Abstract
Adolescent trauma (AT) is a common risk factor for adult-onset posttraumatic stress disorder (PTSD). However, the vulnerability to AT among different individuals varies dramatically, indicating that other cofactors are important. Despite extensive studies, the identification of those cofactors has had little success. Here, we found that after subjected to traumatic stress at postnatal day 25 (P25), a stage that is comparable to the human adolescent period, inducible/reversible forebrain-specific cholecystokinin receptor-2 transgenic (IF-CCKR-2 tg) mice exhibited a significantly higher level of PTSD-like behavior at a later life (adult) stage compared with their wild-type littermates. Moreover, in these traumatized IF-CCKR-2 tg mice, both the glucocorticoid negative feedback inhibition and spatial learning and memory were impaired. Interestingly, if the CCKR-2 transgene was specifically suppressed during the time of AT exposure, these observations were largely diminished, indicating that a temporal association of the elevated CCKergic tone and AT is pathogenically critical. Treatment of traumatized IF-CCKR-2 tg mice with fluoxetine, a selective serotonin reuptake inhibitor, for a period of 4 wk significantly attenuated the PTSD-like behavior and the impaired glucocorticoid negative feedback inhibition, but not the memory deficit, implying that the memory deficit is an independent post-AT clinical entity and not a consequence of PTSD. Taken together, these results reveal a dynamic role of the CCKergic system in the development of post-AT psychopathologies and suggest that a timely antagonism of CCKR-2 activity during AT exposure is a potential preventive strategy for post-AT psychopathologies including PTSD and cognitive dysfunction.
Keywords: anxiety disorder, animals model, face validity, constructive validity, predictive validity
Posttraumatic stress disorder (PTSD), as a predominant form of anxiety disorders, affects 7.8% of people of ages between 15 and 54 y in the United State (1). This prevalence goes up to 32–36% in people who have a history of trauma (2). Particularly, over half of the victims who experienced a preadult trauma such as childhood physical or sexual abuse eventually develop PTSD (3). Given that children, especially early adolescents, have a higher possibility of exposure to traumatic attacks (4), adolescent trauma (AT) is an important risk factor for PTSD.
However, the vulnerability among different individuals to AT is different, and this variability may at least partially be attributed to genetic variations (5). Another important factor is pretrauma stress (6). Stress may dysregulate various neurotransmitter systems in the brain, among which the CCKergic system, including cholecystokinin (CCK) peptides and their receptors, is of a significant importance, based on its dynamic regulation in response to stress (7, 8). Actually, the CCKergic system has long been recognized as an anxiogenic factor (7), and CCK peptides were commonly used to induce anxiety in volunteers (9, 10). CCK peptides and CCK receptor-2 (CCKR-2) widely distribute in the brain, with the highest level in the limbic system (11), the areas that are critically involved in cognition and emotion (12). Our recent transgenic study also showed that overexpression of CCKR-2 in neurons of the forebrain of mice significantly enhanced anxiety-like behavior (13). However, it is still not clear whether or how a higher level of the CCKR-2 expression in the brain contributes to AT vulnerability.
In this study, by using our previously engineered inducible/reversible forebrain-specific cholecystokinin receptor-2 transgenic (IF-CCKR-2 tg; simply dtg hereafter) mice, we demonstrated that a temporal coupling of the elevated CCKergic tone with an AT episode is critical for the development of PTSD-like behavior in mice at a later life stage.
Results
Expression and Function of the CCKR-2 Transgene in the Forebrain of dtg Mice.
As shown in Fig. S1 A–F, our results further confirmed that the expression of the CCKR-2 transgene was forebrain-specific, inducible/reversible, and functional. The enhanced CCK receptor binding activity in the forebrain of dtg mice was observed as early as postnatal day 20 (P20; Fig. S1E).
Effect of the CCKR-2 Transgene and AT on Fear Behavior in a Fear-Conditioning Test.
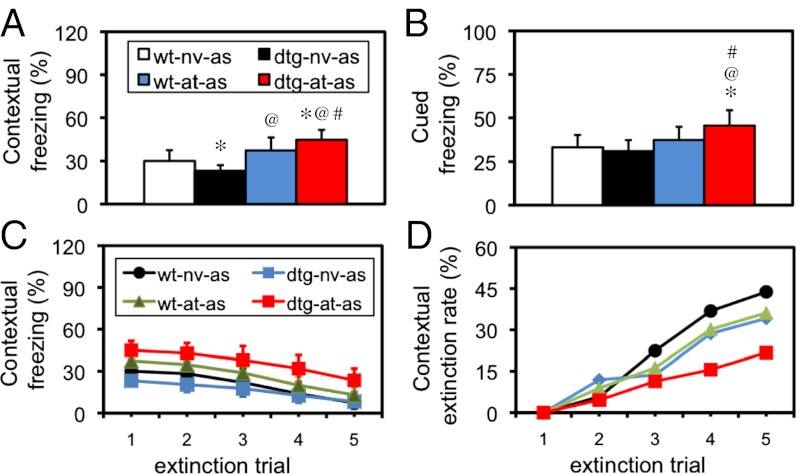
In the fear-conditioning test, as the acute stressor (AS) was actually the unconditioned stimulus (US) that paired the conditioned stimuli such as the context of the shock box, mice without AS (US) would not show any conditioning behavior and thus would not be meaningful to be used as a control. Following AS, naïve (without AT) dtg mice showed an impaired contextual conditioning (P < 0.05; Fig. 1A) but a normal cued conditioning (Fig. 1B) compared with naïve WT mice. Interestingly, following the same AS, dtg mice with AT exhibited an enhanced fear response in both the contextual (Fig. 1A) and cued (Fig. 1B) conditionings compared with that in WT-naïve (P < 0.001), WT-AT (P < 0.001), or dtg-naïve mice (P < 0.05) separately. These results indicated that (i) the CCKR-2 transgene alone impaired the hippocampus-dependent fear memory but not the overall fear responses; and (ii) after subjected to AT, however, dtg mice showed a significantly enhanced fear response in both the contextual and cued conditionings.
Fig. 1.
Enhanced PTSD-like behavior in dtg mice with AT/AS in fear-conditioning and fear-extinction tests. (A) Freezing response in contextual conditioning. *P < 0.05–0.001 compared with WT-naïve-AS mice; @P < 0.001 compared with dtg-naïve-AS mice; #P < 0.05 compared with WT-AT-AS mice; all were Student's t test. (B) Freezing response in cued conditioning. The same group comparisons: *P < 0.05; @P < 0.001; #P < 0.05; all were Student's t tests. as, adult stressor; at, adolescent trauma; nv, naïve. (C) Extinction curve in contextual conditioning following multiple extinction trials. Data above are all expressed as mean ± SD. (D) Overall extinction rate. A lower extinction rate is observed in dtg-AT-AS mice compared with that in any other group. In each group, there were 11 mice (n = 11).
Effect of the CCKR-2 Transgene and AT on Fear Extinction.
Following the experiments above, the same mice were subjected to contextual fear extinction. As shown in Fig. 1C, a within-group one-way ANOVA revealed a significantly less freezing response following extinction trials in WT-naïve-AS [F(4,50) = 19.08, P < 0.001], dtg-naïve-AS [F(4,50) = 17.10, P < 0.001], WT-AT-AS [F(4,50) = 19.55, P < 0.001], and dtg-AT-AS mice [F(4,50) = 12.78, P < 0.001]. Post hoc Fisher’s protected least significant difference (PLSD) tests indicated that the significant difference in extinction rate was observed at the last extinction trial in dtg mice but at the last two trials in all of the other three groups of mice. Using a three-way ANOVA (transgene × AT × extinction trial), we found a significant interaction in fear extinction between transgene and AT [F(1,212) = 18.65, P < 0.001)], but not among all three factors. As summarized in Fig. 1D, the extinction rate in dtg-AT-AS mice, WT-AT-AS/dtg-naïve-AS mice, and WT-naïve-AS mice was lowest, moderate, and highest, respectively. All these results indicated that the interaction between the CCKR-2 transgene and AT impaired the fear extinction. Freezing response in the animals was defined as no any movement, except for respiration.
Effect of the CCKR-2 Transgene and AT on PTSD-Like Behavior in an Elevated-Plus Maze.
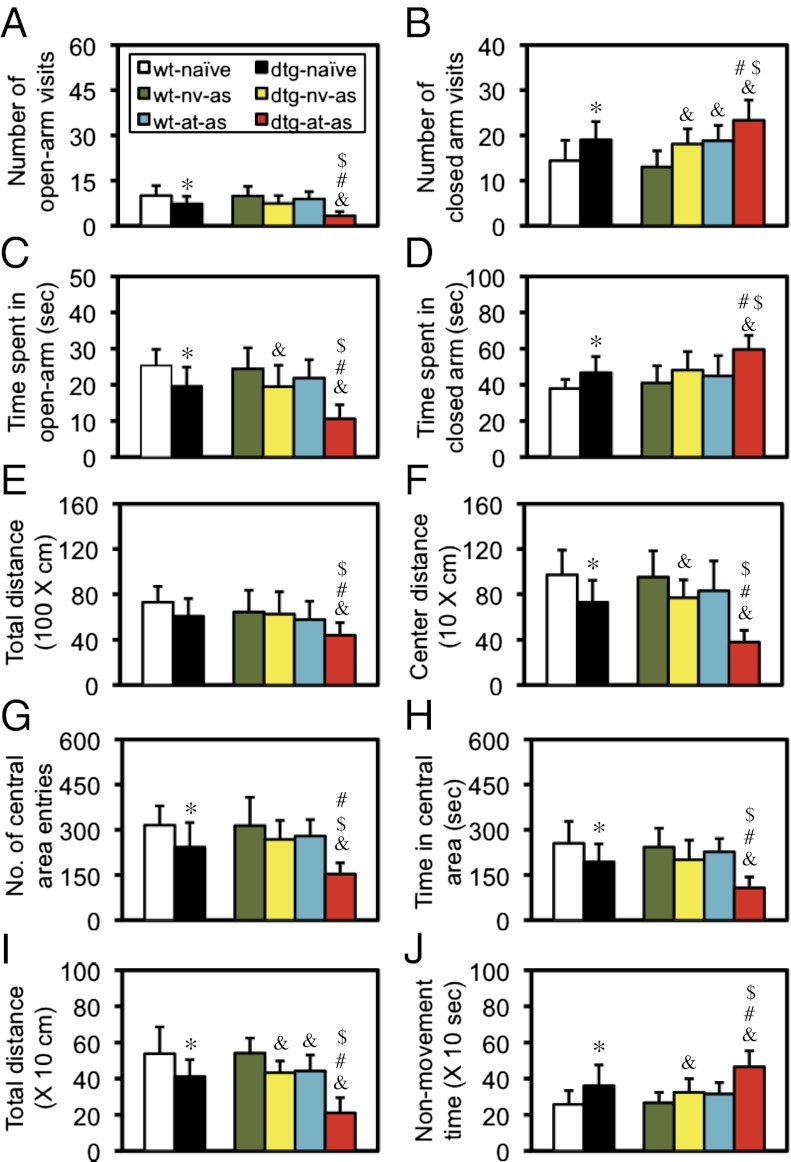
Six groups of mice were examined. A comparison between WT-naïve and dtg-naïve mice showed a significant difference in every index examined (P < 0.05–0.01; Fig. 2 A–D). Analyses of the other four groups with a two-way ANOVA (CCKR-2 transgene × AT) indicated (i) a significant effect of the transgene [F(1,44) = 30.22, P < 0.001], AT [F(1,44) = 11.95, P < 0.01], and interaction [F(3,44) = 4.45, P < 0.05] on the number of open-arm visits (Fig. 2A); (ii) a significant effect of the transgene [F(1,44) = 5.74, P < 0.05] and interaction [F(3,44) = 5.05, P < 0.05], but not AT, on the number of closed arm visits (Fig. 2B); (iii) a significant difference of the transgene [F(1,44) = 28.08, P < 0.001], AT [F(1,44) = 9.45, P < 0.01], and interaction [F(3,44) = 4,31, P < 0.05] on the time spent in open-arms (Fig. 2C); and (iv) a significant effect of the transgene [F(1,44) = 12.22, P < 0.01], AT [F(1,44) = 11.25, P < 0.05], and interaction [F(3,44) = 4.86, P < 0.05] on the time spent in closed arms (Fig. 2D). Detailed post hoc analyses were marked in the figure. These results indicated that the CCKR-2 transgene alone had an anxiogenic effect; AT alone had an anxiogenic effect, but the effect was not consistent; and CCKR-2 facilitated the anxiogenic effect of AT.
Fig. 2.
Enhanced PTSD-like behavior in dtg mice with AT/AS examined by using a battery of behavioral tests. (A–D) Elevated-plus maze test. (A) Number of open-arm visits. (B) Number of closed arm visits. (C) Time spent in open arms. (D) Time spent in closed arms. *P < 0.05–0.01 compared with WT-naïve mice; &P < 0.05–0.001 compared with WT-naïve-AS mice; #P < 0.01–0.001 compared with dtg-naïve-AS mice; $P < 0.05–0.001 compared with WT-AT-AS mice; all were Student's t test. In each group, there were 12 mice (n = 12). as, adult stressor; at, adolescent trauma; nv, naïve. (E–H) Open-field test. (E) Total distance traveled. (F) Distance traveled in the center area. (G) Number of center area entries. (H) Time spent in the center area. The same group comparisons: *P < 0.05; &P < 0.05; #P < 0.01–0.001; $P < 0.05–0.001; all were Student's t test. In each group, there were 11 mice (n = 11). (I and J) Modified tone-fear conditioning test. (I) Total distance traveled. (J) Total nonmovement time. The same group comparisons: *P < 0.05–0.01; &P < 0.05; #P < 0.01–0.001; $P < 0.001; all were Student's t test. In each group, there were 12 mice (n = 12). Data are expressed as mean ± SD.
Effect of the CCKR-2 Transgene and AT on PTSD-Like Behavior in an Open-Field Test.
Another set of six groups of mice was used here. A comparison between WT-naïve and dtg-naïve mice showed a significant difference in every index examined (P < 0.05; Fig. 2 F–H), except for the total distance traveled (Fig. 2E). Analyses of the other four groups with a two-way ANOVA (CCKR-2 transgene × AT) indicated (i) a significant effect of AT alone [F(1,40) = 6.92, P < 0.05], but not the CCKR-2 transgene alone nor the interaction, on the total distance traveled (Fig. 2E); (ii) a significant effect of the transgene [F(1,40) = 27.45, P < 0.001], AT [F(1,40) = 17.45, P < 0.001], and interaction [F(3,40) = 4.89, P < 0.05] on the distance traveled in the center area (Fig. 2F); (iii) a significant effect of the transgene [F(1,40) = 18.78, P < 0.001], AT [F(1,40) = 14.18, P < 0.001], and interaction [F(3,40) = 4,14, P < 0.05] on the number of central area entries (Fig. 2G); and (iv) a significant effect of the transgene [F(1,40) = 24.84, P < 0.001], AT [F(1,40) = 11.38, P < 0.01], and interaction [F(3,40) = 5.83, P < 0.05] on the time spent in central area (Fig. 2H). Detailed post hoc analyses were marked in the figure. These results indicated that both the CCKR-2 transgene and AT had an anxiogenic effect and that a significant interaction existed between these two anxiogenic factors.
Effect of the CCKR-2 Transgene and AT on Fear Generalization in a Modified Tone-Fear Conditioning Test.
A new set of six groups of mice was examined. A comparison between WT-naïve and dtg-naïve mice revealed a significant difference (P < 0.05; Student's t test) in the total distance traveled (Fig. 2I) and nonmovement time (Fig. 2J). Analyses of the other four groups with a two-way ANOVA (CCKR-2 transgene × AT) revealed (i) a significant effect of the transgene [F(1,44) = 51.98, P < 0.001], AT [F(1,44) = 46.02, P < 0.001], and interaction [F(3,44) = 7.74, P < 0.01] on the total distance traveled (Fig. 2I); and (ii) a significant effect the transgene [F(1,44) = 24.58, P < 0.001], AT [F(1,44) = 20.15, P < 0.001], and interaction [F(3,44) = 4.72, P < 0.05] on the total nonmovement time (Fig. 2J). Detailed post hoc analyses were marked in the figure. All these results further confirmed the results observed in the other two behavioral paradigms described above.
Suppression of the CCKR-2 Transgene Expression During AT Exposure Largely Diminished PTSD-Like Behavior.
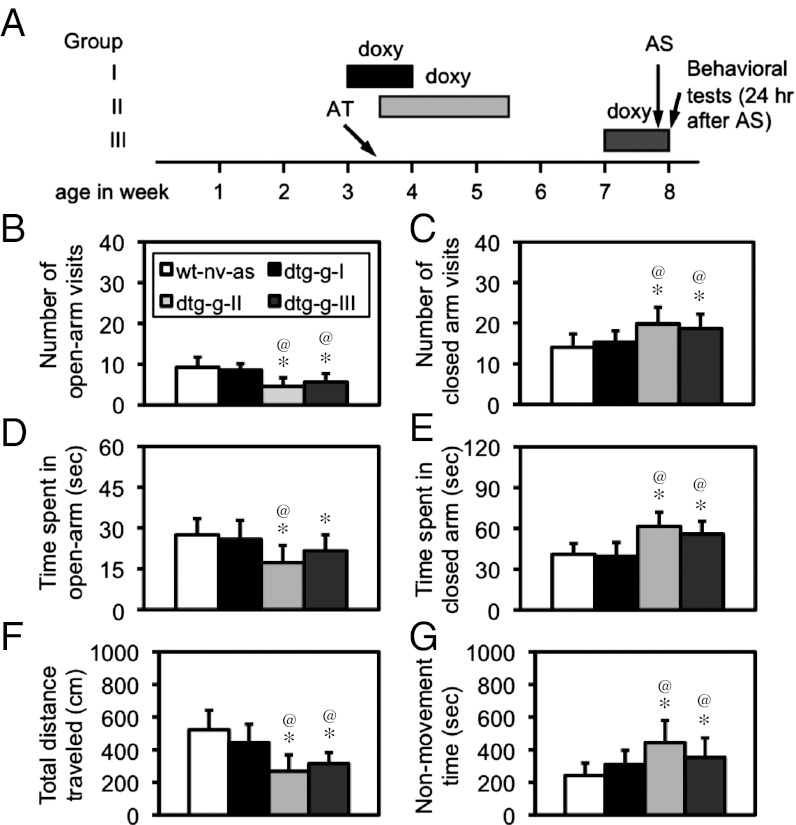
The expression of the CCKR-2 transgene could be almost completely suppressed by treating dtg mice with doxycycline (doxy) for 3 d or restored back by withdrawing doxy for 5 d (Fig. S1 A and B). As shown in Fig. 3A, three groups of dtg mice were treated with doxy for three periods of time: during AT (group I) for 1 wk, from immediately post-AT to the prebehavioral test (group II) for 2 wk, and during behavioral tests (group III) for 1 wk. The expression of the CCKR-2 transgene was suppressed during the AT exposure, post-AT period, and the expression of fear behavior, respectively. PTSD-like behavior was examined 24 h after AS at an adult stage (2 mo old). In an elevated-plus maze test, a one-way ANOVA indicated a significant difference in the number of open-arm visits [F(3,40) = 11.34, P < 0.001; Fig. 3B], number of closed arms visits [F(3,40) = 6.54, P < 0.01; Fig. 3C], time spent in open arms [F(3,40) = 5.26, P < 0.01; Fig. 3D], and time spent in closed arms [F(3,40) =14.53, P < 0.001; Fig. 3E]. Post hoc tests revealed a significant difference (P < 0.05–0.001) in all indices examined between WT-naïve-AS (n = 12) and dtg-doxy-II (n = 12) mice and between WT-naïve-AS and dtg-doxy-III (n = 12) mice but not between WT-naïve-AS and dtg-doxy-I (n = 12) mice. Moreover, a significant difference (P < 0.05–0.001) in every index was observed between dtg-doxy-I and dtg-doxy-II mice, and a significant difference (P < 0.05–0.001) in every index, except for the time spent in open arms, was observed between the dtg-doxy-I and dtg-doxy-III groups.
Fig. 3.
Suppression of CCKR-2 transgene expression during AT largely diminished PTSD-like behavior. (A) Diagram for temporary inhibitions of the CCKR-2 transgene expression in dtg mice. as, adult stressor; at, adolescent trauma; nv, naïve. Groups I, II, and III indicates that dtg mice were treated with doxy to inhibit the CCKR-2 transgene expression from P21 to P28, P25 to P39, and P49 to P56, respectively, which are indicated by bars with different black intensities. (B–E) Elevated-plus maze test. (B) Number of open-arm visits. (C) Number of closed-arm visits. (D) Time spent in open arms. (E) Time spent in closed arms. *P < 0.05–0.01 compared with WT-naïve-AS mice; @P < 0.05–0.01 compared with WT-naïve-AS mice. All were post hoc test following a one-way ANOVA. Data are expressed as mean ± SD. There were 12 mice in each group (n = 12).
To determine whether this phenotype could be observed in another behavioral paradigm, 24 h after the test above, the same mice were reexamined using a modified tone-fear conditioning test. A one-way ANOVA revealed a significant difference in the total distance traveled [F(3,40) = 16.17, P < 0.001; Fig. 3F] and total nonmovement time [F(3,40) = 7.74, P < 0.001; Fig. 3G]. Post hoc tests revealed that the difference in either the total distance traveled (P = 0.064) or total nonmovement time (P = 0.135) between WT-naïve-AS and dtg-doxy-I mice was not significant. Between WT-naïve-AS and dtg-doxy-II mice or between WT-naïve-AS and dtg-doxy-III mice, however, the difference in either index is highly significant (P < 0.01–0.001). A significant difference in either index between dtg-doxy-II and dtg-doxy-III mice could not be identified. All these results indicated that the temporary suppression of the CCKR-2 transgene expression in AT (group I), but not in the other periods of time (group II or III), could largely diminish the AS-triggered PTSD-like behavior.
dtg Mice with AT/AS Were Impaired in Spatial Learning and Memory.
Using a Morris water-maze test, we found that, although spatial learning and memory was not significantly affected by AT alone, nor by the CCKR-2 transgene alone, an interaction between these two factors led to a significant deficit in this cognitive function (Fig. S2 A and B), implying that the development of PTSD may be accompanied by impairment of other cognitive function such as spatial learning and memory.
Interaction Between AT and the CCKR-2 Transgene Prolonged the Activation of the Hypothalamic-Pituitary-Adrenal Axis in Response to AS.
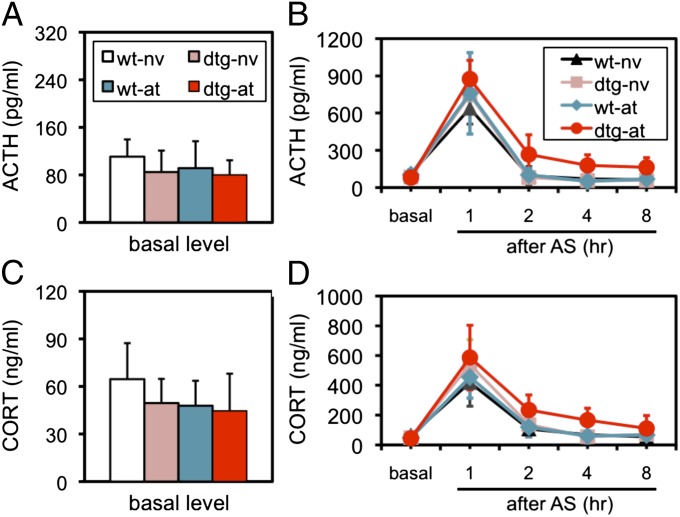
After subjected to AT, both WT and dtg mice were each divided into five groups (n = 12 in each group) for a time course study. The basal level of activity was examined before AS, and the results showed that, although the difference in either the adrenocorticotropic hormone (ACTH; P = 0.067; Fig. 4A) or corticosterone level (CORT; P = 0.06; Fig. 4C) was not significant between WT and dtg mice, a tendency of a lower level in both hormones was noted in these dtg mice. The analysis of the time course with a three-way ANOVA (transgene × AT × time course) revealed (i) a significant effect of the transgene [F(1,220) = 21.04, P < 0.001], AT [F(1,220) = 29.02, P < 0.001], and interaction between transgene and AT [F(1,220) = 14.16, P < 0.05], but not the interaction among the three factors (P = 0.07), on ACTH level (Fig. 4B); and (ii) a significant effect of the transgene [F(1,220) = 23.57, P < 0.001], AT [F(1,220) = 10.08, P < 0.01], and interaction between transgene and AT [F(1,220) = 5.16, P < 0.05], but not the interaction among the three factors (P = 0.448), on CORT level (Fig. 4D). A detailed post hoc analysis revealed that a significantly higher (P < 0.05–0.01) level of both ACTH and CORT was found in dtg-AT-AS mice at 1 (peak level), 2, and 4 h after AS compared with those in any group of mice at the same point of the time course studies. The exact values of all of the groups examined are listed in Tables S1 (ACTH) and S2 (CORT). These results indicated that the interaction between AT and the CCKR-2 transgene did not only increase the peak level of HPA axis activity but also impaired the HPA axis negative feedback inhibition in response to acute stress in the body.
Fig. 4.
Prolonged HPA axis activation in response to AS in dtg mice. (A) Basal serum level of ACTH in naïve WT mice (WT-nv) and naïve dtg mice (dtg-nv). as, adult stressor; at, adolescent trauma; nv, naïve. (B) Time course of ACTH response following the AS. (C) Basal serum level of CORT in naïve WT mice and naïve dtg mice. (D) Time course of CORT response following the AS. Detailed statistical analyses are described in the text. Data in all these figures are expressed as mean ± SD. There were 12 mice in each group (n = 12).
Effect of Chronic Treatment with Fluoxetine on the Phenotypes Observed in AT-Stressed dtg Mice.
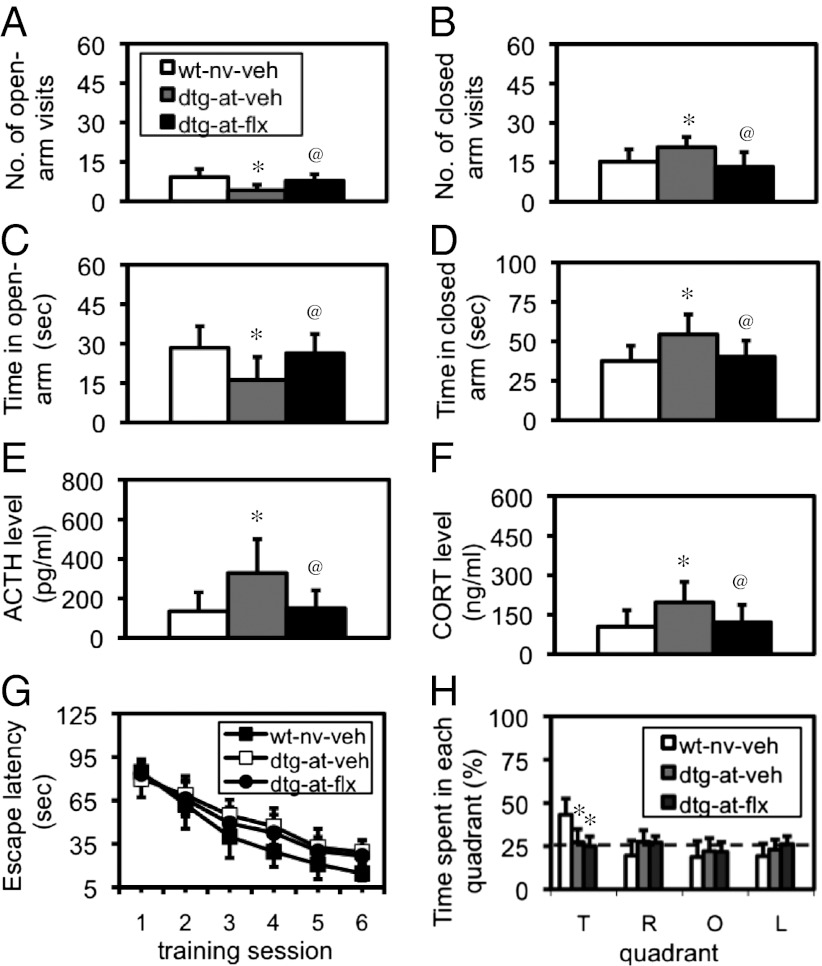
After subjected to AT, WT and dtg mice were treated with fluoxetine (flx; 15 mg/kg per day) or vehicle for 4 wk, and then PTSD-like behavior was examined. In an elevated-plus maze test, a two-group comparison revealed a significant difference (P < 0.05) in the number of open-arm visits (Fig. 5A), number of closed arm visits (Fig. 5B), time spent in open arms (Fig. 5C), and time spent in closed arms (Fig. 5D) between WT-naïve-vehicle and dtg-AT-vehicle mice or between dtg-AT-vehicle and dtg-AT-flx mice, indicating that flx could rescue PTSD-like behavior. Interestingly, the impaired glucocorticoid negative feedback inhibition returned to the normal level after the flx treatment in traumatized dtg mice (Fig. 5 E and F). However, this treatment could not attenuate the deficit in spatial learning and memory, because a one-way ANOVA with repeated measurements revealed a significant difference in the learning curve [F(2,140) = 7.358, P < 0.01; Fig. 5G], and a Student's t test indicated a significant difference in the probe test (P < 0.05; Fig. 5H). All these results indicated that both the PTSD-like behavior and the impaired learning and memory were associated with an interaction between the AT and CCKR-2 transgene, whereas the response to the antidepressant treatment was different.
Fig. 5.
Effect of flx on the PTSD-like behavior, impaired inhibition of HPA reaction in response to AS, and impaired spatial learning and memory in traumatized dtg mice. (A–D) Elevated-plus maze test. (A) Number of open-arm visits. (B) Number of closed arm visits. (C) Time spent in open arms. (D) Time spent in closed arms. *P < 0.05–0.001 compared with WT-naïve-vehicle mice; @P < 0.05–0.01 compared with dtg-AT-vehicle mice; all were Student's t test, with n = 9–11. (E) Serum level of ACTH. (F) Serum level of CORT. The same group comparisons: *P < 0.05–0.01; @P < 0.05–0.01; all were Student's t test, with n = 12–14. (G) Learning curve in a water maze test. The detailed of statistical analyses of the learning curve are described in the text. (H) Probe test in the water maze test. *P < 0.05, data in either dtg-AT-vehicle group (n = 10) or dtg-AT-flx (n = 11) were compared with those in the WT-naïve-vehicle group (n = 10). as, adult stressor; at, adolescent trauma; nv, naïve; L, left quadrant; O, opposite quadrant; R, right quadrant; T, target quadrant.
Discussion
We demonstrated in this study that a higher CCKergic tone in the brain is a cofactor for AT to induce PTSD-like phenotypes in mice. Following AT, dtg mice developed robust PTSD-like behavior, together with a deficit in spatial learning and memory, and prolonged HPA axis activation following AS. Interestingly, a temporal coupling of CCKR-2 transgene expression with AT is critical for the expression of all these phenotypes. Moreover, a chronic treatment of dtg mice with flx rescued both the PTSD-like behavior and the impaired HPA inhibition, but not the memory deficit. These results revealed a dynamic role of the CCKergic system in the pathogenesis of post-AT PTSD and cognitive dysfunctions.
A unique behavioral paradigm used in this study is to model human AT in the mouse via multiple trials of footshock at age P25. Virtually, various stress paradigms have been used to model traumatic stress in the laboratories or even during an early-life stage (P21–P28) in the mouse (14). In contrast to those approaches, the paradigm used here meets many features of a classical traumatic attack in the human such as “sudden,” “intensive,” “helplessness,” and “threatening to death.” In the case of prolonged or repeated constraint stress, for example, the procedure of stress may lead to an adaptive response, so that the effect of stress might gradually fade (15). Another foundational strategy in this study is to trigger PTSD-like behavior by the combination of AT and AS, with an interval of 45 d between them. Clinically, PTSD may occur immediately following a trauma, but in many cases, a time interval may exist between the trauma and onset of the disease (16). To confront a second stress, namely revictimization, is an important etiologic factor for PTSD (17). Thus, the use of AT as an original trauma and AS to mimic revictimization in this study is a comprehensive means to induce PTSD-like behavior. However, based on the results in WT mice, AT/AS alone was still not a reliable way to reproduce PTSD-like behavior in the behavioral tests such as the elevated-plus maze or open-field test. When AT/AS is combined with the CCKR-2 transgene, however, consistent PTSD-like behavior was observed in almost all of the behavioral tests used, indicating that the development of PTSD-like behavior does not only depend on the trauma itself.
One of the most comprehensive efforts in this study is the use of multiple behavioral tests, in an attempt to probe almost all of the aspects of the core symptoms of PTSD in humans. According to DSM-IV, the cluster of the core symptoms of PTSD includes reexperiencing previous traumatic episode/event, persistent avoidance/numbing, and hyperarousal to certain trauma-related cues, together with cognitive deficit choices. The choices of behavioral tests in this study are based on the similarities between the behavioral assessment and the nature of these PTSD core symptoms. For example, to fear a cue that is, in certain way, associated with the previous trauma is a typical sign of reexperiencing in PTSD (18). This type of fear can be readily modeled in the mouse with the fear-conditioning paradigm (19). However, as an enhanced fear response in this test may indicate an enhanced fear memory, another behavioral test, fear extinction (20), was used, to provide a better resolution for behavioral phenotypes. In PTSD patients, impaired fear extinction is a common reexperiencing symptom (21). Thus, the deficit in fear extinction in our dtg mice indicates that the enhanced fear response in the fear-conditioning test is more relevant to an anxiety-related phenotype. The use of the elevated-plus maze and open-field tests apparently strengthens the phenotypical validation, in which a higher avoidance level or a lower exploratory motivation could be considered as a homolog of avoidance/numbing in PTSD patients (22). Similarly, fear to a conditioned cue in the mouse is frequently used to model the hyperarousal observed in PTSD patients (19, 20). In this study, a changed tone-conditioning test was developed, based on the nature of the “hyper”-arousal. Indeed, our results show that this unique paradigm provides a consistent result with other well-established tests, and thus, it is useful for the study of the PTSD-like behavior. In addition to the PTSD-like behavior, the deficit in spatial learning and memory (Fig. S2) provided additional evidence that an impaired cognition is accompanied with the development of PTSD-like behavior. However, in this study, we did not comprehensively explore how this memory function is impaired following AT.
One of the most important findings here is the demonstration that a temporal coupling of the higher CCKergic tone with AT is critical for the development of PTSD-like behavior. This finding is achieved based on the inducible/reversible expression of the CCKR-2 transgene. However, it is still not clear how this coupling occurs, partially due to the fact that the functional significance of the CCKergic system in the brain is still not clear. As G protein–coupled receptors, CCK receptors are associated with Ca2+ release, protein kinase C (PKC) activation, phospholipase A2 activity, and cAMP production (23), whereas the most notable information is from the discovery of its dynamic role in both regulating fear responses and the pathogenesis of anxiety disorders. Following stress, the CCKergic activity in the brain increases (24), and the level of anxiety is correlated with the increased CCKergic activity (25). CCKR-2 agonists only produce, or produce more pronounced, anxiogenic effect in stressed, but not in unstressed, animals (26). More importantly, patients with PTSD are more sensitive to CCKR-2 agonists than controls (27). Hence, our findings do not only confirm that the CCKergic activity is indeed dynamically involved in the pathogenesis of PTSD but also indicate that this dynamic role is particularly relevant to trauma exposure but not the expression of the fear behavior, implying that the CCKergic system may be more importantly related to the pathogenic mechanism. As there are robust interactions between the CCKergic system and other neurotransmitter systems including dopaminergic, serotonergic, and GABAergic systems at both the structural and functional levels (28–30), the mechanism underlying this pathogenic interaction is complex and needs to be further studied.
Another important finding in this study is the discovery of the change in HPA axis activity, which includes (i) a slightly lower basal level of the HPA axis activity in dtg mice compared with control mice, (ii) a synergistic effect of AT and the CCKR-2 transgene on the peak level of HPA axis activity in response to AS; and (iii) a prolonged decay time of HPA axis activity following AS in dtg mice with AT. It has been established that a previous chronic stress in the animals down-regulates HPA axis activity but enhances their response to a novel acute stressor, despite the negative feedback effects (31). Because chronic stress specifically facilitates the release of CCK peptides into the paraventricular nucleus of the hypothalamus (PVN), which projects to the pituitary directly, in response to acute stress (32), the elevated CCKergic tone in our dtg mice may mimic the effect of a chronic stress by working as an “intrinsic stressor” for the animals. Indeed, chronic activation of the HPA axis system is significantly associated with anxiety (33) and early-life stress in humans (34). Therefore, this intrinsic stressor constitutes a basis for the higher vulnerability of dtg mice to AT. At the same time, the impaired AS-induced CORT negative feedback response may, in turn, significantly impair memory in these PTSD mice, which is consistent to findings that memory dysfunction is also observed in PTSD (35).
In animal modeling, predictive validity, a paradigm of whether a treatment that is commonly used for PTSD patients is effective for the observed PTSD-related phenotypes in the animal model, is a necessary validating component. Thus, it is important in this study to confirm whether the overall phenotypes in dtg mice are sensitive to such a treatment. flx, a selective serotonin reuptake inhibitor (SSRI), has great success in the treatment of anxiety disorders (36). Although the effect of flx on PTSD is still in doubt, flx is among the first choices in clinics (37). In animal PTSD models, this compound is also most commonly used to test predictive validity (38). Interestingly, in this study, a chronic treatment of traumatized dtg mice with flx could not only rescue the PTSD-like behavior but also the impaired glucocorticoid negative feedback inhibition, indicating that the effect of flx on PTSD might be related to HPA axis activity, although in this study, we did not have evidence showing how these two systems interact for PTSD. Moreover, this treatment could not attenuate the deficit in spatial learning and memory, indicating that the mnemonic dysfunction is not directly related to PTSD itself, but it is a psychopathology or sequela of the interaction between the elevated CCKergic tone and AT. These results also support our recently established “two-behavior system” in the brain in response to environmental stress (39).
Finally, the currently favorable theory for the pathogenesis of anxiety disorders including PTSD is a gene/environment interaction model (40). Indeed, a twin study of Vietnam veterans revealed that about 37.9% of vulnerability to PTSD was genetically related (41). Clinical association studies have identified more than 10 candidate genes for PTSD (5, 42), whereas the CCKR-2 gene has been repeatedly associated to panic disorder, another major form of anxiety disorders (10). Moreover, polymorphisms in microRNAs that are associated to panic disorder are also functionally related to the CCKergic system (43). It should be noted that panic disorder and PTSD are basically two types of anxiety disorders. Their clinical features, as well as their pathogenic mechanisms, are or might be fundamentally different (44). Thus, our findings in this study do not directly apply to those clinical association results. On the other hand, feeling extensive fear is the core and shared clinical symptom for both panic disorder and PTSD, and the comorbidity rate between them is high (45). Given that a functional polymorphism might directly change the gene expression level or pattern (46), the finding of interaction between the increased CCKR-2 expression and AT event may have some implication in this gene–environmental interaction dogma.
In conclusion, our studies validated a robust PTSD model in the mouse, demonstrated that a higher CCKR-2 expression level in the brain is a cofactor for the pathogenic role of AT in PTSD, and identified a critical time window for the interaction between the elevated CCKergic tone and traumatic insult in the development of PTSD and its related psychopathologies. These results may prove to be valuable for our translational effort on preventing and curing PTSD, a devastating mental disorder in humans.
Materials and Methods
Experimental Animals.
The procedures for the generation of dtg mice are described in SI Materials and Methods. All experimental procedures for the use of animals were previously reviewed and approved by the institutional animal care and use committee at the Louisiana State University Heath Sciences Center at New Orleans, and all experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Real-Time RT-PCR and In Situ Hybridization, CCKR Binding Assay, ELISA, AT, and AS.
Detailed procedures are described in SI Materials and Methods.
PTSD-Like Behavior.
A battery of behavioral tests, as described in SI Materials and Methods, was used to examine PTSD-like behavior. These tests included a fear-conditioning test, fear-extinction test, open-field test, elevated-plus maze test, and a modified tone-conditioning test.
Cognitive Behavior.
As described in SI Materials and Methods, two behavioral tests, fear-conditioning and Morris water maze, were used.
Treatment with flx.
To determine whether PTSD-like behavior was curable, mice were treated with flx (15 mg⋅kg−1⋅d−1; Sigma) for 4 wk in drinking water up to the start of behavioral tests. flx solution was kept in opaque bottles and was changed weekly.
Statistical Analysis.
Both female and male mice were almost equally distributed in each group. Behavioral data were analyzed with one-, two-, or three-way ANOVA or repeated ANOVAs, followed by post hoc tests such as Fisher’s PLSD test or with the Student's t test. Data from the other experiments were analyzed with Student's t test. P < 0.05 was considered significant. MatLab statistical software (R2012B) was used.
Supplementary Material
Acknowledgments
We thank Dr. Elliot S. Gershon and Dr. Sangam Sisodia (University of Chicago) and Dr. Samuel McClugage (Louisiana State University Health Sciences Center) for stimulating discussions. We also thank Dr. Carmen Canavier for help with statistical analyses. This study was partially supported by National Institute of Mental Health Grant MH066243, Alzheimer’s Association Grant NIRG-02-4368, Brain & Behavior Research Foundation, and the Louisiana State Fund (to Y.-P.T.). Matlab expertise was provided by N.Y. as part of the Computational Core for Centers of Biomedical Research Excellence (COBRE) Phase III (1 P30 GM103340-01A1) supported by the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219601110/-/DCSupplemental.
References
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- 3.Cougle JR, Timpano KR, Sachs-Ericsson N, Keough ME, Riccardi CJ. Examining the unique relationships between anxiety disorders and childhood physical and sexual abuse in the National Comorbidity Survey-Replication. Psychiatry Res. 2010;177(1-2):150–155. doi: 10.1016/j.psychres.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Costello EJ, Erkanli A, Fairbank JA, Angold A. The prevalence of potentially traumatic events in childhood and adolescence. J Trauma Stress. 2002;15(2):99–112. doi: 10.1023/A:1014851823163. [DOI] [PubMed] [Google Scholar]
- 5.Cornelis MC, Nugent NR, Amstadter AB, Koenen KC. Genetics of post-traumatic stress disorder: Review and recommendations for genome-wide association studies. Curr Psychiatry Rep. 2010;12(4):313–326. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bomyea J, Risbrough V, Lang AJ. A consideration of select pre-trauma factors as key vulnerabilities in PTSD. Clin Psychol Rev. 2012;32(7):630–641. doi: 10.1016/j.cpr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowers ME, Choi DC, Ressler KJ. Neuropeptide regulation of fear and anxiety: Implications of cholecystokinin, endogenous opioids, and neuropeptide Y. Physiol Behav. 2012;107(5):699–710. doi: 10.1016/j.physbeh.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang C, Luo H, Liu Y, Cao J, Xia H. Plasma hormones facilitated the hypermotility of the colon in a chronic stress rat model. PLoS ONE. 2012;7(2):e31774. doi: 10.1371/journal.pone.0031774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zwanzger P, et al. Effects of tiagabine on cholecystokinin-tetrapeptide (CCK-4)-induced anxiety in healthy volunteers. Depress Anxiety. 2003;18(3):140–143. doi: 10.1002/da.10099. [DOI] [PubMed] [Google Scholar]
- 10.Zwanzger P, Domschke K, Bradwejn J. Neuronal network of panic disorder: The role of the neuropeptide cholecystokinin. Depress Anxiety. 2012;29(9):762–774. doi: 10.1002/da.21919. [DOI] [PubMed] [Google Scholar]
- 11.Dockray GJ. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- 12.Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: Disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51(4):356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Nakajima A, Meacham C, Tang YP. Elevated cholecystokininergic tone constitutes an important molecular/neuronal mechanism for the expression of anxiety in the mouse. Proc Natl Acad Sci USA. 2006;103(10):3881–3886. doi: 10.1073/pnas.0505407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yun J, et al. 2010 Chronic restraint stress impairs neurogenesis and hippocampus-dependent fear memory in mice: Possible involvement of a brain-specific transcription factor Npas4. J Neurochem 114(6):1840–1851. [Google Scholar]
- 15.Zohar J, Sonnino R, Juven-Wetzler A, Cohen H. Can posttraumatic stress disorder be prevented? CNS Spectr. 2009;14(1) Suppl 1:44–51. [PubMed] [Google Scholar]
- 16.Yehuda R, LeDoux J. Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron. 2007;56(1):19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Mouilso ER, Calhoun KS, Gidycz CA. Effects of participation in a sexual assault risk reduction program on psychological distress following revictimization. J Interpers Violence. 2011;26(4):769–788. doi: 10.1177/0886260510365862. [DOI] [PubMed] [Google Scholar]
- 18.Milad MR, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegmund A, et al. Maternal inexperience as a risk factor of innate fear and PTSD-like symptoms in mice. J Psychiatr Res. 2009;43(14):1156–1165. doi: 10.1016/j.jpsychires.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Golub Y, Mauch CP, Dahlhoff M, Wotjak CT. Consequences of extinction training on associative and non-associative fear in a mouse model of posttraumatic stress disorder (PTSD) Behav Brain Res. 2009;205(2):544–549. doi: 10.1016/j.bbr.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kung JC, Chen TC, Shyu BC, Hsiao S, Huang AC. Anxiety- and depressive-like responses and c-fos activity in preproenkephalin knockout mice: Oversensitivity hypothesis of enkephalin deficit-induced posttraumatic stress disorder. J Biomed Sci. 2010;17:29. doi: 10.1186/1423-0127-17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wank SA. Cholecystokinin receptors. Am J Physiol. 1995;269(5 Pt 1):G628–G646. doi: 10.1152/ajpgi.1995.269.5.G628. [DOI] [PubMed] [Google Scholar]
- 24.Harro J, Löfberg C, Rehfeld JF, Oreland L. Cholecystokinin peptides and receptors in the rat brain during stress. Naunyn Schmiedebergs Arch Pharmacol. 1996;354(1):59–66. doi: 10.1007/BF00168707. [DOI] [PubMed] [Google Scholar]
- 25.MacNeil G, Sela Y, McIntosh J, Zacharko RM. Anxiogenic behavior in the light-dark paradigm follwoing intraventricular administration of cholecystokinin-8S, restraint stress, or uncontrollable footshock in the CD-1 mouse. Pharmacol Biochem Behav. 1997;58(3):737–746. doi: 10.1016/s0091-3057(97)00037-3. [DOI] [PubMed] [Google Scholar]
- 26.Cohen H, Kaplan Z, Kotler M. CCK-antagonists in a rat exposed to acute stress: Implication for anxiety associated with post-traumatic stress disorder. Depress Anxiety. 1999;10(1):8–17. [PubMed] [Google Scholar]
- 27.Kellner M, et al. Behavioral and endocrine response to cholecystokinin tetrapeptide in patients with posttraumatic stress disorder. Biol Psychiatry. 2000;47(2):107–111. doi: 10.1016/s0006-3223(99)00118-3. [DOI] [PubMed] [Google Scholar]
- 28.Bradwejn J, de Montigny C. Benzodiazepines antagonize cholecystokinin-induced activation of rat hippocampal neurones. Nature. 1984;312(5992):363–364. doi: 10.1038/312363a0. [DOI] [PubMed] [Google Scholar]
- 29.Zwanzger P, et al. Effects of alprazolam on cholecystokinin-tetrapeptide-induced panic and hypothalamic-pituitary-adrenal-axis activity: A placebo-controlled study. Neuropsychopharmacology. 2003;28(5):979–984. doi: 10.1038/sj.npp.1300131. [DOI] [PubMed] [Google Scholar]
- 30.Rotzinger S, Bush DE, Vaccarino FJ. Cholecystokinin modulation of mesolimbic dopamine function: regulation of motivated behaviour. Pharmacol Toxicol. 2002;91(6):404–413. doi: 10.1034/j.1600-0773.2002.910620.x. [DOI] [PubMed] [Google Scholar]
- 31.Ma S, Morilak DA. Chronic intermittent cold stress sensitises the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol. 2005;17(11):761–769. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- 32.Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: Review and synthesis of four studies. Depress Anxiety. 2007;24(1):66–76. doi: 10.1002/da.20220. [DOI] [PubMed] [Google Scholar]
- 33.Neufeld-Cohen A, et al. Chronic activation of corticotropin-releasing factor type 2 receptors reveals a key role for 5-HT1A receptor responsiveness in mediating behavioral and serotonergic responses to stressful challenge. Biol Psychiatry. 2012;72(6):437–447. doi: 10.1016/j.biopsych.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: A mechanism for later trauma vulnerability. Prog Brain Res. 2008;167:137–149. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geuze E, Vermetten E, Ruf M, de Kloet CS, Westenberg HG. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. J Psychiatr Res. 2008;42(8):659–669. doi: 10.1016/j.jpsychires.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Ravindran LN, Stein MB. The pharmacologic treatment of anxiety disorders: A review of progress. J Clin Psychiatry. 2010;71(7):839–854. doi: 10.4088/JCP.10r06218blu. [DOI] [PubMed] [Google Scholar]
- 37.Asnis GM, Kohn SR, Henderson M, Brown NL. SSRIs versus non-SSRIs in post-traumatic stress disorder: An update with recommendations. Drugs. 2004;64(4):383–404. doi: 10.2165/00003495-200464040-00004. [DOI] [PubMed] [Google Scholar]
- 38.Pinna G, Rasmusson AM. Up-regulation of neurosteroid biosynthesis as a pharmacological strategy to improve behavioural deficits in a putative mouse model of post-traumatic stress disorder. J Neuroendocrinol. 2012;24(1):102–116. doi: 10.1111/j.1365-2826.2011.02234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Q, et al. Bi-directional effect of cholecystokinin receptor-2 overexpression on stress-triggered fear memory and anxiety in the mouse. PLoS ONE. 2010;5(12):e15999. doi: 10.1371/journal.pone.0015999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta D, Binder EB. Gene × environment vulnerability factors for PTSD: The HPA-axis. Neuropharmacology. 2012;62(2):654–662. doi: 10.1016/j.neuropharm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Chantarujikapong SI, et al. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psychiatry Res. 2001;103(2-3):133–145. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- 42.Logue MW, et al. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.113. 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muiños-Gimeno M, et al. Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biol Psychiatry. 2011;69(6):526–533. doi: 10.1016/j.biopsych.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Kross EK, Gries CJ, Curtis JR. Posttraumatic stress disorder following critical illness. Crit Care Clin. 2008;24(4):875–887. doi: 10.1016/j.ccc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Gros DF, Frueh BC, Magruder KM. Prevalence and features of panic disorder and comparison to posttraumatic stress disorder in VA primary care. Gen Hosp Psychiatry. 2011;33(5):482–488. doi: 10.1016/j.genhosppsych.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Etain B, et al. Genetic and functional abnormalities of the melatonin biosynthesis pathway in patients with bipolar disorder. Hum Mol Genet. 2012;21(18):4030–4037. doi: 10.1093/hmg/dds227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.