Abstract
Leukocyte telomere length, representing the mean length of all telomeres in leukocytes, is ostensibly a bioindicator of human aging. The authors hypothesized that shorter telomeres might forecast imminent mortality in elderly people better than leukocyte telomere length. They performed mortality analysis in 548 same-sex Danish twins (274 pairs) aged 73–94 years, of whom 204 pairs experienced the death of one or both co-twins during 9–10 years of follow-up (1997–2007). From the terminal restriction fragment length (TRFL) distribution, the authors obtained the mean TRFL (mTRFL) and the mean values of the shorter 50% (mTRFL50) and shortest 25% (mTRFL25) of TRFLs in the distribution and computed the mode of TRFL (MTRFL). They analyzed the proportions of twin pairs in which the co-twin with the shorter telomeres died first. The proportions derived from the intrapair comparisons indicated that the shorter telomeres predicted the death of the first co-twin better than the mTRFL did (mTRFL: 0.56, 95% confidence interval (CI): 0.49, 0.63; mTRFL50: 0.59, 95% CI: 0.52, 0.66; mTRFL25: 0.59, 95% CI: 0.52, 0.66; MTRFL: 0.60, 95% CI: 0.53, 0.67). The telomere-mortality association was stronger in years 3–4 than in the rest of the follow-up period, and it grew stronger with increasing intrapair difference in all telomere parameters. Leukocyte telomere dynamics might help explain the boundaries of the human life span.
Keywords: aged, leukocytes, mortality, survival analysis, telomere, twins
Shortened mean leukocyte (and lymphocyte) telomere length (LTL) has been found to be associated with a host of aging-related diseases and lifestyle factors, including cardiovascular disease (1–8), dementia (9–11), obesity and insulin resistance (5, 6, 12–14), cigarette smoking (12, 15), psychological stress (16), and low socioeconomic status (17), all of which diminish the human life span (age of death from natural causes). Moreover, both life span (18) and LTL (3, 5, 15, 19, 20) are longer in women than in men. Although telomere dynamics (telomere length and age-dependent attrition rate) apparently contribute to morbidity and mortality in rare Mendelian disorders (21, 22), they are unlikely to be determinants of mortality among persons in the general population who succumb to aging-related diseases at ages substantially younger than their life expectancy. Shortened LTL in a middle-aged male who dies from atherosclerotic coronary heart disease probably is not the cause of his demise but is a lengthy record of underlying processes that have led to his disease.
A fundamental question, however, is whether leukocyte telomere biology influences the life span of persons who have avoided or survived the diseases of aging to join the ranks of the old and very old. Might leukocyte telomeres in some of these persons become critically shortened to impede the proliferative response that is vital for immune function and thereby contribute to their mortality? In this way, leukocyte telomere parameters might limit the life span of some persons who have survived to reach old age because of their genetic endowments and because they have lived under favorable environmental circumstances that reduce extrinsic mortality. However speculative this notion may be, it cannot gain currency without a demonstration of some connection between mortality and LTL in the elderly. Unfortunately, studies that have explored this connection have yielded contrasting findings (7, 11, 22–25).
It is possible, therefore, that there simply is no link between mortality in the elderly and leukocyte (lymphocyte) telomere biology. Alternatively, failure to demonstrate such a link could arise for a number of reasons, including the nature of the cohort (model) and the insensitivity of methods used to measure telomere length to capture subtle differences in telomere parameters among individuals (reviewed by Aviv et al. (26)).
In the present work, using refined methods of measuring telomere length, we explored the links between mortality and not only LTL but also parameters that characterize the shorter telomeres in leukocytes of the elderly, among same-sex Danish twins (i.e., each twin pair was either male or female). We focused our analysis on those twin pairs who had experienced the death of one or both co-twins since leukocyte collection. However, we also performed standard individual-level survival analyses including all 548 twin individuals; that is, we also used the twins who were from pairs in which no deaths occurred over the 9–10 years of follow-up.
Our findings point to an association between shortened leukocyte telomeres and mortality in the elderly, casting a new perspective on the link between telomere biology and the human life span.
MATERIALS AND METHODS
Participants
Leukocyte DNA samples were obtained from the repository of the Longitudinal Study of Aging Danish Twins, which was started in 1995 (27). A total of 689 twins provided blood samples during the 1997 survey of the Longitudinal Study of Aging Danish Twins. Leukocyte telomere parameters were measured in 548 of these twins. This subset had the same age distribution at blood sampling and the same life span as the entire cohort. Age at the time of death was obtained from the Danish civil registration system, which records the date of death of all Danish persons residing in Denmark. This research was approved by the scientific-ethical committee for Vejle and Funen counties, and all participants provided written informed consent.
Measurement and derivation of the terminal restriction fragment parameters
Leukocyte DNA was isolated using a salting-out method (28). The integrity of the DNA was assessed through electrophoresis of 0.5 µg of DNA on 1.0 percent agarose gels (200 V for 2 hours) and staining with ethidium bromide. Samples were digested overnight with the restriction enzyme digest set HphI (3.1 U)/MnlI (3.1 U) (New England Biolabs, Ipswich, Massachusetts). A previous restriction digest set, Hinf I/RsaI (3–6, 11, 21), yielded terminal restriction fragments that included the proximal telomere segments that are not strictly TTAGGG repeats, which extend to the nearest restriction sites on each chromosome. However, the HphI/MnlI restriction digest set cuts DNA at telomere repeat variant TGAGGG and degenerate versions of telomere repeats, which are present in the proximal region of human telomeres (29, 30). In this way, the length of the fragments (telomeres) was measured with a shorter noncanonical telomere segment.
DNA samples (3 µg each) from each twin pair were resolved on the same 0.6 percent agarose gel (20 cm × 20 cm) at 50 V (GNA-200; GE Healthcare, Piscataway, New Jersey). A reference molecular weight DNA ladder (1-kilobase DNA ladder plus λ DNA/HindIII fragments; Invitrogen, Carlsbad, California) was resolved between the two samples, so that the parameters of the terminal restriction fragments from each twin were calculated on the basis of a common adjacent molecular weight DNA ladder. After 16 hours, the DNA was depurinated for 15 minutes in 0.25 n hydrochloric acid, denatured for 30 minutes in sodium hydroxide (0.5 mol/liter)/sodium chloride (1.5 mol/liter), and neutralized for 30 minutes in Tris (0.5 mol/liter), pH 8/sodium chloride (1.5 mol/liter). The DNA was transferred for 1 hour to a positively charged nylon membrane (Roche Applied Science, Indianapolis, Indiana) using a vacuum blotter (Boeckel Scientific, Feasterville, Pennsylvania). The membranes were hybridized at 65°C with the telomeric probe [digoxigenin 3′-end labeled 5′-(CCTAAA)3] overnight in 5× saline-sodium citrate (0.15 mol/liter sodium chloride and 0.015 mol/liter sodium citrate), 0.1 percent N-lauroylsarcosine (Sigma-Aldrich, St. Louis, Missouri), 0.02 percent sodium dodecyl sulfate, and 1 percent blocking reagent (Roche). The membranes were washed three times at room temperature in 2× saline-sodium citrate/0.1 percent sodium dodecyl sulfate (15 minutes) and once in 2× saline-sodium citrate (15 minutes). The digoxigenin-labeled probe was detected by means of the digoxigenin luminescent detection procedure (Roche) and exposed on x-ray film.
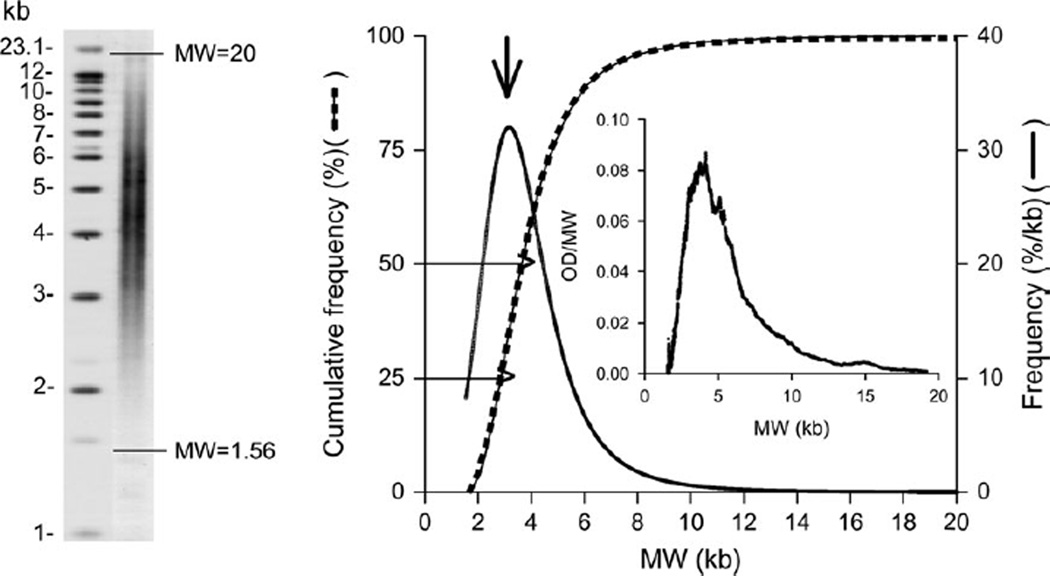
All autoradiographs were scanned, and the terminal restriction fragment length (TRFL) signal was digitized. The optical density values versus DNA migration distances were converted to optical density (adjusted for background)/molecular weight versus molecular weight. The background was fixed as the signal at the nadir of the low molecular weight region. The upper limit was set at a molecular weight of 20 kilobases. In this way we were able to capture a wider distribution of TRFLs, including fragments of very low molecular weight (illustrated in figure 1). The mean TRFL (mTRFL) was calculated and used as a measure of LTL. We also obtained the mean values of TRFLs in the lowest 50 percent (mTRFL50) and the lowest 25 percent (mTRFL25) of the TRFL distribution (figure 1).
FIGURE 1.
Distribution of terminal restriction fragment lengths (TRFLs) among 548 same-sex twins (274 pairs) aged 73–94 years in whom leukocyte telomere parameters were measured, Longitudinal Study of Aging Danish Twins, 1997–2007. The left-hand panel depicts a Southern blot in which the horizontal lines mark the scan limits of the TRFL distribution used for determining TRFL. The inset in the right-hand panel presents the results of a densitometric scan of TRFL (optical density (OD)/molecular weight (MW) vs. molecular weight) from which the cumulative empirical distribution curve was obtained. In the right-hand panel, the dashed line denotes the fitted four-parameter logistic dose-response curve; the solid line represents the data. The two horizontal arrows indicate the cumulative lower 25% and 50% of the TRFL distribution, from which the mean values were obtained. The curve identified by the vertical arrow is the computed first derivative of the logistic dose-response curve, which was used to obtain the mode of the TRFL (denoted by the peak of the curve). kb, kilobases.
We derived an empirical cumulative distribution curve for the DNA sample from each individual and fitted the data by least squares to four-parameter logistic dose-response distribution curves. We then obtained the maximum first derivative of the fitted curve using PROC EXPAND in SAS (SAS Institute, Inc., Cary, North Carolina), which provided the mode (MTRFL) of each individual’s TRFL distribution (figure 1). Because the distribution was skewed, the MTRFL was shifted towards the lower molecular weight, and as such it was more representative than the mTRFL of the contribution of the shorter telomeres to the distribution.
The only information provided to researchers in the laboratory that measured leukocyte telomere parameters was the identification of twins as pairs so that their DNA samples could be resolved in lanes adjacent to the same molecular weight ladder. Otherwise, these researchers were completely blinded to the characteristics of participants. Duplicate measurements (on different gels and different occasions) were obtained for 414 samples. Because of an insufficient amount of DNA or poor autoradiograph quality, single measurements were obtained for 134 samples. Results were electronically transmitted and merged with the covariate data of the Longitudinal Study of Aging Danish Twins. The coefficient of variation for the mTRFL in this elderly cohort derived from the duplicate samples was 3.4 percent.
Statistical analysis
For each individual, we focused our analysis on the mTRFL, mTRFL50, mTRFL25, and MTRFL of the distribution. We computed the proportion of times the co-twin with the shorter telomeres died first. We compared these proportions with the null hypothesis of equality (50 percent/50 percent), using the standard binomial test.
Survival analysis using Cox’s sex-specific proportional hazards model (i.e., with sex-specific baseline hazard functions) was conducted in order to study the relation between telomere length and time from date of blood sampling to death. Since any correlation between twins in a pair could lead to confidence intervals that were too narrow, the analyses were performed using the robust estimator of variance, assuming independence between pairs. First, we estimated hazard ratios using telomere length parameters as independent covariates. Second, we estimated hazard ratios using both telomere variables and age as independent covariates. In addition, we tested models for the interaction of telomere length with age.
All analyses were carried out using Stata, version 9 (Stata Corporation, College Station, Texas).
RESULTS
Of the 274 twin pairs in the study, there were 184 pairs of women, 90 pairs of men, 121 pairs of monozygotic twins, and 153 pairs of dizygotic twins. Table 1 displays the ages and telomere parameters of females and males in these twin pairs.
TABLE 1.
General characteristics of 548 same-sex twins (274 pairs) aged 73–94 years in whom leukocyte telomere parameters were measured, Longitudinal Study of Aging Danish Twins, 1997–2007
| No. of subjects |
Mean age (years) |
Age range (years) |
Leukocyte telomere parameter (kilobases) |
||||
|---|---|---|---|---|---|---|---|
| MTRFL* | mTRFL* | mTRFL50* | mTRFL25* | ||||
| Men | 180 | 78.3 | 73–88 | 3.27 | 4.46 | 2.98 | 2.50 |
| Women | 368 | 79.0 | 73–94 | 3.36 | 4.62 | 3.06 | 2.56 |
MTRFL, mode of terminal restriction fragment length; mTRFL, mean terminal restriction fragment length; mTRFL50, lowest 50% of the terminal restriction fragment length distribution; mTRFL25, lowest 25% of the terminal restriction fragment length distribution.
As of March 2007, after 3,991 years of follow-up, 289 deaths (180 females and 109 males) had occurred among the twins whose leukocyte telomere parameters were measured in this study. The numbers of pairs in which no twin died, one twin died, or both died were 70, 119, and 85, respectively. Hence, at least one twin died in 204 pairs (out of 274).
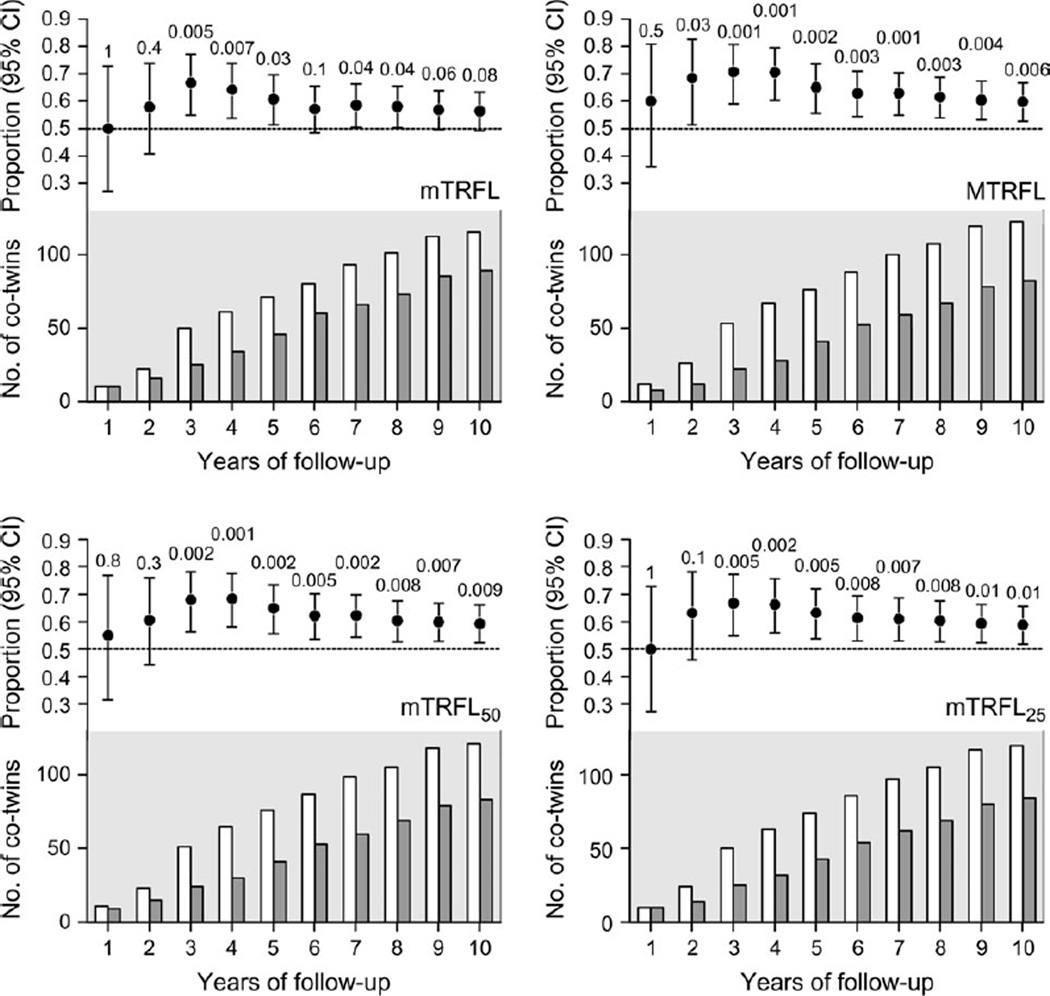
Figure 2 displays, in yearly intervals, the cumulative proportion of pairs in which the co-twin with shorter telomeres died first during the follow-up period. The telomere-mortality association was stronger in the third-to-fourth year than in the rest of follow-up period. Moreover, the proportions of pairs in which the co-twin with the shorter telomeres died first were similar when results were stratified by gender, years of follow-up, or zygosity, although there was more variation and wider confidence intervals because of the smaller sample sizes (data not shown).
FIGURE 2.
Cumulative proportion of 274 same-sex twin pairs (n = 548) in which the co-twin with shorter telomeres died first during a follow-up period of 9–10 years, Longitudinal Study of Aging Danish Twins, 1997–2007. The four panels display data for four leukocyte telomere parameters pertaining to terminal restriction fragment length (TRFL): the mean (mTRFL), the mode (MTRFL), and the means of the shorter 50% (mTRFL50) and shortest 25% (mTRFL25) of the distribution. In each panel, the upper graph displays the cumulative proportion of twin pairs in which the co-twin with the shorter telomeres died first. The lower graph displays the cumulative numbers of co-twins with relatively shorter telomeres (open bars) and longer telomeres (closed bars) who died first during the follow-up period. Bars, 95% confidence interval (CI). The numbers shown above each 95% CI are p values.
Table 2 summarizes the findings obtained at the end of follow-up, showing that the proportions were significantly higher than 50 percent form TRFL50, mTRFL25, and MTRFL. The proportion for mTRFL was of borderline significance.
TABLE 2.
Intrapair comparisons for 204 same-sex twin pairs aged 73–94 years in which one or both co-twins died during follow-up, Longitudinal Study of Aging Danish Twins, 1997–2007
| Leukocyte telomere parameter |
No. in which shorter twin died first* |
Proportion (of 204) |
95% confidence interval |
p value |
|---|---|---|---|---|
| mTRFL† | 115 | 0.56 | 0.49, 0.63 | 0.08 |
| MTRFL† | 122 | 0.60 | 0.53, 0.67 | 0.006 |
| mTRFL50† | 121 | 0.59 | 0.52, 0.66 | 0.009 |
| mTRFL25† | 120 | 0.59 | 0.52, 0.66 | 0.014 |
Number of twin pairs in which the co-twin with the shorter telomeres died first.
mTRFL, mean terminal restriction fragment length; MTRFL, mode of terminal restriction fragment length; mTRFL50, lowest 50% of the terminal restriction fragment length distribution; mTRFL25, lowest 25% of the terminal restriction fragment length distribution.
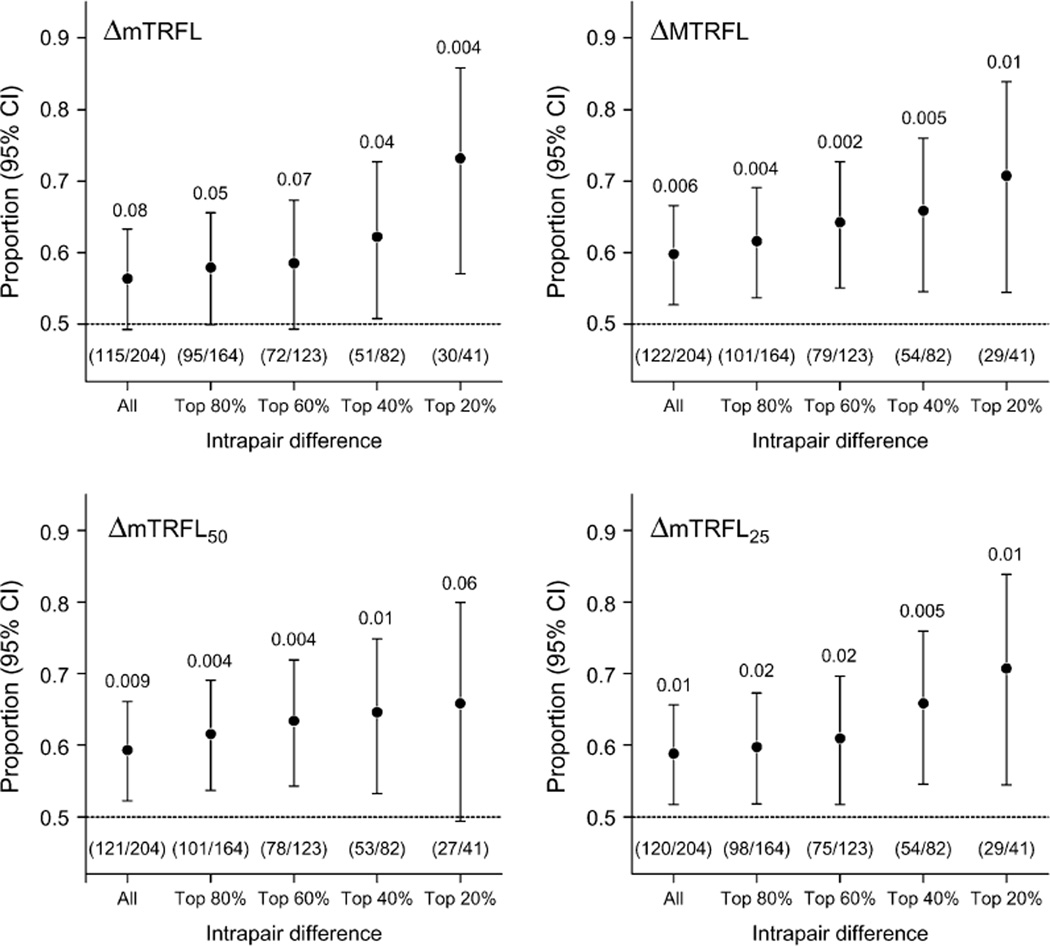
Figure 3 shows that during the entire follow-up period, the increased intrapair difference in telomere parameters was associated with a higher probability that the co-twin with the shorter telomeres would die first. This was particularly apparent for the parameters representing the shorter telomeres in the TRFL distribution (MTRFL, TRFL50, and TRFL25).
FIGURE 3.
Mortality according to intrapair difference in telomere length among 274 same-sex twin pairs (n = 548) during 9–10 years of follow-up, Longitudinal Study of Aging Danish Twins, 1997–2007. The four panels display the proportions of pairs in which the co-twin with the shorter telomeres died first, according to the intrapair difference in one of four leukocyte telomere parameters pertaining to terminal restriction fragment length (TRFL): the mean (mTRFL), the mode (MTRFL), and the means of the shorter 50% (mTRFL50) and shortest 25% (mTRFL25) of the distribution. Numbers of twin pairs are given in parentheses. Bars, 95% confidence interval (CI). The numbers shown above each 95% CI are p values.
The relation of telomere parameters with survival time was also studied in the full sample (n = 548) using Cox regression. The proportional hazards assumption underlying the Cox model was tested (using the Schoenfeld residual test) and was not found to be violated in any of the applied models.
Table 3 displays the hazard ratios for leukocyte telomere parameters in the full sample. When only telomere parameters were included in the model but hazards were allowed to vary between sexes, shorter telomere length was disadvantageous for survival. The hazard ratios for the telomere parameters ranged from 0.54 (95 percent confidence interval (CI): 0.30, 0.98) for mTRFL25 to 0.74 (95 percent CI: 0.52, 1.07) for MTRFL. Age was a significant predictor of survival. When we controlled for effects of age on mortality, which gave a better fit to the data in terms of Akaike’s Information Criterion, the hazard ratios ranged from 0.77 (95 percent CI: 0.49, 1.20) for mTRFL50 to 0.87 (95 percent CI: 0.61, 1.23) for MTRFL; however, they were not significantly different from 1. For mTRFL, the hazard ratio adjusted for age (and sex) was 0.81 (95 percent CI: 0.64, 1.03), with a hazard ratio of 1.10 (95 percent CI: 1.07, 1.13) for age. Variables for interaction and age squared did not improve the fit of the model to the data.
TABLE 3.
Hazard ratio for 548 same-sex twins (274 pairs) aged 73–94 years, according to leukocyte telomere length and age, Longitudinal Study of Aging Danish Twins, 1997–2007*
| Model and leukocyte telomere parameter |
Hazard ratio |
95% confidence interval |
|---|---|---|
| Model 1† | ||
| MTRFL‡ | 0.74 | 0.52, 1.07 |
| mTRFL‡ | 0.72 | 0.55, 0.92 |
| mTRFL50‡ | 0.58 | 0.36, 0.94 |
| mTRFL25‡ | 0.54 | 0.30, 0.98 |
| Model 2§ | ||
| MTRFL | 0.87 | 0.61, 1.23 |
| Age (per year) | 1.10 | 1.08, 1.13 |
| mTRFL | 0.81 | 0.64, 1.03 |
| Age (per year) | 1.10 | 1.07, 1.13 |
| mTRFL50 | 0.77 | 0.49, 1.20 |
| Age (per year) | 1.10 | 1.07, 1.13 |
| mTRFL25 | 0.79 | 0.45, 1.37 |
| Age (per year) | 1.10 | 1.07, 1.13 |
A total of 289 deaths occurred during 3,991 person-years of follow-up. The average duration of follow-up was 7.28 years (range, 0.06–10.3). Baseline hazards were allowed to vary between the sexes.
Hazard ratio per 1-kilobase increase in telomere length.
MTRFL, mode of terminal restriction fragment length; mTRFL, mean terminal restriction fragment length; mTRFL50, lowest 50% of the terminal restriction fragment length distribution; mTRFL25, lowest 25% of the terminal restriction fragment length distribution.
Hazard ratio per 1-kilobase increase in telomere length, adjusted for age at blood sampling (i.e., by adding age as a covariate).
DISCUSSION
The core hypothesis tested in this work was that leukocyte telomere parameters, particularly those representing the shorter telomeres in the telomere length distribution, could predict mortality in elderly twins. On the basis of our analysis of four different but related measures of the TRFL distribution in leukocytes, we feel that we have validated the hypothesis. Our refined method of telomere length measurement and the intrapair comparisons made in same-sex twin pairs were instrumental in proving the hypothesis.
Resorting not only to all-genome TRFL analysis but also to single telomere length analysis, a previous study found that exceptionally old persons (aged 90–104 years) displayed an overrepresentation of ultrashort telomeres (<3 kilobases) in their leukocytes (31). Here we showed that for the entire follow-up period, the association of mortality with mTRFL, which corresponds to LTL, was of only borderline significance, while measures that corresponded to the shorter telomeres in the telomere length distribution (MTRFL, mTRFL50, and mTRFL25) were better predictors of mortality (table 2, figure 2). Moreover, the differences in the telomere parameters between the co-twins displayed a “dose-response” pattern, so that a greater intrapair difference in telomere parameters was associated with a heightened probability of mortality in the co-twin with the shorter telomeres (figure 3).
Five studies have thus far explored the relation between LTL and survival in elderly persons (7, 11, 23–25). In two studies, examining leukocytes, investigators observed that persons with relatively short LTL were more likely to die earlier than their peers (7, 11), but researchers in the other studies (23–25) did not confirm this finding using lymphocytes (23, 25) or leukocytes (24).
Why then is it so important to resolve the controversy as to whether or not LTL can in some way predict mortality in the elderly?
Life expectancy has increased considerably in many countries because of the decline in environmental causes of death during the preceding two centuries and the improvements in medical intervention during the last half century. A sizeable increase in life expectancy in the future may therefore hinge on genetic factors that impose a barrier to the expansion of the human life span (32, 33). One of these factors might be LTL and related telomere parameters.
LTL is a complex genetic trait (19, 20, 34–36). It is highly variable both at birth (37, 38) and afterwards (3–9, 12, 15, 16). Moreover, the rate of age-dependent LTL is also highly variable among individuals (14). On the basis of our findings in elderly twins, we offer the following broad paradigm for the relation between leukocyte telomere parameters and human aging: Birth telomere length is one of the parameters that define the oldest age that might be attained by an individual, based on his/her genetic endowment under favorable environmental circumstances that reduce extrinsic mortality. However, the rate of LTL attrition, which is influenced by both genetic and environmental factors, is an indicator of whether this landmark is reached. Environmental variables such as cigarette smoking (12, 15), obesity (12, 14), and other unhealthy lifestyle factors (17, 39) may accelerate LTL attrition. In this way, not only heredity but also the environment might be a determinant of leukocyte telomere parameters in elderly persons who have avoided or survived the diseases of aging. The associations of these parameters with mortality in the elderly suggest potential telomeric constraints that might hinder sizeable increases in the life span of some persons. These constraints arise from both genetic and environmental factors that account for variation in leukocyte telomere parameters among humans.
We doubt that use of different blood cell types accounted for the discrepancies among previous studies exploring the link between survival and LTL in the elderly (reviewed by Aviv et al. (26)). We note, however, that the demographic characteristics of these studies varied considerably; for example, the cohorts were of different ages and lived in different countries (7, 11, 23–25), and one cohort consisted of persons with dementia (11). In addition, to obtain mean telomere length, four of the studies employed real-time polymerase chain reaction (7, 11, 23, 25), while the fifth used TRFL analysis (24).
Intrapair comparison of co-twins of the same sex is optimal in exploring the relation between mortality and telomere parameters in the elderly. Our present work underscores the power of this approach. While the intrapair comparisons demonstrated strong connections between leukocyte telomere parameters and mortality, survival analysis in the full sample (which included twin pairs with both co-twins still alive during the follow-up period) identified only a trend in age- and sex-adjusted telomeric parameters as predictors of survival. This survival analysis lacks control for genetic and child-rearing environmental factors, and it requires statistical adjustments for both age and sex. Thus, it is considerably less powerful than the intrapair analysis.
In addition, the techniques used to measure telomere length in previous studies might have been insufficiently accurate. The larger the inaccuracy associated with a measurement, the lower its statistical power (37, 41). The Danish twins who were targeted in the present project illustrate this concept. A previous analysis of LTL in these twins provided only marginal and statistically nonsignificant support for an association between survival and LTL in the elderly (24). Given the importance of resolving the question of whether leukocyte telomere parameters can predict mortality in the elderly, we restudied these elderly twins. The different conclusions derived from the two studies reflect crucial differences in methodology and analytical approach. First, results of the present TRFL analysis are much more reproducible and accurate than results of the analysis used originally (the coefficient of variation was 3.4 percent for the present study vs. 12 percent for the previous one). Second, the method we used provided not only the LTL (mTRFL), but also other direct and modeled parameters of the telomere length distribution. In this way, we could assess the relation between mortality and subsets of telomeres of shortened lengths. These additional parameters were, in fact, better predictors of mortality than the mTRFL. Third, we also examined the relation between the magnitude of the intrapair difference in telomere parameters and mortality, showing that the greater the difference, the greater the likelihood that the co-twin with the shorter telomeres would die first. Lastly, the number of co-twins who died during followup was larger and the duration of follow-up was longer in the present study than in its predecessor (24).
A potential shortcoming of our study is that it lacked data regarding the cause of death. Since autopsies are infrequently performed in the general population, death certificates have been used in many studies as a source of information on cause of death. This, for instance, was the case in a previous work that examined the connection between survival and LTL in the elderly (7). However, death certificates are notoriously inaccurate in diagnosing the cause of death (42, 43). Little additional insight would have been gained even if we had attempted to link major causes of death (i.e., cardiovascular disease, cancer, infection, and dementia) with leukocyte telomere parameters, since the immune system, namely leukocytes, may play a role in them all.
What might be the implications of our findings? The weight of the evidence indicates that in the general population, short LTL is not a determinant of either aging or aging-related diseases, including cardiovascular disease (1–8) and dementia (9–11). Rather, LTL is a proxy for underlying mechanisms that bring about aging. In this sense, aging-related diseases, which are associated with shortened LTL, might be regarded as outcomes of accelerated aging.
The question, however, is whether in some elderly persons, LTL may be transformed from a bioindicator of aging to a determinant of life span. If telomere dynamics played such a unique role, they might do so by causing replicative senescence in subsets of hematopoietic stem cells, progenitor cells, or peripheral leukocytes (44, 45), thereby curtailing the immune response and setting a limit on the human life span. Epidemiologic research—which, for obvious reasons, cannot provide evidence of causality—is unlikely to answer this question. We note, however, that in cultured cells it is not the mean length of telomeres but the shortest telomeres that trigger replicative senescence (46, 47). As we have shown here, in the elderly, the shorter telomeres in leukocytes predict mortality better than does LTL.
In conclusion, our findings suggest that short telomeres in leukocytes forecast mortality in the elderly. Since, in any phase of the human life span, LTL is ultimately the product of both genetic endowment and the environment, intensive research should be undertaken to fathom the genes and environmental factors that determine leukocyte telomere parameters at birth and thereafter.
ACKNOWLEDGMENTS
This research was supported by grants AG16592, AG020132, and P01-AG08761 from the US National Institute on Aging and by the Velux Foundation.
Abbreviations
- CI
confidence interval
- LTL
leukocyte telomere length
- TRFL
terminal restriction fragment length
Footnotes
Conflict of interest: none declared.
REFERENCES
- 1.Samani NJ, Boultby R, Butler R, et al. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 2.Brouilette S, Singh RK, Thompson JR, et al. White cell telomere length and risk of premature myocardial infarction. Arterioscler Throm Vasc Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 3.Benetos A, Okuda K, Lajemi M, et al. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 4.Benetos A, Gardner JP, Zureik M, et al. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43:182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 6.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 7.Cawthon RM, Smith KR, O’Brien E, et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 8.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested casecontrol study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 9.von Zglinicki T, Serra V, Lorenz M, et al. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab Invest. 2000;80:1739–1747. doi: 10.1038/labinvest.3780184. [DOI] [PubMed] [Google Scholar]
- 10.Panossian LA, Porter VR, Valenzuela HF, et al. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 11.Honig LS, Schupf N, Lee JH, et al. Shorter telomeres are associated with mortality in those with APOE e4 and dementia. Ann Neurol. 2006;60:181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- 12.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 13.Aviv A, Valdes A, Gardner JP, et al. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin Endocrinol Metab. 2006;91:635–640. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- 14.Gardner JP, Li S, Srinivasan SR, et al. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 15.Nawrot TS, Staessen JA, Gardner JP, et al. Telomere length and possible link to X chromosome. Lancet. 2004;363:507–510. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- 16.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherkas LF, Aviv A, Valdes AM, et al. The effects of social status on biological ageing as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 18.Population Reference Bureau. 2002 world population data sheet of the Population Reference Bureau: demographic data and estimates for the countries and regions of the world. Washington, DC: Population Reference Bureau; 2002. ( http://www.prb.org/pdf/WorldPopulationDS02_Eng.pdf) [Google Scholar]
- 19.Vasa-Nicotera M, Brouilette S, Mangino M, et al. Mapping of a major locus that determines telomere length in humans. Am J Hum Genet. 2005;76:147–151. doi: 10.1086/426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeanclos E, Schork NJ, Kyvik KO, et al. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi H, Calado RT, Ly H, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1481–1483. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 22.Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Ruiz CM, Gussekloo J, van Heemst D, et al. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff C, Petersen HC, Graakjaer J, et al. No association between telomere length and survival among the elderly and oldest old. Epidemiology. 2006;17:190–194. doi: 10.1097/01.ede.0000199436.55248.10. [DOI] [PubMed] [Google Scholar]
- 25.Harris SE, Deary IJ, MacIntyre A, et al. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci Lett. 2006;406:260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 26.Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006;35:1424–1429. doi: 10.1093/ije/dyl169. [DOI] [PubMed] [Google Scholar]
- 27.Bathum L, Petersen HC, Rosholm JU, et al. Evidence for a substantial genetic influence on biochemical liver function test: results from a population based Danish twin study. Clin Chem. 2001;47:81–87. [PubMed] [Google Scholar]
- 28.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baird DM, Britt-Compton B, Rowson J, et al. Telomere instability in the male germline. Hum Mol Genet. 2006;15:45–51. doi: 10.1093/hmg/ddi424. [DOI] [PubMed] [Google Scholar]
- 30.Allshire RC, Dempster M, Hastie ND. Human telomeres contain at least three types of G-rich repeat distributed nonrandomly. Nucleic Acids Res. 1989;17:4611–4627. doi: 10.1093/nar/17.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura M, Barbieri M, Gardner JP, et al. Leukocytes of exceptionally old persons display ultra-short telomeres. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2210–R2217. doi: 10.1152/ajpregu.00615.2007. [DOI] [PubMed] [Google Scholar]
- 32.Couzin J. How much can human life span be extended? Science. 2005;309:83. doi: 10.1126/science.309.5731.83. [DOI] [PubMed] [Google Scholar]
- 33.Abbott A. Ageing: growing old gracefully. Nature. 2004;428:116–118. doi: 10.1038/428116a. [DOI] [PubMed] [Google Scholar]
- 34.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 35.Bischoff C, Graakjaer J, Petersen HC, et al. The heritability of telomere length among the elderly and oldest-old. Twin Res Hum Genet. 2005;8:433–439. doi: 10.1375/183242705774310141. [DOI] [PubMed] [Google Scholar]
- 36.Andrew T, Aviv A, Falchi M, et al. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected, female sibling-pairs. Am J Hum Genet. 2006;78:480–486. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okuda K, Bardeguez A, Gardner JP, et al. Telomere length in the newborn. Pediatr Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Akkad A, Hastings R, Konje JC, et al. Telomere length in small-for-gestational-age babies. Int J Obstet Gynecol. 2006;113:318–323. doi: 10.1111/j.1471-0528.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 39.Bekaert S, De Meyer T, Reitzschel ER, et al. Telomere length and cardiovascular risk factors in middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 40.Lewington S, Thomsen T, Davidsen M, et al. Regression dilution bias in blood total and high-density lipoprotein cholesterol and blood pressure in the Glostrup and Framingham prospective studies. J Cardiovasc Risk. 2003;10:143–148. doi: 10.1097/01.hjr.0000060834.46105.3c. [DOI] [PubMed] [Google Scholar]
- 41.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 42.Lloyd-Jones DO, Martin DM, Larson MG, et al. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;29:1020–1026. doi: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 43.Lenfant C, Friedman L, Thom T. Fifty years of death certificates: The Framingham Heart Study. Ann Intern Med. 1998;129:1066–1067. doi: 10.7326/0003-4819-129-12-199812150-00013. [DOI] [PubMed] [Google Scholar]
- 44.Effros RB, Dagarag M, Spaulding C, et al. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 45.Effros R. Role of T lymphocyte replicative senescence in vaccine efficiency. Vaccine. 2007;25:599–604. doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 46.Hemann MT, Strong MA, Hao LY, et al. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 47.Zou Y, Sfeir A, Gryaznov SM, et al. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol Biol Cell. 2004;15:3709–3718. doi: 10.1091/mbc.E04-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]