Abstract
Objective
To study the screening of essential oils of Skimmia laureola leaves (SLO) for acute toxicity, antinociceptive, antipyretic and anticonvulsant activities in various animal models.
Methods
SLO were extracted using modified Clevenger type apparatus. Acute toxicity test was used in mice to observe its safety level. Antinociceptive activity of SLO was evaluated in acetic acid induced writhing and hot plate tests. Yeast induced hyperthermic mice and pentylenetetrazole induced convulsive mice were used for the assessment of its antipyretic and anticonvulsant profile respectively.
Results
Substantial safety was observed for SLO in acute toxicity test. SLO showed a high significant activity in acetic acid induced writhing test in a dose dependent manner with maximum pain attenuation of 68.48% at 200 mg/kg i.p. However, it did not produce any relief in thermal induced pain at test doses. When challenged against pyrexia evoked by yeast, SLO manifested marked amelioration in hyperthermic mice, dose dependently. Maximum anti-hyperthermic activity (75%) was observed at 200 mg/kg i.p. after 4 h of drug administration. Nevertheless, SLO had no effect on seizures control and mortality caused by pentylenetetrazole.
Conclusions
In vivo studies of SLO showed prominent antinociceptive and antipyretic activities with ample safety profile and thus provided pharmacological base for the traditional uses of the plant in various painful conditions and pyrexia. Additional detail studies are required to ascertain its clinical application.
Keywords: Skimmia laureola leaves, Essential oils, Antinociceptive, Antipyretic activity
1. Introduction
Skimmia laureola (S. laureola) locally named as Nazar Panra belongs to family Rutaceae. In Pakistan, it is common in the Hazara region, Murree Hills, Kashmir, upper Swat and Shangla[1],[2]. Therapeutic values of the plant have been recognized in various cultural of the world. It has been used for the treatment of cold, fever and headache. Smoke of leaves and twig smoke are used as demon repellent[1]. In the form of smoke, dry leaves have been employed for nasal tract clearness. Additionally, leaves of the plant have been recommended for multiple purposes including relieve coughs[3], insecticides, and pesticides[5]. Leaves are commercially harvested and used in food as flavoring agent, in traditional healing and cultural practices, being made into garlands and considered sacred[4]. Essential oils extracted from the plant showed profound anthelmintic activity[6].
Phytochemically, fatty acid ester and quinoline alkaloid have been isolated from the plant with significant inhibition on tyrosinase[10]. Lupene derivatives, chromones, coumarines and triterpine have also been reported[11]–[13]. Three quinoline alkaloids (3-hydroxy, 2, 2, 6-trimethyl-3, 4, 5, 6-tetrahydro-2H-pyrano[3, 2-c] quinoline 5-one, ribalinine and methyl isoplatydesmine) were isolated from the aerial parts of the plant with prominent inhibition against acetylcholinesterase. Additional, methyl isoplatydesmine showed spasmolytic activity possibly mediated through the blockade of voltage-dependent Ca2+ channels[14].
Keeping in mind the outstanding ethno-pharmacological and phytochemical background of S. laureola, the current project was designed to extract the essential oils from the leaves of the plant followed by acute toxicity test, antinociceptive, antipyretic, and anticonvulsant activities in various animal models.
2. Materials and methods
2.1. Chemicals
Paracetamol (Tianjin Bofa Pharmaceutical Co., Lit., China), diclofenac sodium (Suzhou Ausun Chemical Co., Lit., China), acetic acid, Brewer's yeast (Merck Germany), tramadolR (Seale Pakistan Ltd.), and pentylenetetrazole (PTZ) (Sigma Chemical Co., St Louis, USA) were prepared.
2.2. Essential oil extraction
For the extraction of essential oil from the leaves of S. laureola, a modified Clevenger type apparatus was used. Briefly, the leaves of the plant were thoroughly washed, cut into small pieces, placed in distillation flask and subjected to hydro-steam distillation for about 4 h. The steam and vaporized oil were condensed into liquid by a vertical condenser and collected in measuring tube. The volatile oil being immiscible and lighter than water, separated out as an upper layer. The oil was then separated from water and collected in small bottles, dried with anhydrous sodium sulphate. The bottles were sealed, labeled and stored in light resistant vials at 4-6 °C for further use in various experiments[15].
2.3. Animals
NMRI mice were used in various experiments. They were fed with standard laboratory food and water ad libitum. Animals were kept under standard condition of temperature and light. Before the start of experiment, animals were acclimatized with laboratory conditions. All the protocols were approved by Ethics Committee of HEJ Research Institute Karachi. The rulings of the institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council were maintained during all the experiments performed[16].
2.3. Acute toxicity test
Acute toxicity test of essential oils from the leaves of S. laureola (SLO) was performed in NMRI mice (n=6) of either sex to evaluate any possible toxicity. The animals were tested by administering different doses (500, 1 000, 1 500 and 2 000 mg/kg) of SLO, while the control group received normal saline (10 mL/kg). All the groups were observed mortality during 24 h[17].
2.4. Antinociceptive activity
2.4.1. Acetic acid induced writhing test
The analgesic activity was carried out using NMRI mice (18-22 g) of either sex. Animals were divided into five groups (n=6). Group I and II were injected with normal saline (10 mL/kg, i.p.) and ibuprofen (150 mg/kg, i.p.), while remaining groups were treated with SLO (20, 100 and 200 mg/kg, i.p). After the above treatments, animals were injected i.p. with acetic acid (1%). The abdominal constriction (writhing) was counted for 10 min after 5 min of acetic acid injection[18],[19].
2.4.2. Hot plate test
NMRI mice of either sex (n=6) weighing (18-22) g were acclimatized to laboratory conditions for 1 h before the start of experiment with food and water available ad libitum. Animals were then subjected to pre-testing on hot plate (Havard apparatus) maintained at (55.0±0.1) °C. Animals having latency time greater than 15 seconds on hot plate during pre-testing were rejected (latency time). All the animals were grouped each of six. Group I was treated with saline (10 mL/kg), Group II was treated with tramadolR (30 mg/kg i.p). Group III, IV and V were treated with 50, 100 and 200 mg/kg SLO, i.p. respectively. After 30 min of treatment, the animals were placed on hot plate, and the latency time [time for which mouse remains on the hot plate at (55.0±0.1) °C without licking or flicking of hind limb or jumping] was measured in seconds. In order to prevent the tissue damage, a cut-off time of 30 seconds was imposed for all animals[20]. Percentage of analgesia was calculated using the following formula:
Analgesia (%)= (Test latency-Control latency)/(Cut-off time-Control latency)×100
2.5. Antipyretic test
The antipyretic activity was evaluated for SLO using NMRI mice (25-30 g) of either sex. The animals were divided into five groups with each of six. All groups were fasted overnight but allowed free accesses to drinking water. Group I received saline (10 mL/kg) as a negative control, Group II received paracetamol (150 mg/kg) as a standard drug, while the remaining Groups III, IV and V received 50, 100 and 200 mg/kg i.p. SLO respectively. Normal temperature was recorded using digital thermometer, and then pyrexia was induced in all mice using 20% aqueous suspension of Brewer's yeast (10 mL/kg s.c). Rectal temperature was recorded after 24 h and corresponding groups were injected with above doses accordingly[21]. After drugs administration, rectal temperature was again recorded periodically at 1, 2, 3, 4 and 5 h of drugs administration. The percentage of reduction in pyrexia was calculated by the following formula:
Reduction (%)=Ta-Tb/Tb×100
Where Ta represents normal body temperature; Tb temperature after yeast injection.
2.6. Pentylenetetrazole induced anticonvulsant test
The animals were divided into groups of at least six animals. Group I was treated with normal saline (10 mL/kg) as a control, Group II was treated with diazepam (5 mg/kg, i.p.), while remaining groups were treated with SLO (50, 100 and 200 mg/kg i.p). After 30 min of treatment, all the animals were injected PTZ 80 mg/kg s.c. Each animal was observed for onset and mortality[15].
2.7. Statistical analysis
Results were expressed as mean±SEM. One-way ANOVA was used for comparison test of significant differences among groups followed by Dunnet's multiple comparison post test. A level of significance (P<0.05 or P<0.01) was considered for each test.
3. Results
3.1. Acute toxicity test
SLO was found substantially safe in acute toxicity test. As shown in Table 1, gross behaviour changes were not observed even at all test doses (500, 1 000, 1 500 and 2 000 mg/kg i.p). All mice survived at 500 and 1 000 mg/kg. However, 16.67% and 66.67% mortality was noted at 1 500 and 2 000 mg/kg i.p. respectively.
Table 1. Effect of essential oil of S. laureola in acute toxicity test.
| Treatment | Dose | Gross behaviour effect | No. of animal died (n=6) | Mortality (%) |
| Normal saline | 10 mL/kg | No change | 0 | - |
| SLO | 500 mg/kg | No change | 0 | - |
| 1 000 mg/kg | No change | 0 | - | |
| 1 500 mg/kg | No change | 1 | 16.67 | |
| 2 000 mg/kg | No change | 4 | 66.67 |
3.2. Antinociceptive activity
3.2.1. Effect of SLO in acetic acid induced writhing test
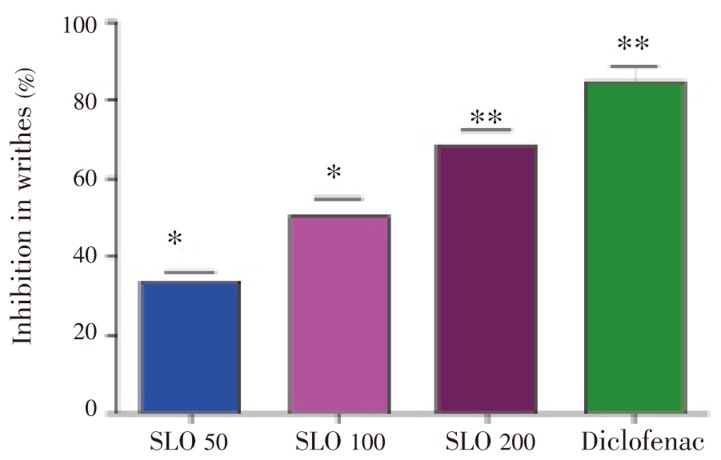
Results of SLO in acetic acid induced writhing test are presented in Table 2. Profound amelioration of abdominal constriction induced in acetic acid pain model was observed in a dose dependent manner. Maximum pain attenuation (68.48%) is noted at 200 mg/kg i.p. (Figure 1). Diclofenac was more prominent in its antinociceptive activity and showed 84.45% pain reversal at 30 mg/kg i.p.
Table 2. Effect of the essential oils of S. laureola in acetic acid-induced writhing in mice.
| Group | Dose | No of writhes |
| Saline | 10 mL/kg | 72.35±2.90 |
| SLO | 50 mg/kg | 48.10±2.35 |
| 100 mg/kg | 35.74±3.10* | |
| 200 mg/kg | 22.80±2.40** | |
| Diclofenac | 10 mg/kg | 11.25±2.50** |
Values were expressed as mean±SEM (n=6). *: P<0.05, **: P<0.01 indicated statistically significant values from control.
Figure 1. Protection (%) of essential oil of S. laureola in acetic acid induced writhing test.
3.2.2. Effect of SLO in hot plat test
The results of SLO in hot plate test are shown in Table 3. The effect was not significant even at the maximum test 200 mg/kg i.p. However, tramadolR used a standard drug, showed profound activity in hot plate test.
Table 3. Effect of essential oil of S. laureola in hot plate test.
| Groups | Dose | Latency time (min) |
|||
| 0 | 60 | 90 | 120 | ||
| Saline | 10 mL/kg | 9.17±0.09 | 9.16±0.05 | 9.19±0.45 | 9.12±0.88 |
| SLO | 50 mg/kg | 9.21±0.50 | 9.20±0.55 | 9.45±0.40 | 9.22±0.33 |
| 100 mg/kg | 9.25±0.70 | 9.28±0.33 | 10.11±0.66 | 9.90±0.44 | |
| 200 mg/kg | 9.29±0.76 | 10.34±0.45 | 10.90±0.77 | 10.20±0.55 | |
| TramadolR | 30 mg/kg | 9.20±0.02 | 25.88±0.06* | 25.80±0.07* | 25.77±0.00* |
Values were expressed as mean±SEM (n=6). *: P<0.001 indicated statistically significant values from control.
3.2.3. Effect of SLO in yeast induced hyperthermic mice
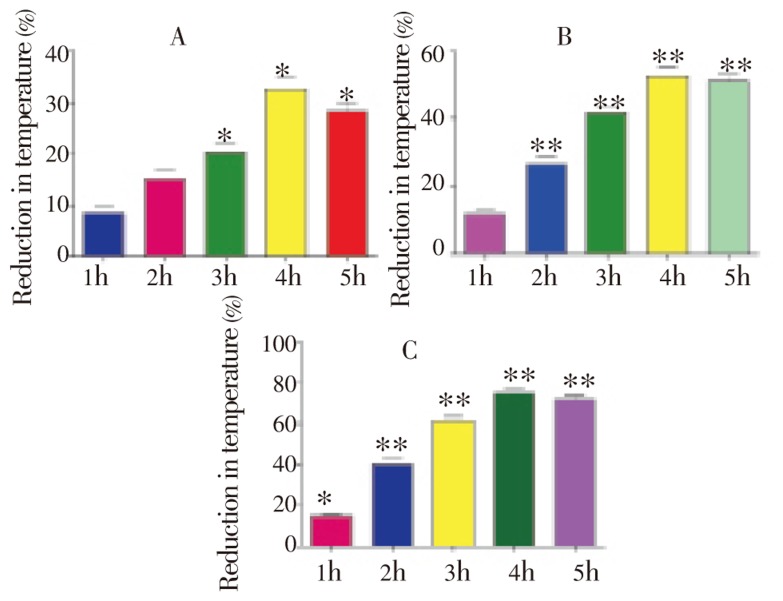
SLO manifested marked antipyretic activity in yeast induced hyperthermic mice as shown in Table 4. It showed profound protection (%) in a dose dependent manner (Figure 2). Maximum protection (80.03%) was observed at 200 mg/kg i.p. (Figure 2c) after 4 h of treatment.
Table 4. Effect of essential oil of S. laureola at 50, 100 and 200 mg/kg i.p. in yeast induced pyrexia.
| Drugs | Dose | Rectal temperature (°C) |
||||||
| Normal (A) | After yeast (B) | 1 h (C1) | 2 h (C2) | 3 h (C3) | 4 h (C4) | 5 h (C5) | ||
| Saline | 10 mL/kg | 37.10±0.17 | 38.44±0.30 | 38.49±0.15 | 38.65±0.10 | 38.80±0.15 | 38.68±0.17 | 38.55±0.21 |
| PRA | 150 mg/kg | 37.00±0.15 | 39.33±0.10 | 38.08±0.12** | 37.58±0.09** | 37.20±0.04** | 37.22±0.06** | 37.27±0.12** |
| SLO | 50 mg/kg | 37.12±0.17 | 39.60±0.22 | 39.39±0.12 | 39.30±0.02 | 39.10±0.15* | 38.79±0.19* | 38.90±0.16* |
| 100 mg/kg | 37.05±0.10 | 39.05±0.14 | 38.81±0.11 | 38.52±0.09** | 38.±1.55** | 38.00±0.15** | 38.03±0.45* | |
| 200 mg/kg | 37.00±0.09 | 39.76±0.23 | 39.34±0.55* | 38.95±0.25** | 38.07±0.35** | 37.69±0.10** | 37.77±0.45** | |
Values were expressed as mean±SEM (n=6). *: P<0.05, **: P<0.01 indicated statistically significant values from control. PRA: Paracetamol.
Figure 2. Protection (%) of SLO in yeast induced hyperthermic mice at A: 50 mg/kg, B: 100 mg/kg, C: 200 mg/kg during various assessment times (1-5 h).
Values are presented as mean±SEM for pyrexia control (n=6).
3.2.4. Effect of SLO in PTZ induced anticonvulsant test
The results of PTZ induced convulsion test are illustrated in Table 5. The results of pentylenetetrazole induced convulsion test are illustrated in Table 5. As shown in Table 5, SLO did not exhibit any protection from seizures and mortality at test doses (50, 100 and 200 mg/kg i.p). However, diazepam (7.5 mg/kg i.p.) being the standard drug, exhibited marked protection from both seizures and mortality.
Table 5. Effect of essential oil of S. laureola on duration and inhibition of seizures and mortality in acute s.c. PTZ-induced seizure model in mice.
| Groups | Dose | Onset of jerks (seconds) | Rearing & falling (seconds) | Hind limb tonic extension (seconds) | Mortality protection (%) |
| PTZ | 90 mg/kg | 210±35 | 465±65 | 850±65 | 0 |
| SLO | 50 mg/kg | 210±43 | 500±55 | 887±82 | 0 |
| 100 mg/kg | 217±65 | 514±35 | 889±58 | 0 | |
| 200 mg kg | 225±43 | 510±40 | 890±80 | 0 | |
| Saline | 0.5 mL/kg | − | − | − | 100 |
| Diazepam | 7.5 mg/kg | 850±23 | 0 | 0 | 100 |
SLO and diazepam were injected i.p. 45 min before the administration of 90 mg/kg of s.c. PTZ. Values were expressed as mean±SEM for the duration of tonic seizures (n=6).
4. Discussion
The present study demonstrated strong antinociceptive (peripherally acting) and antipyretic activities of SLO with substantial safety profile in acute toxicity test. However, it did not show any protection against seizures.
Acetic acid induced writhing test has been primarily used by various research groups for the assessment of antinociceptive of natural compounds worldwide[22],[23]. Acetic acid caused the release of different endogenous noxious mediators such as bradykinin, serotonin, histamine, and substance P[24],[25]. The resulting pain is symbolized by contraction of the abdominal muscle accompanied by an extension of the forelimbs and body elongation. Peripheral nociceptive fibers are sensitive to both narcotics analgesic and non-steroid anti-inflammatory drugs[18],[20]. SLO showed marked reduction in the abdominal constriction provoked by the acetic acid in a dose dependent manner. Consequently, one possible mechanism of antinociceptive activity of SLO could be due to the blockade of the effect or the release of endogenous substances (arachidonic acid metabolites) that excite pain nerve endings.
Hot plate test is principally a spinal reflex and used to test supra-spinal analgesic activity of test articles. For that reason, this test is generally employed for the assessment of centrally acting analgesic drugs, like morphine, while peripheral anti-nociceptive agents are found to be inactive on thermal stimulus[18]. When SLO was subjected to the challenge of hot plate test, it did not show any thermal pain attenuation at test doses. Thus, it could be assumed that the antinociceptive action of SLO was purely mediated through blockade of peripheral pain stimulant without the any involvement of central action.
Fever is the primary feature of disease since time immemorial. The febrile response is coordinated by the central nervous system through endocrine, neurological, immunological and behavioural mechanisms. The initiation, manifestations and regulation of the febrile response are dependent on the pyrogenic and anti-pyrogenic properties of various exogenous and endogenous substances. Medical experts believe that fever is based on consistent rise in body temperature above normal daily fluctuations originating in combination with an elevated thermoregulatory set point[21],[28]. These neurons are sensitive not only to changes in blood temperature but also to cold and warm receptors located in skin and muscle and thus maintain an appropriate balance between the heat production and loss[29].
SLO exhibited profound antipyretic activity against yeast evoked hyperthermic mice when administered intraperitoneally during various assessment times (1-5 h). Pyrexia amelioration was in a dose dependent manner and remained significant up to 5th hour of drug administration. The nonsteroidal antiinflammatory drugs (NSAID) are the drug of choice for the control of febrile in routine practice. Over a period of time after thorough investigation, it is believed that NSAID inhibits prostaglandins synthesis via cyclooxygenase pathway[30]. For that reason, SLO might contain active principle(s) that exhibited inhibitory action on cyclooxygenase. As a result, they produced antipyretic activity by preventing the formation of prostaglandins or by increasing the concentration of body's own antipyretic components[22].
The intraperitoneal administration of SLO did not show any protection from seizures and mortality at various test doses in PTZ induced convulsion test. Consequently, it suggests the absence of such components could exhibit anticonvulsant activity.
In short, SLO showed prominent peripheral antinociceptive and antipyretic activity in animal studies without any anticonvulsant effect. SLO exhibited substantial safety profile in acute toxicity test. Our findings are consistent with the ethnopharmacological uses of the plant in various painful conditions and as antipyretic and thus offered an alternate natural therapeutic modality with considerable safety profile. However, detail studies on the efficacy, potency and safety aspects will guaranteed its clinical utility.
Acknowledgments
We are thankful to the University of Peshawar for financial support to this project [Higier Education of Pakistan (HEC) with Grant no. bm6-071/HEC/Pak].
Comments
Background
Synthetic agents used in the current treatment system are partly healing the diseased condition while largely affecting several other organs. In this condition, traditional therapeutic system could be ideal. Here a traditional therapy is validated in established experimental protocols.
Research frontiers
This research article is based on the preliminary studies on the analgesic and antipyretic potential of essential oil of a viola species. However, mechanism of the said activity is still required to be evaluated.
Related reports
There is not any related report on this particular activity of the plant in literature. So it is first time reported.
Innovations and breakthroughs
In the preliminary stage, it is a very important study that showed yet another natural healing agent for the treatment of very common human agony like pain.
Applications
It could be now used as analgesic and antipyretic in the traditional system of treatment.
Peer review
Generally, the study is a very potential one and is based on the rationalization traditional therapies. It is a good finding and could be useful addition for the journal. So I recommend for publication.
Footnotes
Foundation Project: Supported by Higier Education of Pakistan (HEC) with Grant No. bm6-071/HEC/Pak.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Hamayun M. Traditional uses of some medicinal plants of Swat Valley, Pakistan. Ind J Trad Know. 2007;6(4):636–641. [Google Scholar]
- 2.Nadkarni KM. Indian Materia Medica. Bombay: Popular Prakashan Private Limited (Popular Press); 1976. p. p. 1142. [Google Scholar]
- 3.Baart JLG, Baart-Bremer EL, Sagar MZ. Names of plants in Kalam Kohistani (Pakistan). Work Papers of the Summer Institute of Linguistics, University of North Dakota Session[C] 2004:48. [Google Scholar]
- 4.Shandesh B, Ram C, Robin T. Ethnomedicinal plants used by the people of Manang district, central Nepal. J Ethnobiol Ethnomed. 2006;2:41. doi: 10.1186/1746-4269-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qureshi RA, Ghufran MA, Gilani S, Yousaf Z, Abbas G, Batool A. Indigenous medicinal plants used by local women in southern Himalayan regions of Pakistan. Pak J Bot. 2009;41(1):19–25. [Google Scholar]
- 6.Mehmood F, Qasim M, Khan Z, Iqbal N, Mehmood S, Lateef M, et al. et al. In vitro evaluation of anthelmintic activity of essential oils from different parts of Skimmia laureola (DC.) Zucc. ex Walp., ver. Nair. Pak J Bot. 2011;43(6):2915–2918. [Google Scholar]
- 7.Barkatullah, Ibrar M, Muhammad N. Evaluation of Zanthoxylum armatum DC for in vitro and in vivo pharmacological screening. Afri J Pharm Pharmacol. 2011;5(14):1718–1723. [Google Scholar]
- 8.Rahman S, Ismail M, Muhammad N, Ali F, Chisthi A, Imran M. Evaluation of the stem bark of Pistacia integerrima Stew ex Brandis for its antimicrobial and phytotoxic activities. Afri J Pharm Pharmacol. 2011;5(8):1170–1174. [Google Scholar]
- 9.Raziq N, Muhammad N, Chishti KA, Saeed M, Rahman S, Khan H. Correlation of the antioxidant capacity with the phenolic contents of Hypericum monogynum and Hypericum perforatum. Afri J Pharm Pharmacol. 2011;5(16):1872–1876. [Google Scholar]
- 10.Sultanaa N, Atta-ur-Rahman, Khan TH. Tyrosinase inhibitor fatty ester and a quinoline alkaloid from Skimmia laureola. Z Naturforsch. 2005;60b:1186–1191. [Google Scholar]
- 11.Atta-Ur-Rahman, Sultana N, Khan MR, Choudhary MI. Triterpene and coumarins from Skimmia laureola. Nat Prod Lett. 2002;16(5):305–313. doi: 10.1080/10575630290020613. [DOI] [PubMed] [Google Scholar]
- 12.Waight ES, Razdan TK, Qadri B, Harkar S. Chromones and coumarins from Skimmia laureola. Phytochem. 1987;26(7):2063–2069. [Google Scholar]
- 13.Razdan TK, Harkara S, Qadri B, Qurishi MA, Khuroo MA. Lupenederivatives from Skimmia laureola. Phytochem. 1988;27(6):1890–1892. [Google Scholar]
- 14.Atta-ur-Rahman, Khalid A, Sultana N, Ghayur MN, Mesaik MA, Khan MR, et al. New natural cholinesterase inhibiting and calcium channel blocking quinoline alkaloids. J Enz Inhib Med Chem. 2006;21:6703–6710. doi: 10.1080/14756360600889708. [DOI] [PubMed] [Google Scholar]
- 15.Ibrar M, Muhammad N, Barkatullah, Khan H, Jahan F, Ashraf N. Antinociceptive and anticonvulsant activities of essential oils of Zanthoxylum armatum leaves. Phytopharmacol. 2012;3(1):191–198. [Google Scholar]
- 16.Khan H, Khan MA, Muhammad N, Ashraf N, Gul F, Tariq SA. Antiinflammatory and antioxidant activity of Joshanda partially mediated through inhibition of lipoxygenase. Phytopharmacol. 2012;3(1):19–28. [Google Scholar]
- 17.Khan H, Saeed M, Gilani AH, Khan MA, Dar A, Khan I. The antinociceptive activity of Polygonatum verticillatum rhizomes in pain models. J Ethnopharmacol. 2010;127(2):521–527. doi: 10.1016/j.jep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Khan H, Saeed M, Gilani AH, Khan MA, Khan I, Ashraf N. Anti-nociceptive activity of aerial parts of Polygonatum verticillatum: Attenuation of both peripheral and central pain mediators. Phytother Res. 2011;25(1):1024–1030. doi: 10.1002/ptr.3369. [DOI] [PubMed] [Google Scholar]
- 19.Khan M, Khan H, Khan S, Mahmood T, Khan P, Jabar A. Anti-inflammatory, analgesic and antipyretic activities of Physalis minima Linn. J Enz Inhib Med Chem. 2009;24(3):632–637. doi: 10.1080/14756360802321120. [DOI] [PubMed] [Google Scholar]
- 20.Muhammad N, Saeed M, Khan H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Comple Altern Med. 2012;12:59. doi: 10.1186/1472-6882-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan H, Saeed M, Gilani AH, Muhammad N, Ikram-ul-Haq, Ashraf N, et al. Antipyretic and anticonvulsant activity of Polygonatum verticillatum: comparison of rhizomes and aerial parts. Phytother Res 2012; doi. 2012 doi: 10.1002/ptr.4721. [DOI] [PubMed] [Google Scholar]
- 22.Okokon JE, Nwafor PA. Antiinflammatory, analgesic and antipyretic activities of ethanolic root extract of Croton zambesicus. Pak J Pharm Sci. 2010;23(4):385–392. [PubMed] [Google Scholar]
- 23.Ahmed S, Sultana M, Hasan MMU, Azhar I. Analgesic and antiemetic activity of Cleome viscosa L. Pak J Bot. 2011;43:119–122. [Google Scholar]
- 24.Mazid A, Datta BK, Nahar L, Rashid A, Bachar SC, Bashar S, et al. et al. Analgesic and diuretic properties of santalone from Polygonum flaccidum. Phytother Res. 2010;24(7):1084–1087. doi: 10.1002/ptr.3053. [DOI] [PubMed] [Google Scholar]
- 25.Dellai A. Anticonvulsant and analgesic activities of crude extract and its fractions of the defensive secretion from the Mediterranean sponge, Spongia officinalis. Cancer Cell Int. 2012;12:15. doi: 10.1186/1475-2867-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu B, Descalzi G, Ye HR, Zhuo M, Wang YW. Translational investigation and treatment of neuropathic pain. Mol Pain. 2012;8:15. doi: 10.1186/1744-8069-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhalke RD, Chavan MJ. Analgesic and CNS depressant activities of extracts of Annona reticulata Linn. bark. Phytopharmacol. 2011;1(5):160–165. [Google Scholar]
- 28.Igbe I, Ozolua RI, Okpo SO, Obasuyi O. Antipyretic and analgesic effects of the aqueous extract of the fruit pulp of Hunteria umbellata K Schum (Apocynaceae) Trop J Pharm Res. 2009;8(4):331–336. [Google Scholar]
- 29.Zhu ZZ, Ma KJ, Ran X, Zhang H, Zheng CJ, Han T, et al. et al. Analgesic, anti-inflammatory and antipyretic activities of the petroleum ether fraction from the ethanol extract of Desmodium podocarpum. J Ethnopharmacol. 2011;133(3):1126–1131. doi: 10.1016/j.jep.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 30.Blandizzi C, Tuccori M, Colucci R, Fornai M, Antonioli L, Ghisu N, et al. et al. Role of coxibs in the strategies for gastrointestinal protection in patients requiring chronic non-steroidal anti-inflammatory therapy. Pharmacol Res. 2009;59:90–100. doi: 10.1016/j.phrs.2008.11.004. [DOI] [PubMed] [Google Scholar]