Abstract
Mesenteric ischemic symptoms appear only when two of the three major splanchnic arteries from the abdominal aorta are involved. Recently, we encountered a case of chronic mesenteric ischemia in a 50-year-old female patient caused by atherosclerotic obstruction of the celiac trunk and superior mesenteric artery. She was treated with a retrograde bypass graft from the right common iliac artery to the superior mesenteric artery (SMA) in a C-loop configuration. Complete revascularization is recommended for treatment of intestinal ischemia. When the celiac trunk is a not suitable recipient vessel, bypass grafting to the SMA alone appears to be both an effective and durable procedure for treating intestinal ischemia.
Keywords: Cardiovascular diseases, Chronic mesenteric ischemia, Superior mesenteric artery, Bypass, Surgery
CASE REPORT
A number of reports recommend revascularization of all stenotic splanchnic arteries in patients with mesenteric ischemia [1,2]. However, Foley et al. [3] reported that bypass grafting to the superior mesenteric artery (SMA) alone appears to be both effective and durable for treating intestinal ischemia. We performed a retrograde bypass graft from the right common iliac artery to the SMA only.
A 50-year-old woman was admitted to the hospital with diffuse abdominal pain that had been present for three months and which was aggravated after meals. She could not consume enough food and had experienced moderate weight loss of almost 5 kg in three months. Gastric and colonic endoscopic examinations did not show any abnormal findings. Her symptoms did not improve with conservative medical therapy, and she developed nausea and vomiting. Abdominal computed tomography (CT) showed nonspecific findings. Her symptoms continued to worsen, and she developed cold sweats and abdominal tenderness. Abdominal CT angiography was used to rule out ischemic peritonitis, and the results showed severe stenosis of the celiac trunk with distal obstruction and obstruction (62.3 mm) of the superior mesenteric artery that was too extensive. The imaging enabled visualization of the distal portion of SMA obstruction (2.4 mm) (Fig. 1). We confirmed her symptoms as being caused by mesenteric ischemia. We performed a retrograde bypass graft (6 mm ringed-polytetrafluoroethylene graft) from the right common iliac artery to the SMA in a C-loop configuration with a midline abdominal incision. We did not conduct a bypass operation for the celiac trunk, deciding that it would not have the desired effect as CT angiography showed a critical orifice stenosis of the celiac trunk with distal obstruction. Postoperative reconstructed CT angiography showed good blood flow (Fig. 2). After the SMA bypass operation, her symptoms completely disappeared, and she was discharged on the 13th postoperative day without any complications. Five months have passed since this operation, and the graft has continued to maintain good patency.
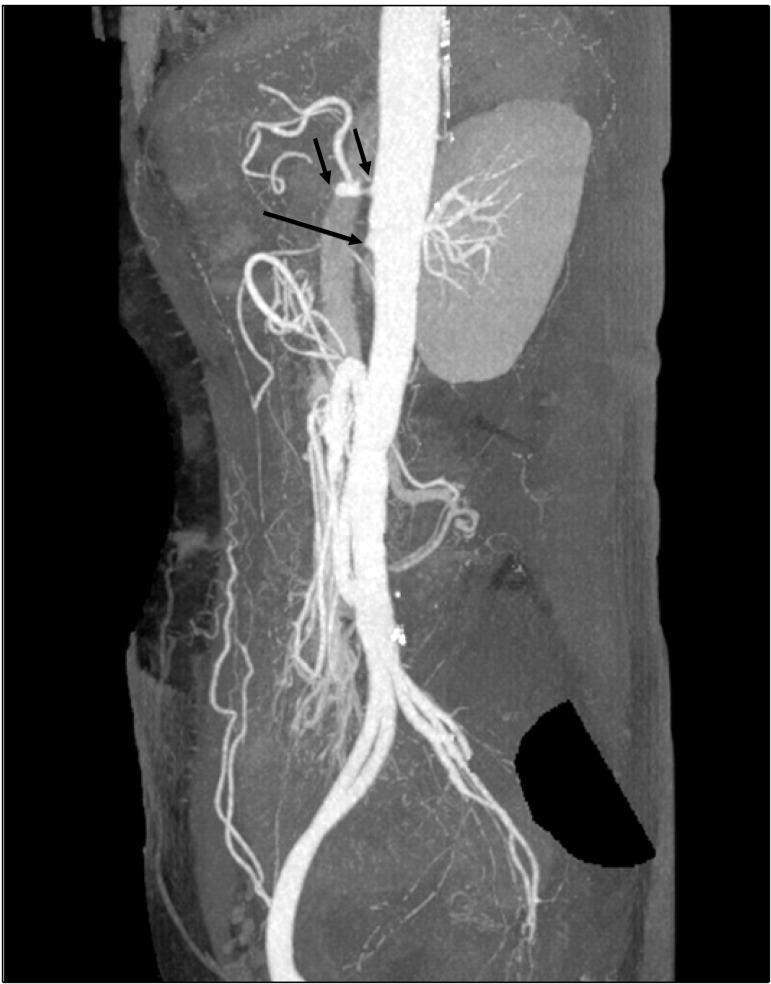
Fig. 1.
Computed tomography angiography showed critical orifice stenosis of the celiac trunk with distal obstruction (short arrow) and orifice obstruction of the superior mesenteric artery (long arrow).
Fig. 2.
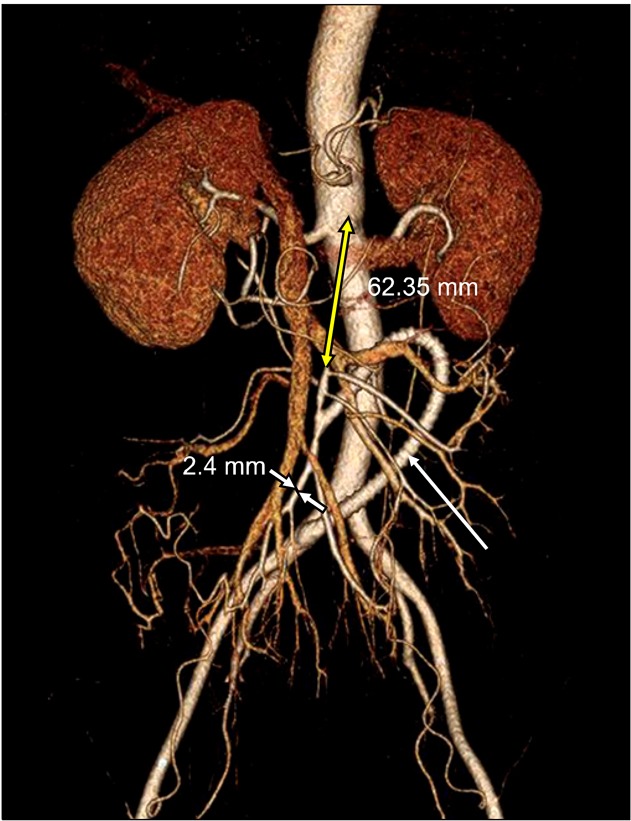
Postoperative reconstructed computed tomography angiography showed the C-shaped polytetrafluoroethylene graft (white arrow) from the right common iliac artery to the superior mesenteric artery (SMA) and good blood flow. The obstructed segment of the SMA was too long (62.35 mm) and the distal SMA was too small (2.4 mm).
DISCUSSION
Chronic mesenteric ischemia (CMI) is an uncommon pathology. It is typically revealed by abdominal pain that lasts from 1 to 4 hours, 15 to 60 minutes after a meal. CMI is associated with nausea, vomiting, and diarrhea. Fear of food leads to significant weight loss. The cause in more than 90% of cases is atherosclerosis. Symptoms appear only when two of the three major splanchnic arteries from the abdominal aorta (celiac, superior, and inferior mesenteric arteries) are involved [4]. Symptomatic patients should be treated without delay as symptoms of CMI are present in 43% of patients who present with acute mesenteric ischemia [5]. Endovascular therapy with angioplasty and/or stent placement is often the first-line treatment, and has a high success rate and a mortality rate lower than that of open surgery (4% vs. 14%). Open mesenteric revascularization with bypass still plays an important role in the treatment of patients with more extensive disease, including long-segment occlusions, small vessel size, multiple tandem lesions, and severe calcification [6]. In our case, the obstructed segment of the SMA was too extensive (62.3 mm) and the distal SMA was small (2.4 mm). One fundamental issue in mesenteric revascularization is the number of vessels to revascularize. In reports from the Mayo Clinic, it was first suggested that complete revascularization resulted in decreased symptomatic recurrence [1] and later that graft patency and survival in patients with three-vessel revascularization were better than with single vessel revascularization [2]. These two studies were limited to patients with chronic mesenteric ischemia and did not use objective methods to determine graft patency. Although these retrospective studies suggest that complete revascularization resulted in fewer recurrences and deaths, their results were not statistically significant [3]. Despite the lack of convincing data to support the necessity of multiple bypass grafts, several authors have advocated complete revascularization [7,8]. In 1984, Baur et al. [9] reported a series of 23 patients who underwent complete revascularization whenever possible. Perioperative mortality was 9%, and during a mean follow-up of 2 years, 9.5% of patients experienced graft failures. We believe that incomplete revascularization may not relieve symptoms in all patients and that progression of disease in nonrevascularized vessels may lead to recurrent symptoms. However, physiologic studies of the celiac and mesenteric arteries have since demonstrated that postprandial hyperemia is limited to the SMA [10-12]. This finding, in combination with the usual disease pattern (ostial lesions) and extensive collaterals, has led us to conclude that in most patients, a single bypass graft to the SMA should alleviate symptoms initially and be durable over time [3]. Proponents of single-vessel revascularization have reported similar long-term results. Series from France have shown SMA reconstruction to be a durable form of treatment for intestinal ischemia [8,13]. In the United States, favorable results for single vessel revascularization have been reported [14,15]. In 1994, Gentile et al. [14] reported 26 patients who had 29 isolated bypass grafts to the SMA for intestinal ischemia. Perioperative mortality was 10%. The mean follow-up was 40 months, and the life table-determined 4 year primary graft patency rate and survival rate were 89% and 82%, respectively. Another fundamental issue in mesenteric revascularization is whether to use antegrade bypass or retrograde bypass. Foley et al. [3] has reported on the use of the distal infrarenal aorta or the infrarenal aorta-right common iliac artery junction as the preferred site for the proximal anastomosis. The key to avoiding graft elongation, angulation, or kinking of the graft is to cut it to length with the SMA in a nearly anatomic position. McMillan et al. [16] noted no significant difference in the long-term graft patency rates for patients undergoing antegrade bypass and retrograde bypass (93% vs. 95% at 36 months). It can be concluded that when the SMA is a suitable recipient vessel, multiple bypass grafts to other splanchnic vessels are unnecessary in the treatment of intestinal ischemia.
ACKNOWLEDGMENTS
This work was supported by the 2010 Inje University research grant.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Hollier LH, Bernatz PE, Pairolero PC, Payne WS, Osmundson PJ. Surgical management of chronic intestinal ischemia: a reappraisal. Surgery. 1981;90:940–946. [PubMed] [Google Scholar]
- 2.McAfee MK, Cherry KJ, Jr, Naessens JM, et al. Influence of complete revascularization on chronic mesenteric ischemia. Am J Surg. 1992;164:220–224. doi: 10.1016/s0002-9610(05)81074-8. [DOI] [PubMed] [Google Scholar]
- 3.Foley MI, Moneta GL, Abou-Zamzam AM, Jr, et al. Revascularization of the superior mesenteric artery alone for treatment of intestinal ischemia. J Vasc Surg. 2000;32:37–47. doi: 10.1067/mva.2000.107314. [DOI] [PubMed] [Google Scholar]
- 4.Garetier M, Delluc C, Rousset J. Chronic mesenteric ischemia. Clin Res Hepatol Gastroenterol. 2011;35:781–782. doi: 10.1016/j.clinre.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Park WM, Gloviczki P, Cherry KJ, Jr, et al. Contemporary management of acute mesenteric ischemia: factors associated with survival. J Vasc Surg. 2002;35:445–452. doi: 10.1067/mva.2002.120373. [DOI] [PubMed] [Google Scholar]
- 6.Oderich GS, Gloviczki P, Bower TC. Open surgical treatment for chronic mesenteric ischemia in the endovascular era: when it is necessary and what is the preferred technique? Semin Vasc Surg. 2010;23:36–46. doi: 10.1053/j.semvascsurg.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Calderon M, Reul GJ, Gregoric ID, et al. Long-term results of the surgical management of symptomatic chronic intestinal ischemia. J Cardiovasc Surg (Torino) 1992;33:723–728. [PubMed] [Google Scholar]
- 8.Kieny R, Batellier J, Kretz JG. Aortic reimplantation of the superior mesenteric artery for atherosclerotic lesions of the visceral arteries: sixty cases. Ann Vasc Surg. 1990;4:122–125. doi: 10.1007/BF02001365. [DOI] [PubMed] [Google Scholar]
- 9.Baur GM, Millay DJ, Taylor LM, Jr, Porter JM. Treatment of chronic visceral ischemia. Am J Surg. 1984;148:138–144. doi: 10.1016/0002-9610(84)90301-5. [DOI] [PubMed] [Google Scholar]
- 10.Moneta GL, Taylor DC, Helton WS, Mulholland MW, Strandness DE., Jr Duplex ultrasound measurement of postprandial intestinal blood flow: effect of meal composition. Gastroenterology. 1988;95:1294–1301. doi: 10.1016/0016-5085(88)90364-2. [DOI] [PubMed] [Google Scholar]
- 11.Nicholls SC, Kohler TR, Martin RL, Strandness DE., Jr Use of hemodynamic parameters in the diagnosis of mesenteric insufficiency. J Vasc Surg. 1986;3:507–510. doi: 10.1067/mva.1986.avs0030507. [DOI] [PubMed] [Google Scholar]
- 12.Jager K, Bollinger A, Valli C, Ammann R. Measurement of mesenteric blood flow by duplex scanning. J Vasc Surg. 1986;3:462–469. doi: 10.1067/mva.1986.avs0030462. [DOI] [PubMed] [Google Scholar]
- 13.Cormier JM, Fichelle JM, Vennin J, Laurian C, Gigou F. Atherosclerotic occlusive disease of the superior mesenteric artery: late results of reconstructive surgery. Ann Vasc Surg. 1991;5:510–518. doi: 10.1007/BF02015274. [DOI] [PubMed] [Google Scholar]
- 14.Gentile AT, Moneta GL, Taylor LM, Jr, Park TC, McConnell DB, Porter JM. Isolated bypass to the superior mesenteric artery for intestinal ischemia. Arch Surg. 1994;129:926–931. doi: 10.1001/archsurg.1994.01420330040009. [DOI] [PubMed] [Google Scholar]
- 15.Stanton PE, Jr, Hollier PA, Seidel TW, Rosenthal D, Clark M, Lamis PA. Chronic intestinal ischemia: diagnosis and therapy. J Vasc Surg. 1986;4:338–344. [PubMed] [Google Scholar]
- 16.McMillan WD, McCarthy WJ, Bresticker MR, et al. Mesenteric artery bypass: objective patency determination. J Vasc Surg. 1995;21:729–740. doi: 10.1016/s0741-5214(05)80004-7. [DOI] [PubMed] [Google Scholar]