Abstract
Objective
To describe the occurrence of various spinal deformations in a captive-bred wild line of Poecilia wingei (P. wingei).
Methods
Fish belonging to a wild line of P. wingei caught from Laguna de Los Patos, Venezuela, were bred in an aquarium home-breeding system during a period of three years (2006-2009). The spinal curvature was observed to study spinal deformities in P. wingei.
Results
Out of a total of 600 fish, 22 showed different types of deformities (scoliosis, lordosis, kyphosis), with a higher incidence in females. Growth, swimming and breeding of deformed fish were generally normal.
Conclusions
Possible causes for spinal curvature in fish are discussed on the basis of the current literature. While it is not possible to determine the exact cause(s) of spinal deformities observed in the present study, traumatic injuries, nutritional imbalances, genetic defects or a combination of these factors can be supposed to be involved in the pathogenesis of such lesions.
Keywords: Poecilia wingei, Spinal deformities, Scoliosis, Lordosis, Kyphosis
1. Introduction
Skeletal deformities are commonly encountered in both cultured and wild fish[1]–[8], with a higher frequency in hatchery populations. Such anomalies can cause economic loss to fish farmers; in addition, when occurring in wild species, they are used as indicators of water pollution because of their high incidence in polluted areas[7], [9]–[11]. Evidence suggest that such abnormalities are induced during the embryonic and post-embryonic periods of life and it has been proposed that the condition has a multifactorial aetiology[12]. Spinal malformations are the most frequent type of deformity seen in fish, mainly represented by dorso-ventral deviation (kyphosis and lordosis) or curvature in the coronal plane (scoliosis), which can be variably associated. Affected fish do not usually swim efficiently, are less capable of acquiring food, are at a greater risk of predation, as well as are more susceptible to physiological imbalances[2]. To the best of our knowledge, occurrence of spinal deformations in Poecilia wingei (P. wingei)[13], an endemic little livebearer from Laguna de Los Patos, Laguna La Malaguena, and Laguna Buena Vista in the North of Venezuela, has not yet been reported. In this survey, we report several cases of spinal deformities occurring in a wild line of P. wingei bred in a home fish farm during a period of three years. Discussion on the possible causes for spinal curvature in fish is also made on the basis of a literature review.
2. Materials and methods
Fish specimens, belonging to a wild line of P. wingei caught from Laguna de Los Patos near the city of Cumaná, Venezuela, by Dr. Roman Slaboch in 2001 and successively bred in Italy by one of the authors, were collected from an aquarium home-breeding system during a period of three years (2006-2009). Four different tanks were used: one was represented by a planted 200 L aquarium (pH 7.9; Gh 10; Kh 6; PO4−3 0.3 mg/L; O2 7 mg/L; CO2 10 mg/L; Fe 0.07 mg/L; NO2−1 0 mg/L; NO3−1 7.5 mg/L), whereas 3 tanks (50 L) were used for breeding, as well as fry growing (pH 7.4; Gh 8-8.5; Kh 5; PO4−3 0.5 mg/L; O2 7-8 mg/L; NO2−1 0 mg/L; NO3−1 12.5 mg/L). Each aquarium was equipped with its own internal filtering, heating (26 °C) and water pump systems. A weekly 50% water change and 2 times a day feeding were also applied, using frozen Artemia salina, as well as a wide range of high quality dry foods, supplemented with vitamins A, C, D3, E, highly unsaturated fatty acids and beta glucan. Since the spine of P. wingei is visible without magnification, fish were evaluated for curvature from the side and above while in a glass tank and then photographed with a digital camera (Nikon Coolpix E5200).
3. Results
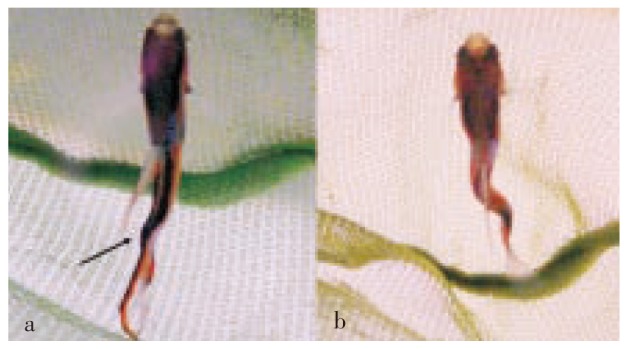
Out of a total of 600 fish, 22 (3.6%) showing spinal deformations were detected: 2 fry, 2 adult males, and 18 adult females. The predominant type of spinal abnormalities was scoliosis, which was observed in 20 fish (2 fry, 2 males, 16 females) with variable degree of curvature (Figure 1 and 2). Of 18 adult fish with scoliosis, 1 female also had secondary kyphosis (Figure 3a), 2 females also had secondary lordosis (Figure 3b), whereas 2 females also showed both kyphosis and lordosis. Two females only exhibited primary lordosis. All but 1 fish were born with apparently normal spines and developed scoliosis within the 2-3 weeks past birth, whereas lordosis and kyphosis only became macroscopically evident after sexual maturity. Curvatures were generally observed in the posterior half of the spinal column. All but 1 fish showed normal growth, swimming and breeding behaviour. A unique female fish born with scoliosis and lordosis displayed abnormal swimming, characterized by a vertical position, which did not prevent it from acquiring food. However, it did not appear to be able to reproduce.
Figure 1. P. wingei.
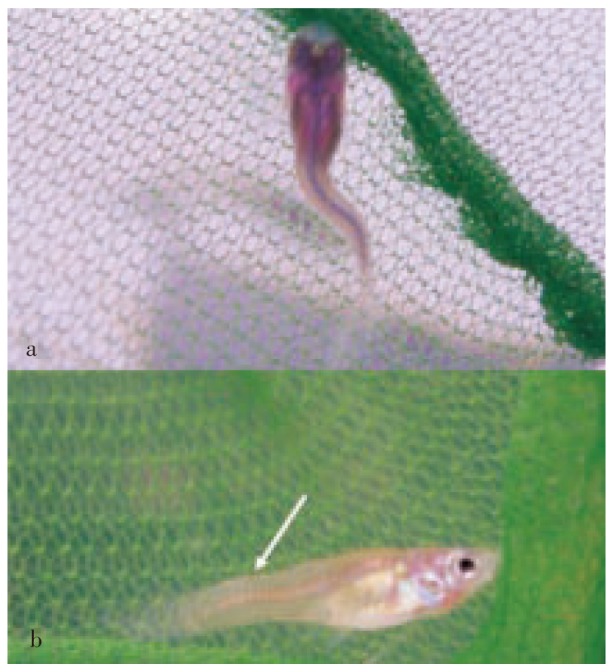
a: Dorsal; b: lateral (arrow) view of a fish fry showing severe scoliosis.
Figure 2. P. wingei.
Two adult males showing a: mild (arrow) b: severe scoliosis.
Figure 3. P. wingei.

a: Adult pregnant female showing kyphosis (arrow); b: Adult female showing lordosis (arrow); this fish displayed abnormal swimming.
4. Discussion
Spinal deformities in fish can be caused by a variety of injuries which can be classified as physical, environmental, nutritional, infectious, and genetic/heritable[14]. In addition, many experimental conditions have been shown to be able to induce skeletal malformations, such as pinealectomy, exposure to radiations, or electricity[15]–[17]. The persistent request of new fish varieties has also leaded to selective breeding of several lineages showing characteristic spinal abnormalities such as the so called “Balloon Kissing Gourami” (Helostoma temminkii, Cuvier 1829) “Balloon Molly” (Poecilia velifera, Regan 1914), “Blood Parrot Cichlid” (Heros severus x Amphilophus citrinellus hybrid)[18] and ornamental goldfish (Carassius auratus L.).
Several chemical/physical water parameters are known to be responsible for spinal malformations in fish[19], including pH and heat shock[12],[20], low dissolved oxygen[21], herbicides, organophosphate and organochlorine pesticides[22], heavy metals[11],[23]–[28] and other pollutants[11],[29]–[32]. These potential causes do not seem to be related to our cases since the water of the farm was previously filtered in a reverse osmotic system in order to block heavy metals and other potential dangerous chemical agents, and then added with specific salts and water conditioner (Sera Aquatan®). The physical/chemical parameters were also weekly measured (Hanna ph®, Tetra Tetratest®) and the temperature was controlled by means of electronic water heaters.
As far as microbial diseases are concerned, fish mycobacteriosis caused by Mycobacterium marinum, Mycobacterium fortuitum and Mycobacterium chelonei has been recognized to be responsible for the development of spinal defects. Other external clinical signs include anorexia, lethargy, emaciation, tendency to remain in one corner of the aquarium tank, exophthalmus, scale loss and dermal ulceration, pigmentary changes, and ascites[33]. On the other hand, the most important parasitic infection known to be associated with the induction of spinal deformities is that caused by Myxobolus spp. In particular, Myxobolus acanthogobii (Myxobolus buri) is a myxosporean parasite recognized to cause scoliosis of cultured yellowtail Seriola quinqueradiata and Japanese mackerel Scomber japonicus[34], whereas Myxobolus cerebralis, one of the best known parasites of salmonids, is the causative agent of “Whirling disease”[35], which is characterized by deformed head and spine due to cartilage necrosis, as well as by pigmentation changes (“Black tail”) due to damage to sympathetic nerves adjacent to spine.
A strong association between vaccine side effects and spinal deformity in harvest-sized Atlantic salmon, Salmo salar L. has also been reported[36],[37]. In addition, multiple stressful handling procedures, usually occurring in intensive farming conditions of salmon aquaculture, can cause extreme mechanical loading to the spine, which may potentially induce local inflammation leading to changes in the normal pattern of growth and remodelling of the spine, that end in a spinal deformity[38]–[40].
Nutritional imbalances are other factors which can be involved in the development of gill, operculum and spinal malformations in fish[41]. The most important are vitamin C[1],[42]–[45], aminoacidic (tryptophan)[45] and phosphorous[46],[47] deficiencies. On the contrary, excess of vitamin A is known to induce skeletal abnormalities in fish and such vitamin A-induced lesions also represent a popular model for studying the development of skeleton in fish larvae[48]. In our survey an aetiology linked to vitamin C imbalance cannot be excluded since a vitamin analysis was not performed and there is no information about the request of vitamin C in growing fry of the Family of Poeciliidae. However, vitamin C enriched high quality foods were used to feed fish in our study in order to avoid the occurrence of vitamin C deficiency. At the same time, such deficiency also causes decrease in collagen content preventing the formation of physiologically normal cartilage[44], as well as jaw, snout and operculum deformities associated with distortion of gill filaments in young, rapidly growing fish[45]. In our survey, we did not observe skeletal abnormalities other than spinal deformities.
Other causes inducing vertebral deformity in fish are represented by the absence of a functional swim bladder[49], as well as by traumatic injuries, which may occur during capture and/or transport[44]. Traumas may also be related to a strong water current[12]. Such traumatic aetiology must be considered for our cases, particularly when scoliosis is concerned, even though the water flowing was obtained with a regulatable recirculation system based on water pumps. In particular, a 640 L/h pump was used for the biotopic 200 L tank, whereas for each of three 50 L breeding tanks a manually downregulated 360 L/h pump was applied. In this connection, it is important to underline that we did not observe the occurrence of spinal abnormalities among a total population of about 290 fry (age from 1 to 70 days) of P. wingei bred in an external planted pond without water pumps during every summer (from June to September) from 2006 to 2009 (data not shown).
Finally, a genetic basis has also been proposed for spinal malformations[50]–[55], since spontaneous spinal curvature mutants exist. Particularly, the so-called Mutant Guppy (P. reticulata) Syndrome Curveback, characterized by a primary sagittal lordosis with some individual exhibiting posterior kyphosis and /or coronal deviation, appears to be a possible animal model to study the pathogenesis of human idiopathic scoliosis. This Curveback lineage, originated from a curved male crossed to a normal female, followed by full-sib mating, showed a female bias for curves of high magnitude[48]. It is interesting to notice that both founders were from a population collected in Cumaná, Venezuela[57] which has been proposed to be an established local population of P. wingei[13]. In addition, malformations due to a genetic alteration do not seem to prevent fish from achieving a normal size[58].
In conclusion, even though the exact cause of spinal deformities observed in the present study can not be determined, traumatic injuries, nutritional imbalances, genetic defects or a combination of these factors can be supposed to be involved in the pathogenesis of such lesions in this captive-bred wild line of P. wingei.
Comments
Background
This study describes the occurrence of spinal deformities of a wild line of P. wingei bred in aquarium-home breeding system for three years. Skeletal deformities in experimental fish are frequently used as indicators for different pollutions and toxic exposures. It is necessary to understand and quantify the type and degree of deformities naturally present in the experimental model fish. In this context this study will provide a naturally occurring deviation from the normal morphology of vertebral curvature in fish.
Research frontiers
Studies on the spinal deformities of wild fish kept in aquarium-home system is going to generate a new set of information which will be helpful to quantify the frequency of naturally occurring deformities from those developed due to exposure to different toxic substances.
Related Reports
The materials and methods and results of the present study indicate the deformities which have already been described by many workers. However, other workers have reported these studies in face of exposure to different pollutants.
Innovations and breakthroughs
No previous study described the morphological changes in aq wild species in environmentally controlled aquarium- home system.
Applications
This study is generating an important data which may be helpful to evaluate the pollution induced deformities keeping in view the skeletal abnormalities which may occur spontaneously in captive-bred fish without any exposure to toxic substances/polutants.
Peer review
It is a good study with interesting and novel information about skeletal deformities in a wild fish species kept in aquarium environment. The results will be helpful to evaluate the abnormalities occurring by toxic substances experimental studies made on this of other fish species.
Footnotes
Foundation Project: Supported by a grant from University of Teramo (Research Projects 60%/2009).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Al-Harbi AH. Skeletal deformities in cultured Common carp Cyprinus carpio L. Asian Fish Sci. 2001;14:247–254. [Google Scholar]
- 2.Silverstone AM, Hammel L. Spinal deformities in farmed Atlantic salmon Salmo salar. Can Vet J. 2002;43:782–784. [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan M, Hammond G, Roberts RJ, Manchester NJ. Spinal deformation in commercially cultured Atlantic salmon, Salmo salar L.: a clinical and radiological study. J Fish Dis. 2007;30(12):745–752. doi: 10.1111/j.1365-2761.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 4.Aunsmo A, Øvretveit S, Breck O, Valle PS, Larssen RB, Sandberg M. Modelling sources of variation and risk factors for spinal deformity in farmed Atlantic salmon using hierarchical- and cross-classified multilevel models. Prevent Vet Med. 2009;90:137–145. doi: 10.1016/j.prevetmed.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Yadegari M, Raissy M, Ansari M. A radiographical study on skeletal deformities in cultured rainbow trout (Oncorhynchus mykiss) in Iran. Global Veterinaria. 2011;7(6):601–604. [Google Scholar]
- 6.Messaudi I, Kessabi K, Kacem A, Saïd K. Incidence of spinal deformities in natural populations of Aphanius fasciatus Nardo, 1827 from the Gulf of Gabes, Tunisia. Afr J Ecol. 2009;47(3):360–366. [Google Scholar]
- 7.Chang CW, Wangm YT, Tzeng WN. Morphological study on vertebral deformity of the thornfish Terapon jarbua in the thermal effluent outlet of a nuclear power plant in Taiwan. J Fish Soc Taiwan. 2010;37(1):1–11. [Google Scholar]
- 8.Alaya HB, Galzin R, Quignard JP, Trabelsi M. Spinal deformities in the black-striped pipefish Syngnathus abaster (Pisces, Syngnathidae) from the Tunis North Lake, Tunisia. Chemosphere. 2011;82(3):318–320. doi: 10.1016/j.chemosphere.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 9.Yershov PN. The vertebral abnormalities in eelpout Zoarces viviparus (Linnaeus, 1758) (Pisces, Zoarcidae) Proc Zool Inst RAS. 2008;312(1/2):74–82. [Google Scholar]
- 10.Kessabi K, Navarro A, Casado M, Saïd K, Messaoudi I, Piña B. Evaluation of environmental impact on natural populations of the Mediterranean killifish Aphanius fasciatus by quantitative RNA biomarkers. Mar Environ Res. 2010;70:327–333. doi: 10.1016/j.marenvres.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Kessabi K, Annabi A, Navarro A, Casado M, Hwas Z, Saïd K, et al. et al. Structural and molecular analysis of pollution-linked deformities in a natural Aphanius fasciatus (Valenciennes, 1821) population from the Tunisian coast. J Environ Monit. 2012;14(8):2254–2260. doi: 10.1039/c2em30329a. [DOI] [PubMed] [Google Scholar]
- 12.Eissa AE, Moustafa M, El-Husseiny IN, Saeid S, Saleh O, Borhan T. Identification of some skeletal deformities in freshwater teleosts raised in Egyptian aquaculture. Chemosphere. 2009;77:419–425. doi: 10.1016/j.chemosphere.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 13.Poeser FN, Kempkes M, Insbrucker IJH. Description of Poecilia (Acanthophacelus) wingei n.sp. from the Paria peninsula, Venezuela, including notes on Acanthophacelus Eigenmann, 1907 and other subgenera of Poecilia Bloch and Schneider, 1801 (Teleostei, Cyprinodontiformes, Poecilidae) Contrib Zool. 2005;74:97–115. [Google Scholar]
- 14.Gormann KF, Breden F. Teleosts as model for human vertebral stability and deformity. Comp Biochem Physiol, Part C. 2007;145:28–38. doi: 10.1016/j.cbpc.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Fjelldal PG, Grotmol S, Kryvi H, Gjerdet NR, Taranger GL, Hansen T, et al. et al. Pinealectomy induces malformation of the spine and reduces the mechanical strength of the vertebrae in Atlantic salmon, Salmo salar. J Pineal Res. 2004;36(2):132–139. doi: 10.1046/j.1600-079x.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 16.Schroder H. Inheritance of radiation-induced spinal curvature in the guppy Lebistes reticulata. Can J Genet Citol. 1969;11:937–947. doi: 10.1139/g69-109. [DOI] [PubMed] [Google Scholar]
- 17.DeVore PW, Eaton JG. An investigation of spinal deformities of trout, Salmo sp. in the Brule river, Wisconsin. J Great Lakes Res. 1983;9:69–73. [Google Scholar]
- 18.Bassler G. Belgium: Bassler biofish, West Meerbeek; 2006. The new illustrated guide to fish diseases. [Google Scholar]
- 19.Davidson J, Good C, Welsh C, Summerfelt ST. Abnormal swimming behavior and increased deformities in rainbow trout Oncorhynchus mykiss cultured in low exchange water recirculating aquaculture systems. Aquacult Eng. 2011;45:109–117. [Google Scholar]
- 20.Ytteborg E, Baeverfjord G, Torgersen J, Hjelde K, Takle H. Molecular pathology of vertebral deformities in hyperthermic Atlantic salmon (Salmo salar) BMC Physiol. 2010;10:12. doi: 10.1186/1472-6793-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro SR, Bustos Obregon E, Rojas RM. Vertebral column deformity and hypoxia in Salmo salar. Int J Morphol. 2011;29(4):1291–1295. [Google Scholar]
- 22.Çelik ES, Kaya H, Yılmaz S. Effects of phosalone on mineral contents and spinal deformities in common carp (Cyprinus carpio, L. 1758) Turk J Fish Aquat Sci. 2012;12:259–264. [Google Scholar]
- 23.Messaoudi I, Deli T, Kessabi K, Barhoumi S, Kerkeni A, Saïd K. Association of spinal deformities with heavy metal bioaccumulation in natural populations of grass goby, Zosterisessor ophiocephalus Pallas, 1811 from the Gulf of Gabès (Tunisia) Environ Monit Assess. 2009;156(1–4):551–560. doi: 10.1007/s10661-008-0504-2. [DOI] [PubMed] [Google Scholar]
- 24.Kessabi K, Kerkeni A, Saïd K, Messaoudi I. Involvement of Cd bioaccumulation in spinal deformities occurrence in natural populations of Mediterranean killifish. Biol Trace Elem Res. 2009;128:72–81. doi: 10.1007/s12011-008-8255-z. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Cao L, Liu J, Lin L, Dou S. Short-term mercury exposure affecting the development and antioxidant biomarkers of Japanese flounder embryos and larvae. Ecotoxicol Environ Saf. 2010;73:1875–1883. doi: 10.1016/j.ecoenv.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Sassi A, Annabi A, Kessabi K, Kerkeni A, Saïd K, Messaoudi I. Influence of high temperature on cadmium-induced skeletal deformities in juvenile mosquitofish (Gambusia affinis) Fish Physiol Biochem. 2010;36(3):403–409. doi: 10.1007/s10695-009-9307-9. [DOI] [PubMed] [Google Scholar]
- 27.Ayed N, Faure E, Quignard JP, Trabelsi M. Determination of P, Ca, Zn, Cd and Pb concentrations in muscle, gills, liver, gonads and skeletons of two natural populations of Atherina lagunae in North Tunis Lake, Tunisia. JWARP. 2011;3:421–428. [Google Scholar]
- 28.Barjhoux I, Baudrimont M, Morin B, Landi L, Gonzalez P, Cachot J. Effects of copper and cadmium spiked-sediments on embryonic development of Japanese medaka (Oryzias latipes) Ecotoxicol Environ Saf. 2012;79:272–282. doi: 10.1016/j.ecoenv.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Madureira TV, Cruzeiro C, Rocha MJ, Rocha E. The toxicity potential of pharmaceuticals found in the Douro River estuary (Portugal)-Experimental assessment using a zebrafish embryo test. Environ Toxicol Pharmacol. 2011;32:212–217. doi: 10.1016/j.etap.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Zhou Q, Li H, Liu W, Wang T, Jiang G. Effects of silver nanoparticles on the development and histopathology biomarkers of Japanese medaka (Oryzias latipes) using the partial-life test. Aquat Toxicol. 2010;100:160–167. doi: 10.1016/j.aquatox.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Adema-Hannes R, Shenker J. Acute lethal and teratogenic effects of tributyltin chloride and copper chloride on mahi mahi (Coryphaena hippurus) eggs and larvae. Environ Toxicol Chem. 2008;27(10):2131–2135. doi: 10.1897/07-369.1. [DOI] [PubMed] [Google Scholar]
- 32.Boudreau M, Sweezey MJ, Lee K, Hodson PV, Courtenay SC. Toxicity of Orimulsion-400 to early life stages of Atlantic herring (Clupea harengus) and mummichog (Fundulus heteroclitus) Environ Toxicol Chem. 2009;28(6):1206–1217. doi: 10.1897/08-280.1. [DOI] [PubMed] [Google Scholar]
- 33.Gauthier DT, Rhodes MW. Mycobacteriosis in fishes: a review. Vet J. 2009;180:33–47. doi: 10.1016/j.tvjl.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama H, Freeman MA, Itoh N, Fukud Y. Spinal curvature of cultured Japanese mackerel Scomber japonicus associated with a brain myxosporean, Myxobolus acanthogobii. Dis Aquat Org. 2005;66:1–7. doi: 10.3354/dao066001. [DOI] [PubMed] [Google Scholar]
- 35.Roberts RJ. Fish pathology. London: W.B.Saunders; 2001. [Google Scholar]
- 36.Aunsmo A, Guttvik PJ, Midtlyng RB, Larssen O, Skjerve E. Association of spinal deformity and vaccine-induced abdominal lesions in harvest-sized Atlantic salmon, Salmo salar L. J Fish Dis. 2008;31:515–524. doi: 10.1111/j.1365-2761.2007.00899.x. [DOI] [PubMed] [Google Scholar]
- 37.Berg A, Yurtseva A, Hansen T, Lajus D, Fjelldal PG. Vaccinated farmed Atlantic salmon are susceptible to spinal and skull deformities. J Appl Ichthyol. 2012;28(3):446–452. [Google Scholar]
- 38.Gil Martens L, Lock EJ, Fjelldal PG, Wargelius A, Araujo P, Torstensen BE, et al. et al. Dietary fatty acids and inflammation in the vertebral column of Atlantic salmon, Salmo salar L., smolts: a possible link to spinal deformities. J Fish Dis. 2010;33(12):957–972. doi: 10.1111/j.1365-2761.2010.01201.x. [DOI] [PubMed] [Google Scholar]
- 39.Gil-Martens L. Inflammation as a potential risk factor for spinal deformities in farmed Atlantic salmon (Salmo salar L.) J Appl Ichthyol. 2010;26(2):350–354. [Google Scholar]
- 40.Gil-Martens L, Fjelldal PG, Lock EJ, Wargelius A, Wergeland H, Witten PE, et al. et al. Dietary phosphorus does not reduce the risk for spinal deformities in a model of adjuvant-induced inflammation in Atlantic salmon (Salmo salar) postsmolts. Aquacult Nutr. 2012;18:12–20. [Google Scholar]
- 41.Cahu C, Infante Z, Takeuchi T. Nutritional components affecting skeletal development in fish larvae. Aquaculture. 2003;227:245–258. [Google Scholar]
- 42.Darias MJ, Mazurais D, Koumoundouros G, Cahu CL, Zambonino-Infante JL. Overview of vitamin D and C requirements in fish and their influence on the skeletal system. Aquaculture. 2011;315(1-2):49–60. [Google Scholar]
- 43.Zhou Q, Wang L, Wang H, Xie F, Wang T. Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum) Fish Shellfish Immunol. 2012;32(6):969–975. doi: 10.1016/j.fsi.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 44.Preziosi R, Gridelli S, Borghetti P, Diana A, Parmeggiani A, Fioravanti ML, et al. et al. Spinal deformity in a sandtiger shark, Carcharias taurus Rafinesque: a clinical-pathological study. J Fish Dis. 2006;29:49–60. doi: 10.1111/j.1365-2761.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 45.Lall SP. Disorders of nutrition and metabolism. In: Leatherland JF, Woo PTK, editors. Fish diseases and disorders. Vol. 2: Non-infectious disorders. 2nd ed. Oxfordshire: CAB International; 2010. pp. 202–237. [Google Scholar]
- 46.Sullivan M, Reid SW, Ternent H, Manchester NJ, Roberts RJ, Stone DA, et al. et al. The aetiology of spinal deformity in Atlantic salmon, Salmo salar L.: influence of different commercial diets on the incidence and severity of the preclinical condition in salmon parr under two contrasting husbandry regimes. J Fish Dis. 2007;30(12):759–767. doi: 10.1111/j.1365-2761.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 47.Fjelldal PR, Hansen T, Albrektsen S. Inadequate phosphorus nutrition in juvenile Atlantic salmon has a negative effect on long-term bone health. Aquaculture. 2012;334-337:117–123. [Google Scholar]
- 48.Haga Y, Du SJ, Satoh S, Kotani T, Fushimi H, Takeuchi T. Analysis of the mechanism of skeletal deformity in fish larvae using a vitamin A-induced bone deformity model. Aquaculture. 2011;315(1-2):26–33. [Google Scholar]
- 49.Chatain B. Abnormal swimbladder development and lordosis in sea bass (Dicentrarchus labrax) and sea bream (Sparus auratus) Aquaculture. 1994;119:371–379. [Google Scholar]
- 50.Kolstad K, Thorland I, Refstie T, Gjerde B. Body weight, sexual maturity, and spinal deformity in strains and families of Atlantic cod (Gadus morhua) at two years of age at different locations along the Norwegian coast. ICES J Mar Sci. 2006;63:246–252. [Google Scholar]
- 51.Sullivan M, Guy D, Roberts RJ, Manchester NJ. The aetiology of spinal deformity in Atlantic salmon, Salmo salar L.: influence of genetic factors on the frequency and severity in freshwaters stages. J Fish Dis. 2007;30:753–758. doi: 10.1111/j.1365-2761.2007.00888.x. [DOI] [PubMed] [Google Scholar]
- 52.Bardon A, Vandeputte M, Dupont-Nivet M, Chavanne H, Haffray P, Vergnet A, et al. et al. What is the heritable component of spinal deformities in the European sea bass (Dicentrarchus labrax)? Aquaculture. 2009;294(3-4):194–201. [Google Scholar]
- 53.Evans M, Neff BD. Non-additive genetic effects contribute to larval spinal deformity in two populations of Chinook salmon (Oncorhynchus tshawytscha) Aquaculture. 2009;296:169–173. [Google Scholar]
- 54.Grimmett SG, Chalmers HJ, Wolf JC, Bowser PR. Spinal deformity in triploid grass carp Ctenopharyngodon idella (Valenciennes) J Fish Dis. 2011;34(3):217–225. doi: 10.1111/j.1365-2761.2010.01229.x. [DOI] [PubMed] [Google Scholar]
- 55.Tomasiewicz HG, Liu XC, Tassone C, North P, Thometz J. A line of zebrafish with increased incidence of spinal deformities. Stud Health Technol Inform. 2012;176:454. [Google Scholar]
- 56.Gorman KF, Tredwell SJ, Breden F. The mutant guppy syndrome curveback as a model for human hereditable spinal curvature. Spine. 2007;32:735–741. doi: 10.1097/01.brs.0000259081.40354.e2. [DOI] [PubMed] [Google Scholar]
- 57.Alexander HJ, Breden F. Sexual isolation and extreme morphological divergence in the Cumaná guppy: a possible case of incipient speciation. J Evol Biol. 2004;17:1238–1254. doi: 10.1111/j.1420-9101.2004.00788.x. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Romero J, Càrdenas L, Pérez Urbiola JC, Hinohuye Rivera R, Silva Hernandez MA. A spinal column malformation in the creolefish Paranthias colonus (Osteichtyes: Serranidae) Rev Biol Trop. 2001;49:1267–1268. [PubMed] [Google Scholar]


