SUMMARY
An aetiopathogenetic analysis of non-endemic nasopharyngeal carcinoma (NPC) in European and Southern American patient groups was performed. Specifically, the study sought to determine the proportion of Epstein-Barr Virus (EBV)-positive tumour cells in NPC patients in two very different populations (Europe and South America) in areas not associated with a high incidence of NPC. Clinical data (age, sex and onset of clinical disease) were also analyzed. A total of 50 NPC samples, 24 from a European hospital (EH) and 26 from two South American hospitals (SAH), were included. Nuclear staining for Epstein-Barr virus–encoded small RNA (EBER) was performed by in situ hybridization (ISH). Latent membrane protein 1 (LMP1) expression was measured by immunohistochemical (IHC) analysis. A higher incidence of NPC was observed in patients > 40 years of age in EH; in SAH, by contrast, the incidence was higher in patients aged ≤ 40 years. Cervical lymph node metastasis was detected in 31 patients (of whom 84.6% were from SAH). A total of 72% of samples were EBERpositive; the incidence of EBER positivity was greater in type 3 NPCs. EBV was detected in a large proportion of epithelial cells in samples from both EH and SAH (75% vs. 69.2%, respectively). An association was found between EBER detection in lymphocytes and patient origin (p = 0.0001). LMP1 expression was detected in 64% of patients. ISH for the detection of EBER is the most sensitive technique for demonstrating EBV in tumour tissue. The incidence of EBV was not significantly greater in either of the study populations, but was significantly higher in patients with type 3 NPC. Definitive histological diagnosis of NPC was reached earlier in EH than in SAH, where metastases were more frequently diagnosed, suggesting that the disease had reached a more advanced stage by the time treatment was started.
KEY WORDS: Nasopharyngeal carcinoma (NPC), Caucasian, Epstein-Barr virus (EBV), LMP1
RIASSUNTO
Scopo del nostro studio è stato confrontare i risultati dell'analisi eziopatogenetica sul carcinoma nasofaringeo non endemico (NPC) in pazienti europei e del Sud America ed in particolare determinare l'incidenza di cellule tumorali positive per Epstein-Barr Virus (EBV) nelle due diverse popolazioni. Sono stati inoltre analizzati i dati clinici (età, sesso e caratteristiche dell'insorgenza della malattia) al fine di evidenziare analogie e differenze nel confronto con i dati abitualmente rilevati nelle ampie casistiche disponibili. Sono stati analizzati un totale di 50 casi di NPC: 24 da ospedali europei e 26 da ospedali Sud Americani. La marcatura nucleare dell'RNA codificato dall'Epstain-Barr virus è stata ottenuta mediante ibridizzazione in situ. L'espressione della proteina latente di membrana (LMP1), è stata invece misurata mediante analisi immunoistochimica. Nei pazienti europei è stata osservata un'incidenza più alta di NPC nei casi di età superiore a 40 anni, nei pazienti del Sud America, invece, l'incidenza era maggiore nei casi di età inferiore/uguale a 40 anni di età. In 31 pazienti sono state riscontrate metastasi latero-cervicali linfonodali (di cui l'84.6% provenivano dal Sud America). Il 72% dei campioni è risultato positivo alla marcatura nucleare dell'RNA codificato dall'Epstain-Barr virus, con un'incidenza più alta nei casi di NPC di tipo 3. L'EBV è stato rilevato nella gran parte delle cellule epiteliali dei campioni provenienti sia dagli ospedali europei che sud americani (75% vs. 69.2%). Una correlazione statisticamente significativa è stata riscontrata fra la positività alla marcatura nucleare dell'RNA codificato dall'Epstain-Barr virus e le origini del paziente (p = 0.0001). L'espressione della LMP1 è stata rilevata nel 64% dei pazienti. In conclusione la detezione della marcatura nucleare dell'RNA codificato dall'Epstain Barr Virus mediante ibridizzazione è la metodica più sensibile per dimostrare la presenza di EBV nei campioni tumorali. In nessuna delle popolazioni studiate si è osservata una incidenza più alta, statisticamente significativa, di EBV, evidenziata, invece, nei pazienti con NPC di tipo 3. La diagnosi istologica definitiva è stata raggiunta prima negli ospedali Europei piuttosto che in quelli sud americani, nei quali alla diagnosi erano più frequentemente presenti metastasi laterocervicali, indicative di uno stadio tumorale più avanzato al momento dell'inizio del trattamento.
Introduction
Nasopharyngeal carcinoma (NPC) accounts for 2% of all head and neck cancers in Europe and North America; the annual incidence in the United States is less than 1 case/100,000 inhabitants/year. However, NPC is much more common in southern China, Taiwan, South-East Asia, North Africa, Greenland and Alaska, where rates of 30 to 50 cases/100,000 inhabitants/year have been reported 1. This remarkable geographical distribution pattern has led to the identification of high-risk (southern China, Taiwan, South-East Asia), intermediate-risk (Maghreb and a number of African countries) and lowrisk areas.
The pathogenesis of NPC is multifactorial. Major contributors include genetic and dietary factors, as well as demonstrated exposure to environmental carcinogens 2-5. The present study, comprising patients from two continents, sought to obtain data on the pathogenesis of NPC outside Far East Asia, where EBV is thought to play a major role in tumourigenesis. The association between EBV and NPC has been confirmed by identification of the virus genome in tumour cells. The EBV genome is widely found in cells involved in dysplasia or preinvasive lesions 6, indicating that EBV infection is a key event in the development of NPC.
The World Health Organization classifies NPC based on histology 7. Type 1, keratinizing squamous carcinoma, is characterized by well-differentiated cells that produce keratin. Type 2, non-keratinizing squamous carcinoma, varies in cell differentiation but does not produce keratin. Type 3 is also non-keratinizing, but is less differentiated, with highly variable cell types (clear cell, spindle cell, anaplastic). Type 2 and 3 NPC are EBV-associated, and have a better prognosis than type 1; EBV infection is generally absent in type 1, especially in non-endemic areas 8. However, more recent data indicate that almost all NPC, regardless of histological subtype, have comorbid EBV infections, which is strong evidence for EBV as the aetiological origin of NPC. This close association with EBV makes NPC unique among head and neck cancers.
Few studies have addressed the epidemiology, biological behaviour and prognostic factors of NPC outside Asia, so knowledge of this lesion remains limited in Western countries. Because of its very low incidence, epidemiological and clinical studies – particularly in Europe – are uncommon, although a limited amount of aetiopathogenetic research has been carried out in small patient series 9 10. The present study sought to determine the proportion of EBVpositive tumour cells in NPC patients drawn from two very different populations (Europe and South America) in areas not associated with a high incidence of NPC. Clinical data (age, sex and onset of clinical disease) were also compared to data in large patient series.
Material and methods
Formalin-fixed, paraffin-embedded tissue sections from 21 nasopharyngeal mucosa specimens and 29 cervical lymph nodes obtained during cavum biopsy or cervical lymph node dissection were retrieved from the files of the Hospital Universitario Virgen Macarena in Seville, Spain (HUVM) (24 cases), the Hospital Sor María Ludovica La Plata in Buenos Aires, Argentina (HSML) (6 cases) and the Consultoría en Patología in Sao Paulo, Brazil (CPSP) (20 cases) between January 2007 and June 2010. All laboratory work was performed in the pathology department of the HUVM.
Histological evaluation was performed by two pathologists using haematoxylin/eosin staining. All NPC patients were classified according to WHO criteria (1975). Patient clinical and pathological data, including a number of epidemiological features, are summarized in Table I. This study was approved by the appropriate hospital ethics committees, and informed consent was obtained from all patients.
Table I.
Clinical data and histological classification.
| Age (years) | Sex (f/m) | Risk factors* | Histological classification | Metastasis | Hospital origin | |
|---|---|---|---|---|---|---|
| 1 | 25 | M | No | Type 3 | Yes | HUVM |
| 2 | 48 | F | No | Type 3 | Yes | HUVM |
| 3 | 75 | M | No | Type 2 | Yes | HUVM |
| 4 | 70 | M | Yes | Type 3 | No | HUVM |
| 5 | 55 | M | No | Type 3 | Yes | HUVM |
| 6 | 55 | M | No | Type 1 | No | HUVM |
| 7 | 56 | F | No | Type 3 | No | HUVM |
| 8 | 66 | F | No | Type 3 | Yes | HUVM |
| 9 | 53 | M | No | Type 2 | No | HUVM |
| 10 | 47 | M | Yes | Type 3 | Yes | HUVM |
| 11 | 71 | M | Yes | Type 3 | No | HUVM |
| 12 | 57 | M | No | Type 3 | No | HUVM |
| 13 | 56 | M | No | Type 3 | Yes | HUVM |
| 14 | 71 | M | No | Type 3 | Yes | HUVM |
| 15 | 45 | M | No | Type 3 | No | HUVM |
| 16 | 75 | M | Yes | Type 3 | No | HUVM |
| 17 | 44 | F | No | Type 3 | No | HUVM |
| 18 | 85 | M | No | Type 3 | No | HUVM |
| 19 | 46 | M | No | Type 3 | No | HUVM |
| 20 | 52 | M | No | Type 3 | No | HUVM |
| 21 | 85 | F | Yes | Type 3 | Yes | HUVM |
| 22 | 44 | M | No | Type 3 | No | HUVM |
| 23 | 35 | M | No | Type 2 | No | HUVM |
| 24 | 53 | M | No | Type 3 | No | HUVM |
| 25 | 12 | M | Yes | Type 3 | No | HSML |
| 26 | 18 | F | No | Type 3 | Yes | HSML |
| 27 | 18 | M | No | Type 3 | No | HSML |
| 28 | 12 | M | No | Type 3 | Yes | HSML |
| 29 | 8 | F | No | Type 1 | No | HSML |
| 30 | 33 | M | No | Type 3 | No | HSML |
| 31 | 24 | M | No | Type 3 | Yes | CPSP |
| 32 | 43 | F | No | Type 3 | Yes | CPSP |
| 33 | 16 | F | Yes | Type 3 | Yes | CPSP |
| 34 | 15 | M | No | Type 3 | Yes | CPSP |
| 35 | 31 | M | No | Type 3 | Yes | CPSP |
| 36 | 48 | M | No | Type 2 | Yes | CPSP |
| 37 | 42 | M | No | Type 3 | Yes | CPSP |
| 38 | 14 | M | No | Type 3 | Yes | CPSP |
| 39 | 18 | F | No | Type 3 | Yes | CPSP |
| 40 | 41 | F | No | Type 3 | Yes | CPSP |
| 41 | 77 | M | Yes | Type 3 | Yes | CPSP |
| 42 | 54 | F | No | Type 3 | Yes | CPSP |
| 43 | 49 | M | No | Type 3 | Yes | CPSP |
| 44 | 50 | F | No | Type 3 | Yes | CPSP |
| 45 | 35 | F | No | Type 3 | Yes | CPSP |
| 46 | 39 | M | No | Type 3 | Yes | CPSP |
| 47 | 26 | F | No | Type 3 | Yes | CPSP |
| 48 | 47 | M | No | Type 3 | Yes | CPSP |
| 49 | 51 | M | Yes | Type 3 | Yes | CPSP |
| 50 | 17 | M | No | Type 3 | Yes | CPSP |
HUVM: Hospital Universitario Virgen Macarena, Spain; HSML = Hospital Sor María Ludovica, La Plata, Buenos Aires, Argentina; CPSP: Consultoría En Patología, Sao Paulo, Brazil; f/m: female/male;
alcohol and/or tobacco consumption, or high-risk occupation.
In-situ hybridization (ISH) was performed using an Epstein-Barr virus–encoded small RNA (EBER) PNAFITC probe (DAKO, Glostrup, Denmark), following the manufacturer's instructions. Briefly, after sections were dewaxed, dehydrated, and digested with proteinase K, hybridization solution containing EBER/PNA-FITC was applied. After washing, DAKO anti-FITC solution was applied. The DAKO LSAB peroxidase-based visualization kit and DAB were used as chromogens. Dark brown nuclear staining identified a positive hybridization signal. Results were expressed differently depending on the cell type. Epithelial cells were classed as EBER-positive when nuclear staining was detected; lymphocytes were deemed positive when staining was detected in > 5% of lymphocytes in the infiltrate. Positive and negative control slides were run for each specimen by replacing the EBV probe with a positive control fluorescein-conjugated PNA probe (DAKO, Glostrup, Denmark).
Paraffin-embedded tissue blocks from 50 NPC patients were cut into 3 µm sections and placed on APES pre-coated slides. Sections were dewaxed in xylene and rehydrated in alcohol and water. Immunohistochemical (IHC) staining was performed using the peroxidase-antiperoxidase technique following microwave antigen retrieval procedure. Anti-LMP1 antibody (Abcam, USA) was overlaid on NPC tissue array sections and incubated overnight at 4°C. Secondary antibody incubation was performed at room temperature for 30 min. Two pathologists independently scored the results of immunohistochemical staining, and any discrepant scores were re-examined to obtain a consensus score. Samples were considered LMP1 positive when membrane staining was detected. Negative controls were obtained by replacing the primary antibodies with PBS.
Results were expressed as mean ± SEM or as percentage of total cases. Student's t test was used to check inter-group age differences, and Fisher's test was used to analyze associations between variables. A p value < 0.05 was considered statistically significant.
Results
The 50 patients with NPC were aged between 8 and 77 (mean 44 ± 10). The percentage of patients diagnosed in Europe and in South America was similar (48% vs. 52%). There was a clear predominance of male patients (70%) regardless of patient origin (Fig. 1B). European patients were older than their South American counterparts (57 ± 7.5 vs. 32 ± 8.5, p = 0.0001). Sixty-four per cent of all patients were over 40 years. An association was observed between origin and patient age (p = 0.0001). In the European hospital, most patients were over 40 years (91.6% of diagnosed cases), while in the South American hospitals most were under 40 years (61.5% of diagnosed cases; Fig. 1C). A total of 62% of patients had cervical lymph node metastasis, while the disease remained localized in the remaining 38% of cases. An association was observed between patient origin and presence of metastases at diagnosis (p = 0.0001). Metastases were more common in patients in South American hospitals than in the European Hospital (84.6% vs. 37.5%); Fig. 1D).
Fig. 1.
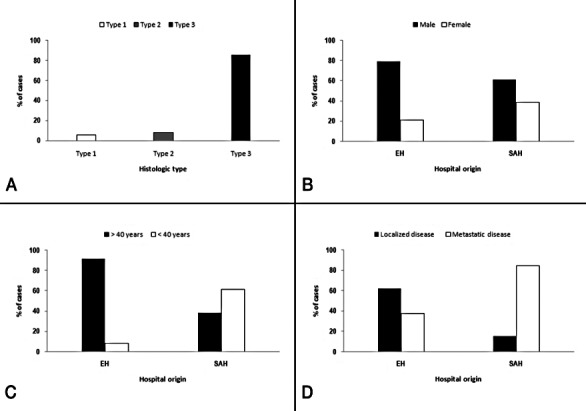
(A) Histological type. (B) Sex by hospital origin. (C) Age by hospital origin. (D) Localized or metastatic disease by hospital origin. Data are expressed as means (%).
Alcohol consumption was reported in < 15% of patients, and only 18% were smokers. None of the patients reported high consumption of salted foods, and none worked in jobs associated with higher exposure to this type of tumour.
All NPC patients were classified according to WHO criteria (1975) (Fig. 1A). Two patients (4%) had keratinizing carcinomas (type 1), four patients (8%) had differentiated non-keratinizing squamous carcinomas (type 2) and the remaining 44 patients (88%) had undifferentiated nonkeratinizing carcinoma (type 3). All patients received radiotherapy plus chemotherapy (RT+CT). No correlation was found between clinical data (age, sex, recurrence, metastasis and mortality), histological type or mitotic numbers.
Epstein-Barr virus was detected in a large proportion of epithelial cells in samples from both European and South American hospitals (75% vs. 69.2%; Fig. 2A). An association was found between EBER detection in lymphocytes and patient origin (p = 0.0001). In 79.2% of European patients, EBV was detected in less than 5% of lymphocytes, while in 88.5% of South American patients, EBV was detected in over 5% of lymphocytes (Fig. 2B). Associations were also found between EBER detection in lymphocytes and both patient age (p = 0.036) and metastatic disease (p = 0.001). Patients in whom EBER was detected in less than 5% of lymphocytes were mostly aged over 40 years (81.8%), while most patients in whom EBER was detected in over 5% of lymphocytes displayed metastatic disease (82.1%).
Fig. 2.
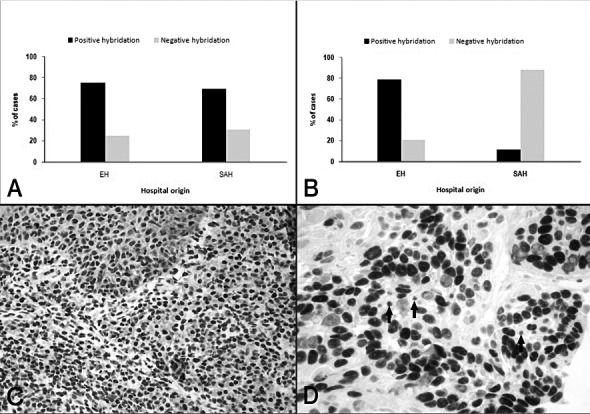
EBER identification by ISH: (A). Epithelial cells. (B) Lymphocytes. Data are expressed as mean percentage of positive-staining cells (%). (C) (20×). (D) (40×). Positive hybridization of epithelial cells and lymphocytes.
Positive membrane staining for LMP-1 in epithelial tumour cells was recorded in 64% of patients (Fig. 3); positive staining in European patients was slightly – though not significantly – higher than in South American patients (70.9% vs. 57.7%, respectively). An association was noted between LMP1 detection in lymphocytes and patient origin (p = 0.0001). In 86.4% of European patients, LMP1 was detected in less than 5% of lymphocytes, while in 82.1% of South American patients, LMP1 was detected in over 5% of lymphocytes (Fig. 2B). Associations were also found between LMP1 detection in lymphocytes and patient age (p = 0.036), and metastatic disease (p = 0.001). Most patients in whom LMP1 was detected in less than 5% of lymphocytes were over 40 years (81.8%), while in patients displaying LMP1 in over 5% of lymphocytes metastatic disease was common (74.2%).
Fig. 3.
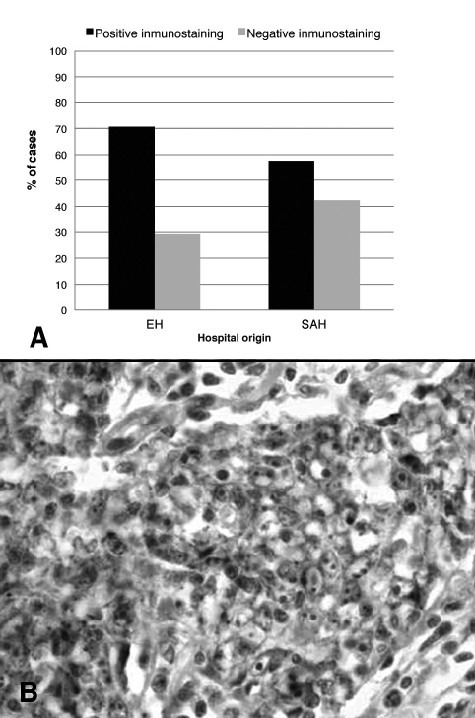
LMP1 expression by IHC. (A) Data expressed as mean percentage of positive-staining cells (%). (B) (40×).
Neither EBER positivity nor LMP 1 positivity correlated significantly with clinical stage or histological type.
Discussion
The pathogenesis of NPC is multifactorial. A number of epidemiological and experimental studies point to the involvement of dietary factors in the aetiology of the disease. Consumption of salted foods rich in volatile nitrosamines has been identified as a proven risk factor 2 11. The tumour has also been linked to occupational factors, including exposure to building materials, paint and inflammable products, but only exposure to wood residues has so far been demonstrated to constitute a significant risk 4 5. Unlike other tumours of the head and neck, smoking appears to play a less important role in the development of NPC 12-14. No link with any risk factor was observed in the patients studied here.
In epidemiological terms, the relatively low incidence of NPC in the study countries means that the present results can only be meaningfully compared with findings for patient series from areas with a similar incidence 9 10. However, an attempt has been made to compare tumour pathogenesis in the two non-endemic areas.
In the present study of 50 patients, 64% were over 40 years old, a finding also reported for other patient groups in countries with a low incidence of NPC 15-17. Nevertheless, in the European hospital most patients were over 40 years (91%), while in the South American hospitals only 38.5% were over 40. Although this finding is not easy to interpret, any attempt to seek a genetic or environmental explanation must start from the assumed involvement of factors characteristic of countries where NPC is relatively endemic. Most patients in the two South American hospitals were from Brazil, a country with a larger Asian population than Spain, and more particularly Seville, where the European hospital is located. Various conclusions may be drawn from the findings of the present study. Firstly, despite the histologically-aggressive appearance of the NPCs (mainly type 3) and despite reports in the literature highlighting their considerable ability to spread 18-20, most cases were diagnosed in the early stages of the disease, when involvement was only locoregional. Secondly, metastases were observed in 62% of patients, while the disease was localized in the remaining 38%. This would suggest that NPC is a fairly silent, locally-aggressive tumour with a prolonged clinical period facilitating invasion of the lymphatic plexus and the appearance of metastases with no other clinical signs or symptoms striking enough to lead to an earlier diagnosis. Finally, attention is drawn to the fact that this study included 50 cases drawn from hospitals on different continents. The incidence of metastasis was significantly greater in South American patients (84.6% vs. 37.5%). This difference reflects a certain degree of bias, in that one of the two South American hospitals mainly receives cases referred from other centres for exhaustive analysis and a decision on treatment. Moreover, access to healthcare facilities is more restricted in South America than in Spain, so diagnosis is often delayed.
The present study sought to demonstrate the involvement of EBV in NPC by immunohistochemical analysis of LMP1 expression, and also used the ISH technique to test for EBV latency in tumour cells. This technique has been described as highly sensitive (98%) and highly specific (100%) in this type of tumour 21, although lower figures are sometimes reported in the literature. Most studies report a 90-100% association of EBV with type 3 NPC, and a less marked association with more differentiated forms of NPC (types 1 and 2) 22.
Here, 72% of patients were EBER-positive. No significant correlation was observed with clinical stage or histological type, although the highest EBER-positive rate was found in patients with type 3 NPC, which is consistent with the presence of cervical lymph node metastases. Similar results (65% EBER positive) were reported by Gabusi et al. 9 in a group of 26 NPC patients of Italian origin, most of whom had type 3 NPC. A study of NPC in Tunisian patients also found that almost 100% of patients with type 3 NPC were EBER-positive 10. All this seems to suggest greater involvement of EBV in the genesis of type 3 (undifferentiated) NPC; this is the predominant type in the Asian population, where EBV is virtually endemic.
In this study, the results obtained for IHC detection of LMP1 were similar to those reported in other non-Asian patients, although 62% of patients were LMP1-positive, compared with around 10% of patients in other studies in the Mediterranean area 21. However, this included all histological types of NPC, rather than just type 3. There are striking exceptions to this generally uniform trend; in a study carried out in Virginia (USA), all NPC patients tested were found to be negative for LMP1 23.
In general, however, the present results confirm the correlation between the two viral infection markers, LMP1 and EBER, in roughly the same proportion of patients (around two-thirds) reported in other studies 18 24. It should be noted, however, that ISH for the detection of EBER in paraffin- embedded tissues is both more sensitive and more specific for demonstrating EBV than IHC techniques aimed at detecting LMP1. The relationship between EBV detection and differences in patient origin suggests the need for further epidemiological and virological research.
To conclude, mean patient age was significantly higher in the European group than in the South American group. The definitive histological diagnosis of NPC was reached earlier in European than in South American hospitals, where metastases were more frequently diagnosed, suggesting that the disease had reached a more advanced stage by the time treatment was started. A very high rate of joint presentation of NPC and EBV infection is reported in patients from high-risk countries, where type 3 NPC (lymphoepithelioma) displays a predominance approaching 100%. Joint presentation of NPC and EBV was less marked in South American and Spanish patients, among whom type 3 NPC was slightly less predominant.
References
- 1.Aparicio J, Llorca C, Montalar J. Carcinoma de nasofaringe. In: Gonzalez Barón M, editor. Cáncer de cabeza y cuello. Madrid:: Ergón; 1995. pp. 207–222. [Google Scholar]
- 2.Yu MC. Diet and nasopharyngeal carcinoma. FEMS Microbiol Immunol. 1990;2:235–242. doi: 10.1111/j.1574-6968.1990.tb03524.x. [DOI] [PubMed] [Google Scholar]
- 3.Poirier S, Ohshima H, The G, et al. Volatile nitrosamine levels in common foods from Tunisia, south China and Greenland, high-risk areas for nasopharyngeal carcinoma (NPC) Int J Cancer. 1987;39:293–296. doi: 10.1002/ijc.2910390305. [DOI] [PubMed] [Google Scholar]
- 4.Wu YT, Luo HL, Johnson DR. Effect of nickel sulfate on cellular proliferation and Epstein-Barr virus antigen expression in lymphoblastoid cell lines. Cancer Lett. 1986;32:171–179. doi: 10.1016/0304-3835(86)90116-3. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong RW, Imery PB, Lye MS, et al. Nasopharyngeal carcinoma in Malaysian Chinese: occupational exposures to particles, formaldehyde and heat. Int J Epidemiol. 2000;29:991–998. doi: 10.1093/ije/29.6.991. [DOI] [PubMed] [Google Scholar]
- 6.Song-Qing F, Jian M, Jie Z, et al. Differential expression of Epstein Barr virus encoded RNA and several tumor related genes in various types of nasopharyngeal epithelial lesions and nasopharyngeal carcinoma using tissue microarray analysis. Human Pathology. 2006;37:593–605. doi: 10.1016/j.humpath.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Shanmugaratnam K, Sobin L. Histologic typing of tumours of the upper respiratory tract and ear. 2nd ed. Geneva: WHO; 1991. [DOI] [PubMed] [Google Scholar]
- 8.Marks JE, Phillips JL, Menck HR. The National Cancer Data Base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer. 1998;83:582–588. doi: 10.1002/(sici)1097-0142(19980801)83:3<582::aid-cncr29>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Gabusi E, Lattes C, Fiorentino M, et al. Expression of Epstein- Barr virus-encoded RNA and biological markers in Italian nasopharyngeal carcinomas. J Exp Clin Cancer Res. 2001;20:371–376. [PubMed] [Google Scholar]
- 10.Hila L, Farah F, Ayari H, et al. Epidemiological study, immunohistochemistry and in situ hybridization studies of nasopharyngeal carcinomas: A Tunisian report. Pathol Biol. 2009;57:427–429. doi: 10.1016/j.patbio.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Poirier S, Bouvier G, Malaveille C, et al. Volatile nitrosamine levels and genotoxicity of food samples from high-risk areas for nasopharyngeal carcinoma before and after nitrosation. Int J Cancer. 1989;44:1088–1094. doi: 10.1002/ijc.2910440625. [DOI] [PubMed] [Google Scholar]
- 12.Fandi A, Altun M, Azli N, et al. Nasopharyngeal cancer: epidemiology, staging and treatment. Semin Oncol. 1994;21:382–397. [PubMed] [Google Scholar]
- 13.Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:421–429. doi: 10.1016/s1044579x02000858. [DOI] [PubMed] [Google Scholar]
- 14.Jmal A, Boussen H, Gara S, et al. Le cancer du nasopharynx de l'enfant en Tunisie: étude rétrospective épidémiologique, clinique et biologique it propos de 48 cas. Bull Cancer. 2005;92:977–981. [PubMed] [Google Scholar]
- 15.Bray F, Haugen M, Moger TA, et al. Age incidence curves of nasopharyngeal carcinoma worlwide: bimodality in low-risk populations and aetiologic implications. Cancer Epidemiol Biomarkers Prev. 2008;17:2356–2365. doi: 10.1158/1055-9965.EPI-08-0461. [DOI] [PubMed] [Google Scholar]
- 16.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 17.Curado MP, Edwards B, Shin HR, et al. IARC Scientific Publications no. 160. Vols IX. Lyon: IARC; 2007. Cancer incidence in five continents. [Google Scholar]
- 18.Friedrich E, Friedrich S, Lobeck H, et al. Epstein-Barr virus DNA and epithelial markers in nasopharyngeal carcinoma. Med Microbiol Immunol. 2003;192:141–144. doi: 10.1007/s00430-002-0134-1. [DOI] [PubMed] [Google Scholar]
- 19.Spano JP, Busson P, Atlan D, et al. Nasopharyngeal carcinomas: an update. Eur J Cancer. 2003;39:2121–2135. doi: 10.1016/s0959-8049(03)00367-8. [DOI] [PubMed] [Google Scholar]
- 20.Andrea T, Deuryp MD. Epstein Barr virus associated epithelial and mesenchymal neoplasms. Human Pathology. 2008;39:473–483. doi: 10.1016/j.humpath.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Tsai S, Jin Y, Mann R, et al. Epstein-Barr virus detection in nasopharyngeal tissues of patients with suspected nasopharyngeal carcinoma. Cancer. 1998;82:1449–1453. [PubMed] [Google Scholar]
- 22.Mc Dermot AL, Dutt SN, Watkinson JC. The aetiology of nasopharyngeal carcinoma. Clin Otroralyngol. 2001;26:82–92. doi: 10.1046/j.1365-2273.2001.00449.x. [DOI] [PubMed] [Google Scholar]
- 23.Dawson CW, Tramountains G, Eliopoulus AG, et al. Epstein Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem. 2003;278:3694–3704. doi: 10.1074/jbc.M209840200. [DOI] [PubMed] [Google Scholar]
- 24.Zubizarreta P, D'Antonio G, Raslawski E, et al. Nasopharyngeal carcinoma in childhood and adolescence. Cancer. 2000;89:690–695. [PubMed] [Google Scholar]


