Abstract
Protein targeting to the endoplasmic reticulum is mediated by signal or signal-anchor sequences. They also play an important role in protein topogenesis, because their orientation in the translocon determines whether their N- or C-terminal sequence is translocated. Signal orientation is primarily determined by charged residues flanking the hydrophobic core, whereby the more positive end is predominantly positioned to the cytoplasmic side of the membrane, a phenomenon known as the “positive-inside rule.” We tested the role of conserved charged residues of Sec61p, the major component of the translocon in Saccharomyces cerevisiae, in orienting signals according to their flanking charges by site-directed mutagenesis by using diagnostic model proteins. Mutation of R67, R74, or E382 in Sec61p reduced C-terminal translocation of a signal-anchor protein with a positive N-terminal flanking sequence and increased it for signal-anchor proteins with positive C-terminal sequences. These mutations produced a stronger effect on substrates with greater charge difference across the hydrophobic core of the signal. For some of the substrates, a charge mutation in Sec61p had a similar effect as one in the substrate polypeptides. Although these three residues do not account for the entire charge effect in signal orientation, the results show that Sec61p contributes to the positive-inside rule.
INTRODUCTION
Targeting of secretory and membrane proteins to the endoplasmic reticulum (ER) is mediated by signal sequences (Walter and Johnson, 1994). Their main feature is a stretch of 7-25 apolar residues that is recognized by the signal recognition particle (SRP) (Keenan et al., 2001). On SRP binding to the signal sequence, the ribosome-nascent chain complex is targeted to the ER by interaction with the SRP receptor, and the signal is transferred into the translocation and integration channel, the translocon. In addition to their function in targeting, signal sequences play an important role in protein topogenesis by orienting themselves in the translocon and ultimately the membrane (Spiess, 1995). The signal sequence of a secretory protein will assume an Ncyt/Cexo orientation (cytoplasmic N and exoplasmic C terminus) and thereby initiate the translocation of its C-terminal sequence into the lumen of the ER. Noncleavable signal-anchors of membrane proteins translocate either their C-terminal or their N-terminal end. Several parameters determine signal orientation in the membrane. The best established one is the distribution of charged residues on either side of the hydrophobic core of the signal. As was first discovered in bacteria, flanking sequences with positive charges are predominantly localized to the cytoplasmic side of the membrane, a phenomenon known as the “positive-inside rule” (von Heijne, 1986). For ER signals, the charge difference between the two flanking regions of the signal core, rather than the positive charge per se, correlates with signal sequence orientation (Hartmann et al., 1989). Because there is no general electrical potential across the ER membrane, the charge rule is likely to be due to interactions of charges in the signal with charges at or near the translocon.
Additional features besides flanking charges have been shown to affect signal orientation. Folding of sequences N-terminal to an internal signal may sterically hinder translocation of the N terminus irrespective of the charge distribution (Denzer et al., 1995), and increasing length and hydrophobicity of the apolar signal core favors translocation of the N terminus into the ER lumen (Sakaguchi et al., 1992; Wahlberg and Spiess, 1997; Eusebio et al., 1998; Harley et al., 1998; Rösch et al., 2000).
To study the mechanism of signal orientation, we have recently analyzed model proteins with an N-terminal signal-anchor consisting of a long and hydrophobic core of 22 leucines (Goder and Spiess, 2003). Despite a positive N terminus and a negative C-terminal flanking sequence, only a fraction of the molecules inserted with the expected Ncyt/Cexo orientation. This fraction increased with increasing length of the protein and with increasing time of translation, indicating that the signal initially inserts in an Nexo/Ccyt orientation and inverts with time according to the charge distribution. As expected, the kinetics of signal reorientation increased with decreasing absolute charge difference Δ(C-N). In contrast, increasing hydrophobicity of the signal core slowed down signal inversion, probably because of tighter interaction with the signal binding site in the translocon.
In the present study, we investigated the contribution of the major translocon component in the yeast S. cerevisiae, Sec61p, in reading the orientation information encoded in the flanking charges of signal sequences. Sec61p is the largest subunit in the heterotrimeric membrane protein complex (composed of Sec61p, Sbh1p, and Sss1p, corresponding to Sec61α, β, and γ in mammalian cells) that forms the protein conducting channel across the ER membrane (Sanders et al., 1992; Görlich and Rapoport, 1993; Heinrich et al., 2000). The protein is essential in S. cerevisiae and conserved throughout the kingdoms (Deshaies and Schekman, 1987; Pohlschröder et al., 1997). Numerous cross-linking experiments demonstrated that signal sequences of secretory proteins and signal-anchors of membrane proteins are in contact with Sec61p or its mammalian homolog Sec61α during the insertion process (High et al., 1993a,b; Mothes et al., 1994; Pilon et al., 1998; Plath et al., 1998). We show that specific charged residues of Sec61p in lumenal and cytoplasmic loops of the protein contribute to orienting signal sequences according to the positive-inside rule in the ER membrane.
MATERIALS AND METHODS
Alignment of Sec61p Orthologues
The amino acid sequence of Sec61p (S. cerevisiae/Swiss-Prot:P32915) was aligned with that of 10 orthologues (Candida albicans/Q9P8E3; Candida lipolytica/P78979; Homo sapiens/P57726; Mus musculus/Q9JLR1; Canis familiaris/P38377; Caenorhabditis elegans/O18239; Arabidopsis thalia/Q9ZV90; Drosophila melanogaster/Q9VMD1; Methanobacterium thermoautotrophicum/O26134; Pyrococcus abyssi/Q9V1V8) and the homolog Ssh1p (S. cerevisiae/P38353) by using the multiple alignment program ClustalW (http://www.ch.embnet.org/software/ClustalW.html). The 10 transmembrane domains of Sec61p have been localized using the prediction program TMpred (http://www.ch.embnet.org/software/TMPRED_form.html). The predicted topology is in agreement with the prediction for Sec61α (Görlich et al., 1992) and is supported by experimental data by Wilkinson et al. (1996), except for the exact position of TM5, due to ambiguous data.
Yeast Strains and Sec61p Mutagenesis
The yeast strain RSY1293 (matα, can1-100, leu2-3122, his3-11,15, trp1-1, ura3-1, ade2-1, sec61::HIS3, [pDQ1]) was a gift from R. Schekman (University of California, Berkeley, Berkeley, CA) (Pilon et al., 1997). pDQ1 (LEU2 CEN SEC61) was exchanged by YCplac33 (URA3 CEN) containing wild-type SEC61. This resulting strain VGY61 was used as a parental strain to introduce mutant sec61 cloned into YCplac111 (LEU2 CEN) by plasmid shuffling using 5-fluoro-orotic acid. Site-directed mutagenesis of SEC61 was performed by polymerase chain reaction using pDQ1 as a template and Vent polymerase (New England Biolabs, Beverly, MA).
To create a Δssh1 mutant strain for our analysis, strain VGY61 was crossed with BWY465 (ssh1::TRP1; from B. Wilkinson, University of Manchester, Manchester, UK) (Wilkinson et al., 2001). The resulting diploid was sporulated, subjected to tetrad dissection, and screened for the Trp/Ura phenotype. The Δssh1 phenotype was verified by immunoblot analysis with antibodies against Ssh1p (from T. Sommer, Berlin, Germany) in comparison with wild-type cells. This strain was subsequently used for plasmid shuffling with sec61 mutant plasmids. The Δssh1 strains were frozen immediately after generation. Experiments were made within a few days upon thawing out aliquots. At the time of the experiments, the majority of the cells were still respiration competent.
Model Protein Constructs
All model proteins were engineered by polymerase chain reaction by using Vent polymerase (New England Biolabs). [Leu16](-3)CPY consists of the 38 N-terminal residues of H1ΔLeu16 (Wahlberg and Spiess, 1997), fused via a Val-Asp linker (corresponding to a SalI site) to residues 5-211 of mature carboxypeptidase Y (CPY), followed by an Ala-Cys linker (SphI site) fused to a C-terminal triple hemagglutinin (HA)-tag. 40[Leu16](+5) consists of the 74 N-terminal residues of H1-4Leu16 (Wahlberg and Spiess, 1997) fused to CPY-HA as described above. 60[H1](+1)CPY consists of residues 1-33 of Ste2p, including an N-glycosylation site fused via a Ser-Arg-Leu linker to residues 14-74 of construct H1-3 (Beltzer et al., 1991), followed by CPY-HA as in the other constructs mentioned above. Flanking charges were mutated as indicated in the text. Model proteins were expressed using the 2 μ/URA3 plasmid pRS426 with a glycerol-3-phosphate dehydrogenase promoter and phosphoglycerate kinase terminator (from N. Kralli, Scripps Institute, San Diego, CA).
Labeling and Immunoprecipitation
For in vivo pulse labeling, yeast cells were grown overnight at 30°C in minimal medium with histidine, adenine, leucine, and tryptophan; diluted; and grown to OD600 nm of ∼1. Cells equivalent to 1.5 OD were resuspended in 200 μl of medium, incubated for 15 min at 30°C, and labeled for 5 min with 100 μCi/ml [35S]methionine (Amersham Biosciences, Piscataway, NJ). For a chase, the cells were further incubated with excess (0.2 mM) unlabeled methionine. Cells were then supplemented with 5 mM azide, transferred to ice, washed twice with phosphate-buffered saline, and lysed with glass beads for 7 min in a bead-beater. The lysate was supplemented with 1% SDS and heated at 95°C for 5 min. Cell remnants were removed by centrifugation for 10 min in a Microfuge, and the supernatant was used for immunoprecipitation by using monoclonal antibodies against the HA-tag. The immune complexes were isolated with protein A-Sepharose (Amersham Biosciences) and analyzed by SDS-PAGE and autoradiography. Signals were quantified by PhosphorImager (Molecular Dynamics, Sunnyvale, CA). To analyze endogenous Gas1p, the amount of cells was upscaled 2.5-fold, and cells were labeled for 3 min by using 400 μCi/ml [35S]methionine.
For deglycosylation, the immune complexes were released from protein A-Sepharose by boiling in 50 mM Na citrate, pH 6, 1% SDS, and incubated with 1 mU endo-β-d-N-acetyl glucosaminidase H for 5 h at 37°C, before gel electrophoresis. For protease protection experiments, pulse-labeled cells were washed and spheroplasted by incubation with 2 mg of zymolyase 20T (Seikagaku America, Rockville, MD) per milliliter of packed yeast cells for 30 min at 30°C in 50 mM Tris-HCl, pH 7.5, in the presence of 30 mM dithiothreitol. Cells were then transferred to ice and lysed with 20 strokes in a Dounce homogenizer. The homogenate was split and incubated for 30 min with or without 100 μg/ml trypsin in presence or absence of 0.4%Triton X-100. All reactions were supplemented with 500 μg/ml trypsin inhibitor and processed as described above for immunoprecipitation. For alkaline extraction, cells were grown to OD600 nm of 1.5 and the equivalent of 3.0 OD600 nm units was transferred to ice, washed twice with phosphate-buffered saline, and lysed with glass beads. The cells were split in two aliquots, one of which was kept on ice. The other aliquot was incubated at pH 11.5 for 10 min on ice and centrifuged through a sucrose cushion using an Airfuge as described (Wessels et al., 1991). The supernatant was precipitated with 10% trichloroacetic acid. Total, supernatant, and pellet were subjected to SDS-PAGE and immunoblot analysis.
RESULTS
Model Proteins to Detect Changes in Signal Orientation
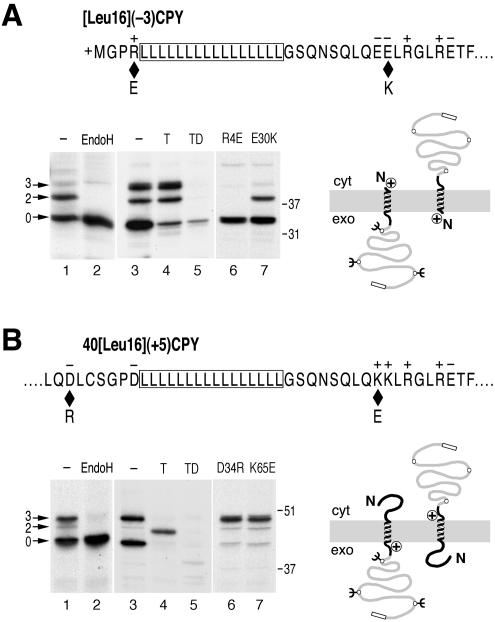
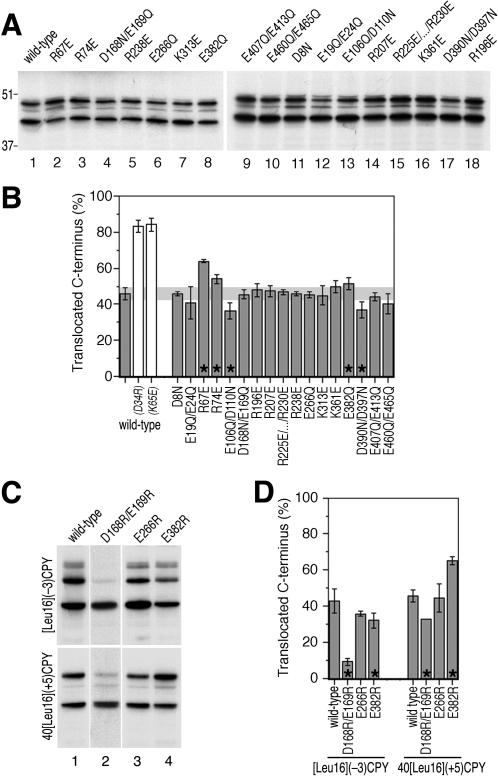
To monitor the effect of Sec61p mutants on signal orientation, we made use of model proteins that insert with mixed orientations in wild-type yeast cells. The model protein [Leu16](-3)CPY has an N-terminal signal-anchor of 16 leucine residues and modified N- and C-terminal flanking regions with a charge difference Δ(C-N) (calculated according to Hartmann et al., 1989) of -3 (Figure 1A). This sequence was fused to a portion of the yeast secretory protein CPY, to ensure good expression, and an HA-tag for immunoprecipitation. Three sites for N-linked glycosylation serve as reporters of C-terminal translocation. On expression in cells with wild-type Sec61p and labeling with [35S]methionine for 5 min, only a fraction of ∼45% of [Leu16](-3)CPY was glycosylated, as verified by sensitivity to endoglycosidase H (Figure 1A, lanes 1 and 2). The rest of the products were unglycosylated, consistent with an Nexo/Ccyt orientation. Incubation of spheroplasted and homogenized cells with trypsin resulted in degradation of the nonglycosylated protein fraction except for a small amount that was resistant even in the presence of detergent (Figure 1A, lanes 3-5). In contrast, the glycosylated protein fraction was protected from protease in the absence, but was completely digested in the presence of detergent. This experiment confirmed that the glycosylation pattern represents protein orientation.
Figure 1.
Model substrates to detect changes in signal orientation. (A) The N-terminal sequence of [Leu16](-3)CPY with a signal-anchor of 16 consecutive leucine residues (boxed), positive N- and net negative C-terminal flanking regions is shown. Diamonds indicate the positions of mutations R4E and E30K that change the charge difference Δ(C-N) from -3 to -1. Cells with wild-type Sec61p expressing [Leu16](-3)CPY were labeled with [35S]methionine for 5 min. The protein was immunoprecipitated and analyzed by SDS-gel electrophoresis and autoradiography (lane 1). Three products with zero, two, or three glycans (arrows) were produced, as confirmed by endoglycosidase H (EndoH) sensitivity (lane 2). In a protease protection experiment (lanes 3-5), labeled cells were homogenized and incubated without protease (-), with trypsin (T), or with trypsin in the presence of detergent (TD), before immunoprecipitation and analysis. Protected glycosylated products and trypsin-sensitive unglycosylated products correspond to Ncyt/Cexo and Nexo/Ccyt orientations, respectively, as illustrated schematically on the right. The C-terminal box indicates the HA-tag. Reduced charge difference by mutations R4E (lane 6) or E30K (lane 7) resulted in an increased unglycosylated population with Nexo/Ccyt orientation. (B) The internal signal-anchor sequence of 40[Leu16](+5)CPY (residues 32-74) are shown. Diamonds indicate the positions of mutations D34R and K65E that reduce the charge difference Δ(C-N) from +5 to +3. 40[Leu16](+5)CPY was expressed in cells with wild-type Sec61p and subjected to endoglycosidase H sensitivity and protease protection assays as in A. Reduced charge difference by mutations D34R (lane 6) or K65E (lane 7) resulted in an increased glycosylated population with Ncyt/Cexo orientation. The positions of molecular weight markers are indicated to the right of the autoradiographs. Cyt, cytoplasm; exo, exoplasm.
Interestingly, threefold glycosylation was increased in cells prepared for the protease protection assay compared with cells lysed immediately after labeling. This is most likely due to posttranslational modification of the third glycosylation site during the 30 min needed to spheroplast the labeled cells. This is also supported by the glycosylation pattern at steady state where the completely modified species was the predominant glycosylated product, and in a pulse-chase experiment (see below; Figures 3C, lane 1; and 5B).
Figure 3.
Specific charge mutations in Sec61p affect the orientation patterns of [Leu16](-3)CPY. (A) [Leu16](-3)CPY was expressed in Sec61p wild-type and mutant strains, pulse-labeled with [35S]methionine for 5 min, immunoprecipitated and analyzed by gel electrophoresis and autoradiography. The mutant R225E/K226E/K228E/K229E/R230E is abbreviated as R225E/... /R230E. (B) The fractions of glycosylated products (Ncyt/Cexo orientation) were quantified by PhosphorImager analysis. The average and SD of at least three individual determinations were plotted. The white bars show the orientation patterns of the mutated model proteins with wild-type Sec61p (see Figure 1A, lanes 6 and 7). Asterisks indicate a significant difference to values obtained with wild-type cells according to Student's t test (p ≤ 0.05). (C) Membrane integration of [Leu16](-3)CPY in cells expressing wild-type Sec61p and selected mutants was tested by alkaline extraction (lanes 1-15). Supernatant (S) and membrane pellet (P), as well as starting material (T, total) were analyzed by immunoblotting using anti-HA antibody. As a soluble control protein, CPY was analyzed in parallel (lanes 16-18). The positions of molecular weight markers (in kilodaltons) are indicated.
Figure 5.
Product stability and glycosylation efficiency in Sec61p mutant strains. (A) To test glycosylation efficiency, the model protein 60[H1](+1) with an N-terminal portion of yeast Ste2p that contains a glycosylation site was expressed in strains with selected Sec61p mutants, labeled for 5 min with [35S]methionine and immunoprecipitated (lanes 1-9). The number of attached glycans is indicated. In addition, the glycosylation efficiency for endogenous Gas1p was analyzed in cells with different Sec61p mutants by labeling for 3 min with [35S]methionine and immunoprecipitation (lanes 10-15). An aliquot from wild-type cells was subjected to deglycosylation by endoglycosidase H (endoH) before analysis (lane 10). (B) [Leu16](-3)CPY was expressed in Sec61p wild-type and mutant strains, pulse-labeled for 5 min with [35S]methionine and chased in the presence of excess unlabeled methionine for 0-40 min, before immunoprecipitation and analysis. The apparent half-lives are >90 min for the combined glycosylated forms and 30-40 min for the unglycosylated form in all strains. Note the gradual shift of two- to threefold glycosylated form during the chase period.
[Leu16](-3)CPY inserts with mixed orientations despite a charge difference Δ(C-N) of -3, which according to the positive-inside rule predicts C-terminal translocation. This is because signal reorientation, driven by a local electric potential, is slowed down by the hydrophobic Leu16 core of the signal (Goder and Spiess, 2003). Mutation of charged residues in Sec61p, which contribute to the potential, are expected to reduce the driving force of signal inversion and to generate a reduced population of glycosylated Ncyt/Cexo molecules. To have an idea of the topology changes to be expected by such a mutation, we mutated flanking charges in the substrate protein [Leu16](-3)CPY: the N-terminal arginine-4 was mutated to glutamate (R4E) or the C-terminal glutamate-30 to lysine (E30K), in both cases reducing the absolute charge difference of the model protein by 2 (Δ(C-N) = -1). On expression in cells with wild-type Sec61p, the fraction of glycosylated products was reduced from ∼45% to ∼5 and ∼26%, respectively (Figure 1A, lanes 6 and 7). The effect is larger in the R4E mutant, most likely because residue 4 is closer to the hydrophobic signal core than residue 30.
N-Terminal signals with an inverted charge distribution [i.e., a positive Δ(C-N)] insert uniformly with Nexo/Ccyt orientation, because there is no force for signal reorientation. If the signal is internal, however, the N-terminal hydrophilic domain may lead to mixed orientations also with inverted flanking charges (Beltzer et al., 1991; Denzer et al., 1995; Wahlberg and Spiess, 1997), because folding of the N-terminal sequence competes with its translocation and may cause signal inversion. As a second diagnostic substrate protein we used 40[Leu16](+5)CPY (Figure 1B). It differs from [Leu16](-3)CPY in that the Leu16 core is preceded by the 40-amino acid N-terminal domain of the asialoglycoprotein receptor H1, and the two closest flanking charges on each side are mutated to residues of opposite charge (two N-terminal arginines to aspartic acids and two C-terminal glutamic acids to lysines). The resulting charge difference Δ(C-N) is thus +5. Reduction of the charge difference by mutation of aspartate-34 to arginine or of lysine-68 to glutamate increased the fraction of glycosylated, Ncyt/Cexo products from 47 to ∼84% (Figure 1B, lanes 6 and 7), confirming that the topology of this model protein depends on charge interactions to a similar extent as [Leu16](-3)CPY.
Specific Charge Mutations in Sec61p Decrease C-Terminal Translocation of [Leu16](-3)CPY
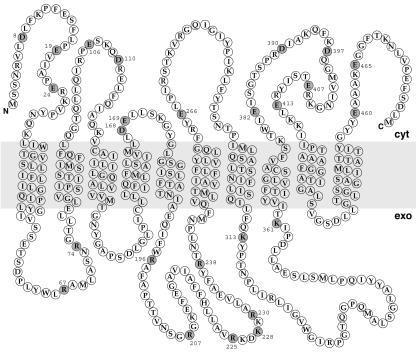
Sec61p spans the membrane 10 times, with both its N and C terminus facing the cytoplasmic side of the membrane (Wilkinson et al., 1996) (Figure 2). To identify charged residues in Sec61p that might interact with flanking charges of signal sequences during the orientation process, the S. cerevisiae sequence was aligned to its homolog Ssh1p and to 10 orthologues (see MATERIALS AND METHODS). We screened for conserved charged residues opposed to the positive-inside rule (i.e., negatively charged residues facing the cytoplasmic and positively charged residues facing the lumenal side of the membrane). As shown in Figure 2, we identified 25 residues fulfilling these criteria.
Figure 2.
Conserved residues of Sec61p potentially involved in decoding the flanking charges of signal sequences. The amino acid sequence and membrane topology of S. cerevisiae Sec61p is shown. Charged residues opposed to the positive-inside rule and well conserved among Sec61p orthologues are represented by filled circles.
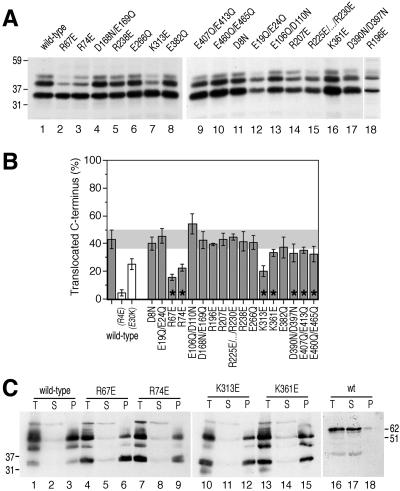
To test the contribution of these residues for signal orientation, they were altered by site-directed mutagenesis. To minimize potential conformational effects, glutamate and aspartate were mutated to the structurally related glutamine and asparagine, respectively, increasing the cytosolic net charge by 1 unit. Because structurally similar residues do not exist for arginine or lysine, these residues were mutated to amino acids of opposite charge, reducing exoplasmic net charge by 2 units. However, in a first series of constructs, closely spaced acidic residues were mutated together. The majority of Sec61p mutants thus contained a net charge change of 2 units. The resulting mutant plasmids were introduced into the yeast strain VGY61, which lacks a chromosomal copy of SEC61, replacing the plasmid of wild-type SEC61 by plasmid shuffling. The newly transformed yeast strains were screened for a growth phenotype. None of the mutant strains displayed an obvious growth defect on rich media plates at 15, 30, or 37°C, indicating that the Sec61p mutants are all functional.
[Leu16](-3)CPY was expressed in yeast strains harboring mutant Sec61p, pulse-labeled for 5 min with [35S]methionine, immunoprecipitated, and analyzed by gel electrophoresis and autoradiography (Figure 3A). The model protein exhibited altered glycosylation patterns in several mutant strains. On quantitation, seven mutants showed a significant decrease in the glycosylated fraction, suggesting the expected shift toward Nexo/Ccyt orientation (Figure 3B; p ≤ 0.05 according to Student's t test; marked by asterisks). For three of them, R67E, R74E, and K313E, the extent of the change was similar to that observed for the charge mutations in the substrate proteins [Leu16](-1)CPY(R4E) and (E30K). By using alkaline extraction, it was confirmed for these three mutants of Sec61p and for K361E that all products were stably integrated into the ER membrane (Figure 3C). The decrease in glycosylated protein is therefore not due to inefficient membrane integration. It could also be shown that the mutations in Sec61p did not reduce the efficiency of glycosylation, e.g., by disturbing the recruitment of the glycosylation machinery to the translocon (see below; Figure 5A). The observed changes in glycosylation patterns thus reflect a shift toward Nexo/Ccyt topology.
R67E, R74E, and E382R Inversely Affect Signal-Anchors with Inverted Flanking Charges
An increased population of Nexo/Ccyt [Leu16](-3)CPY molecules is a necessary, but not a sufficient criterion for the identification of charged residues involved in signal orientation, because mutations in Sec61p that sterically hinder signal reorientation or protein translocation will affect topology in the same direction. Charged residues that contribute to the local electrical potential driving signal orientation are expected to cause the opposite effect on a signal with an inverted, i.e., positive, charge difference Δ(C-N), as in construct 40[Leu16](+5)CPY.
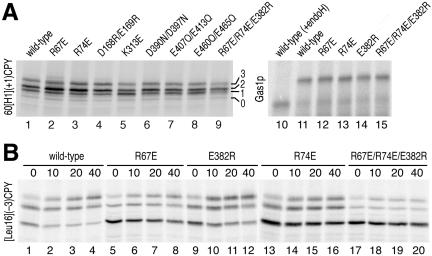
Expressing 40[Leu16](+5)CPY in Sec61p mutant strains revealed a smaller variation in glycosylation patterns compared with [Leu16](-3)CPY (Figure 4, A and B). Of those Sec61p mutations that showed a shift toward N-terminal translocation for [Leu16](-3)CPY, R67E and R74E showed the opposite effect, a shift toward C-terminal translocation, for 40[Leu16](+5)CPY, which supports a role for these residues in signal orientation via the flanking charges. The effects with 40[Leu16](+5)CPY, however, were rather weak compared with those of charge mutations in the substrate protein. K313E and K361E, which promoted N-terminal translocation of [Leu16](-3)CPY, did not do so for 40[Leu16](+5)CPY. Because these residues did not show a significant change in the opposite direction either, their role in signal orientation is not clear. In contrast, the mutants D390N/D397N, E407Q/E413Q, and E460Q/E465Q resulted in a consistent decrease in the glycosylated fractions of both substrate proteins, suggesting a general defect in signal reorientation or translocation.
Figure 4.
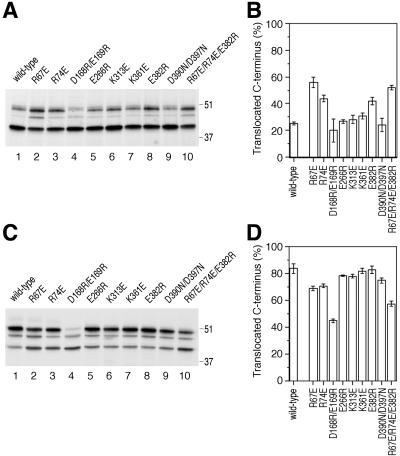
The Sec61p mutants R67E, R74E, and E382R inversely affect orientation of 40[Leu16](+5)CPY, a substrate with inverted flanking charges. (A and B) Insertion patterns of 40[Leu16](+5)CPY in Sec61p wild-type and mutant strains were determined and quantified as for [Leu16](-3)CPY in Figure 3, A and B. The positions of molecular weight markers (in kilodaltons) are indicated. (C and D) Additional Sec61p mutants, D168R/E169R, E266R, and E382R, were analyzed for orientation effects for the substrates [Leu16](-3)CPY and 40[Leu16](+5)CPY.
E382Q is an interesting Sec61p mutation, because it resulted in a small but significant increase in the glycosylated population of 40[Leu16](+5)CPY and possibly a small decrease in the glycosylated population of [Leu16](-3)CPY. Because the net charge change of this mutation is only 1, we also tested the mutation E382R, in which the negative charge is replaced by a positive one. Similarly, we generated E266R and D168R/E169R. Whereas E266R behaved like wild-type Sec61p and D168R/E169R reduced C-terminal translocation for both substrates (accompanied by a growth defect at all temperatures), E382R significantly reduced the C-terminally translocated fraction of [Leu16](-3)CPY and increased that of 40[Leu16](+5)CPY (Figure 4, C and D). This result supports the conclusion that E382 contributes to orienting signal sequences by interaction with flanking charges.
As an additional control that the glycosylation patterns also reflect the topologies of substrates with an internal signal-anchor, we tested a subset of the Sec61p mutants with the substrate protein 60[H1](+1)CPY. This protein consists of the 60-amino acid N-terminal portion of the α-factor receptor Ste2p, the hydrophobic core of the signal-anchor domain of H1, and the same C-terminal sequence as 40[Leu16](+5)CPY. The charge difference Δ(C-N) is only +1, but combined with a less hydrophobic signal. Because the Ste2p domain contains a glycosylation site, this protein is glycosylated upon membrane insertion in either orientation: two- or threefold when the C terminus is translocated, and once when the N terminus is translocated. On expression in cells with wild-type or mutant Sec61p (Figure 5A, lanes 1-9), only a minor and constant fraction of ∼4% of 60[H1](+1)CPY remained unglycosylated, indicating that the efficiency of glycosylation and of targeting and membrane integration is not compromised by the Sec61p mutations tested. In addition, mutations R67E and R74E clearly increased the fraction with multiple glycosylation (i.e., Nexo/Ccyt orientation) compared with wild-type Sec61p, confirming the results with 40[Leu16](+5)CPY. Similarly, glycosylation of the endogenous glycoprotein Gas1p after a 3-min [35S]methionine labeling was equal and efficient in wild-type as well as Sec61p mutant strains (Figure 5A, lanes 10-15). Glycosylation is thus not generally slowed down in cells expressing the critical mutants of Sec61p.
To rule out that the Sec61p mutations might differentially affect the stabilities of glycosylated and unglycosylated products, a pulse-chase experiment was performed. [Leu16](-3)CPY was expressed in cells with wild-type or mutant Sec61p, labeled for 5 min, and chased for up to 40 min before immunoprecipitation and analysis (Figure 5B). Except for a posttranslational shift from two- to threefold glycosylated species, the glycosylated forms were very stable in all strains tested, whereas the unglycosylated form had a half-life of ∼30 min. The results show that the decreased fraction of glycosylated [Leu16](-3)CPY in cells with specific Sec61p mutants is not the result of increased degradation of these products. The degradation rates of either form are not significantly affected by the mutations in Sec61p.
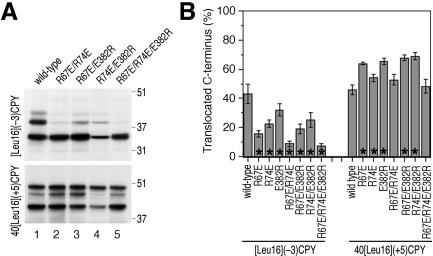
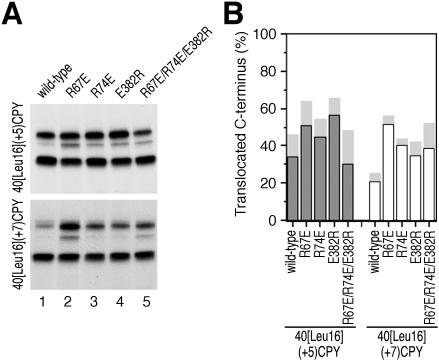
Based on the experiments described above, amino acids R67, R74, and E382 fulfilled the criteria for charged residues in Sec61p to be involved in orienting signal sequences according to the positive-inside rule. The mutations R67E, R74E, and E382R were then combined in all possible combinations to test whether their effects on the topologies of [Leu16](-3)CPY and 40[Leu16](+5)CPY are additive. As is shown in Figure 6, A and B, this was only partially the case. Particularly, mutant Sec61p with the combinations R67E/R74E and R67E/R74E/E382R did not show a significant increase of C-terminal translocation for the substrate 40[Leu16](+5)CPY but did show a strong decrease for [Leu16](-3)CPY. This might be explained by an additional structural defect in Sec61p that inhibits signal reorientation and is superimposed on the charge effect, adding to it for [Leu16](-3)CPY and subtracting from it for 40[Leu16](+5)CPY. A structural defect might already be present to some extent in some of the single mutations. If this is the case, one might expect the charge effect of Sec61p mutations to be the more prominent the larger the charge difference of the substrate protein. To test this, we analyzed a selection of the Sec61p mutants using the substrates 40[Leu16](+7)CPY and 40[Leu16](+3)CPY, two variants of 40[Leu16](+5)CPY in which the charge difference was increased by mutation of glutamate-72 to lysine or decreased by mutation of lysine-65 to glutamate, respectively. Indeed, as is shown in Figure 7, the Sec61p mutants R67E, R74E, and E382R stimulated C-terminal translocation of 40[Leu16](+7)CPY much more strongly (A and B) than they did for 40[Leu16](+5)CPY, whereas the charge effect seemed to be overruled by structural defects for 40[Leu16](+3)CPY (C and D).
Figure 6.
Combining charge mutations R67E, R74E, and E382R. (A and B) Insertion patterns of [Leu16](-3)CPY or 40[Leu16](+5)CPY in strains with combined Sec61p mutations were determined and quantified as in Figure 3, A and B. For comparison, the values for protein orientation in single mutants have been included.
Figure 7.
Sec61p mutations affect signal orientation both by charge effects and by structural effects. Insertion patterns of 40[Leu16](+7)CPY (A and B) and of 40[Leu16](+3)CPY (C and D) in Sec61p wild-type and mutant strains were determined and quantified as in Figure 3 (A and B). 40[Leu16](+7)CPY corresponds to 40[Leu16](+5)CPY in which the charge difference was increased by mutation E72K. In 40[Leu16](+3)CPY, the charge difference was reduced by mutation K65E.
Effects of Charge Mutations R67E, R74E, and E382R Are Independent of the Presence or Absence of Ssh1p
Besides the “classical” translocon made of Sec61p, Sbh1p, and Sss1p, yeast expresses a second type of translocon complex consisting of Ssh1p (Sec sixty-one homolog) with ∼30% identity to Sec61p, Sbh2p, and Sss1p (Finke et al., 1996). The fact that certain mutations in Sec61p affect the topologies of our model substrates shows that Sec61p is involved in their membrane integration. Yet, it cannot be excluded that to some extent the substrates also utilize the Ssh1p translocon, which might have different properties with respect to orienting our diagnostic constructs. It is therefore conceivable that mutations in Sec61p affect the topologies of a substrate protein not by altering the local potential in the Sec61p complex, but by altering the ratio at which Sec61p and Ssh1p translocons are used. To test this possibility, the effect of the Sec61p mutations R67E, R74E, E382R, and R67E/R74E/E382R on the topologies of 40[Leu16](+5)CPY and 40[Leu16](+7)CPY was measured in cells lacking Ssh1p. As shown in Figure 8, the Sec61p mutations shifted the topologies in the same direction and to similar extents in the absence of Ssh1p as in its presence. The observed effects are thus on topogenesis in the Sec61p translocon. However, the topologies were all shifted toward less C-terminal translocation in ΔSSH1 cells. This might indicate that a constant fraction of the substrates is integrated by the Ssh1p translocons, which yield less C-terminal translocation or, more likely, that the lack of Ssh1p indirectly affected orientation of the model proteins. Indeed, ΔSSH1 cells have a significant growth defect and displayed sustained unfolded protein response (Wilkinson et al., 2001).
Figure 8.
Effects of mutations R67E, R74E, and E382R in Sec61p are independent of Ssh1p. Insertion patterns of 40[Leu16](+5)CPY and 40[Leu16](+7)CPY in strains lacking Ssh1p were determined and quantified as in Figure 3, A and B. The average of two independent experiments is plotted. For comparison, the values determined in the presence of Ssh1p in previous figures are indicated by light gray bars.
DISCUSSION
Statistical and experimental data provide a strong indication that electrostatic forces are important for correctly orienting the polypeptide in the plasma membrane of bacteria and in the ER membrane of eukaryotes either by the effect of an electrical potential across the membrane and/or by direct charge interactions. In prokaryotes, the electrochemical potential across the plasma membrane was indeed shown to stimulate the translocation of chain segments containing negatively charged residues and to inhibit translocation of positively charged regions (Andersson and von Heijne, 1994). In addition, negatively charged phospholipids were found to prevent transmembrane passage of positive charges (van Klompenburg et al., 1997). Reduction of the anionic lipid content in Escherichia coli from 25% to <10% by repressing phosphatidylglycerophosphate synthase expression favored translocation of N-terminal-positive charges in diagnostic variants of leader peptidase, without affecting the membrane potential or SecA function. This finding suggested that the positive charges interact with acidic lipids at the cytosolic surface of the membrane and are retained there, allowing the less positive end of the signal/transmembrane segment to be translocated.
In eukaryotic cells, there is no general potential across the ER membrane. Our recent study with an N-terminal signal-anchor with a hydrophobic core of 22 leucines showed the signal to initially insert with an Nexo/Ccyt orientation and to subsequently invert orientation depending on the flanking charges (Goder and Spiess, 2003). This observation argues against retention of the more positive flanking sequence of this signal on the cytoplasmic surface but suggests that a local potential at the translocon serves as the driving force for reorientation.
The present study now shows that specific charged residues in Sec61p contribute to signal orientation according to the positive-inside rule. This conclusion is based on the effect of selective charge mutations in Sec61p on the orientation of diagnostic model proteins. Mutation of R67, R74, or E382 weakened the charge rule, reducing C-terminal translocation of a signal-anchor protein with a negative charge difference Δ(C-N) and increasing it for signal-anchor proteins with a positive charge difference. These inverse effects on substrates with opposite charge difference are an important criterion that allows distinction between an electrostatic influence on the substrate from indirect, steric inhibition of signal reorientation, and/or polypeptide translocation. Indeed, mutation of R67, R74, or E382 caused slight translocation defects that were superimposed on the electrostatic effect, even concealing it for a substrate with a small charge difference (such as 40[Leu16](+3)CPY).
Charged residues of the translocon interacting with flanking charges of signal sequences are expected to be positioned close to the lumenal and cytoplasmic ends of the signal binding site of the translocon. R67 and R74 are localized to the first lumenal loop of Sec61p just preceding transmembrane domain 2, and E382 at the cytosolic end of transmembrane domain 8 (Figure 2). Conspicuously, transmembrane domain 2 has already been shown to be part of the signal sequence binding site. Cross-linking studies indicated that the signal sequence of prepro-α-factor bound to the Sec61 complex by intercalating between transmembrane domains 2 and 7 (Plath et al., 1998).
Although residues R67, R74, and E382 contribute to orienting signal sequences, they are clearly not solely responsible for the positive-inside rule. This is obvious from the fact that the triple mutant R67E/R74E/E382R is viable, albeit with a growth defect. Elimination or even inversion of the charge rule should have more serious consequences. Candidates for additional contributors are the other subunits of the translocon complex, Sbh1p and Sss1p, as well as Sec62p and Sec63p. A contribution by lipids is also conceivable. The signal of arrested nascent polypeptides that are just long enough to enter the translocon could be cross-linked not only to Sec61p/Sec61α but also to phospholipids (Martoglio et al., 1995; Mothes et al., 1998; Plath et al., 1998). Close, perhaps even asymmetric access of charged lipids to the signal binding site of the translocon could also play an important role in signal orientation.
Most signal sequences are less hydrophobic than the ones analyzed here and are therefore more sensitive to electrostatic forces acting on them. It cannot be excluded that many signals with lower affinity to the hydrophobic signal binding site may respond to negative charges at the cytosolic ER surface even before engaging with the binding site in the translocon. Residues R67, R74, and E382 of Sec61p might be important to rescue signals that have inserted in the wrong orientation and therefore, when mutated, might seriously affect only a subset of proteins.
In a recent study (Tipper and Harley, 2002), yeast mutants were selected that cause inefficient orientation of an Nexo/Ccyt signal-anchor (the N terminus and first transmembrane segment of Ste2p with a charge difference of +5) fused to a cleavage site for Kex2p protease and to invertase. Two genes, SPF1, encoding a P-type ATPase in the ER or Golgi, and STE24, a metalloprotease of the ER, were identified to increase invertase secretion from ∼3 to 9-12% of wild-type cells when mutated. The mechanism by which these genes affect signal orientation is unknown. It was speculated that they might be involved in ER Ca2+ homeostasis or in controlling ER membrane lipid composition. It is conceivable that several mechanisms are involved in generating the positive-inside rule. Our results show that one of them is the translocon protein Sec61p itself.
Acknowledgments
We thank Dr. Robbie Loewith for critically reading the manuscript, and Drs. E. Hartmann, N. Kralli, R. Schekman, T. Sommer, and B. Wilkinson for strains and reagents. This work was supported by grant 31-061579.00 from the Swiss National Science Foundation.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-08-0599. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-08-0599.
Abbreviations used: Abbreviations used: CPY, carboxypeptidase Y; ER, endoplasmic reticulum; SRP, signal recognition particle.
References
- Andersson, H., and von Heijne, G. (1994). Membrane protein topology: effects of delta mu H+ on the translocation of charged residues explain the `positive inside' rule. EMBO J. 13, 2267-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltzer, J.P., Fiedler, K., Fuhrer, C., Geffen, I., Handschin, C., Wessels, H.P., and Spiess, M. (1991). Charged residues are major determinants of the transmembrane orientation of a signal-anchor sequence. J. Biol. Chem. 266, 973-978. [PubMed] [Google Scholar]
- Denzer, A.J., Nabholz, C.E., and Spiess, M. (1995). Transmembrane orientation of signal-anchor proteins is affected by the folding state but not the size of the N-terminal domain. EMBO J. 14, 6311-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies, R.J., and Schekman, R. (1987). A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J. Cell Biol. 105, 633-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio, A., Friedberg, T., and Spiess, M. (1998). The role of the hydrophobic domain in orienting natural signal sequences within the ER membrane. Exp. Cell Res. 241, 181-185. [DOI] [PubMed] [Google Scholar]
- Finke, K., Plath, K., Panzner, S., Prehn, S., Rapoport, T.A., Hartmann, E., and Sommer, T. (1996). A second trimeric complex containing homologs of the Sec61p complex functions in protein transport across the ER membrane of S. cerevisiae. EMBO J. 15, 1482-1494. [PMC free article] [PubMed] [Google Scholar]
- Goder, V., and Spiess, M. (2003). Molecular mechanism of signal sequence orientation in the endoplasmic reticulum. EMBO J. 22, 3645-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich, D., Prehn, S., Hartmann, E., Kalies, K.U., and Rapoport, T.A. (1992). A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell 71, 489-503. [DOI] [PubMed] [Google Scholar]
- Görlich, D., and Rapoport, T.A. (1993). Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell 75, 615-630. [DOI] [PubMed] [Google Scholar]
- Harley, C.A., Holt, J.A., Turner, R., and Tipper, D.J. (1998). Transmembrane protein insertion orientation in yeast depends on the charge difference across transmembrane segments, their total hydrophobicity, and its distribution. J. Biol. Chem. 273, 24963-24971. [DOI] [PubMed] [Google Scholar]
- Hartmann, E., Rapoport, T.A., and Lodish, H.F. (1989). Predicting the orientation of eukaryotic membrane-spanning proteins. Proc. Natl. Acad. Sci. USA 86, 5786-5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich, S.U., Mothes, W., Brunner, J., and Rapoport, T.A. (2000). The Sec61p complex mediates the integration of a membrane protein by allowing lipid partitioning of the transmembrane domain. Cell 102, 233-244. [DOI] [PubMed] [Google Scholar]
- High, S., Andersen, S.S., Görlich, D., Hartmann, E., Prehn, S., Rapoport, T.A., and Dobberstein, B. (1993a). Sec61p is adjacent to nascent type I and type II signal-anchor proteins during their membrane insertion. J. Cell Biol. 121, 743-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High, S., Martoglio, B., Görlich, D., Andersen, S.S., Ashford, A.J., Giner, A., Hartmann, E., Prehn, S., Rapoport, T.A., Dobberstein, B., et al. (1993b). Site-specific photocross-linking reveals that Sec61p and TRAM contact different regions of a membrane-inserted signal sequence. J. Biol. Chem. 268, 26745-26751. [PubMed] [Google Scholar]
- Keenan, R.J., Freymann, D.M., Stroud, R.M., and Walter, P. (2001). The signal recognition particle. Annu. Rev. Biochem. 70, 755-775. [DOI] [PubMed] [Google Scholar]
- Martoglio, B., Hofmann, M.W., Brunner, J., and Dobberstein, B. (1995). The protein-conducting channel in the membrane of the endoplasmic reticulum is open laterally toward the lipid bilayer. Cell 81, 207-214. [DOI] [PubMed] [Google Scholar]
- Mothes, W., Jungnickel, B., Brunner, J., and Rapoport, T.A. (1998). Signal sequence recognition in cotranslational translocation by protein components of the endoplasmic reticulum membrane. J. Cell Biol. 142, 355-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes, W., Prehn, S., and Rapoport, T.A. (1994). Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J. 13, 3973-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon, M., Römisch, K., Quach, D., and Schekman, R. (1998). Sec61p serves multiple roles in secretory precursor binding and translocation into the endoplasmic reticulum membrane. Mol. Biol. Cell 9, 3455-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon, M., Schekman, R., and Römisch, K. (1997). Sec61p mediates export of a misfolded secretory protein from the endoplasmic reticulum to the cytosol for degradation. EMBO J. 16, 4540-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath, K., Mothes, W., Wilkinson, B.M., Stirling, C.J., and Rapoport, T.A. (1998). Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell 94, 795-807. [DOI] [PubMed] [Google Scholar]
- Pohlschröder, M., Prinz, W.A., Hartmann, E., and Beckwith, J. (1997). Protein translocation in the three domains of life: variations on a theme. Cell 91, 563-566. [DOI] [PubMed] [Google Scholar]
- Rösch, K., Naeher, D., Laird, V., Goder, V., and Spiess, M. (2000). The topogenic contribution of uncharged amino acids on signal sequence orientation in the endoplasmic reticulum. J. Biol. Chem. 275, 14916-14922. [DOI] [PubMed] [Google Scholar]
- Sakaguchi, M., Tomiyoshi, R., Kuroiwa, T., Mihara, K., and Omura, T. (1992). Functions of signal and signal-anchor sequences are determined by the balance between the hydrophobic segment and the N-terminal charge. Proc. Natl. Acad. Sci. USA 89, 16-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, S.L., Whitfield, K.M., Vogel, J.P., Rose, M.D., and Schekman, R.W. (1992). Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell 69, 353-365. [DOI] [PubMed] [Google Scholar]
- Spiess, M. (1995). Heads or tails—what determines the orientation of proteins in the membrane. FEBS Lett. 369, 76-79. [DOI] [PubMed] [Google Scholar]
- Tipper, D.J., and Harley, C.A. (2002). Yeast genes controlling responses to topogenic signals in a model transmembrane protein. Mol. Biol. Cell 13, 1158-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Klompenburg, W., Nilsson, I., von Heijne, G., and de Kruijff, B. (1997). Anionic phospholipids are determinants of membrane protein topology. EMBO J. 16, 4261-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne, G. (1986). Net N-C charge imbalance may be important for signal sequence function in bacteria. J. Mol. Biol. 192, 287-290. [DOI] [PubMed] [Google Scholar]
- Wahlberg, J.M., and Spiess, M. (1997). Multiple determinants direct the orientation of signal-anchor proteins: the topogenic role of the hydrophobic signal domain. J. Cell Biol. 137, 555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, P., and Johnson, A.E. (1994). Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu. Rev. Cell Biol. 10, 87-119. [DOI] [PubMed] [Google Scholar]
- Wessels, H.P., Beltzer, J.P., and Spiess, M. (1991). Analysis of protein topology in the endoplasmic reticulum. Methods Cell Biol. 34, 287-302. [DOI] [PubMed] [Google Scholar]
- Wilkinson, B.M., Critchley, A.J., and Stirling, C.J. (1996). Determination of the transmembrane topology of yeast Sec61p, an essential component of the endoplasmic reticulum translocation complex. J. Biol. Chem. 271, 25590-25597. [DOI] [PubMed] [Google Scholar]
- Wilkinson, B.M., Tyson, J.R., and Stirling, C.J. (2001). Ssh1p determines the translocation and dislocation capacities of the yeast endoplasmic reticulum. Dev. Cell 1, 401-409. [DOI] [PubMed] [Google Scholar]