Abstract
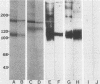
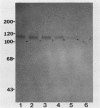
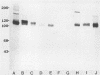
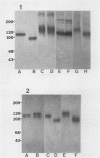
A monoclonal antibody (MAb 30B6) was recently described by Rogalski and Singer (J. Cell Biol. 101:785-801, 1985) which identified an integral membrane glycoprotein of chicken cells that was associated with a wide variety of sites of actin microfilament attachments to membranes. In this report, we present a further characterization of this integral protein. An immunochemical comparison was made of MAb 30B6 binding properties with those of two other MAbs, JG9 and JG22, which identify a component of a membrane protein complex that interacts with extracellular matrix proteins including fibronectin. We showed that the 110-kilodalton protein recognized by MAb 30B6 in extracts of chicken gizzard smooth muscle is identical, or closely related, to the protein that reacts with MAbs JG9 and JG22. These 110-kilodalton proteins are also structurally closely similar, if not identical, to one another as demonstrated by 125I-tryptic peptide maps. However, competition experiments showed that MAb 30B6 recognizes a different epitope from those recognized by MAbs JG9 and JG22. In addition, the 30B6 antigen is part of a complex that can be isolated on fibronectin columns. These results together establish that the 30B6 antigen is the same as, or closely similar to, the beta-chain of the protein complex named integrin, which is the complex on chicken fibroblast membranes that binds fibronectin. Although the 30B6 antigen is present in a wide range of tissues, its apparent molecular weight on gels varies in different tissues. These differences in apparent molecular weight are due, in large part, to differences in glycosylation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada S. S., Yamada K. M. Characterization of a 140-kD avian cell surface antigen as a fibronectin-binding molecule. J Cell Biol. 1986 Feb;102(2):442–448. doi: 10.1083/jcb.102.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C. A., Shea E., Duggan K., Horwitz A. F. Integrin (the CSAT antigen): functionality requires oligomeric integrity. J Cell Biol. 1986 Dec;103(6 Pt 1):2421–2428. doi: 10.1083/jcb.103.6.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. E. Characterization of a 140Kd cell surface glycoprotein involved in myoblast adhesion. J Cell Biochem. 1984;25(2):109–121. doi: 10.1002/jcb.240250206. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Greve J. M., Gottlieb D. I., Singer S. J. Immunocytochemical localization of 140 kD cell adhesion molecules in cultured chicken fibroblasts, and in chicken smooth muscle and intestinal epithelial tissues. J Histochem Cytochem. 1985 Jun;33(6):576–586. doi: 10.1177/33.6.3889142. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Hasegawa E., Hasegawa T., Weinstock C., Yamada K. M. Development of cell surface linkage complexes in cultured fibroblasts. J Cell Biol. 1985 Apr;100(4):1103–1114. doi: 10.1083/jcb.100.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Bradley D., Buck C. A., Horwitz A. F. Distribution of the cell substratum attachment (CSAT) antigen on myogenic and fibroblastic cells in culture. J Cell Biol. 1985 May;100(5):1528–1539. doi: 10.1083/jcb.100.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Gottlieb D. I. Monoclonal antibodies which alter the morphology of cultured chick myogenic cells. J Cell Biochem. 1982;18(2):221–229. doi: 10.1002/jcb.1982.240180209. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Knudsen K. A., Horwitz A. F., Buck C. A. A monoclonal antibody identifies a glycoprotein complex involved in cell-substratum adhesion. Exp Cell Res. 1985 Mar;157(1):218–226. doi: 10.1016/0014-4827(85)90164-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neff N. T., Lowrey C., Decker C., Tovar A., Damsky C., Buck C., Horwitz A. F. A monoclonal antibody detaches embryonic skeletal muscle from extracellular matrices. J Cell Biol. 1982 Nov;95(2 Pt 1):654–666. doi: 10.1083/jcb.95.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Rogalski A. A., Singer S. J. An integral glycoprotein associated with the membrane attachment sites of actin microfilaments. J Cell Biol. 1985 Sep;101(3):785–801. doi: 10.1083/jcb.101.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Vuento M., Engvall E. Interaction of fibronectin with antibodies and collagen in radioimmunoassay. Biochim Biophys Acta. 1978 Jun 21;534(2):210–218. doi: 10.1016/0005-2795(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Schroeder T. E. Dynamics of the contractile ring. Soc Gen Physiol Ser. 1975;30:305–334. [PubMed] [Google Scholar]
- Tokuyasu K. T., Maher P. A., Singer S. J. Distributions of vimentin and desmin in developing chick myotubes in vivo. I. Immunofluorescence study. J Cell Biol. 1984 Jun;98(6):1961–1972. doi: 10.1083/jcb.98.6.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]