SUMMARY
The role of PET-CT imaging in head and neck squamous cell carcinoma during pre-treatment staging, radiotherapy planning, treatment response assessment and post-therapy follow-up is reviewed with focus on current evidence, controversial issues and future clinical applications. In staging, the role of 18F-FDG PET-CT is well recognized for detecting cervical nodal involvement as well as for exclusion of distant metastases and synchronous primary tumours. In the evaluation of treatment response, the high negative predictive value of 18F-FDG PET-CT performed at least 8 weeks from the end of radio-chemotherapy allows prevention of unnecessary diagnostic invasive procedures and neck dissection in many patients, with a significant impact on clinical outcome. On the other hand, in this setting, the low positive predictive value due to possible post-radiation inflammation findings requires special care before making a clinical decision. Controversial data are currently available on the role of PET imaging during the course of radio-chemotherapy. The prognostic role of 18F-FDG PET-CT imaging in head and neck squamous cell carcinoma is recently emerging, in addition to the utility of this technique in evaluation of the tumour volume for planning radiation therapy. Additionally, new PET radiopharmaceuticals could provide considerable information on specific tumour characteristics, thus overcoming the limitations of 18F-FDG.
KEY WORDS: 18F-FDG, PET-CT, Carcinoma, Squamous cell, Head and neck tumours
RIASSUNTO
In questa review è analizzato il ruolo della PET-CT nei carcinomi squamosi del distretto testa-collo in fase di stadiazione, nella pianificazione del trattamento radiante, nella valutazione della risposta al trattamento radio-chemioterapico e nel follow-up, tenendo conto dei dati attualmente disponibili, delle questioni controverse e delle future applicazioni cliniche. In fase di stadiazione, è ampiamente riconosciuto il ruolo della PET-CT con 18F-FDG nella valutazione del coinvolgimento linfonodale, nonché nella esclusione della presenza di metastasi a distanza e di tumori primitivi sincroni. Nella valutazione della risposta al trattamento, l'elevato valore predittivo negativo della 18F-FDG PET-CT, effettuata almeno 8 settimane dopo la fine del trattamento radio-chemioterapico, consente di evitare in molti pazienti inutili procedure diagnostiche invasive nonché la dissezione del collo, con conseguente significativo impatto clinico. D'altra parte, in questa fase il basso valore predittivo positivo della metodica, causato dai possibili falsi positivi secondari alla concomitante flogosi post-attinica, deve essere tenuto in particolare considerazione prima di prendere una decisione clinica. Dati controversi sono attualmente disponibili sul ruolo dell'imaging PET durante il trattamento radio-chemioterapico. Negli ultimi anni, è emerso il ruolo prognostico della 18F-FDG PET-CT nei carcinomi a cellule squamose del distretto testa-collo, così come l'utilità di questa tecnica nella valutazione del volume del tumore per la pianificazione del trattamento radiante. Inoltre, nuove prospettive provengono dall'impiego dei nuovi radiofarmaci PET che potrebbero fornire notevoli informazioni su caratteristiche biologiche specifiche del tumore, superando di conseguenza i noti limiti del 18F-FDG.
Introduction
18F-fluorodeoxy-D-glucose positron emission tomography- computed tomography (18F-FDG PET-CT) has become an important diagnostic tool for evaluation of head and neck squamous cell carcinomas (HNSCCs), and is applied in various clinical settings, ranging from pre-treatment staging to radiotherapy planning, treatment response assessment and post-therapy follow-up 1 2. Although 18F-FDG is the most commonly used PET tracer for oncologic purposes, its use in HNSCCs suffers from some limitations due to the complex anatomy of this region and the small size of the anatomical structures, as well as the physiological uptake of 18F-FDG in normal organs that may influence image interpretation. 18F-FDG uptake reflects glucose metabolism and can be observed in several normal tissues with wide variability of the normal pattern, including brain, vocal cords, salivary glands, cervical muscles, lymphoid tissue and brown fat, as well as in various benign tumours, such as common Warthin's tumour 3. Moreover, the inflammatory processes that occur in patients submitted to surgery or radiotherapy are a frequent cause of false positive PET results, since the activated inflammatory cells show increased 18F-FDG uptake 4 5. Finally, artefacts related to patient movement or metal dental prostheses may further limit interpretation of PET images, thus requiring non-attenuation corrected PET data evaluation 6 7. PET-CT scanners currently allow quick and high resolution imaging, and can correlate anatomical location with functional information. The recent introduction of whole-body PET-magnetic resonance imaging (MRI) in clinical practice offers new opportunities for integrated functional-anatomic imaging 8 9. This review will focus on the use of 18F-FDG PET-CT in various clinical scenarios of HNSCCs with attention to PET radiopharmaceuticals other than 18F-FDG, and the new perspectives offered by PET-MRI.
Pre-treatment staging
Accurate assessment of disease extension is essential to plan the most appropriate treatment, with important implications for patient outcomes. In clinical practice, the conventional diagnostic strategy of HNSCC patients includes detailed physical examination and endoscopy followed by imaging modalities such as neck ultrasound, neck MRI and neck-chest CT for the assessment of disease extent and diagnosis of synchronous second primary tumours (SPTs). Recent studies have shown that 18F-FDG PET-CT is more accurate than conventional staging in HNSCCs, thus resulting in a change of therapeutic management in about one-third of patients 10-12.
Primary tumour assessment
Even though 18F-FDG PET-CT detects primary HNSCC with high sensitivity (> 95%), primary tumour assessment is generally performed with clinical examination, endoscopy and MRI 13 14. The main limitation of standard PET-CT, if performed with low-dose unenhanced CT, is its inability to accurately assess the site, extent of tumour spread and relationship between the tumour and adjacent structures. This limitation can only partially be overcome by with contrast-enhanced PET-CT 12. Initial reports on the use of integrated PET-MRI in HNSCCs showed better tumour delineation and good correlation between the metabolic parameters obtained using PET-MRI and PETCT 9 15. In patients with cervical lymph node metastasis from a carcinoma of unknown origin, 18F-FDG PET-CT represents a useful diagnostic tool to detect the primary tumour, with a detection rate of 25-38.5% 16-18. Future studies with PET-MRI will determine whether the technique can improve the detection rate of occult primary head and neck tumours 9.
Cervical lymph node assessment
The main indication of 18F-FDG PET-CT in newly diagnosed HNSCCs is detection of cervical lymph node involvement, which is one of the most important prognostic factors. With regards to node involvement, the main limitation of all imaging techniques is the high rate of false negative results, staged as cN0, which turn out to be pN+ after neck dissection. Sentinel lymph node biopsy has several limitations that limit its routine clinical employment in the head and neck 19.
This awareness is the basis of the indications for prophylactic neck treatment by international guidelines 20; however, this approach may lead clinicians to unnecessarily treat a number of necks with relevant morbidity. Data from the literature support the superiority of PET-CT over morphologic imaging in detecting lymph node involvement 12. A clear advantage of functional imaging is that an alteration in the size or structure of lymph nodes is not required to detect lesions (Fig. 1). However, it must be stressed that small lymph node lesions may be missed (possible false negative results), and that inflamed lymph nodes may take up the tracer (possible false positive results). In particular, the finite spatial resolution of a PET-CT scanner (4-6 mm) limits its sensitivity for microscopic disease that is detectable only by histopathology after neck dissection. For this reason, a negative 18F-FDG PET-CT scan for lymph node involvement does not justify a "wait and see" approach in all cases; the decision to perform neck dissection in patients with negative morphological and functional imaging still relies essentially on the evaluation of risk factors and tumour characteristics such as T-stage and histopathological features 12 21 22. Preliminary data with PET-MRI report good sensitivity (85%) and specificity (92%) for nodal staging, higher than PET-CT, but with the same limitation of under-staging a certain number of patients with micrometastases 9.
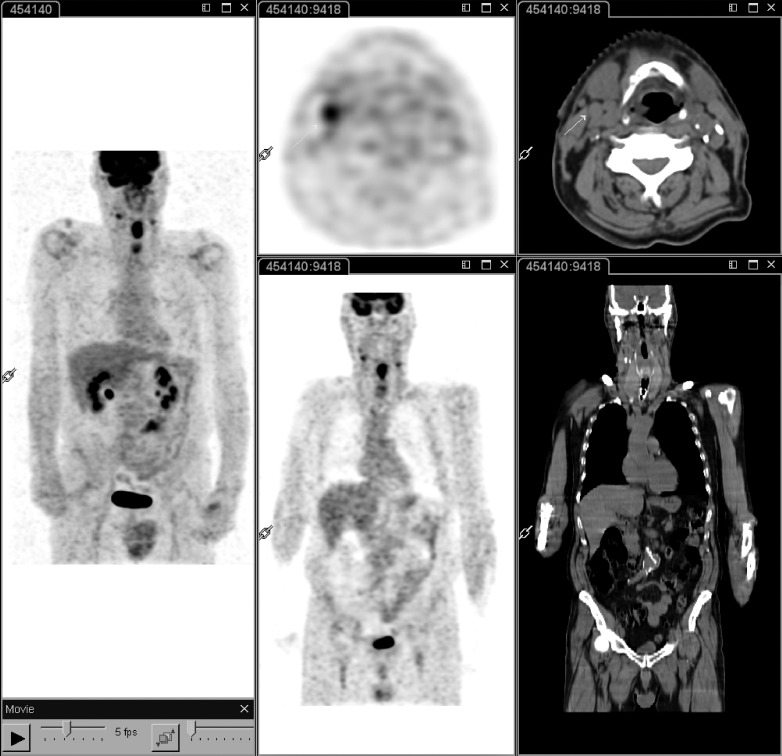
Fig. 1.
18F-FDG PET-CT performed at staging in a patient with laryngeal carcinoma. Whole body PET-CT (left panel) shows intense 18F-FDG uptake of the primary tumour as well as two lateral cervical nodes, one of which on the right side demonstrates a short axis less than 10 mm in the transaxial plane of CT (middle and right panels; white arrow). These findings have both prognostic and therapeutic implications: the patient is candidate for radiochemotherapy with bilateral cervical nodal irradiation.
Distant metastasis assessment
The screening for distant metastases is important in patients with advanced disease, especially with nodal involvement, and in naso- and hypopharyngeal carcinomas. The most common sites are lungs, bone and liver (Fig. 2). In this context, a higher accuracy of 18F-FDG PET-CT than CT for detection of distant metastasis has been clearly demonstrated, except for small lung lesions. Moreover, in approximately 13% of cases the improvement in detection of distant lesions leads to a change of management with important consequences for patient survival 23-25.
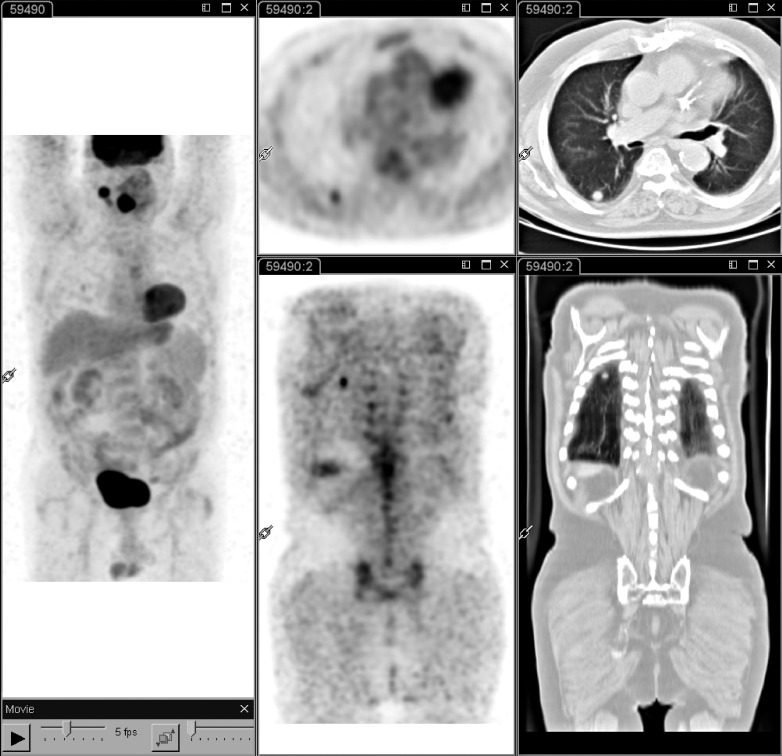
Fig. 2.
18F-FDG PET-CT performed at staging in a patient with laryngeal carcinoma. Whole body PET-CT (left panel) shows intense 18F-FDG uptake of the primary tumour and a right lateral cervical lymphadenopathy. In addition, a round solid pulmonary nodule with high 18F-FDG uptake is evident in the apical segment of the lower lobe of the right lung, suggesting a metastatic lesion (middle and right panels).
Second primary tumour assessment
Second primary tumours (SPTs) can occur in 5-10% of HNSCC patients and are more frequent in the head and neck region, oesophagus and lungs (Fig. 3). SPTs are notably the first cause of death with a decisive impact on overall survival rates of early stage HNSCC patients 26. 18F-FDG PET-CT is an accurate method to detect second primaries, with a high negative predictive value and a relatively lower positive predictive value as inflammation and benign hyperplasia in the head and neck region, and benign or precancerous intestinal polyps can result in false positive PET findings 12 27. Nonetheless, PET-CT, by detecting second primary lesions, can impact both the treatment choice and, most of all, the overall survival of early stage HNSCCs that already have a very good disease specific survival 28.
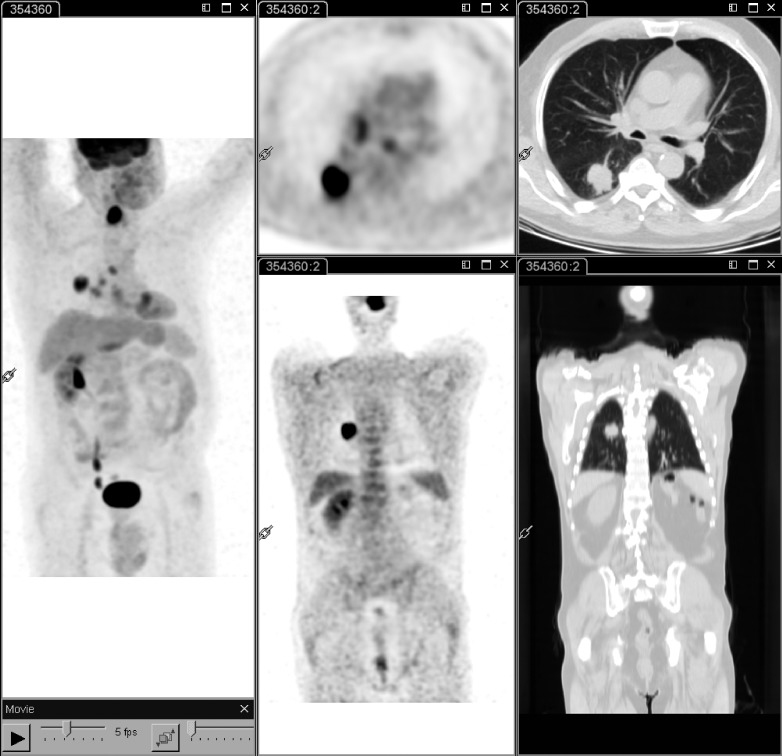
Fig. 3.
18F-FDG PET-CT performed at staging in a patient with laryngeal carcinoma. Whole body PET-CT (left panel) shows intense 18F-FDG uptake in the primary tumour as well as in a lung mass located in the apical segment of the right lower lobe; mediastinal lymph nodes with increased activity are also associated (left and middle panels). These findings suggest a second primary tumour.
Prognostic significance of pre-treatment PET-CT
Despite the substantial changes in treatment strategies over the past two decades, disease control in HNSCCs is still heterogeneous, since patients with similar clinicopathological features undergoing the same treatment may differ widely in response to treatment and prognosis 29. Thus, the identification of further prognostic factors along with TNM staging could help to identify high-risk patients who respond poorly to conventional therapy, and could benefit from intensification or switch of treatment modality. In this context, the prognostic value of various 18F-FDG pre-treatment parameters including maximal and mean standardized uptake value (SUVmax and SUV mean), metabolic tumour volume (MTV) and total lesion glycolysis (TLG) is under investigation 30. The utility of these parameters has been evaluated with conflicting results 30-32. The main criticism is the variable behaviour of the different metabolic parameters, each showing potential pitfalls in terms of calculation and reproducibility.
Radiotherapy planning
Molecular imaging with 18F-FDG provides a unique opportunity for radiation treatment planning in HNSCCs in terms of selection and delineation of target volumes as well as dose planning 33. Looking at target volume selection, the added value of 18F-FDG PET-CT is identification of the occult primary tumour in patients with cervical lymph node metastasis with the possibility of decreasing the radiotherapy target volume, and consequently the acute and late side effects of radiotherapy, as well as detection of distant metastases with high sensitivity 16-18. For accurate delineation of target volumes and organs at risk, the goal of using molecular imaging is to optimize the treatment plan thanks to its superior sensitivity and contrast resolution over anatomical imaging techniques 34. In the initial experience of Daisne et al., gross tumour volume (GTV) delineated from 18F-FDG PET was closest to the pathologic GTV from surgical specimens, and significantly smaller than GTV delineated by CT and MRI 35. However, subsequent data were less consistent 34. The main drawback is the lack of a standardized method for functional volume segmentation, which heavily influences the volume and shape of the resulting GTV 34-36. Further applications of 18F-FDG PET-CT for radiotherapy planning are under clinical investigation, and include the possibility of directing dose escalation to 18F-FDG-avid sub-volumes of the tumour as well as adapting the radiotherapy plan during treatment thanks to the information on the biological and molecular tumour changes induced by therapy 34-37. Moreover, the possibility of targeting radiation-resistant regions within the tumour on the basis of biologic information of molecular imaging is under investigation, for example the identification of the hypoxic volume within the GTV by using hypoxia-related PET tracers to deliver higher doses to hypoxic cells 38.
Treatment response assessment
An accurate evaluation of response is essential in the management of patients with HNSCC treated with radio- chemotherapy. 18F-FDG PET-CT is commonly used to assess treatment response, since it identifies viable tumour within residual masses, thus overcoming the known limitations of morphological imaging modalities 39-42. In particular, 18F-FDG PET-CT has a high negative predictive value (> 95%) that can spare the patient of unnecessary diagnostic invasive procedures and neck dissection in many cases (about 75%), with a significant impact on patient outcome and morbidity, and a low risk of under-treatment (about 2%) 12 41. Several studies have demonstrated that in patients with a complete metabolic response neck dissection can be avoided, even in the presence of residual node abnormalities by conventional imaging 43 44 (Fig. 4); considering the additional morbidity of neck dissections in irradiated necks, this approach can be considered a relevant achievement in head and neck oncology. Unfortunately, the positive predictive value is low, due to the high number of false positive results related to post-radiation inflammation 45. To reduce the number of false positive findings, it is crucial to accurately select high risk patients (i.e. HPV negative), to know radiation treatment volume and choose the timing for PET-CT post-treatment assessment. There is general consensus to set the optimum time for 18F-FDG PET-CT at 8-12 weeks, thereby reducing both false positive and false negative findings, while the the latter is related to the presence of undetectable microscopic residual disease. 18F-FDG PET-CT was considered to be useful in selective cases to assess response to induction treatment in patients enrolled in larynx preservation trials to assess the risks and benefits of treatment 46. Correlating the post-treatment metabolic response with clinical outcomes, the data indicate that 18F-FDG PETCT performed at the end of radio-chemotherapy provides prognostic information, as it strongly correlates with local and regional control and survival. However, controversial results are reported regarding the role of PET-CT performed early during treatment 5 29 47 48. In a series of 26 patients treated with radio-chemotherapy for HNSCC, our group did not find a significant correlation between the "early" changes of FDG uptake in the primary tumour and lymph node involvement and local and regional control, respectively, or between the overall metabolic response at PET-CT and clinical outcome 29. Our results on the unreliability of 18F-FDG PET-CT for the early assessment of response to radiochemotherapy in HNSCC are in contrast with the recent study by Hentschel et al. who showed that a decrease of 50% or more of SUVmax from the beginning (0 Gy) to week 1 or 2 of treatment (10 or 20 Gy) is a potential prognostic marker for patients with HNSCC 48.
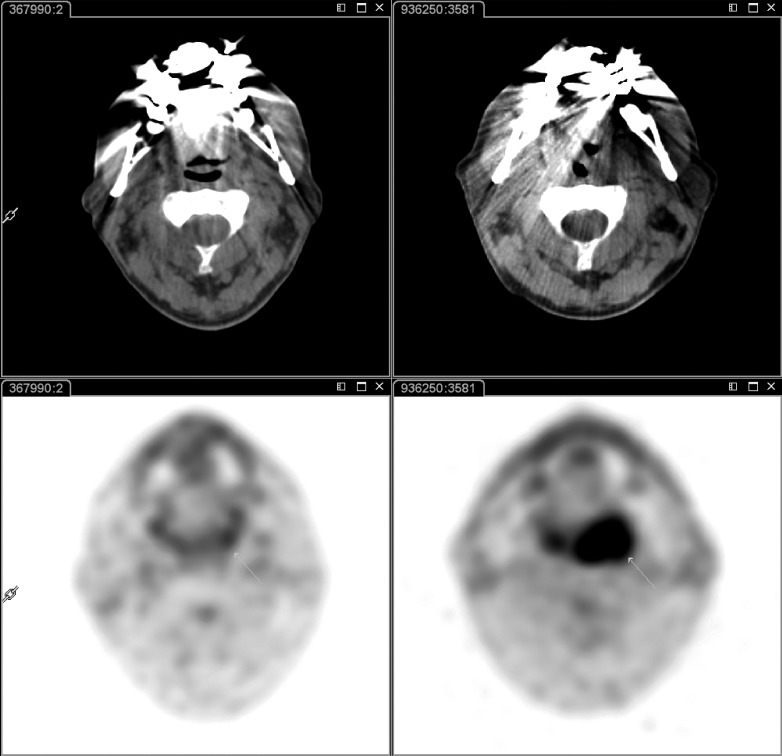
Fig. 4.
18F-FDG PET-CT at baseline (right panel) and 8 weeks after the end of radio-chemotherapy (left panel). At baseline, intense 18F-FDG uptake is evident in the left oropharyngeal region (white arrow); after radio-chemotherapy, 18F-FDG uptake was no longer visible, suggesting complete metabolic response to treatment.
Post-therapy follow-up
Despite initial aggressive treatment, loco-regional or distant recurrence can occur in HNSCC patients, especially within the first year; early detection of loco-regional disease may improve survival by increasing the effectiveness of salvage therapy, which is the most effective modality in this setting; in contrast, the real advantage of early detection of distant metastases in asymptomatic patients is still unclear 12. At morphologic imaging and physical examination, detection of loco-regional recurrence or residual disease may be difficult due to the presence of treatment-induced changes that could not be differentiated from residual/recurrent disease. Several studies involving both patients with suspected recurrence and those without clinical symptoms have demonstrated that PET-CT imaging is superior to physical examination and conventional imaging to detect recurrent loco-regional disease, as well as distant lesions and metachronous primary tumours 42 49-53. For patients with a PET positive result, biopsy is recommended due to a relatively high false positive rate related to post-treatment inflammation 12. A recent systematic review and meta-analysis of studies assessing the diagnostic performance of 18F-FDG PET and PET-CT in response assessment and surveillance imaging of HNSCC patients reported a high NPV (> 94%) and a suboptimal PPV (< 60%) both for the primary site and cervical nodes 54.
PET radiopharmaceuticals other than 18F-FDG
It is well known that 18F-FDG is not a specific marker of malignancy, since 18F-FDG uptake in normal or active inflammatory tissues may occur, thus limiting image interpretation. To overcome these drawbacks, more specific PET tracers reflecting specific biologic tumour characteristics may be useful to differentiate tumours by inflammation; these tracers, which are under clinical investigation, include 18F-fluorothymidine for DNA synthesis (18F-FLT), 18F-fluoroethyl-L-thyrosine (18F-FET) and L-methyl-11Cmethionine (11C-MET) for amino acid uptake and protein synthesis 55-57. Among these tracers, 18F-FLT seems to be the most promising because of its specificity 33. Moreover, tracers that allow in vivo detection and quantification of tumour hypoxia such as 18F-fluoromisonidazole (18F-FMISO) or 18F-fluoroazomycin-arabinofluranoside (18F-FAZA) and 18F-3-[F]fluoro-2-(4-((2-nitro-1H-imidazol-1-yl)methyl)-1H-1,2,3,-triazol-1-yl)-propan-1-ol (18F-HX4) are of growing interest; in fact, hypoxia is not a rare event in HNSCCs with important consequences on treatment response since hypoxic cells are more resistant to the cytotoxic effects of ionizing radiation 55 57-59 (Table I).
Table I.
PET radiopharmaceuticals other than 18F-FDG.
| PET Radiopharmaceutical | Molecular Target | Indications | Clinical application | References |
|---|---|---|---|---|
| 18F-FLT | Proliferation | Staging-restaging Response evaluation Adaptive radiotherapy |
Experimental | 56, 60-62 |
|
18F-FET 11C-MET |
Protein synthesis | Staging-restaging Adaptive radiotherapy |
Experimental Early clinical |
63, 64 |
|
18F-FMISO 18F-FAZA 18F-HX4 |
Hypoxia | Staging Response Evaluation Adaptive radiotherapy |
Experimental | 59, 65-70 |
| 68Zr/124I-anti EGFR | Targeting of EGFR | Targeted therapies | Experimental | 71 |
| 18F-Annexin | Apoptosis | Response evaluation | Experimental | 72 |
| 18F-Galacto-RGD | Neoangiogenesis | Response evaluation Targeted therapies |
Experimental | 73 |
FLT = fluorothymidine; FET = fluoroethyl-L-thyrosine; MET = L-methyl-methionine; MISO = fluoromisonidazole; FAZA = fluoroazomycin-arabinofluranoside; HX4 = 3-[F]fluoro- 2-(4-((2-nitro-1H-imidazol-1-yl)methyl)-1H-1,2,3,-triazol-1-yl)-propan-1-ol; EGFR = epidermal growth factor receptor; RGD = arginine-glycine-aspartate.
Our attitude in clinical practice
In our Centre, we usually perform pre-treatment 18F-FDG PET-CT evaluation in all patients with HNSCC who are candidates for primary irradiation (± chemotherapy), with the exception of early glottic lesions, and in all cases with high risk of distant metastases (hypopharyngeal and rhinopharyngeal primary tumours, cN3 cases). The most relevant clinical information coming from this pre-treatment scan is refinement of neck staging and evaluation of distant metastases or second primaries; both inquiries have a substantial impact on treatment planning. We also prescribe 18F-FDG PET-CT in patients with cervical metastases and no primary site at conventional assessment.
For treatment response evaluation, we usually perform a PET-CT scan 8-12 weeks after the end of radio-chemotherapy. Patients with a complete metabolic response are referred to periodic follow-up, whereas patients showing a positive scan after treatment undergo subsequent evaluation including, when indicated, biopsy.
During follow-up, especially in the first year when most recurrences occur, we perform 18F-FDG PET-CT when a recurrence is suspected and a confirmatory biopsy would be associated with significant morbidity, as in case of neck node enlargement, tissue deep under a reconstructive flap, significant persistent post-irradiation toxicity and, in general, difficult exposure requiring general anaesthesia for an adequate biopsy sampling.
Conclusions
18F-FDG PET-CT is particularly useful for staging, restaging and radiotherapy planning as well as for assessment of treatment response in HNSCC patients, due to its superior accuracy over clinical examination and conventional anatomic imaging. The main limitations, especially in the post-treatment setting, are possible false positive results due to inflammation and the inability to detect microscopic disease. In the future, new tracers other than 18F-FDG, as well as PET-MRI imaging, will provide clear advantages in several clinical scenarios.
References
- 1.Oyen WJ, Marres HA, Kaanders JH. Progress in nuclear medicine procedures in head and neck oncology. Q J Nucl Med Mol Imaging. 2011;55:485–486. [PubMed] [Google Scholar]
- 2.Corvò R. Evidence-based radiation oncology in head and neck squamous cell carcinoma. Radiother Oncol. 2007;85:156–170. doi: 10.1016/j.radonc.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz E, Hurlimann S, Soyka JD, et al. FDG-positive Warthin's tumors in cervical lymph nodes mimicking metastases in tongue cancer staging with PET/CT. Otolaryngol Head Neck Surg. 2009;140:134–135. doi: 10.1016/j.otohns.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Kubota R, Yamada S, Kubota K, et al. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–1980. [PubMed] [Google Scholar]
- 5.Hentschel M, Appold S, Schreiber A, et al. Serial FDG-PET on patients with head and neck cancer: implications for radiation therapy. Int J Radiat Biol. 2009;85:796–804. doi: 10.1080/09553000903039180. [DOI] [PubMed] [Google Scholar]
- 6.Wong RJ. Current status of FDG-PET for head and neck cancer. J Surg Oncol. 2008;97:649–652. doi: 10.1002/jso.21018. [DOI] [PubMed] [Google Scholar]
- 7.Burrell SC, Abbeele AD, et al. 2-Deoxy-2-[F-18]fluoro- D-glucose-positron emission tomography of the head and neck: an atlas of normal uptake and variants. Mol Imaging Biol. 2005;7:244–256. doi: 10.1007/s11307-005-4112-z. [DOI] [PubMed] [Google Scholar]
- 8.Loeffelbein DJ, Souvatzoglou M, Wankerl V, et al. PET-MRI fusion in head-and-neck oncology: current status and implications for hybrid PET/MRI. J Oral Maxillofac Surg. 2012;70:473–483. doi: 10.1016/j.joms.2011.02.120. [DOI] [PubMed] [Google Scholar]
- 9.Buchbender C, Heusner TA, Lauenstein TC, et al. Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis. J Nucl Med. 2012;53:928–938. doi: 10.2967/jnumed.112.105338. [DOI] [PubMed] [Google Scholar]
- 10.Czernin J, Allen-Auerbach M, Schelbert HR. Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006. J Nucl Med. 2007;48(Suppl 1):78S–88S. [PubMed] [Google Scholar]
- 11.Connell CA, Corry J, Milner AD, et al. Clinical impact of, and prognostic stratification by, F-18 FDG PET/CT in head and neck mucosal squamous cell carcinoma. Head Neck. 2007;29:986–995. doi: 10.1002/hed.20629. [DOI] [PubMed] [Google Scholar]
- 12.Mak D, Corry J, Lau E, et al. Role of FDG-PET/CT in staging and follow-up of head and neck squamous cell carcinoma. Q J Nucl Med Mol Imaging. 2011;55:487–499. [PubMed] [Google Scholar]
- 13.Roh JL, Yeo NK, Kim JS, et al. Utility of 2-[18F] fluoro-2- deoxy-D-glucose positron emission tomography and positron emission tomography/computed tomography imaging in the preoperative staging of head and neck squamous cell carcinoma. Oral Oncol. 2007;43:887–893. doi: 10.1016/j.oraloncology.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Baek CH, Chung MK, Son YI, et al. Tumor volume assessment by 18F-FDG PET/CT in patients with oral cavity cancer with dental artifacts on CT or MR images. J Nucl Med. 2008;49:1422–1428. doi: 10.2967/jnumed.108.051649. [DOI] [PubMed] [Google Scholar]
- 15.Boss A, Stegger L, Bisdas S, et al. Feasibility of simultaneous PET/MR imaging in the head and upper neck area. Eur Radiol. 2011;21:1439–1446. doi: 10.1007/s00330-011-2072-z. [DOI] [PubMed] [Google Scholar]
- 16.Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer. 2004;101:2641–2649. doi: 10.1002/cncr.20687. [DOI] [PubMed] [Google Scholar]
- 17.Johansen J, Petersen H, Godballe C, et al. FDG-PET/CT for detection of the unknown primary head and neck tumor. Q J Nucl Med Mol Imaging. 2011;55:500–508. [PubMed] [Google Scholar]
- 18.Wong WL, Sonoda LI, Gharpurhy A, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the assessment of occult primary head and neck cancers-- an audit and review of published studies. Clin Oncol (R Coll Radiol) 2012;24:190–195. doi: 10.1016/j.clon.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Antonio JK, Santini S, Politi D, et al. Sentinel lymph node biopsy in squamous cell carcinoma of the head and neck: 10 years of experience. Acta Otorhinolaryngol Ital. 2012;32:18–25. [PMC free article] [PubMed] [Google Scholar]
- 20.Pfister DG, Ang KK, Brizel DM, et al. Head and neck cancers. J Natl Compr Canc Netw. 2011;9:596–650. doi: 10.6004/jnccn.2011.0053. [DOI] [PubMed] [Google Scholar]
- 21.Schoder H, Carlson DL, Kraus DH, et al. 18F-FDG PET/CT for detecting nodal metastases in patients with oral cancer staged N0 by clinical examination and CT/MRI. J Nucl Med. 2006;47:755–762. [PubMed] [Google Scholar]
- 22.Stoeckli SJ, Steinert H, Pfaltz M, et al. Is there a role for positron emission tomography with 18F-fluorodeoxyglucose in the initial staging of nodal negative oral and oropharyngeal squamous cell carcinoma. Head Neck. 2002;24:345–349. doi: 10.1002/hed.10057. [DOI] [PubMed] [Google Scholar]
- 23.Brouwer J, Senft A, Bree R, et al. Screening for distant metastases in patients with head and neck cancer: is there a role for (18)FDG-PET? Oral Oncol. 2006;42:275–280. doi: 10.1016/j.oraloncology.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Ng SH, Chan SC, Liao CT, et al. Distant metastases and synchronous second primary tumors in patients with newly diagnosed oropharyngeal and hypopharyngeal carcinomas: evaluation of (18)F-FDG PET and extended-field multi-detector row CT. Neuroradiology. 2008;50:969–979. doi: 10.1007/s00234-008-0426-2. [DOI] [PubMed] [Google Scholar]
- 25.Lonneux M, Hamoir M, Reychler H, et al. Positron emission tomography with [18F]fluorodeoxyglucose improves staging and patient management in patients with head and neck squamous cell carcinoma: a multicenter prospective study. J Clin Oncol. 2010;28:1190–1195. doi: 10.1200/JCO.2009.24.6298. [DOI] [PubMed] [Google Scholar]
- 26.Narayana A, Vaughan AT, Fisher SG, et al. Second primary tumors in laryngeal cancer: results of long-term follow-up. Int J Radiat Oncol Biol Phys. 1998;42:557–562. doi: 10.1016/s0360-3016(98)00250-8. [DOI] [PubMed] [Google Scholar]
- 27.Strobel K, Haerle SK, Stoeckli SJ, et al. Head and neck squamous cell carcinoma (HNSCC)--detection of synchronous primaries with (18)F-FDG-PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:919–927. doi: 10.1007/s00259-009-1064-6. [DOI] [PubMed] [Google Scholar]
- 28.Dinapoli N, Parrilla C, Galli J, et al. Multidisciplinary approach in the treatment of T1 glottic cancer. The role of patient preference in a homogenous patient population. Strahlenther Onkol. 2010;186:607–613. doi: 10.1007/s00066-010-2142-1. [DOI] [PubMed] [Google Scholar]
- 29.Castaldi P, Rufini V, Bussu F, et al. Can "early" and "late"18F-FDG PET-CT be used as prognostic factors for the clinical outcome of patients with locally advanced head and neck cancer treated with radio-chemotherapy? Radiother Oncol. 2012;103:63–68. doi: 10.1016/j.radonc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Higgins KA, Hoang JK, Roach MC, et al. Analysis of pretreatment FDG-PET SUV parameters in head-and-neck cancer: tumor SUVmean has superior prognostic value. Int J Radiat Oncol Biol Phys. 2012;82:548–553. doi: 10.1016/j.ijrobp.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 31.Xie P, Li M, Zhao H, et al. 18F-FDG PET or PET-CT to evaluate prognosis for head and neck cancer: a meta-analysis. J Cancer Res Clin Oncol. 2011;137:1085–1093. doi: 10.1007/s00432-010-0972-y. [DOI] [PubMed] [Google Scholar]
- 32.Schinagl DA, Span PN, Oyen WJ, et al. Can FDG PET predict radiation treatment outcome in head and neck cancer? Results of a prospective study. Eur J Nucl Med Mol Imaging. 2011;38:1449–1458. doi: 10.1007/s00259-011-1789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregoire V, Chiti A. Molecular imaging in radiotherapy planning for head and neck tumors. J Nucl Med. 2011;52:331–334. doi: 10.2967/jnumed.110.075689. [DOI] [PubMed] [Google Scholar]
- 34.Arens AI, Troost EG, Schinagl D, et al. FDG-PET/CT in radiation treatment planning of head and neck squamous cell carcinoma. Q J Nucl Med Mol Imaging. 2011;55:521–528. [PubMed] [Google Scholar]
- 35.Daisne JF, Duprez T, Weynand B, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology. 2004;233:93–100. doi: 10.1148/radiol.2331030660. [DOI] [PubMed] [Google Scholar]
- 36.Schinagl DA, Vogel WV, Hoffmann AL, et al. Comparison of five segmentation tools for 18F-fluoro-deoxy-glucosepositron emission tomography-based target volume definition in head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:1282–1289. doi: 10.1016/j.ijrobp.2007.07.2333. [DOI] [PubMed] [Google Scholar]
- 37.Troost EG, Schinagl DA, Bussink J, et al. Clinical evidence on PET-CT for radiation therapy planning in head and neck tumours. Radiother Oncol. 2010;96:328–334. doi: 10.1016/j.radonc.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Lee NY, Mechalakos JG, Nehmeh S, et al. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2008;70:2–13. doi: 10.1016/j.ijrobp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kao J, Vu HL, Genden EM, et al. The diagnostic and prognostic utility of positron emission tomography/computed tomography- based follow-up after radiotherapy for head and neck cancer. Cancer. 2009;115:4586–4594. doi: 10.1002/cncr.24493. [DOI] [PubMed] [Google Scholar]
- 40.Passero VA, Branstetter BF, Shuai Y, et al. Response assessment by combined PET-CT scan versus CT scan alone using RECIST in patients with locally advanced head and neck cancer treated with chemoradiotherapy. Ann Oncol. 2010;21:2278–2283. doi: 10.1093/annonc/mdq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber WA. Assessing tumor response to therapy. J Nucl Med. 2009;50(Suppl 1):1S–10S. doi: 10.2967/jnumed.108.057174. [DOI] [PubMed] [Google Scholar]
- 42.Isles MG, McConkey C, Mehanna HM, et al. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol. 2008;33:210–222. doi: 10.1111/j.1749-4486.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- 43.Ware RE, Matthews JP, Hicks RJ, et al. Usefulness of fluorine- 18 fluorodeoxyglucose positron emission tomography in patients with a residual structural abnormality after definitive treatment for squamous cell carcinoma of the head and neck. Head Neck. 2004;26:1008–1017. doi: 10.1002/hed.20097. [DOI] [PubMed] [Google Scholar]
- 44.Porceddu SV, Pryor DI, Burmeister E, et al. Results of a prospective study of positron emission tomography-directed management of residual nodal abnormalities in node-positive head and neck cancer after definitive radiotherapy with or without systemic therapy. Head Neck. 2011;33:1675–1682. doi: 10.1002/hed.21655. [DOI] [PubMed] [Google Scholar]
- 45.Yao M, Smith RB, Hoffman HT, et al. Clinical significance of postradiotherapy [18F]-fluorodeoxyglucose positron emission tomography imaging in management of head-andneck cancer-a long-term outcome report. Int J Radiat Oncol Biol Phys. 2009;74:9–14. doi: 10.1016/j.ijrobp.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 46.Lefebvre JL, Ang KK. Larynx preservation clinical trial design: key issues and recommendations-a consensus panel summary. Int J Radiat Oncol Biol Phys. 2009;73:1293–1303. doi: 10.1016/j.ijrobp.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 47.Farrag A, Ceulemans G, Voordeckers M, et al. Can 18FFDG- PET response during radiotherapy be used as a predictive factor for the outcome of head and neck cancer patients? Nucl Med Commun. 2010;31:495–501. doi: 10.1097/MNM.0b013e3283334e2b. [DOI] [PubMed] [Google Scholar]
- 48.Hentschel M, Appold S, Schreiber A, et al. Early FDG PET at 10 or 20 Gy under chemoradiotherapy is prognostic for locoregional control and overall survival in patients with head and neck cancer. Eur J Nucl Med Mol Imaging. 2011;38:1203–1211. doi: 10.1007/s00259-011-1759-3. [DOI] [PubMed] [Google Scholar]
- 49.Abgral R, Querellou S, Potard G, et al. Does 18F-FDG PET/ CT improve the detection of posttreatment recurrence of head and neck squamous cell carcinoma in patients negative for disease on clinical follow-up? J Nucl Med. 2009;50:24–29. doi: 10.2967/jnumed.108.055806. [DOI] [PubMed] [Google Scholar]
- 50.Liu T, Xu W, Yan WL, et al. FDG-PET, CT, MRI for diagnosis of local residual or recurrent nasopharyngeal carcinoma, which one is the best? A systematic review. Radiother Oncol. 2007;85:327–335. doi: 10.1016/j.radonc.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Lowe VJ, Boyd JH, Dunphy FR, et al. Surveillance for recurrent head and neck cancer using positron emission tomography. J Clin Oncol. 2000;18:651–658. doi: 10.1200/JCO.2000.18.3.651. [DOI] [PubMed] [Google Scholar]
- 52.Krabbe CA, Pruim J, Dijkstra PU, et al. 18F-FDG PET as a routine posttreatment surveillance tool in oral and oropharyngeal squamous cell carcinoma: a prospective study. J Nucl Med. 2009;50:1940–1947. doi: 10.2967/jnumed.109.065300. [DOI] [PubMed] [Google Scholar]
- 53.Terhaard CH, Bongers V, Rijk PP, et al. F-18-fluoro-deoxy- glucose positron-emission tomography scanning in detection of local recurrence after radiotherapy for laryngeal/ pharyngeal cancer. Head Neck. 2001;23:933–941. doi: 10.1002/hed.1135. [DOI] [PubMed] [Google Scholar]
- 54.Gupta T, Master Z, Kannan S, et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38:2083–2095. doi: 10.1007/s00259-011-1893-y. [DOI] [PubMed] [Google Scholar]
- 55.Bussink J, Herpen CM, Kaanders JH, et al. PET-CT for response assessment and treatment adaptation in head and neck cancer. Lancet Oncol. 2010;11:661–669. doi: 10.1016/S1470-2045(09)70353-5. [DOI] [PubMed] [Google Scholar]
- 56.Troost EG, Bussink J, Hoffmann AL, et al. 18F-FLT PET/ CT for early response monitoring and dose escalation in oropharyngeal tumors. J Nucl Med. 2010;51:866–874. doi: 10.2967/jnumed.109.069310. [DOI] [PubMed] [Google Scholar]
- 57.Gornik G, Weber W. New tracers beyond FDG in head and neck oncology. Q J Nucl Med Mol Imaging. 2011;55:529–540. [PubMed] [Google Scholar]
- 58.Loon J, Janssen MH, Ollers M, et al. PET imaging of hypoxia using [18F]HX4: a phase I trial. Eur J Nucl Med Mol Imaging. 2010;37:1663–1668. doi: 10.1007/s00259-010-1437-x. [DOI] [PubMed] [Google Scholar]
- 59.Chen L, Zhang Z, Kolb HC, et al. 18F-HX4 hypoxia imaging with PET/CT in head and neck cancer: a comparison with 18F-FMISO. Nucl Med Commun. 2012;33:1096–1102. doi: 10.1097/MNM.0b013e3283571016. [DOI] [PubMed] [Google Scholar]
- 60.Hoshikawa H, Nishiyama Y, Kishino T, et al. Comparison of FLT-PET and FDG-PET for visualization of head and neck squamous cell cancers. Mol Imaging Biol. 2011;13:172–177. doi: 10.1007/s11307-010-0331-z. [DOI] [PubMed] [Google Scholar]
- 61.Hoshikawa H, Kishino T, Mori T, et al. Comparison of (18) F-FLT PET and ( 18 ) F-FDG PET for detection of cervical lymph node metastases in head and neck cancers. Acta Otolaryngol. 2012;132:1347–1354. doi: 10.3109/00016489.2012.709319. [DOI] [PubMed] [Google Scholar]
- 62.Kishino T, Hoshikawa H, Nishiyama Y, et al. Usefulness of 3'-deoxy-3'-18F-fluorothymidine PET for predicting early response to chemoradiotherapy in head and neck cancer. J Nucl Med. 2012;53:1521–1527. doi: 10.2967/jnumed.111.099200. [DOI] [PubMed] [Google Scholar]
- 63.Haerle SK, Fischer DR, Schmid DT, et al. 18F-FET PET/ CT in advanced head and neck squamous cell carcinoma: an intra-individual comparison with 18F-FDG PET/CT. Mol Imaging Biol. 2011;13:1036–1042. doi: 10.1007/s11307-010-0419-5. [DOI] [PubMed] [Google Scholar]
- 64.Hasebe M, Yoshikawa K, Ohashi S, et al. A study on the prognostic evaluation of carbon ion radiotherapy for head and neck adenocarcinoma with C-11 methionine PET. Mol Imaging Biol. 2010;12:554–562. doi: 10.1007/s11307-010-0318-9. [DOI] [PubMed] [Google Scholar]
- 65.Zygogianni A, Kyrgias G, Kouvaris J, et al. A new role of PET/ CT for target delineation for radiotherapy treatment planning for head and neck carcinomas. Hell J Nucl Med. 2012;15:139–143. [PubMed] [Google Scholar]
- 66.Hendrickson K, Phillips M, Smith W, et al. Hypoxia imaging with [F-18] FMISO-PET in head and neck cancer: potential for guiding intensity modulated radiation therapy in overcoming hypoxia-induced treatment resistance. Radiother Oncol. 2011;101:369–375. doi: 10.1016/j.radonc.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bowen SR, Kogel AJ, Nordsmark M, et al. Characterization of positron emission tomography hypoxia tracer uptake and tissue oxygenation via electrochemical modeling. Nucl Med Biol. 2011;38:771–780. doi: 10.1016/j.nucmedbio.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kikuchi M, Yamane T, Shinohara S, et al. 18F-fluoromisonidazole positron emission tomography before treatment is a predictor of radiotherapy outcome and survival prognosis in patients with head and neck squamous cell carcinoma. Ann Nucl Med. 2011;25:625–633. doi: 10.1007/s12149-011-0508-9. [DOI] [PubMed] [Google Scholar]
- 69.Yamane T, Kikuchi M, Shinohara S, et al. Reduction of [(18) F]fluoromisonidazole uptake after neoadjuvant chemotherapy for head and neck squamous cell carcinoma. Mol Imaging Biol. 2011;13:227–231. doi: 10.1007/s11307-010-0365-2. [DOI] [PubMed] [Google Scholar]
- 70.Wang W, Lee NY, Georgi JC, et al. Pharmacokinetic analysis of hypoxia (18)F-fluoromisonidazole dynamic PET in head and neck cancer. J Nucl Med. 2010;51:37–45. doi: 10.2967/jnumed.109.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niu G, Li Z, Xie J, et al. PET of EGFR antibody distribution in head and neck squamous cell carcinoma models. J Nucl Med. 2009;50:1116–1123. doi: 10.2967/jnumed.109.061820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu S, Kiesewetter DO, Zhu L, et al. Longitudinal PET imaging of doxorubicin-induced cell death with (18)F-Annexin V. Mol Imaging Biol. 2012;14:762–770. doi: 10.1007/s11307-012-0551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beer AJ, Grosu AL, Carlsen J, et al. 18Fgalacto-RGD positron emission tomography for imaging of alphavbeta3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6610–6616. doi: 10.1158/1078-0432.CCR-07-0528. [DOI] [PubMed] [Google Scholar]