Abstract
OBJECTIVE
To describe activity patterns in pregnant women with type 1 diabetes and evaluate the impact of increased structured physical activity on glucose control.
RESEARCH DESIGN AND METHODS
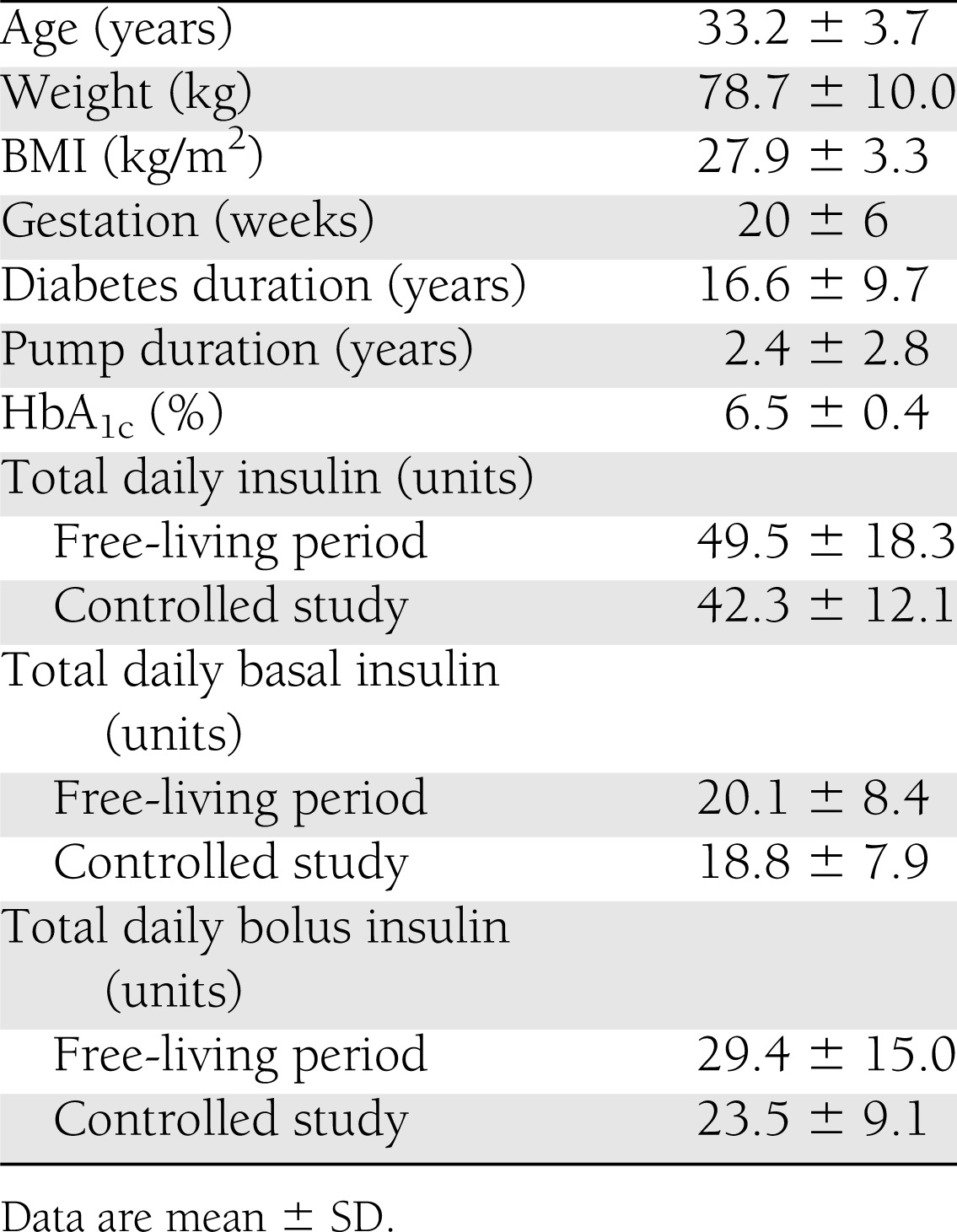
Physical activity energy expenditure (PAEE) and glucose levels (continuous glucose monitoring) were measured in 10 pregnant women with type 1 diabetes (age 33.2 years, gestation 20 weeks, BMI 27.9 kg/m2, diabetes duration 16.6 years, HbA1c 6.5% [48 mmol/mol], insulin pump duration 2.4 years) during a day at home (free-living) and during a 24-h visit incorporating controlled diet and structured physical activity with light intensity activity (three 20-min self-paced walks) and moderate intensity activity (two 50-min sessions of brisk treadmill walking). PAEE was evaluated through individually calibrated combined heart rate and movement sensing.
RESULTS
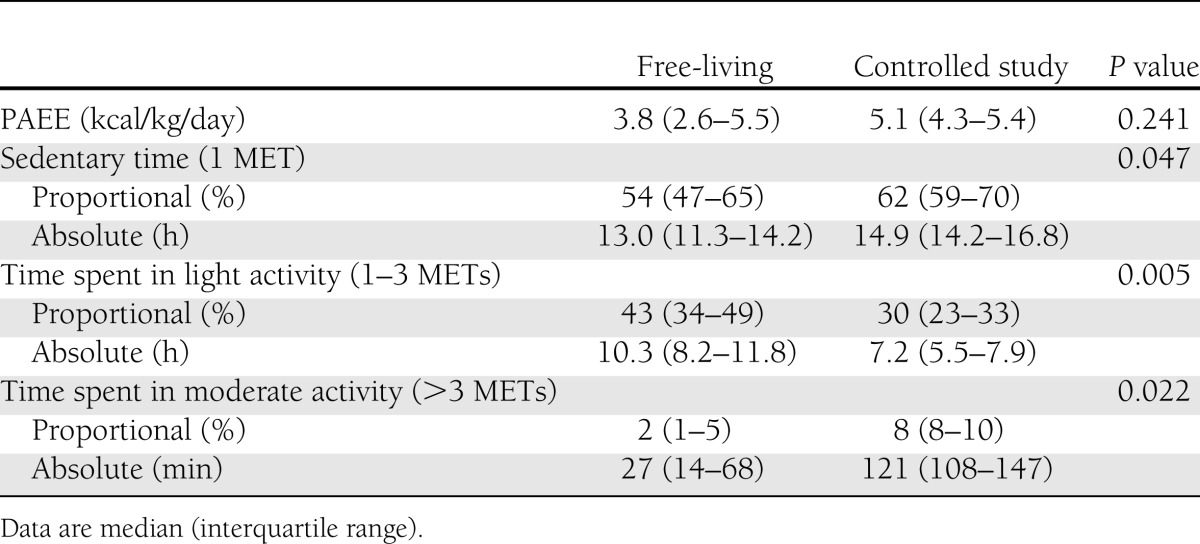
Free-living PAEE was comparable to that under controlled study conditions (3.8 and 5.1 kcal/kg/day, P = 0.241), with women achieving near to the recommended 30 min of moderate physical activity (median 27 min [interquartile range 14–68]). During the free-living period, more time was spent in light activity (10.3 vs. 7.2 h, P = 0.005), with less sedentary time (13.0 vs. 14.9 h, P = 0.047) and less moderate activity (27 vs. 121 min, P = 0.022). The free-living 24-h mean glucose levels by continuous glucose monitoring were significantly higher (7.7 vs. 6.0 mmol/L, P = 0.028). The effect of controlled diet and exercise persisted overnight, with significantly less time spent hyperglycemic (19 vs. 0%, P = 0.028) and less glucose variability (glucose SD 1.3 vs. 0.7 mmol/L, P = 0.022).
CONCLUSIONS
A controlled diet and structured physical activity program may assist women with type 1 diabetes in achieving optimal glucose control during pregnancy.
In general health, regular physical activity is associated with physiological benefits, including improvements in cardiovascular fitness, body composition, blood pressure, and lipid profiles. In type 2 diabetes, exercise has a beneficial effect on glycemic control associated with enhanced peripheral insulin sensitivity (1). In type 1 diabetes, physical activity levels are inversely related to the development of complications and mortality risk (2), although there is less evidence of glycemic benefit, perhaps because of reduced insulin dose or increased caloric intake to prevent hypoglycemia (3).
During pregnancy, additional advantages of exercise include limitation of excess maternal weight gain, attenuation of pregnancy-induced insulin resistance (4), and lessening of delivery-related complications (5). Beneficial effects on body weight, fat mass, and cardiovascular profile may persist for several years after pregnancy (6). The American College of Obstetricians and Gynecologists recommends at least 30 min of moderate intensity exercise on most days of the week in healthy pregnant women (7). Physical activity levels decline with advancing gestation in healthy pregnancy, compensating for the increased energy cost of exercise (8). Fear of hypoglycemia may limit activity levels further in type 1 diabetes pregnancy.
Previous studies evaluated physical activity in women with or at risk for gestational diabetes, demonstrating improved insulin sensitivity (9) and glycemic control (10) and a reduced risk of developing diabetes (11). Only one study, carried out more than 25 years ago, evaluated physical activity in type 1 diabetes pregnancy; it suggested that postprandial walking during the second and third trimesters improved lipid profiles with no change in glycemic control (12).
There are no objective data on physical activity energy expenditure (PAEE) and daily activity patterns in type 1 diabetes pregnancy. Our aim was to prospectively investigate the impact of increased physical activity, relating PAEE to glycemic control recorded by continuous glucose monitoring (CGM) in women with type 1 diabetes in their second trimester of pregnancy through data collected during a period of directed exercise under controlled conditions and during normal (free-living) activity.
RESEARCH DESIGN AND METHODS
Study subjects
Physical activity patterns and glucose control were prospectively investigated during a free-living day and a controlled study day in 10 pregnant women with well-controlled type 1 diabetes. Women were recruited from two antenatal diabetes clinics (Cambridge and London, U.K.) between April 2010 and April 2011. They were all established insulin pump users participating in a study of closed-loop insulin delivery (13). The study protocol was approved by the local Research Ethics Committee, and all participants gave written, informed consent.
Study procedures
The protocols for evaluating the performance of closed-loop insulin delivery have been previously described (13,14). This report examines data collected during the open loop days, when conventional continuous subcutaneous insulin infusion therapy was continued. In brief, inclusion criteria were type 1 diabetes (World Health Organization criteria) for at least 12 months, current insulin pump therapy, and a viable singleton pregnancy. Women with poor glycemic control (HbA1c >10%), BMI ≥35 kg/m2, insulin resistance (total daily dose ≥1.5 units/kg), or severe medical or psychological comorbidities were excluded (13).
Controlled study conditions.
A FreeStyle Navigator CGM (Abbott Diabetes Care, Alameda, CA) was inserted one to two days before the study, either by a research nurse or the patient herself after training. The CGM data were visible to women at home but not during controlled study conditions. On arrival at the clinical research facility (Cambridge, U.K.) at noon, an intravenous sampling cannula was inserted. Venous samples were obtained every 15 to 30 min from 1400 h until study end at noon on day 2. Plasma glucose concentration was measured immediately with a Yellow Springs Instruments analyzer (YSI2300 STAT Plus; YSI, Farnborough, U.K.). Each woman’s usual insulin infusion pump was replaced, without interruption of insulin infusion, with a standard study pump (Animas 2020; Johnson & Johnson, New Brunswick, NJ) before administration of the lunchtime insulin bolus.
After a light lunch (choice of 35-g carbohydrate tuna, cheese, or ham salad sandwich and 15-g carbohydrate snack of yogurt, fruit, biscuits, or chocolate), a noninvasive combined heart rate and accelerometer device (Actiheart; Cam Ntech Ltd., Papworth, U.K.) was attached to the anterior chest wall with two standard electrocardiographic pads. A ramped step test, consisting of 8 min of stepping on an elevated platform at progressively increasing frequency from 0.25 Hz (one foot placement per second) up to 0.55Hz, was performed to enable individual calibration (15). The monitor was then set to record at 30-s intervals and worn continuously throughout the study. The Actiheart has been validated outside of pregnancy, with reported intradevice and interdevice coefficients of variation for movement and heart rate of 0.5 and 0.03% and 5.7 and 0.03%, respectively (16).
Physical activities.
The activity schedule included three 20-min self-paced postprandial walks (after breakfast, lunch, and dinner) and two sessions of brisk treadmill walking (in the afternoon and morning). The afternoon treadmill exercise incorporated 25 min of walking at 4.8 km/h followed by a 5-min rest interval and 25 min at 2.6 km/h at 10% incline. The morning exercise involved two 25-min sessions of walking at 3.9 km/h with no gradient, separated by a 5-min rest. The Borg rating of perceived exertion was used to assess effort level during exercise (17). This scale ranges from 6 (no effort) to 20 (maximal exertion). Between scheduled activities, women undertook sedentary tasks, such as working on a computer, watching television, or reading.
Meals and snacks.
Women chose their preferred options from a selection of standardized 60-g carbohydrate evening meals (chicken breast with mashed potato, beef lasagna with garlic bread, spaghetti with pesto, macaroni and cheese, or spinach and ricotta cannelloni) and standardized 50-g carbohydrate breakfasts (whole grain toast with jam or fruit or whole grain breakfast cereal and fruit with milk), all freshly prepared by study dietitians. All study meals had a similar macronutrient composition of 50% carbohydrate, 35% fat, and 15% protein. Various additional snacks were consumed according to capillary glucose measured before, during, and after treadmill exercise sessions, aiming to maintain glucose levels above 6 mmol/L (108 mg/dL). A 15-g carbohydrate snack was given for capillary glucose ≥6 mmol/L, whereas a 30-g carbohydrate snack was given for values <6 mmol/L. An additional 15-g carbohydrate snack (choice of yogurt, fruit, biscuits, or chocolate) was provided at 2100 h.
Hypoglycemia.
During controlled study conditions, hypoglycemic episodes, defined as plasma glucose ≤3.0 mmol/L (54 mg/dL) with symptoms or ≤2.5 mmol/L (45 mg/dL) without symptoms, were treated with 15-g oral carbohydrate (90 mL Lucozade Energy Original; GlaxoSmithKline UK Ltd., Middlesex, U.K.). Plasma glucose levels were repeated every 15 min until hypoglycemia resolved, with additional treatments as required.
Insulin dose adjustment.
Meals were accompanied by an insulin bolus calculated from each patient’s usual insulin to carbohydrate ratio. The bolus was administered 10 min before eating except in cases of hypoglycemia with capillary glucose <3.5 mmol/L (63 mg/dL), in which case it was given immediately before the meal. Dose adjustments were made by the women on the basis of capillary glucose tests (up to seven per day) without access to CGM glucose data and without medical input, aiming to maintain glucose levels within the National Institute for Health and Clinical Excellence (NICE) recommended target glucose range of 3.5–7.8 mmol/L (63–140 mg/dL) (18). Women continued their standard insulin pump regimen, reducing basal insulin infusion rates for exercise or reducing preprandial insulin boluses as per normal practice.
Free-living study conditions.
On completion of the controlled study day, women continued wearing the CGM and Actiheart monitor at home for up to 3 days. The women were now able to access the CGM data in real time, and training, including written instructions on device calibration and data interpretation, was provided. The Actiheart, which is waterproof, was worn continuously throughout the day and overnight. During the recording period, women were asked to consume meals and carry out physical activity according to their usual daily routine (ad libitum), aiming for NICE glucose control targets for pregnancy. Standardized dietary recommendations were for a low-carbohydrate (30–40 g) or a protein-based breakfast and 50–60 g carbohydrate for lunch and evening meals, with between-meal or pre-exercise snacks (15 g carbohydrate per 30 min physical activity). During this part of the study, biochemical hypoglycemia was defined as sensor glucose ≤2.5 mmol/L for ≥20 min. Subjective symptomatic episodes were not formally documented. At the end of the free-living recording period, women returned the monitoring devices, which were downloaded by means of software packages provided by the manufacturers.
Study measures
PAEE.
Heart rate data were processed with robust Gaussian regression to handle potential measurement noise (19). Sleeping heart rate, calculated as the highest of the 60 lowest heart rate recordings during a 24-h period, was used with the step test–derived calibration parameters to individualize heart rate–energy expenditure equations. PAEE was calculated from these parameters with a branched equation model (20). The proportion of time spent at various intensities was described in metabolic equivalents (METs), which express the energy cost of physical activity as a multiple of resting metabolic rate (21). METs are conventionally categorized as sedentary (≤1 MET), light (1−3 METs), or moderate to vigorous (>3 METs). To reduce any persisting effect of the controlled study, the first 24 h of free-living PAEE recordings was not considered for analysis. A 22-h consecutive period of PAEE recordings under free-living conditions at home, matched in time against the controlled study visit (1400–1200 h), was evaluated.
CGM.
The FreeStyle Navigator (Abbott Diabetes Care) used has a 1-h calibration period, after which it starts measuring interstitial glucose. This device has been validated in pregnancy, with median absolute relative difference between sensor and plasma glucose of 11.4 and 94.6% values within the clinically acceptable accuracy range (Clarke error grid zones A and B) (14). The device was calibrated with capillary glucose measurements according to manufacturer’s instructions. Glycemic outcomes were calculated from the CGM sensor glucose readings for overall (1400–1200 h) and overnight (2300–0730 h) periods. These included time in target (3.5–7.8 mmol/L) (18), time spent below and above target, mean glucose, SD of glucose, and the low blood glucose index (22), assessing the duration and extent of hypoglycemia.
Statistical analyses
Power calculations were not performed for this early phase investigation of PAEE in type 1 diabetes pregnancy. Values are given as median (interquartile range) or mean ± SD. Significant differences (P ≤ 0.05) were identified with nonparametric t tests for paired data. Analyses were conducted with SPSS (version 15; IBM Corporation, Armonk, NY).
RESULTS
Demographic data
The study participants included seven primiparous and three multiparous women, all in employment (seven full-time ≥35 h/week and three part-time <35 h/week), living with a partner, nonsmoking, and of white European ethnicity. Compliance with CGM and Actiheart device wear was 100% during both controlled study and free-living periods. Baseline biomedical characteristics and insulin doses are summarized in Table 1.
Table 1.
Baseline characteristics of study participants and total daily insulin during free-living and controlled study conditions
Activity patterns and energy expenditure
The free-living PAEE was 3.8 kcal/kg/day (interquartile range 2.6–5.5), with the wide interquartile range indicating a large variation in intensity of physical activity at home (Table 2). During both free-living and controlled study periods, most of the day was spent sedentary, with less sedentary time during the free-living period: 13.0 h (30%) vs. 14.9 h (43%, P = 0.047). Women spent 10.3 h (43%) in light intensity activity and 27 min (2%) in moderate intensity activity during the free-living period, compared with 7.2 h (30%) and 121 min (8%) during controlled study conditions (P = 0.005 and P = 0.022), respectively.
Table 2.
PAEE and time spent in sedentary, light intensity, and moderate intensity activity during free-living and controlled study conditions
During the free-living period, the highest PAEE was observed during the morning between 0730 and 0900 h, with the lowest PAEE in the evening between 2100 and 2230 h (Fig. 1). During the controlled study, the highest PAEE was recorded during scheduled activity sessions, with peak PAEE levels during moderate intensity treadmill walking (1500 and 0930 h) only slightly higher than during the self-paced postprandial walks (1400 h, 19:30 h, and 09:00 h). For the moderate intensity treadmill exercise, the Borg score ratings ranged from 7 to 15, with median scores of 10 and 9 during the afternoon and morning treadmill sessions, respectively, indicating that the exercise was generally very well tolerated.
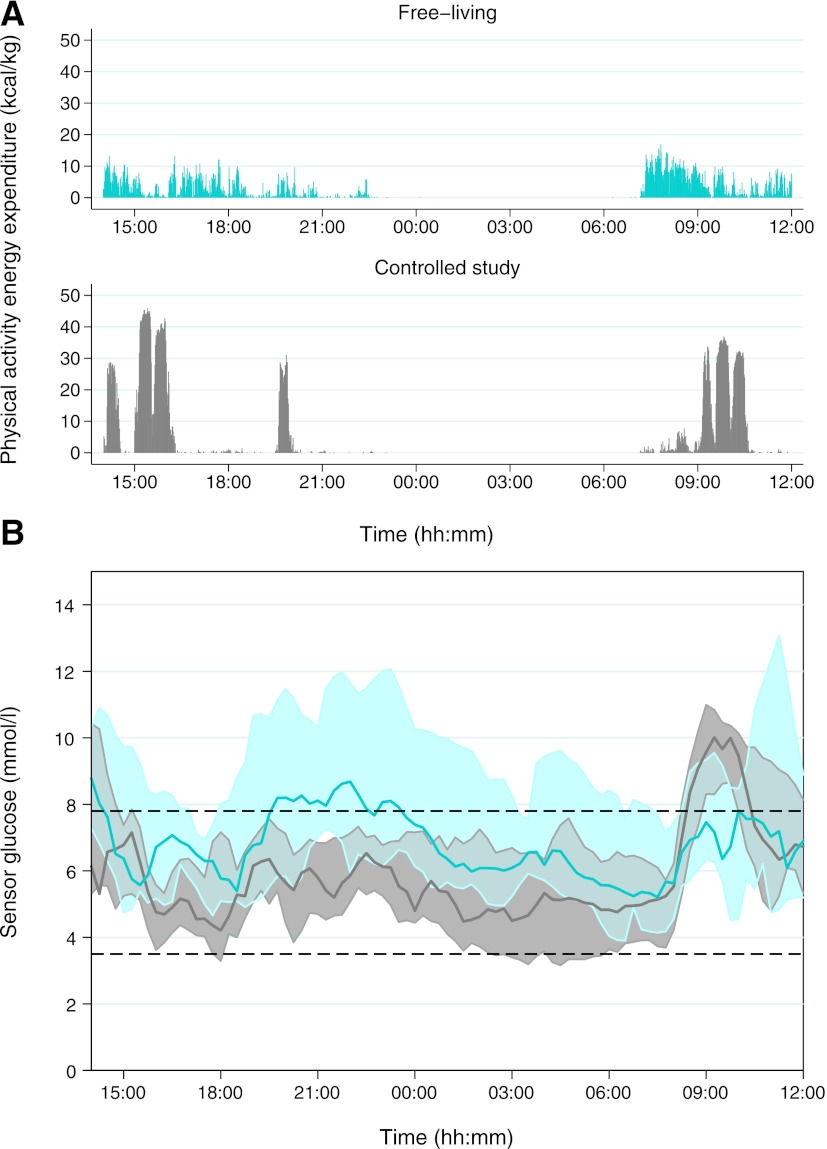
Figure 1.
PAEE and sensor glucose levels during free-living and controlled study conditions. Data are shown as median and interquartile range, with free-living values in blue and controlled study values in gray. A: PAEE measured by combined heart rate and accelerometry. B: Sensor glucose levels measured by CGM.
Glycemic control
Overall.
Overall mean CGM glucose was higher during the free-living period than during the controlled study: 7.7 mmol/L (139 mg/dL) vs. 6.0 mmol/L (108 mg/dL) (P = 0.028). There was also a trend toward more time spent above target: 28 vs. 17% (P = 0.059) (Table 3). The median CGM glucose profile (Fig. 1) was generally higher during the free-living period, with glucose levels above target and with greater glucose variability, as indicated by the larger interquartile range, particularly during the evening (1900–2300 h). It is also notable, however, that during the morning (0700–1000 h) glucose levels were closer to target during the free-living period. During the controlled study, glucose levels remained in target range for most of the day, except after the 50-g carbohydrate breakfast, which preceded the morning walk and treadmill session.
Table 3.
Sensor glucose outcomes determined by CGM during the overall and overnight study periods
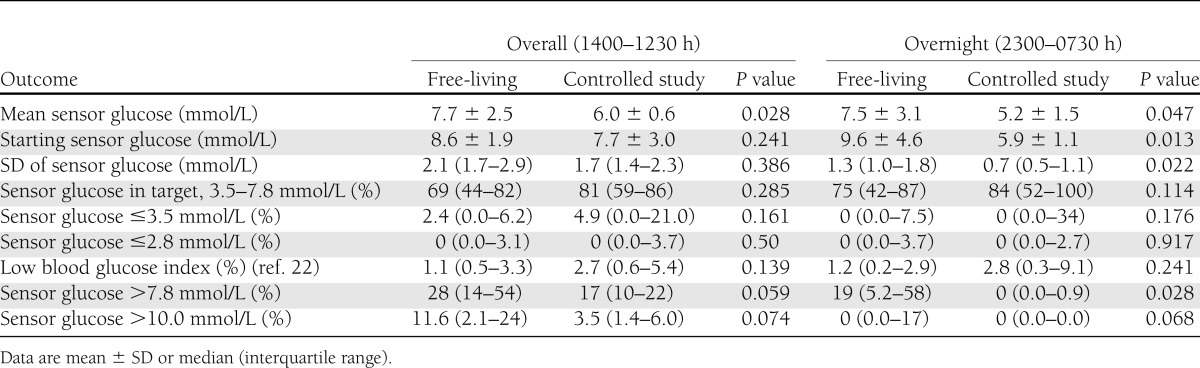
There was no difference in the overall time spent in hypoglycemia (2 vs. 5%, P = 0.161). During the free-living period, CGM data revealed two episodes of biochemical hypoglycemia in two subjects, occurring at 0215 and 1100 h. During the controlled study, there were 17 episodes of symptomatic hypoglycemia (1.7 episodes per subject per day) requiring treatment with 15–30 g oral carbohydrate. None of the events necessitated intravenous dextrose treatment. Eleven (65%) episodes occurred during or within two hours of the moderate intensity treadmill sessions despite setting of temporary basal infusion rates (70% of women) or reduction in preprandial insulin boluses and consumption of supplementary snacks (total carbohydrates consumed ranged from 15–75 g).
Overnight.
Overnight mean sensor glucose level was 7.5 mmol/L (135 mg/dL) during the free-living period, compared with 5.2 mmol/L (94 mg/dL) during the controlled study (P = 0.047) (Table 3). At home, women had a significantly higher mean glucose level before going to bed (2300 h): 9.6 mmol/L (173 mg/dL) vs. 5.9 mmol/L (106 mg/dL, P = 0.013), with 19% of the time (approximately 2 h) spent hyperglycemic overnight compared with 0% during controlled study conditions (P = 0.028). The SD of glucose, representing nocturnal glucose variability, was almost halved after controlled diet and exercise: 0.7 mmol/L (12.6 mg/dL) vs. 1.3 mmol/L (23.4 mg/dL, P = 0.022). This is illustrated by the narrow interquartile range of overnight CGM glucose during the controlled study (Fig. 1). Despite lower mean glucose values, there was only one episode of hypoglycemia after midnight in the controlled study, occurring in a subject who had experienced four earlier episodes after the afternoon treadmill exercise.
CONCLUSIONS
This is the first study to describe daily activity in a cohort of pregnant women with type 1 diabetes, together with the impact of a structured increase in moderate intensity physical exercise on immediate glucose control, by using continuous PAEE and CGM data collected during a day of controlled diet and exercise and a day of free-living conditions. The period of controlled diet and enhanced exercise lowered plasma glucose, with the effect persisting overnight, while avoiding significant hypoglycemia, suggesting that more attention to daily dietary and physical activity patterns may be warranted to assist women with type 1 diabetes in achieving optimal glucose control during pregnancy.
During the free-living period, the majority of waking time (10.3 h, or 43%) was spent in light intensity activity, which included standing, housework, and slow walking, with 27 min (2%) spent in moderate intensity physical activity. This compares favorably with a recent English study conducted in obese and overweight nondiabetic pregnant women, which found that only 2 h out of a total 12.4 daytime hours (16%) was spent in light intensity activities, with 9.7 h (81%) spent sedentary, although an equivalent of 33 min were spent in moderate intensity exercise (23). Studies of healthy pregnancy from Switzerland (24) and Sweden (25) also reported lesser amounts of light intensity exercise (5.3 and 4.1 h, respectively), although the women in those studies spent rather more time in moderate to vigorous intensity exercise (57 and 63 min, respectively).
During the day of structured diet and exercise, women accumulated 121 min of moderate intensity activity. Although this was achieved without physical discomfort, it was not without adverse sequelae, with 17 episodes of hypoglycemia (on average 1.7 episodes per woman per day). Eleven episodes of postexercise hypoglycemia required oral carbohydrates, despite careful adherence to recommended exercise treatment algorithms. The controlled diet and exercise conditions were, however, also accompanied by significantly lower overall mean glucose levels, with optimal glucose control before bed persisting overnight and notably no time spent hypoglycemic or hyperglycemic during the night. Nocturnal glycemic variability was also reduced, which is important because fluctuating glucose levels are associated with an increased risk of severe hypoglycemia (26). It is of interest that in our study peak PAEE values during the treadmill sessions and the self-paced walks were comparable. This suggests that encouraging women to walk for 20 min after each meal may be a practical way of increasing activity levels rather more than the current general recommendation of 30 min moderate intensity exercise daily.
Controlling their diabetes with frequent home monitoring and use of insulin pump therapy during the free-living period, women spent more than 6 h/day hyperglycemic, despite almost achieving the recommended 30 min of moderate activity daily. Glucose levels were most strikingly above target in the evening corresponding to the least physically active period of the day, and were nearer to target during the morning, when women were more active in household tasks and getting ready for work. It is remarkable that even a cohort of women with well-controlled diabetes (mean HbA1c 6.5% [48 mmol/mol]) who received antenatal diabetes care at specialist insulin pump clinics and had access to structured education and frequent home glucose monitoring results struggled to match their insulin doses to postprandial glucose levels, particularly after the evening meal. Although the women also had real-time access to CGM data during the study, it should be noted that they were not habitual CGM users.
It is possible that during the controlled study period women were more engaged in diabetes self-management and had more time to consider insulin dose adjustment without the distractions of everyday life; however, it is more likely that the improved glucose control is attributable to the increased physical activity and strict dietary compliance. Postprandial hyperglycemia observed in the morning during the controlled study may have been related to the higher carbohydrate breakfast provided in anticipation of morning exercise. Nonetheless, a similar if less striking increase in glucose was seen during the free-living day, suggesting that diurnal variations in insulin sensitivity or meal absorption are also likely to play a role.
These data provide some of the first insights into the amount and intensity of PAEE in type 1 diabetes pregnancy. An important strength of our study is the use of an objective measure of physical activity, rather than self-reported questionnaires, which may be confounded by subjective overestimation of activity levels (27,28). Additionally, a combined heart rate and movement sensor was used, which provides a more accurate estimate of PAEE than does either heart rate or accelerometry alone (16,29). Although initially validated in nonpregnant populations (16), the Actiheart has been used in two recent studies in healthy pregnant women (24,25). Moreover, we individually calibrated heart rate response to energy expenditure, which reduces estimation error in general and is particularly relevant in this study because of the physiological effects of pregnancy on heart rate.
These data were obtained from a larger study designed to evaluate closed-loop insulin delivery. Additional limitations include the small number of subjects studied and their demographic characteristics (subjects were all employed, all living with a partner, predominantly primiparous, and all in their second trimester), which may influence activity levels or self-efficacy in diabetes management. Although the recorded free-living period was always at least 24 h after the controlled study, it is still possible that there may have been a persisting effect on glucose control of the controlled diet and exercise conditions. The evaluation of a single free-living day also may not be representative of usual activity patterns. Furthermore, active monitoring of glucose and activity levels may have motivated women to be more physically active and vigilant in self-management. A record of meals consumed and prandial insulin boluses administered at home was not maintained. Because food intake as well as exercise was mandated during the controlled study, it is not possible to differentiate among the synergistic effects on glucose control of controlled diet, insulin administered, and structured physical activity.
In summary, we have objectively described 24-h free-living physical activity patterns in combination with glucose levels in pregnant women with type 1 diabetes. Despite having well-controlled diabetes treated by insulin pump therapy and almost achieving the goal of 30 min of moderate intensity exercise daily, these women had average glucose levels above the recommended targets. We have also described the impact of a structured increase in physical activity with controlled diet, suggesting a potentially beneficial impact on evening and overnight glucose control. Comparable activity levels achieved during postprandial self-paced walks and brisk treadmill walking suggest that 20 min of walking after meals may be a gestationally appropriate and practical approach to enhance physical activity during pregnancy. Incorporation of accelerometers and other physical activity measures into newer-generation insulin pumps may allow further individual tailoring of insulin dose adjustment in the future.
Hypoglycemia remains a limitation to exercising during pregnancy, and new strategies to predict and minimize the risk of exercise-related hypoglycemia are required. Although larger studies of physical activity and its longer term effects on glucose control are required to inform evidence-based exercise guidelines, our preliminary pilot investigation suggests that structured diet and exercise can further improve glycemic control in type 1 diabetes pregnancy.
Acknowledgments
This work was funded by a Diabetes UK Project Grant BDA 07/003551. The research was conducted with support from JDRF, the Investigator-Initiated Study Program of Animas Corporation (Animas 2020 insulin pump), Medical Research Council Centre for Obesity and Related Metabolic Diseases, National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre, and Addenbrooke’s Clinical Research Facility (Cambridge, U.K.).
The funders had no role in the study design, data collection/analysis/interpretation or manuscript preparation.
The research was conducted with support from Abbott Diabetes Care (Freestyle Navigator CGM and sensors) and the Investigator-Initiated Study Program of Animas Corporation (Animas 2020 insulin pump). M.E.W. has received license fees from Becton Dickenson and has received patent applications. S.A.A. has received consultancy and lecture fees from Medtronic, Roche, Novo Nordisk, and Eli Lilly. R.H. has received honoraria for speaking engagements from Medtronic, LifeScan, Novo Nordisk, Eli Lilly, Animas, and the Medtronic Advisory Panel; has received license fees from Becton Dickenson; and has received patent applications. H.R.M. has received honoraria for speaking engagements from Medtronic, Roche, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
K.K., S.A.A., R.H., and H.R.M. designed the study. K.K., J.M.A., and K.C. recruited participants. K.K., D.E., J.M.A., K.C., and H.R.M. performed studies. K.K. and M.N. analyzed data. P.R.-B. contributed to the design of the study. K.K., R.H., and H.R.M. interpreted data. K.K. drafted the manuscript. K.W., S.B., M.E.W., S.A.A., R.H., and H.R.M. reviewed and edited the manuscript. All authors approved the final version. K.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
These data were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank Laura Watson, Addenbrooke’s Clinical Research Facility, Cambridge, U.K., and Kate Westgate, MRC Epidemiology, Cambridge, U.K., for PAEE data; Angie Watts, University of Cambridge, Cambridge U.K., for laboratory support, and Stephen Luzio, University of Swansea, Swansea, U.K. The authors also thank all the participating women and their families and partners for their generous support.
Footnotes
Clinical trial reg. no. ISRCTN50385583, www.isrctn.org.
Ethical approval: Essex 2 Research Ethics Committee 09/H0302/113.
References
- 1.LaMonte MJ, Blair SN, Church TS. Physical activity and diabetes prevention. J Appl Physiol 2005;99:1205–1213 [DOI] [PubMed] [Google Scholar]
- 2.Moy CS, Songer TJ, LaPorte RE, et al. Insulin-dependent diabetes mellitus, physical activity, and death. Am J Epidemiol 1993;137:74–81 [DOI] [PubMed] [Google Scholar]
- 3.Chimen M, Kennedy A, Nirantharakumar K, Pang TT, Andrews R, Narendran P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia 2012;55:542–551 [DOI] [PubMed] [Google Scholar]
- 4.Wolfe LA, Heenan AP, Bonen A. Aerobic conditioning effects on substrate responses during graded cycling in pregnancy. Can J Physiol Pharmacol 2003;81:696–703 [DOI] [PubMed] [Google Scholar]
- 5.Melzer K, Schutz Y, Boulvain M, Kayser B. Physical activity and pregnancy: cardiovascular adaptations, recommendations and pregnancy outcomes. Sports Med 2010;40:493–507 [DOI] [PubMed] [Google Scholar]
- 6.Clapp JF, 3rd. Long-term outcome after exercising throughout pregnancy: fitness and cardiovascular risk. Am J Obstet Gynecol 2008;199:489 e1-6 [DOI] [PMC free article] [PubMed]
- 7.Committee on Obstetric Practice ACOG committee opinion. Exercise during pregnancy and the postpartum period. Number 267, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 2002;77:79–81 [DOI] [PubMed] [Google Scholar]
- 8.Goldberg GR, Prentice AM, Coward WA, et al. Longitudinal assessment of energy expenditure in pregnancy by the doubly labeled water method. Am J Clin Nutr 1993;57:494–505 [DOI] [PubMed] [Google Scholar]
- 9.Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metab 2009;35:418–421 [DOI] [PubMed] [Google Scholar]
- 10.de Barros MC, Lopes MA, Francisco RP, Sapienza AD, Zugaib M. Resistance exercise and glycemic control in women with gestational diabetes mellitus. Am J Obstet Gynecol 2010;203:556 e1-6 [DOI] [PubMed]
- 11.Dempsey JC, Sorensen TK, Williams MA, et al. Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. Am J Epidemiol 2004;159:663–670 [DOI] [PubMed] [Google Scholar]
- 12.Hollingsworth DR, Moore TR. Postprandial walking exercise in pregnant insulin-dependent (type I) diabetic women: reduction of plasma lipid levels but absence of a significant effect on glycemic control. Am J Obstet Gynecol 1987;157:1359–1363 [DOI] [PubMed] [Google Scholar]
- 13.Murphy HR, Kumareswaran K, Elleri D, et al. Safety and efficacy of 24-h closed-loop insulin delivery in well-controlled pregnant women with type 1 diabetes: a randomized crossover case series. Diabetes Care 2011;34:2527–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy HR, Elleri D, Allen JM, et al. Closed-loop insulin delivery during pregnancy complicated by type 1 diabetes. Diabetes Care 2011;34:406–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brage S, Ekelund U, Brage N, et al. Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. J Appl Physiol 2007;103:682–692 [DOI] [PubMed] [Google Scholar]
- 16.Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr 2005;59:561–570 [DOI] [PubMed] [Google Scholar]
- 17.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–381 [PubMed] [Google Scholar]
- 18.Guideline Development Group Management of diabetes from preconception to the postnatal period: summary of NICE guidance. BMJ 2008;336:714–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stegle O, Fallert SV, MacKay DJ, Brage S. Gaussian process robust regression for noisy heart rate data. IEEE Trans Biomed Eng 2008;55:2143–2151 [DOI] [PubMed] [Google Scholar]
- 20.Brage S, Brage N, Franks PW, et al. Branched equation modeling of simultaneous accelerometry and heart rate monitoring improves estimate of directly measured physical activity energy expenditure. J Appl Physiol 2004;96:343–351 [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(Suppl):S498–S504 [DOI] [PubMed] [Google Scholar]
- 22.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 1998;21:1870–1875 [DOI] [PubMed] [Google Scholar]
- 23.McParlin C, Robson SC, Tennant PW, et al. Objectively measured physical activity during pregnancy: a study in obese and overweight women. BMC Pregnancy Childbirth 2010;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melzer K, Schutz Y, Boulvain M, Kayser B. Pregnancy-related changes in activity energy expenditure and resting metabolic rate in Switzerland. Eur J Clin Nutr 2009;63:1185–1191 [DOI] [PubMed] [Google Scholar]
- 25.Löf M. Physical activity pattern and activity energy expenditure in healthy pregnant and non-pregnant Swedish women. Eur J Clin Nutr 2011;65:1295–1301 [DOI] [PubMed] [Google Scholar]
- 26.Ringholm L, Pedersen-Bjergaard U, Thorsteinsson B, Damm P, Mathiesen ER. Hypoglycaemia during pregnancy in women with type 1 diabetes. Diabet Med 2012;29:558–566 [DOI] [PubMed] [Google Scholar]
- 27.Stein AD, Rivera JM, Pivarnik JM. Measuring energy expenditure in habitually active and sedentary pregnant women. Med Sci Sports Exerc 2003;35:1441–1446 [DOI] [PubMed] [Google Scholar]
- 28.Harrison CL, Thompson RG, Teede HJ, Lombard CB. Measuring physical activity during pregnancy. Int J Behav Nutr Phys Act 2011;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crouter SE, Churilla JR, Bassett DR., Jr Accuracy of the Actiheart for the assessment of energy expenditure in adults. Eur J Clin Nutr 2008;62:704–711 [DOI] [PubMed] [Google Scholar]