Myriad extracellular and intracellular signals constantly poke or prod cells in order to influence the vast number of functions required of cells to maintain homeostasis. Selective reception of these signals and of their subsequent translation and transduction are tasks coordinated by cellular signal-transduction systems. Two of these systems, the Ras/Raf/MEK (mitogen-activated ERK-kinase)/ERK cascade and the cAMP/PKA signaling system, each regulate numerous cellular functions and their influences on these continue to be the subject of intense study (1). Interestingly, research confirms that rather than functioning in mutually independent “tracks,” the Ras/Raf/MEK/ERK cascade and the cAMP/PKA system often interact and numerous reports document where activation of cAMP/PKA signaling either amplified or antagonized Ras/Raf/MEK/ERK-mediated events, both in physiological settings as well as in pathologies, including cancers (1, 2). Although numerous points of intersection between these systems have been described (1, 2), one critical point of interplay involves an inhibitory PKA-mediated phosphorylation of Raf-1 (V-raf-1 murine leukemia viral oncogene homolog 1, also known as C-Raf) at S259. Raf-1 is one of the three known MEK kinases, along with A-Raf and B-Raf, which can stimulate MEK-catalyzed ERK phosphorylation in this cascade. In PNAS, Brown et al., (3) provide mechanistic insights into the manner by which PKA-mediated phosphorylation and inhibition of Raf-1 is regulated in cells, and they highlight a role for localized hydrolysis of cAMP in this important process.
The unique mechanism elaborated in Brown et al., (3) fits perfectly in an emerging paradigm in which the selectivity of intracellular cAMP-signaling is achieved through the creation of several distinct nonoverlapping intracellular cAMP-signaling compartments, and the ability of cells to differentially task these compartments with the control of distinct cAMP-regulated cellular functions. Although early researchers who proposed the existence of cellular signaling compartments were largely ignored, currently this concept is rapidly gaining broad acceptance. In a cellular context, these compartments are defined by the unique integration of several proteins, including—but not limited to—scaffolding proteins (such as A-kinase anchoring proteins), cAMP effectors (PKA, exchange proteins activated by cAMP, or cyclic nucleotide-regulated ion channels), and cAMP-hydrolyzing phosphodiesterases (PDEs) into signaling complexes, and the subsequent subcellular tethering of these complexes (termed “signalosomes”) into defined subcellular domains. Localized cAMP-signaling is thus largely achieved through activation of individual cAMP-effectors in selected compartments, a process dynamically regulated, and often limited, through the local hydrolysis of cAMP by the cAMP-PDEs located in said compartment. Although there are relatively few distinct cAMP effectors, given the large number of scaffolding proteins [15 AKAP families (4, 5)] and PDEs [11 gene families (PDE1–PDE11) encoding no fewer than 80 distinct enzymes (6)], the number of individual signaling complexes and compartments that a given cell can generate should be numerous. In addition to providing a model that explains the selective actions of distinct cAMP-elevating agents, signal compartmentation also provides support for the therapeutically relevant proposition that disruption of cAMP-mediated events selectively within individual compartments may provide increased selectivity over that offered by agents that do not discriminate but rather affect cAMP-signaling globally. In their study, Brown et al. (3) identify a unique protein–protein interaction between Raf-1 and a cAMP-hydrolyzing PDE, namely phosphodiesterase 8A (PDE8A). PDE8 family members are enzymes characterized by their relatively high affinity and specificity for cAMP, as opposed to cGMP, and their insensitivity to the otherwise pan-PDE inhibitor, 3-isobutyl-1-methylxanthine. Currently, the possibility that targeting PDE family enzymes selectively within signalosomes may provide novel therapeutic options for treating several human diseases is being tested.
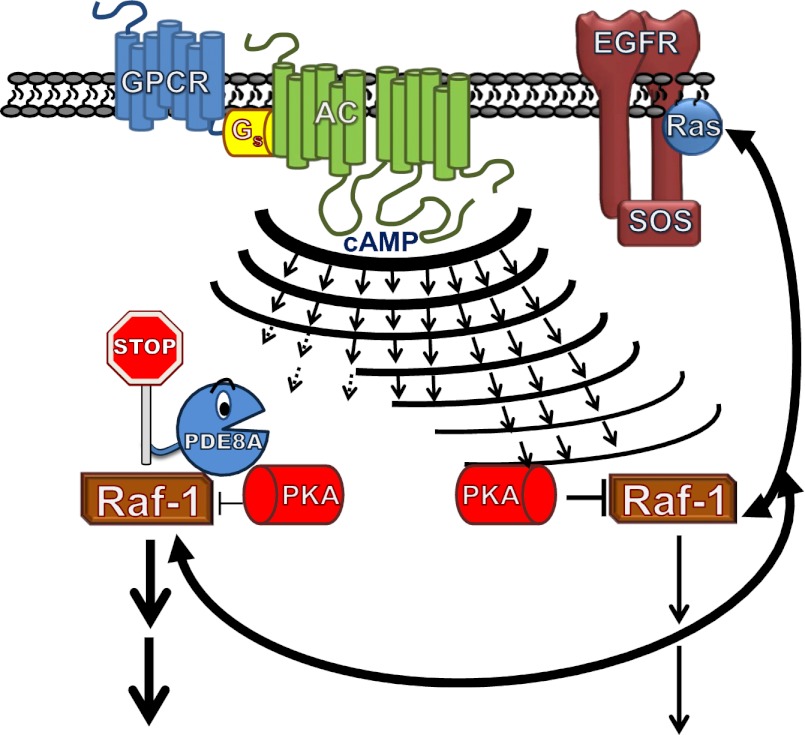
Using several complementary approaches, including peptide array-based binding studies, Brown et al. (3) demonstrate that Raf-1 and PDE8A interact directly without the need of accessory proteins or lipids, and identify a peptide motif in PDE8A (R454 RLSGNEY461) critical for this interaction. Interestingly, although the affinity of many protein–protein interactions tends to be weak, in this instance PDE8A/Raf-1 binding was very tight (Kd < 61 pM), and was not affected by changes in cellular cAMP concentrations or the presence in cells of PDE8 inhibitors. The influence of PDE8A activity, which reportedly accounted for less than 5% of total cAMP PDE activity in the cells studied, on PKA-mediated phosphorylation of Raf-1 at S259 was studied using several approaches. First, HEK293 cells were made to overexpress either native PDE8A or a mutated version of PDE8A engineered to be unable to hydrolyze cAMP (i.e. catalytically “dead”). Although overexpression of PDE8A decreased PKA-dependent Raf-1 phosphorylation at S259, overexpression of the “dead” PDE8A promoted Raf-1 S259 phosphorylation. By analogy to their previous work (7), the ability of the “dead” PDE8A to promote Raf-1 S259 phosphorylation was suggested to result from displacement of native PDE8A from Raf-1 and a resultant increase in cAMP within this compartment. Because overexpression of the PDE8A variants also impacted Raf-1 S259 phosphorylation and its effect on downstream of ERK phosphorylation, in response to global increases in cAMP (using forskolin), the authors concluded that their data were consistent with the idea that Raf-1–bound PDE8A generated a compartment of reduced cAMP around Raf-1, which in turn also reduced PKA-mediated phosphorylation in this compartment (Fig. 1). Also consistent with this model, a cell-permeable peptide containing the PDE8A-based, Raf-1 binding sequence “RRLSGNEY,” but not a scrambled control peptide, was able to promote PKA-mediated phosphorylation of Raf-1 at S259 and to impact subsequent ERK phosphorylation. Of import, this system was shown to be operational and to control basal Raf-1–mediated phosphorylation of ERK when tested in wild-type and PDE8-null Drosophila, as well as in Leydig cells isolated from wild-type and PDE8-null mice. Although previous studies have shown that PDE3- or PDE4-family enzymes can be counted on to control localized cAMP-dependent events in cells (8–12), Brown et al.’s study (3) shows that local control of Raf-1 activity can be added to the list of important signaling events that are regulated in cells by the hyperlocalized hydrolysis of cAMP by compartmented PDEs.
Fig. 1.
Activation of a host of GPCRs and their subsequent interaction with the stimulatory G-protein, Gs, promotes formation of the second messenger, cAMP, by membrane-associated adenylyl cyclases (ACs). The small and hydrophilic cAMP can then diffuse rapidly throughout the cell to activate its effectors, including PKA. In cells, local compartments, populated by certain PDEs can buffer these increases in cAMP and, by so doing, act locally to limit access of cAMP to its effector. As described in the text, a picomolar affinity interaction between a cAMP-selective PDE, namely PDE8A, and the MEK kinase (Raf-1) in cells promotes formation of a PDE8A/Raf-1 complex that allows PDE8A to limit PKA phosphorylation of Raf-1 at S259 and to antagonize the inhibition of MAPK signaling that is the consequence of this phosphorylation. SOS, Son of Sevenless; EGRF, epidermal growth-factor receptor.
Given the space limitations imposed on authors of manuscripts reporting unique and important findings, such as those found in Brown et al. (3), the interested reader is almost always left wanting more. For example, although the reported PDE8A/Raf-1 complex was clearly sufficient to explain effects brought about by addition of PDE8 inhibitors, or of forskolin, the efficiency of this
Brown et al. demonstrate that Raf-1 and PDE8A interact directly without the need of accessory proteins or lipids.
system at integrating cAMP signals generated upon activation of distinct stimulatory G protein-coupled G protein-coupled receptors (GPCRs) was not tested. Given that distinct GPCRs selectively modulate individual cAMP-regulated cellular functions in many cell types, assessing how well this mechanism accounts for distinct sources of cAMP could highlight differences in the relative contribution of this system. Furthermore, results of future studies aimed at localizing the PDE8A-regulated cAMP-signaling cellular compartment using FRET or other applicable technologies, and aiming to define whether compartmentation of PKA is also involved or necessary for this mechanism to function efficiently should further define the nature of this compartment and elucidate its importance in cells. Of specific relevance in the context of potential future studies is an earlier report in which PKA was shown to bind Raf-1 (reviewed in ref. 2), as well as a recent report in which AKAP-Lbc and the scaffolding protein kinase suppressor of Ras (KSR-1) were shown to foster integration between the Raf/MEK/ERK cascade and cAMP/PKA system by coordinating PKA phosphorylation of a regulatory site on KSR-1 (13). Determining whether PDE8A forms part of the AKAP-Lbc and KSR-1 complex, or regulates a “pool” of Raf- 1 distinct from that associated with this complex, could have significant ramifications on how best to use cAMP-elevating agents to control Raf-1 activity in cells. Moreover, because Ras, the upstream regulator of Raf-1, can also be differentially targeted to distinct sites in cells, and because its activation within these alternate domains can temporally and spatially control its downstream effects (14), localizing the domain in which PDE8A regulates Raf-1 inhibition may also assist efforts to further define how Ras operates in cells and identify whether pools of Ras activate distinct pools of Raf-1 in certain cells. Finally, because Raf isoform switching has been identified as an important factor affecting the therapeutic value of certain novel Raf-targeted antineoplastic agents (reviewed in ref. 2), assessing whether PDE8A also associates with B-Raf or A-Raf, or impacts Raf switching, may assist efforts to limit the undesired effects of these agents in these cancers.
Footnotes
The author declares no conflict of interest.
See companion article on page E1533.
References
- 1.Gerits N, Kostenko S, Shiryaev A, Johannessen M, Moens U. Relations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: Comradeship and hostility. Cell Signal. 2008;20(9):1592–1607. doi: 10.1016/j.cellsig.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Dumaz N. Mechanism of RAF isoform switching induced by oncogenic RAS in melanoma. Small GTPases. 2011;2(5):289–292. doi: 10.4161/sgtp.2.5.17814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown KM, et al. Phosphodiesterase-8A binds to and regulates Raf-1 kinase. Proc Natl Acad Sci USA. 2013;110:E1533–E1542. doi: 10.1073/pnas.1303004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tröger J, Moutty MC, Skroblin P, Klussmann E. A-kinase anchoring proteins as potential drug targets. Br J Pharmacol. 2012;166(2):420–433. doi: 10.1111/j.1476-5381.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott JD, Santana LF. A-kinase anchoring proteins: Getting to the heart of the matter. Circulation. 2010;121(10):1264–1271. doi: 10.1161/CIRCULATIONAHA.109.896357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiol Rev. 2011;91(2):651–690. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- 7.McCahill A, et al. In resting COS1 cells a dominant negative approach shows that specific, anchored PDE4 cAMP phosphodiesterase isoforms gate the activation, by basal cyclic AMP production, of AKAP-tethered protein kinase A type II located in the centrosomal region. Cell Signal. 2005;17(9):1158–1173. doi: 10.1016/j.cellsig.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: A molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007;100(7):950–966. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- 9.Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35(2):91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Wilson LS, et al. A phosphodiesterase 3B-based signaling complex integrates exchange protein activated by cAMP 1 and phosphatidylinositol 3-kinase signals in human arterial endothelial cells. J Biol Chem. 2011;286(18):16285–16296. doi: 10.1074/jbc.M110.217026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rampersad SN, et al. Cyclic AMP phosphodiesterase 4D (PDE4D) Tethers EPAC1 in a vascular endothelial cadherin (VE-Cad)-based signaling complex and controls cAMP-mediated vascular permeability. J Biol Chem. 2010;285(44):33614–33622. doi: 10.1074/jbc.M110.140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosenden R, Taskén K. Cyclic AMP-mediated immune regulation—Overview of mechanisms of action in T cells. Cell Signal. 2011;23(6):1009–1016. doi: 10.1016/j.cellsig.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Smith FD, et al. AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade. Nat Cell Biol. 2010;12(12):1242–1249. doi: 10.1038/ncb2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casar B, et al. Ras subcellular localization defines extracellular signal-regulated kinase 1 and 2 substrate specificity through distinct utilization of scaffold proteins. Mol Cell Biol. 2009;29(5):1338–1353. doi: 10.1128/MCB.01359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]