with Ctrl mice (Fig. 3B). At 6 mo after 4OHT injection, neutrophils appeared to dominate hematopoiesis in the BM of CKO mice (Fig. 3B).
Fig. 3.
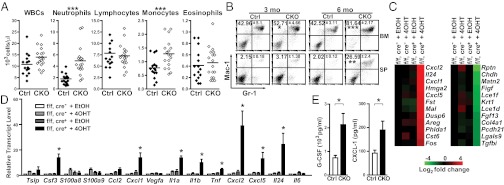
Enhanced neutrophil-dominated inflammatory reactions in CKO mice. (A) The numbers of leukocytes measured with an automated hematology analyzer in Ctrl and CKO mice at 6 mo after 4OHT injection. The mean number of each cell type is indicated by a horizontal line (Ctrl, n = 15; CKO, n = 18). (B) Flow cytometry analysis of the frequencies of Gr-1+Mac-1+ cells in BM cells and splenocytes (SP) of Ctrl and CKO mice. The numbers shown represent the percentage of Gr-1+Mac-1+ neutrophils in each upper-right quadrant. (C) Heat map of microarray analysis using indicated keratinocytes cultured with 4OHT or EtOH. Data were normalized to Prdm1f/f (f/f), K5-CreER(Cre)− + EtOH group, and the 12 genes with the greatest changes are shown. (D) RNA from keratinocytes cultured as described in C was used for qRT-PCR analysis of cytokine/chemokine gene expression. (E) The Luminex assay was used to detect the increased levels of G-CSF and CXCL-1 in serum of CKO mice at 6 mo after 4OHT injection. Results are mean ± SEM (n = 3, 4, and 6 in B, D, and E, respectively). *P < 0.05; **P < 0.01; ***P < 0.005.
Regarding adaptive immune responses, after the onset of skin disease, the numbers of CD4+ T, CD8+ T, and B220+ B cells in the spleen decreased in CKO mice, owing to the increased numbers of neutrophils (Fig. S4 A and B). Of note, the numbers of CD62L+CD44lo naïve CD4+ and CD8+ T cells were reduced in CKO spleen and cervical lymph nodes (Fig. S4 C and D). In addition, CKO mice exhibited an increased frequency of B220loCD138+ plasma cells in the secondary lymphoid organs (Fig. S4E), in agreement with the increased amounts of IgG2a, IgG2b, and IgE isotypes in CKO mouse sera (Fig. S4F). Thus, Blimp-1 deficiency in keratinocytes led to aberrant chronic immunologic reactions, including greatly enhanced granulopoiesis in the BM, increased neutrophils and monocytes in the periphery, and more active adaptive immune responses.
Increased Expression of Cytokines/Chemokines in Prdm1-Deficient Keratinocytes.
To understand the mechanisms underlying spontaneous skin inflammation in CKO mice, we used microarrays to examine the differential gene expression profiles of keratinocytes with deleted or WT Prdm1. Keratinocytes isolated from Prdm1f/f, K5-CreER+ mice cultured in the presence of 4OHT showed effective deletion of Prdm1 in vitro (Fig. S5A). Ethanol (EtOH) solvent-treated keratinocytes from Prdm1f/f, K5-CreER+ mice and 4OHT- or EtOH-treated keratinocytes from Prdm1f/f, K5-CreER− mice were used as controls. Microarray analyses revealed 93 up-regulated genes and 109 down-regulated genes that were altered by more than twofold in the mice with deleted Prdm1 (Tables S1 and S2). Genes that showed the most significant changes after normalization to the Prdm1f/f, K5-CreER− + EtOH group were arranged using heat map analysis (Fig. 3C). We found several corneocyte-related genes, including repetin (Rptn), late cornified envelope (Lce) 1f, keratin 1 (Krt1), and Lce1d., that were significantly down-regulated on Prdm1 deletion in vitro. Interestingly, among the top 12 up-regulated genes, in addition to a known Blimp-1 target gene, Fos (7), we identified four genes encoding cytokines/chemokines, C-X-C chemokine ligand (Cxcl) 1, Cxcl2, Cxcl5, and Il24, each of which encodes a neutrophil/macrophage attractant (11).
We next analyzed a panel of cytokine/chemokine transcripts using cDNA from Prdm1-deleted keratinocytes. Several other cytokine/chemokine genes not present in our microarray data, including Csf3, Il1a, Il1b, and Tnf, were also up-regulated in Prdm1-deleted keratinocytes (Fig. 3D). We confirmed that the mRNA levels of Cxcl1, Cxcl2, Cxcl5, and Il24 in the microarray were increased on deletion of Prdm1 in vitro (Fig. 3D). A Luminex assay revealed secretion of the foregoing cytokines/chemokines, including granulocyte colony-stimulating factor (G-CSF), IL-1α, and C-X-C chemokine ligand 1 (CXCL-1), into the culture medium (Fig. S5B). Furthermore, CKO mice exhibited significantly increased levels of G-CSF and CXCL-1 (Fig. 3E), as well as IL-17 and IL-1β (Fig. S5C), in serum. Thus, keratinocytes lacking Blimp-1 showed increased secretion of cytokines/chemokines.
Blimp-1 Indirectly Regulates Csf3 in Keratinocytes by Repressing Fos and Fosl1.
We next studied whether the foregoing cytokine/chemokine genes are repressed by Blimp-1 in cultured keratinocytes transduced with a lentiviral vector encoding Blimp-1 fused with GFP (Blimp-1–GFP) or GFP alone. As demonstrated by qRT-PCR, most of these cytokine/chemokine transcripts, including Csf3, Cxcl1, Cxcl5, Il24, and Il1b, were suppressed by Blimp-1–GFP in keratinocytes compared with the GFP control (Fig. 4A). We detected several potential Blimp-1 consensus binding sites (12) spanning 5 kb upstream or downstream of the transcriptional start site of these cytokine/chemokine genes (Fig. S6A). ChIP results showed that Blimp-1 binds to a known Blimp-1 target gene, Fos, in keratinocytes (9), as expected (Fig. S6B). However, no significant binding of Blimp-1 to the candidate cytokine/chemokine genes was detected compared with the negative control locus, snail3 (9) (Fig. S6B), suggesting that these cytokine/chemokine genes may be indirectly regulated by Blimp-1.
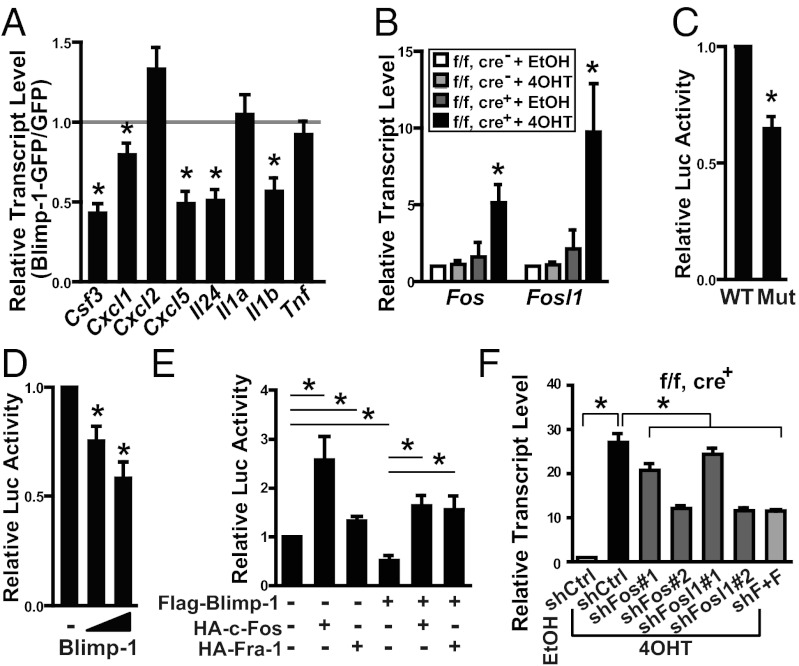
Fig. 4.
Blimp-1 indirectly regulates G-CSF expression by suppressing Fos and Fosl1. (A) Keratinocytes from neonatal mice were cultured and transduced with lentiviral vectors expressing Blimp-1–GFP or GFP for 2 d. The expression of the indicated genes was analyzed with qRT-PCR. Data were normalized to levels of the internal control, Actb, and are presented as the ratio of the indicated genes in Blimp-1–GFP to GFP-expressing keratinocytes. (B) qRT-PCR of Fos and Fosl1 mRNA levels using RNA prepared from keratinocytes cultured as described in Fig 3C. (C) A dual-luciferase reporter assay conducted in primary keratinocytes to compare the activities of the Csf3 promoter containing either the WT or the mutated (Mut) AP-1–binding element. (D and E) Dual-luciferase reporter assays of Csf3 promoter activities. Firefly luciferase reporter driven by the WT Csf3 promoter was used in a cotransfection experiment with Blimp-1 (D) or the indicated expression vectors (E) into primary keratinocytes. Here “−” indicates transfected with empty vector. (F) qRT-PCR of Csf3 mRNA from keratinocytes treated with EtOH or 4OHT at 5 d after transduction with lentiviral vectors producing control shRNA (shCtrl), shRNA against Fos (shFos), shRNA against Fosl1 (shFosl1), or shRNA against both Fos and Fosl1 (shF+F). Here “1” and “2” represent two different shRNAs used. Data are mean ± SEM (n ≥3 in all panels). *P < 0.05.
Because Blimp-1 directly suppresses Fos, and activator protein 1 (AP-1) family proteins are involved in the transcriptional regulation of numerous cytokine genes in inflammatory skin diseases (13), we analyzed the mRNA levels of AP-1 family members in Prdm1-deleted keratinocytes. In addition to Fos, the expression of another AP-1 family gene, Fosl1, was increased in Prdm1-deleted keratinocytes in our microarray (Table S1). Indeed, the ChIP assay showed that Blimp-1 binds to at least two sites in the Fosl1 locus in keratinocytes (Fig. S6B). The mRNA levels of Fos and Fosl1 (Fig. 4B), but not of other genes (Fig. S7A), were increased in Prdm1-deleted keratinocytes. Accordingly, FBJ osteosarcoma oncogene (c-Fos) and fos-related antigen 1 (Fra-1) proteins (encoded by Fos and Fosl1, respectively) were increased in Prdm1-deleted keratinocytes (Fig. S7B).
We next asked whether Blimp-1 indirectly suppresses these cytokine/chemokine genes through c-Fos or Fra-1. To do so, we conducted a luciferase reporter assay using a luciferase reporter carrying the −3,886 to +20 region of the Csf3 promoter. In primary keratinocytes, activity of the Csf3 promoter-driven luciferase reporter was reduced when a known AP-1–binding site (14) was mutated (Fig. 4C). In the presence of exogenous Blimp-1, c-Fos, or Fra-1 (Fig. S7 C and D), Csf3 promoter-driven luciferase activity was suppressed by Blimp-1 in a dose-dependent manner (Fig. 4D), but was enhanced by c-Fos or Fra-1 (Fig. 4E). The suppressive effect of Blimp-1 on the Csf3 promoter was compensated for by c-Fos or Fra-1 (Fig. 4E), suggesting that Csf3 is indirectly repressed by Blimp-1 via down-regulation of AP-1 family genes. Furthermore, de-repression of Fos and Fosl1 after Prdm1 deletion in keratinocytes appears to be crucial for the enhanced production of cytokines/chemokines, given that knockdown of Fos, Fosl1, or both by lentiviral vectors in Prdm1-deleted keratinocytes (Fig. S7E) dampened the increased Csf3 expression (Fig. 4F) and affected the expression of other cytokines/chemokines (Fig. S7F).
G-CSF Is Responsible for the Systemic Neutrophilia in CKO Mice.
We next investigated the role of G-CSF in Blimp-1 deficiency-induced skin inflammation and systemic granulopoiesis, because elevated expression was prominently detected in CKO sera (Fig. 3E). CKO mice were bred with Csf3-deficient mice to create double-KO (DKO) mice. As expected, the increased number of neutrophils in peripheral blood was reduced in DKO mice compared with Ctrl and CKO mice (Fig. 5A). In addition, the numbers of neutrophils in spleen and BM of DKO mice decreased to levels comparable to those in Ctrl mice (Fig. 5B). Interestingly, inflammatory skin lesions were partially ameliorated, owing to a thinner epidermis and fewer infiltrating immune cells, including lymphocytes, in DKO skin compared with CKO skin (Fig. 5C). Therefore, increased G-CSF production apparently can be attributed, at least in part, to the inflammatory symptoms in CKO mice.
Fig. 5.
G-CSF contributes to inflammatory diseases in CKO mice. (A) The number of neutrophils in peripheral blood of the indicated mice at 5–6 mo after 4OHT injection was measured with an automated hematology analyzer. Data are mean ± SEM (n = 4). ***P < 0.005. (B) Populations of Gr-1+Mac-1+ neutrophils in BM and splenocytes (SP) of the indicated mice shown in a representative dot plot from two independent experiments. (C) H&E staining of neck skin from the indicated mice at 6 mo after 4OHT injection. The enlarged images in the boxed areas of the upper panels are shown in the lower panels. Arrowheads indicate lymphocytes. (Scale bars: 100 μm in upper panels; 15 μm in lower panels.)
Discussion
The etiology of skin inflammatory disorders is poorly understood. In particular, whether skin inflammatory diseases result primarily from dysregulation of immune cells or of epidermal keratinocytes remains controversial (1, 15). We found that ablation of Prdm1 in keratinocytes triggered the release of several cytokines/chemokines, suggesting that Blimp-1 in the epidermis may participate in restraining immunologic responses under basal conditions. Supporting this hypothesis, in some cases of clinical eczema, Blimp-1 expression was reduced even though granular layers were thickened. We do not know why some eczema cases exhibited increased Blimp-1 levels, however; it may reflect the etiologic complexity of skin inflammatory diseases or perhaps a secondary effect. Our findings are also consistent with a previous report suggesting that Prdm1 deletion in keratinocytes during mouse development triggered skin lesions that might have resulted from the mice scratching irritated/itchy skin areas (9). Furthermore, CKO mice demonstrated more severe immune responses in the DNFB-induced contact hypersensitivity experimental model and the tape-stripping test, indicating that the absence of Blimp-1 in the epidermis may predispose to inflammatory reactions.
Interestingly, Blimp-1 protein levels in keratinocytes were reduced after treatment with LPS or TNF-α; this reduction seems to reflect regulation via a posttranscriptional mechanism, given the lack of significant change in Blimp-1 mRNA levels in keratinocytes after each treatment. This finding is also unique for keratinocytes, because signaling through Toll-like receptors and cytokine receptors is known to increase Blimp-1 mRNA and protein in several other cell types, including T cells, B cells, and dendritic cells (6, 16, 17).
Our CKO mice developed a complex immune response including increased serum levels of IgG2a, IgG2b, and IgE. Moreover, the in vitro deletion of Prdm1 led to increased expression of several cytokine/chemokine genes, including Csf3, Cxcl1, Il1a, Il1b, Tnfa, Cxcl2, Cxcl5, and Il24, along with significantly increased serum G-CSF and CXCL-1 levels, in CKO mice. These findings indicate the development of enhanced innate immune responses, because all these cytokines/chemokines are associated with the activation or recruitment of macrophages/neutrophils. Inducible expression of PKC family members, such as PKCε or PKCα, in the epidermis leads to the development of systemic neutrophilia and increased levels of chemoattractants for neutrophils and macrophages, including CXCL-1 and G-CSF (18, 19), and this situation most resembles the innate immune responses of CKO mice in the present study. Interestingly, CKO mice showed some similar features of an orphan disease known as Sweet syndrome, which involves neutrophilic dermatosis and systemic neutrophilia and is associated with elevated serum G-CSF and IL-6 levels (20–22). Moreover, some patients with Sweet syndrome also have an inflammatory bowel disease, such as Crohn disease or ulcerative colitis (22). T-cell–specific Prdm1-deficient mice develop inflammatory bowel disease (23). However, although Il-6 is directly suppressed by Blimp-1 in dendritic cells (6), and IL-6 mRNA levels were increased in CKO neck skin (Fig. S5D), Prdm1 deletion in keratinocytes did not affect IL-6 mRNA expression (Fig. 3D), arguing against Il-6 as a direct target of Blimp-1 in keratinocytes. Nevertheless, the determination of whether genetic alternation of Blimp-1 pathways is associated with human inflammatory diseases requires future genome-wide analysis.
In addition to genes encoding chemoattractants for neutrophils and macrophages, among the genes differentially expressed in our microarray study, Fos and Fosl1 are direct targets of Blimp-1. Increased FBJ murine osteosarcoma viral oncogene (v-Fos) expression in a transgenic mouse model results in hyperplasia and hyperkeratosis (24). Other up-regulated genes, such as follistatin (Fst), myelin and lymphocyte protein (Mal), and amphiregulin (Areg), are involved in regulating epidermal proliferation (25–27). Pleckstrin homology-like domain, family A, member 1 (Phlda1) is a putative epithelial cell marker (28). Cystatin M/E (Cst-6) is abnormally expressed in human skin diseases, such as atopic dermatitis and psoriasis (29). Regarding the down-regulated genes, repetin (Rptn) is an epidermal differentiation protein (30), and Matrillin-2 (Matn2) is involved in wound healing (31). Late cornified envelope (Lce) is a stratum-corneum protein involved in barrier formation (32). Galectin-9 (Lgals9) reduces Th1-mediated skin inflammation and thus inhibits contact hypersensitivity and psoriatic reactions in experimental animal models (33). The absence of Blimp-1 in keratinocytes, leading to altered expression of the foregoing genes, may account for the abnormal growth and cornification of keratinocytes, as well as skin inflammation.
G-CSF, a proinflammatory cytokine, regulates neutrophil activation, recruitment, and homeostasis (34) and can promote keratinocyte proliferation (35). We reasoned that G-CSF–mediated excessive infiltration of neutrophils to the dermis is related, at least in part, to the pathology in CKO mice. Csf3 deletion did not fully abrogate skin inflammation in CKO mice, however, suggesting that additional effectors may participate in skin inflammation induced by ablation of Blimp-1. Although Blimp-1 can reduce the expression of G-CSF, our ChIP assay did not detect direct binding of Blimp-1 to Csf3. Csf3 is suppressed by JunB (14). In our case, JunB apparently did not contribute to the increased G-CSF levels in CKO mice, given that the expression of JunB was unchanged in Prdm1-deleted keratinocytes. Fos and Fosl1 are directly regulated by Blimp-1 and contribute to elevated levels of cytokines/chemokines, especially G-CSF, in CKO keratinocytes. In contrast to a previous report demonstrating that the AP-1–binding site of the Csf3 promoter, which is located in the −652 to −646 region, negatively regulates Csf3 transcription (14), we found that this site was induced by c-Fos and Fra-1. This discrepancy may be related to the differing compositions of AP-1 family members resulting from the deletion of different genes in the two studies. Along with its regulation by Blimp-1, Csf3 induced by activated c-Fos can be controlled by metalloproteinase 17 (ADAM17)−Notch pathways (36), suggesting that multiple pathways may be involved in cytokine synthesis and immune barrier maintenance.
Materials and Methods
Mice and Reagents.
Prdm1f/f mice (37) were bred with K5-CreER mice (38) on a mixed 129SvJ/C57BL/6 background. Sex-matched Prdm1f/f, K5-CreER+ and littermate control mice (4–5 wk old) were injected i.p. three times over 3 d with 4–5 mg/kg body weight 4OHT (Sigma-Aldrich) in 0.25 mL of sunflower seed oil. Here we refer to Prdm1f/f, K5-CreER+ and littermate control mice that received 4OHT injection as CKO and Ctrl mice, respectively. To generate mice deficient in both Blimp-1 and G-CSF, we crossed Prdm1f/f, K5-CreER+/− mice with Csf3-deficient mice (kindly provided by Dr. Margaret L. Hibbs, Ludwig Institute for Cancer Research, Victoria, Australia) (39). Detailed information on maintenance of mice and cell lines is provided in SI Materials and Methods.
Isolation and Culture of Primary Keratinocytes.
Primary human, neonatal mouse, and adult mouse tail keratinocytes were prepared as described previously (40). Details are provided in SI Materials and Methods.
Histology, Immunofluorescence, and IHC Staining.
Mouse skin samples were fixed in 10% (vol/vol) phosphate-buffered formalin, embedded in paraffin, and cut into 3-μm-thick sections for H&E or IHC staining. Human skin biopsy specimens with eczema were used for Blimp-1 staining as described previously (41). More information is provided in SI Materials and Methods.
DNFB-Induced Contact Dermatitis (Contact Hypersensitivity) and Tape Stripping.
Mice injected with 4OHT for 2 mo were subjected to the tape-stripping test and induction of contact dermatitis essentially as described previously (42). Details are provided in SI Materials and Methods.
Flow Cytometry.
Cells from spleen, lymph nodes, and BM were isolated and stained as described previously (6). Fluorescence intensity was analyzed using a FACScanto flow cytometer (BD Biosciences), and results were analyzed using FCS Express 3.0 software (De Novo Software). Antibodies are described in SI Materials and Methods.
Other Methods.
Please see SI Materials and Methods.
Statistics.
Statistical significance was determined using the two-tailed Student t test. Data are reported as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Chien-Mei Yen and Yuan-Hsuan Chan for technological assistance. This work was supported by Academia Sinica (Grant AS-99-CDA-L12, to K.-I.L.) and by the Taiwan National Science Council (Grant 100-2628-B-001-015-MY4, to K.-I.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE34586).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219462110/-/DCSupplemental.
References
- 1.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9(10):679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groves RW, Mizutani H, Kieffer JD, Kupper TS. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1 alpha in basal epidermis. Proc Natl Acad Sci USA. 1995;92(25):11874–11878. doi: 10.1073/pnas.92.25.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths CE, Nickoloff BJ. Keratinocyte intercellular adhesion molecule-1 (ICAM-1) expression precedes dermal T lymphocytic infiltration in allergic contact dermatitis (Rhus dermatitis) Am J Pathol. 1989;135(6):1045–1053. [PMC free article] [PubMed] [Google Scholar]
- 4.Davis MM. Blimp-1 over Budapest. Nat Immunol. 2007;8(5):445–447. doi: 10.1038/ni0507-445. [DOI] [PubMed] [Google Scholar]
- 5.Shaffer AL, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17(1):51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 6.Chan YH, et al. Absence of the transcriptional repressor Blimp-1 in hematopoietic lineages reveals its role in dendritic cell homeostatic development and function. J Immunol. 2009;183(11):7039–7046. doi: 10.4049/jimmunol.0901543. [DOI] [PubMed] [Google Scholar]
- 7.Martins GA, Cimmino L, Liao J, Magnusdottir E, Calame K. Blimp-1 directly represses Il2 and the Il2 activator Fos, attenuating T cell proliferation and survival. J Exp Med. 2008;205(9):1959–1965. doi: 10.1084/jem.20080526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang DH, Cattoretti G, Calame KL. The dynamic expression pattern of B lymphocyte induced maturation protein-1 (Blimp-1) during mouse embryonic development. Mech Dev. 2002;117(1-2):305–309. doi: 10.1016/s0925-4773(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 9.Magnúsdóttir E, et al. Epidermal terminal differentiation depends on B lymphocyte-induced maturation protein-1. Proc Natl Acad Sci USA. 2007;104(38):14988–14993. doi: 10.1073/pnas.0707323104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horsley V, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126(3):597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol. 2011;11(8):505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo TC, Calame KL. B lymphocyte-induced maturation protein (Blimp)-1, IFN regulatory factor (IRF)-1, and IRF-2 can bind to the same regulatory sites. J Immunol. 2004;173(9):5556–5563. doi: 10.4049/jimmunol.173.9.5556. [DOI] [PubMed] [Google Scholar]
- 13.Foletta VC, Segal DH, Cohen DR. Transcriptional regulation in the immune system: All roads lead to AP-1. J Leukoc Biol. 1998;63(2):139–152. doi: 10.1002/jlb.63.2.139. [DOI] [PubMed] [Google Scholar]
- 14.Meixner A, et al. Epidermal JunB represses G-CSF transcription and affects haematopoiesis and bone formation. Nat Cell Biol. 2008;10(8):1003–1011. doi: 10.1038/ncb1761. [DOI] [PubMed] [Google Scholar]
- 15.Bos JD, De Rie MA. The pathogenesis of psoriasis: Immunological facts and speculations. Immunol Today. 1999;20(1):40–46. doi: 10.1016/s0167-5699(98)01381-4. [DOI] [PubMed] [Google Scholar]
- 16.Calame K. Activation-dependent induction of Blimp-1. Curr Opin Immunol. 2008;20(3):259–264. doi: 10.1016/j.coi.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Lin KI, et al. Reishi polysaccharides induce immunoglobulin production through the TLR4/TLR2-mediated induction of transcription factor Blimp-1. J Biol Chem. 2006;281(34):24111–24123. doi: 10.1074/jbc.M601106200. [DOI] [PubMed] [Google Scholar]
- 18.Cataisson C, et al. CXCR2 ligands and G-CSF mediate PKCalpha-induced intraepidermal inflammation. J Clin Invest. 2006;116(10):2757–2766. doi: 10.1172/JCI27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wheeler DL, et al. Overexpression of protein kinase C-epsilon in the mouse epidermis leads to a spontaneous myeloproliferative-like disease. Am J Pathol. 2005;166(1):117–126. doi: 10.1016/s0002-9440(10)62237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuss-Borst MA, Müller CA, Waller HD. The possible role of G-CSF in the pathogenesis of Sweet’s syndrome. Leuk Lymphoma. 1994;15(3-4):261–264. doi: 10.3109/10428199409049722. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami T, et al. Elevated serum granulocyte colony-stimulating factor levels in patients with active phase of sweet syndrome and patients with active Behcet disease: Implication in neutrophil apoptosis dysfunction. Arch Dermatol. 2004;140(5):570–574. doi: 10.1001/archderm.140.5.570. [DOI] [PubMed] [Google Scholar]
- 22.Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martins GA, et al. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7(5):457–465. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- 24.Greenhalgh DA, et al. Hyperplasia, hyperkeratosis and benign tumor production in transgenic mice by a targeted v-fos oncogene suggest a role for fos in epidermal differentiation and neoplasia. Oncogene. 1993;8(8):2145–2157. [PubMed] [Google Scholar]
- 25.Antsiferova M, et al. Keratinocyte-derived follistatin regulates epidermal homeostasis and wound repair. Lab Invest. 2009;89(2):131–141. doi: 10.1038/labinvest.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieg P, et al. Tumor promoters induce a transient expression of tumor-associated genes in both basal and differentiated cells of the mouse epidermis. Carcinogenesis. 1988;9(1):95–100. doi: 10.1093/carcin/9.1.95. [DOI] [PubMed] [Google Scholar]
- 27.Lin MH, Chang KW, Lin SC, Miner JH. Epidermal hyperproliferation in mice lacking fatty acid transport protein 4 (FATP4) involves ectopic EGF receptor and STAT3 signaling. Dev Biol. 2010;344(2):707–719. doi: 10.1016/j.ydbio.2010.05.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakthianandeswaren A, et al. PHLDA1 expression marks the putative epithelial stem cells and contributes to intestinal tumorigenesis. Cancer Res. 2011;71(10):3709–3719. doi: 10.1158/0008-5472.CAN-10-2342. [DOI] [PubMed] [Google Scholar]
- 29.Zeeuwen PL, van Vlijmen-Willems IM, Egami H, Schalkwijk J. Cystatin M/E expression in inflammatory and neoplastic skin disorders. Br J Dermatol. 2002;147(1):87–94. doi: 10.1046/j.1365-2133.2002.04785.x. [DOI] [PubMed] [Google Scholar]
- 30.Krieg P, et al. Repetin (Rptn), a new member of the “fused gene” subgroup within the S100 gene family encoding a murine epidermal differentiation protein. Genomics. 1997;43(3):339–348. doi: 10.1006/geno.1997.4818. [DOI] [PubMed] [Google Scholar]
- 31.Ichikawa T, Suenaga Y, Koda T, Ozaki T, Nakagawara A. DeltaNp63/BMP-7-dependent expression of matrilin-2 is involved in keratinocyte migration in response to wounding. Biochem Biophys Res Commun. 2008;369(4):994–1000. doi: 10.1016/j.bbrc.2008.02.128. [DOI] [PubMed] [Google Scholar]
- 32.Jackson B, et al. Late cornified envelope family in differentiating epithelia—response to calcium and ultraviolet irradiation. J Invest Dermatol. 2005;124(5):1062–1070. doi: 10.1111/j.0022-202X.2005.23699.x. [DOI] [PubMed] [Google Scholar]
- 33.Niwa H, et al. Stable form of galectin-9, a Tim-3 ligand, inhibits contact hypersensitivity and psoriatic reactions: A potent therapeutic tool for Th1- and/or Th17-mediated skin inflammation. Clin Immunol. 2009;132(2):184–194. doi: 10.1016/j.clim.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Panopoulos AD, Watowich SS. Granulocyte colony-stimulating factor: Molecular mechanisms of action during steady-state and “emergency” hematopoiesis. Cytokine. 2008;42(3):277–288. doi: 10.1016/j.cyto.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawada A, et al. Granulocyte and macrophage colony-stimulating factors stimulate proliferation of human keratinocytes. Arch Dermatol Res. 1997;289(10):600–602. doi: 10.1007/s004030050246. [DOI] [PubMed] [Google Scholar]
- 36.Murthy A, et al. Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity. 2012;36(1):105–119. doi: 10.1016/j.immuni.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro-Shelef M, et al. Blimp-1 is required for the formation of immunoglobulin-secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19(4):607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 38.Liang CC, You LR, Chang JL, Tsai TF, Chen CM. Transgenic mice exhibiting inducible and spontaneous Cre activities driven by a bovine keratin 5 promoter that can be used for the conditional analysis of basal epithelial cells in multiple organs. J Biomed Sci. 2009;16:2. doi: 10.1186/1423-0127-16-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hibbs ML, et al. Mice lacking three myeloid colony-stimulating factors (G-CSF, GM-CSF, and M-CSF) still produce macrophages and granulocytes and mount an inflammatory response in a sterile model of peritonitis. J Immunol. 2007;178(10):6435–6443. doi: 10.4049/jimmunol.178.10.6435. [DOI] [PubMed] [Google Scholar]
- 40.Redvers RP, Kaur P. Serial cultivation of primary adult murine keratinocytes. Methods Mol Biol. 2005;289:15–22. doi: 10.1385/1-59259-830-7:015. [DOI] [PubMed] [Google Scholar]
- 41.Angelin-Duclos C, Cattoretti G, Lin KI, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J Immunol. 2000;165(10):5462–5471. doi: 10.4049/jimmunol.165.10.5462. [DOI] [PubMed] [Google Scholar]
- 42.Hsu DK, et al. Endogenous galectin-3 is localized in membrane lipid rafts and regulates migration of dendritic cells. J Invest Dermatol. 2009;129(3):573–583. doi: 10.1038/jid.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.