Abstract
Objective
To investigate clinical, pathological and mycological findings in canaries, in which pox lesions and Aspergillus fumigatus (A. fumigatus) infection were observed simultaneously.
Methods
This study was performed on a breeding colony (about 100 canaries) affected by fatal wasting disease. Necropsy was undertaken on 10 severely affected canaries, and gross lesions were recorded. Samples from internal organs displaying lesions were obtained for histopathological evaluation. Tracheal swap samples of internal organs of the all infected animals with lesions at necropsy were cultured in Sabouraud Dextrose Agar for mycological examination.
Results
At necropsy, caseous foci were determined in the lungs, on the air sacs, liver, spleen, heart. Swelling of the eyelids, diffuse hemorrhages in the subcutaneous tissue with small papular lesions of the skin were other typical necropsy findings. Histopathologically, pathognomonic eosinophilic intracytoplasmic inclusion bodies, which called Bollinger bodies, in both skin cells and vacuolated air way epithelial cells confirmed canary pox infection. Moreover, histopathological examination of the white-yellowish caseous foci revealed necrotic granulomatous reaction consisting of macrophages, heterophil leukocytes and giant cells encapsulated with a fibrous tissue. After the culture of the tissue samples, the formation of bluish green colonies confirmed A. fumigatus infection.
Conclusions
Canary pox has been known as the disease that can result in high losses in a short time, as a re-emerging disease that has not been present during recent years in canary flocks in Iran. So, the current paper provides useful information to prevent misdiagnosed of canary pox disease which can cause secondary mycotic infection.
Keywords: Canary, Aspergilosis, Diagnosis, Pox infection, Re-emerging disease, Mycotic infections
1. Introduction
Canaries are regarded by cage-bird fanciers to be their hardiest species, although severe losses can be sustained by infection with canary pox[1]. Canary pox virus, a virulent avipoxvirus, generally presents as a systemic infection and can cause up to 100% mortality in susceptible canary flocks[2]. It can enter human cells but cannot survive and multiply there[3]. Upper respiratory tract disease, pneumonia, air sacculitis, and splenomegaly may be evident[4].
On the other hand, fungal infections caused by Aspergillus species are a major cause of morbidity and mortality among captive birds. The filamentous and saprophytic Aspergillus fumigatus (A. fumigatus) is the predominant species found in infected birds. This fungus produces huge numbers of airborne conidia, which are found ubiquitously in the environment[5],[6]. In young animals, the infection is usually acute and severe, presenting high morbidity and mortality, whereas the infection usually appears sporadic and chronic in adult birds[7]. Aspergillosis mainly locates in the respiratory system and is characterized with lesions in the internal organs, eyes and in certain cases, the brain in poultry[8]–[10]. It can cause various disease manifestations, including life-threatening invasive aspergillosis in animals and humans[11]. Macroscopic findings may range from miliary to larger granulomatous foci which are grey-yellow-white in color and dry in consistency[7],[12]. Cases of aspergillosis have already been reported in chickens[13], turkeys[14], ostriches[15], great rhea[16], penguins[17], coturnix quail[18], as well as free-flying wild birds[19]. In these studies, histopathological findings have been determined, agent isolation has been performed and transfer of infection to other animal species has been attempted. The aim of the present study was to investigate clinical, pathological and mycological findings in canaries, in which pox lesions and A. fumigatus infection were observed simultaneously.
2. Materials and methods
This study was performed on a breeding colony (about 100 canaries) with a fatal wasting disease that was referred to the veterinary hospital of Shahid Bahonar University of Kerman, Kerman, Iran. Birds that were affected early in the outbreak were found dead and no signs of disease had been observed. Most of the birds that were affected later in the outbreak showed gasping which continued for hours or days. Necropsy was performed on 10 severely affected canaries and gross lesions were recorded. Samples from internal organs displaying lesions were fixed in 10% (v/v) buffered formalin. Following trimming and blocking in paraffin, 5–6 µm thick cross sections were prepared and stained with hematoxylin-eosin (H&E) and periodic acid schiff for specific evaluation of polysaccharides of fungi. Tracheal swap samples of internal organs of all the infected animals with lesions at necropsy were cultured in sabouraud dextrose agar (Oxoid, Hampshire, UK) for mycological examination. Tissue samples were inoculated on sabouraud dextrose agar and incubated at 25 °C at aerobic conditions for isolation of the agent. In order to avoid bacterial contamination, 20 IU/mL penicillin G potassium and 40 µg/mL streptomycine sulphate (Exir Pharmaceutical Co) were added to the media. Lactophenol cotton blue stain was used for the microscopic examination of isolates and 200 g/L potassium hydroxide was used for direct microscopic examination of internal organ samples and tracheal swaps.
3. Results
3.1. Macroscopic lesions
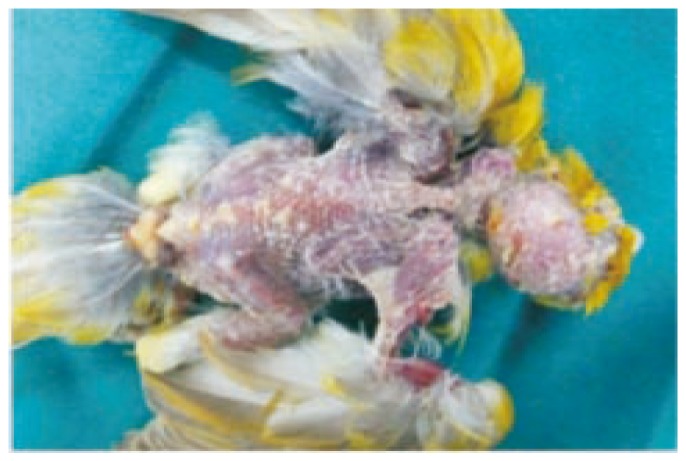
Dehydration and enlargement of the spleen were the only constant findings in birds that died suddenly at the outset of the infection. As more chronic cases died, the following lesions were common: heavy mucoid tracheal exudate with tracheitis; consolidation of the lungs (Figure 1); thickening of the air sacs which contained caseous, yellow material; fibrinous exudate on the surface of a slightly enlarged liver; enlargement of the spleen; caseous pericarditis; and unilateral or bilateral swelling of the eyelids with edema and diffuse hemorrhages in the subcutaneous tissue with small papular lesions of the skin (Figure 2).
Figure 1. Prominent consolidation of the lungs.
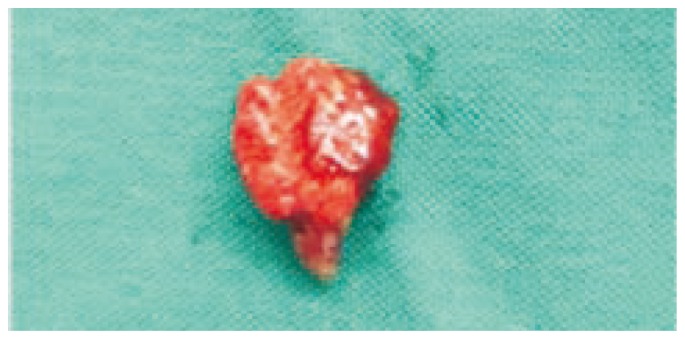
Figure 2. Multiple papular lesions on the skin of the head and back.
3.2. Histopathological lesions
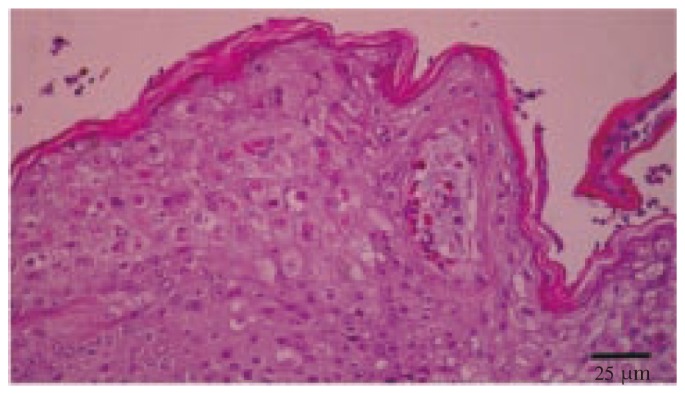
Histopathological examination of numerous tissues, including the liver, spleen, gizzard, cornea, trachea, air sacs and skin lesions, revealed changes a typical severe septicemic infection. Histopathological examination of the skin lesions revealed hyperkeratosis, acanthosis, ballooning degeneration of keratinocytes, and pathognomonic eosinophilic intracytoplasmic inclusion bodies which called Bollinger bodies (Figure 3). Histologically, epithelial hyperplasia and vacuolar degeneration of airway epithelial cells associated with small numbers of mixed inflammatory cells and typical Bollinger bodies in the cytoplasm of vacuolized epithelial cells were observed. On the basis of these findings, canary pox infection was diagnosed.
Figure 3. Hyperkeratosis, acanthosis, ballooning degeneration of keratinocytes, and Bollinger bodies of the skin (H&E).
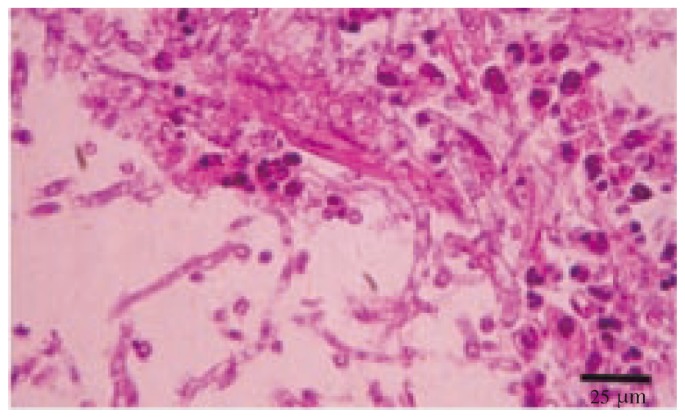
Moreover, histological examination revealed the presence of early lesions in the lungs characterized with infiltration of partly lymphocytes, a small number of macrophages, and giant cells as well as heterophil leukocyte with central necrosis. Fungal hyphae and spores were also observed in these foci (Figure 4). In larger granulomatous areas, necrotic regions infiltrated with macrophages, giant cells, lymphocytes, and heterophil leukocyte were surrounded by fibrous tissue from the outside. These structures were found to be located mainly near the parabronchi. Short and thin fungal hyphae with septa and fungal spores were determined within these granulomatous foci (Figure 5). Further thrombosis in the lumina of blood vessels and haemorrhages in the parenchyma were also detected.
Figure 4. Numerous fungal hyphae surrounded by granulomatous reaction in the lung (H&E).
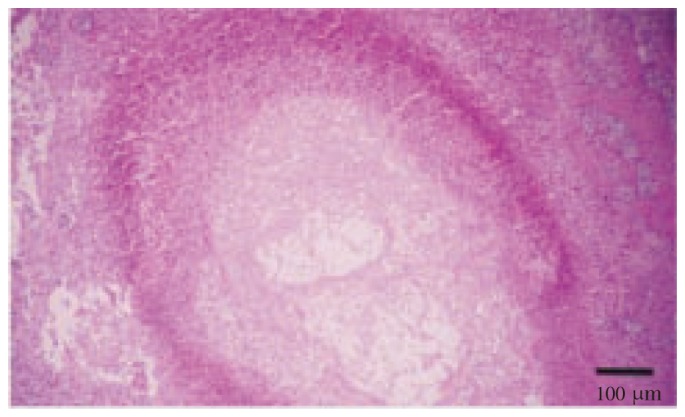
Figure 5. Short and thin septate fungal hyphae within granulomatous reaction in the lung (H&E).
3.3. Mycological findings
After the culture of the tissue samples collected during the necropsy of all dead birds (lungs, air sacs, myocardium, thoracic wall and abdominal serosa), the formation of bluegreen colonies was observed and A. fumigatus was diagnosed according to the prescribed mycological examination.
4. Discussion
Canary pox has been known as a re-emerging disease that has not been present during recent years in canary flocks in Iran. The emergence of new diseases and the re-emergence of recognized diseases are familiar events in the annals of poultry medicine. Some of these emerging diseases could have been present earlier but were not recognized because of low prevalence, mild signs and lesions, lack of diagnostic techniques, or misdiagnosis[20]. Method of transmission by which canary pox virus to this aviary is not known, but a few mites (Dermanyssus gallinae) were found on one of the first canaries affected. Some of the post-mortem changes found in the above birds were similar to previous studies, particularly the changes involving the spleen, liver, lungs and the pericardium and intracellular inclusion bodies found on histopathology. Some canary pox viruses caused a condition showing little resemblance to fowl pox, and that canary pox viruses had been found to be specific for the canary, while others are transmissible to other species of birds[1]. Coulston and Manwell described a canary pox with gasping in the early stages and eye swelling in later cases[21]. The above mentioned cases showed symptoms similar to this and also feature of canary pox. In some cases, the location and size of the pox lesion can cause severe debilitation. In general, a preponderance of pox lesions on the eyes may contribute to death, because the birds may not be able to find food. Aspergillosis is reported to cause great economic loss mainly in chicken pullets[13], turkeys[14], quails[18] and pigeons[22]. Similar to the reports of other researchers, in the present study, typical caseous nodules, which ranged from pin point to chickpea in size, were observed in the lungs, air sacs, myocardium, thoracic wall and abdominal serosa. Contrary to some reports, in the present study, no lesions were detected in the brain, eyes, glandular stomach, bursa of fabricius and skin[23],[24]. The macroscopic appearance of caseous nodules may be confused with tuberculosis, Marek's disease and tumours. Exact differential diagnosis can be made according to detection of fungal hyphae and spores upon histopathological examination[25],[26]. Similar to the results of the present study, other researchers have observed the presence of Aspergillus hyphae and spores in caseous necrotic masses, as well as cell infiltrations consisting of giant cells, heterophils and macrophages together with necrotic granulomatous foci surrounded with an outer layer of connective tissue[27]–[29]. Macroscopic, microscopic and mycological findings determined on canaries in this study were found to be similar to lesions detected in various poultry species diagnosed with aspergillosis. To the best of our knowledge, this is the first study carried out on canaries in which an A. fumigatus infection and canary pox lesion were observed simultaneously. In conclusion, considering canary pox as a re-emerging disease which can cause high losses in a short time in canary flocks and A. fumigatus as an opportunistic factor, particular attention is needed to prevent misdiagnosis of these diseases.
Acknowledgments
This work was financially supported by a grant for Scientific Research from Vice Chancellor of Research of Shahid Bahonar University of Kerman, Iran (Grant No. MP/342/41).
Comments
Background
Canary pox has been known as the disease which can result in high losses in a short time, as a re-emerging disease which has not been present during recent years in canary flocks in Iran. The goal of the present study was to prevent misdiagnosis of canary pox disease which can cause secondary mycotic infections.
Research frontiers
Studies are being performed to identify pathogenesis, diagnosis and the exact transmission ways of these two important diseases to prevent high mortality in canary flocks.
Related reports
Thiel et al. described a simple polymerase chain reaction–based method for identification and discrimination of avipoxvirus strains similar to the fowlpox or canarypox viruses. On the other hand, Beernaert et al. examied the impact of the use of different inoculation routes and immunosuppression on the course of an infection with A. fumigatus in racing pigeons. In the present study, the author described clinical, pathological and mycological findings due to these two important diseases in canaries. To the best of our knowledge, this is the first study in which pox lesions and A. fumigates infection (as an opportunistic agent) was observed simultaneously in canaries.
Innovations and breakthroughs
The emergence of new diseases and the re-emergence of recognized diseases are familiar events in the annals of poultry medicine. This study assesses clinical and pathological findings of concurrent poxvirus lesions and aspergillosis infection in canaries.
Applications
Some of these emerging diseases could have been present earlier but were not recognized because of low prevalence, mild signs and lesions, lack of diagnostic techniques, or misdiagnosis. The results of the present study suggest the best ways for diagnosis, control and prevent of such important diseases.
Peer review
This is a very interesting study in which authors valuated clinical and pathological findings of concurrent poxvirus lesions and aspergillosis infection in canaries. Practitioner in avian medicine can use these diagnostic keys to find exact diseases when clinical signs are the same.
Footnotes
Foundation Project: Supported by a grant for Scientific Research from Vice Chancellor of Research of Shahid Bahonar University of Kerman, Iran (Grant No. MP/342/41).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Saif YM, Fadly AM, Glisson JR. Diseases of poultry. USA: Blackwell Publishing; 2008. pp. 253–269. [Google Scholar]
- 2.Thiel T, Whiteman NK, Tirapé A, Baquero MI, Cedeño V, Walsh T, et al. et al. Characterization of canarypox-like viruses infecting endemic birds in the Galápagos Islands. J Wildl Dis. 2005;41(2):342–353. doi: 10.7589/0090-3558-41.2.342. [DOI] [PubMed] [Google Scholar]
- 3.Murphy FA, Gibbs El J, Horzinek MC, Studdert MJ. Veterinary Virology. USA: E- Publishing; 2012. p. 231. [Google Scholar]
- 4.Tomas N, Tully JR. Hand book of avian medicine. 2nd ed. USA: Saunders; 2009. [Google Scholar]
- 5.Van Waeyenberghe L, Pasmans F, D'Herde K, Ducatelle R, Favoreel H, et al. et al. Germination of Aspergillus fumigatus inside avian respiratory macrophages is associated with cytotoxicity. Vet Res. 2012;43:32. doi: 10.1186/1297-9716-43-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beernaert LA, Pasmans F, Baert K, Van Waeyenberghe L, Chiers K, Haesebrouck F, et al. et al. Designing a treatment protocol with voriconazole to eliminate Aspergillus fumigatus from experimentally inoculated pigeons. Vet Microbiol. 2009;139:393–397. doi: 10.1016/j.vetmic.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Cacciuttolo E, Rossi G, Nardoni S, Legrottaglie R, Mani P. Anatomopathological aspects of avian aspergillosis. Vet Res Commun. 2009;33:521–527. doi: 10.1007/s11259-008-9199-7. [DOI] [PubMed] [Google Scholar]
- 8.Templeton SP, Buskirk AD, Green BJ, Beezhold DH, Schmechel D. Murine models of airway fungal exposure and allergic sensitization. Med Mycol. 2010;48:217–228. doi: 10.3109/13693780903420658. [DOI] [PubMed] [Google Scholar]
- 9.Mutua PM, Gicheru MM, Makanya AN, Kiama SG. Comparative quantitative and qualitative attributes of the surface respiratory macrophages in the domestic duck and the rabbit. Int J Morphol. 2011;29:353–362. [Google Scholar]
- 10.Manafi H, Bagheri H. Coccidiosis in combination with Aspergillus ochraceus (NRRL 3174) in commercial broiler chicks. Res Opin Anim Vet Sci. 2011;1(Suppl):S33–S35. [Google Scholar]
- 11.Olias P, Gruber AD, Hafez HM, Lierz M, Slesiona S, Brock M, et al. et al. Molecular epidemiology and virulence assessment of Aspergillus fumigatus isolates from white stork chicks and their environment. Vet Microbiol. 2011;148:348–355. doi: 10.1016/j.vetmic.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Beernaert LA, Pasmans F, Van Waeyenberghe L, Haesebrouck F, Martel A. Aspergillus infections in birds: a review. Avian Pathol. 2010;39(5):325–331. doi: 10.1080/03079457.2010.506210. [DOI] [PubMed] [Google Scholar]
- 13.Sharma VD, Sethi MS, Joshi HC. Acute aspergillosis in chick. Indian Vet J. 1979;56:151–152. [Google Scholar]
- 14.Ononiwu JC, Momoh MA. An outbreak of aspergillosis in turkey poults. Bull Anim Health Prod Africa. 1983;31:75–77. [Google Scholar]
- 15.Perelman B, Kuttin ES. Aspergillosis in ostriches. Avian Pathol. 1992;21:159–163. doi: 10.1080/03079459208418830. [DOI] [PubMed] [Google Scholar]
- 16.Reissig EC, Uzal FA, Schettino A, Robles CA. Pulmonary aspergillosis in a great rhea (Rhea americana) Avian Dis. 2002;46:754–756. doi: 10.1637/0005-2086(2002)046[0754:PAIAGR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez-Perez S, Mateos A, Dominguez L, Martinez-Nevado E, Blanco JL, Garcia ME. Polyclonal Aspergillus fumigatus infection in captive penguins. Vet Microbiol. 2010;144:444–449. doi: 10.1016/j.vetmic.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Ghori HM, Edgar SA. Comparative susceptibility of chickens, turkeys, and coturnix quail to aspergillosis. Poult Sci. 1973;52:2311–2315. doi: 10.3382/ps.0522311. [DOI] [PubMed] [Google Scholar]
- 19.Perrott JK, Armstrong DP. Aspergillus fumigatus densities in relation to forest succession and edge effects: Implications for wildlife health in modifie environments. Ecohealth. 2011;8(3):290–300. doi: 10.1007/s10393-011-0716-8. [DOI] [PubMed] [Google Scholar]
- 20.Saif YM, Fadly AM, Glisson JR. Diseases of poultry. USA: Blackwell Publishing; 2008. pp. 1163–1179. [Google Scholar]
- 21.Coulston F, Manwell RD. Successful chemotherapy of a virus disease of the canary. Am J Vet Res. 1941;2:101. [Google Scholar]
- 22.Beernaert LA, Pasmans F, Haesebrouck F, Martel A. Modelling Aspergillus fumigatus infections in racing pigeons (Columba livia domestica) Avian Pathol. 2008;37:545–549. doi: 10.1080/03079450802382280. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Perez S, Blanco JL, Alba P, Garcia ME. Mating type and invasiveness are significantly associated in Aspergillus fumigatus. Med Mycol. 2010;48:273–277. doi: 10.1080/13693780903095414. [DOI] [PubMed] [Google Scholar]
- 24.Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobsen ID, Grosse K, Slesiona S, Hube B, Berndt A, Brock M. Embryonated eggs as an alternative infection model to investigate Aspergillus fumigatus virulence. Infect Immun. 2010;78:2995–3006. doi: 10.1128/IAI.00268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verstappen FALM, Dorrestein GM. Aspergillosis in Amazon parrots after corticosteroid therapy for smoke-inhalation injury. J Avian Med Surg. 2005;19(2):138–141. [Google Scholar]
- 27.Bain JM, Tavanti A, Davidson AD, Jacobsen MD, Shaw D, Gow NAR, et al. et al. Multilocus sequence typing of the pathogenic fungus Aspergillus fumigates. J Clin Microbiol. 2007;45:1469–1477. doi: 10.1128/JCM.00064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tell LA. Aspergillosis in mammals and birds: impact on veterinary medicine. Med Mycol. 2005;43:71–73. doi: 10.1080/13693780400020089. [DOI] [PubMed] [Google Scholar]
- 29.Kiama SG, Adekunle JS, Maina JN. Comparative in vitro study of interactions between particles and respiratory surface macrophages, erythrocytes, and epithelial cells of the chicken and the rat. J Anat. 2008;213:452–463. doi: 10.1111/j.1469-7580.2008.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


