Abstract
OBJECTIVE
Two meta-analyses of randomized trials of statins found increased risk of type 2 diabetes. One possible explanation is bias due to differential survival when patients who are at higher risk of diabetes survive longer under statin treatment.
RESEARCH DESIGN AND METHODS
We used electronic medical records from 500 general practices in the U.K. and included data from 285,864 men and women aged 50–84 years from January 2000 to December 2010. We emulated the design and analysis of a hypothetical randomized trial of statins, estimated the observational analog of the intention-to-treat effect, and adjusted for differential survival bias using inverse-probability weighting.
RESULTS
During 1.2 million person-years of follow-up, there were 13,455 cases of type 2 diabetes and 8,932 deaths. Statin initiation was associated with increased risk of type 2 diabetes. The hazard ratio (95% CI) of diabetes was 1.45 (1.39–1.50) before adjusting for potential confounders and 1.14 (1.10–1.19) after adjustment. Adjusting for differential survival did not change the estimates. Initiating atorvastatin and simvastatin was associated with increased risk of type 2 diabetes.
CONCLUSIONS
In this sample of the general population, statin therapy was associated with 14% increased risk of type 2 diabetes. Differential survival did not explain this increased risk.
Recent analyses of randomized clinical trials of statins have shown a higher incidence of diabetes in the treatment arm (1,2). A meta-analysis of 13 placebo-controlled randomized trials showed 9% (95% CI 2–17%) higher odds of diabetes in the statin arm. Another meta-analysis of five secondary prevention trials that compared more- versus less-intensive statin treatment reported 12% (4–22%) higher odds of diabetes in the more-intensive treatment arms (2). One observational study also reported a positive association between statin use and the subsequent risk of diabetes (3), although another study reported different associations (null, positive, or negative) depending on the particular statins used (4). The collective evidence led the Food and Drug Administration to require that the labels of all statin drugs be changed to indicate the potential increased risk of diabetes as a side effect.
Part of the observed association between statin use and risk of diabetes in both randomized trials and observational studies might be attributable to bias from differential survival. Because statins reduce the risk of death from cardiovascular diseases (CVD) (5), survivors who were treated with statins could have a worse risk profile for CVD than untreated survivors. Therefore, the treated survivors might be at higher risk of diabetes simply because CVD risk factors such as overweight/obesity, smoking, and poor diet are also risk factors for type 2 diabetes (Fig. 1). Indeed, a meta-regression analysis of randomized trials found a stronger harmful effect in trials that enrolled older participants or those with a higher baseline mean BMI, which suggests potential bias due to differential survival (1). Neither the randomized trials nor the observational studies addressed this possibility, perhaps because the absolute difference in survival in the randomized trials was only 1.4 percentage points (1).
Figure 1.
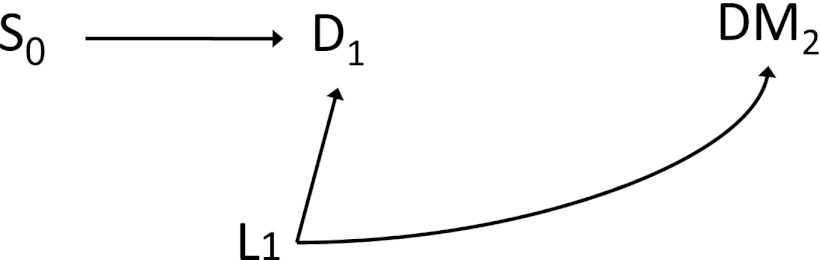
Simplified directed acyclic graph presenting the potential for differential survival. D1, death during follow-up (e.g., due to cardiovascular disease); DM2, diabetes mellitus by the end of follow-up; L1, a vector of common risk factors of diabetes and mortality such as age, obesity, and smoking; S0, statin therapy at baseline.
We set out to examine the role of bias due to differential survival by applying inverse probability weights to data from a database of electronic medical records from the U.K.
RESEARCH DESIGN AND METHODS
We emulated the design and analysis of a randomized trial of statins using observational data as described in detail elsewhere (6). We used data from the Health Improvement Network (THIN), which is a large computerized database of anonymized longitudinal medical records from >500 primary care practices in the U.K. The database includes demographic information as well as symptoms and diagnoses, summaries of referrals and hospitalizations, outpatient prescriptions, test results, and lifestyle information for every registered patient.
We started the follow-up on 1 January 2000 and followed patients through 31 December 2010. We included men and women 50–84 years of age who did not have diabetes (type 1, type 2, or gestational diabetes), had not been prescribed any statins in the past 2 years, had at least 2 years of continuous recording in the database (operationalized as at least 2 years since the first recorded prescription for any drug), and at least one health contact within the past 2 years. We excluded patients who had cancer, chronic liver, or kidney disease, schizophrenia, or used antipsychotic drugs at baseline.
The intervention of interest was being prescribed any statin (atorvastatin, fluvastatin, pravastatin, rosuvastatin, and simvastatin). As several previous studies had indicated that effects may depend on the type of statins (7), we also compared the effects of different statins versus no treatment.
The outcome of interest was incident type 2 diabetes. The diagnosis was based on the Read classification codes assigned by the general practitioners or use of hypoglycemic drugs or insulin; the date of incident diabetes was the earliest of diabetes codes or diabetes medication. For the majority of cases, the type of diabetes was specifically reported by the physician. If the physician used an unspecific diagnostic code (e.g., diabetes mellitus), we reviewed the patient’s medical record back to 1 year before the diagnosis including any referral letters and physicians’ free-text comments to assign the type of diabetes. If the age of onset was ≤35 years and the patient had 1 or more prescriptions for insulin and <1 year of oral hypoglycemic treatment, the case was classified as type 1 diabetes. Conversely, if the age of onset was ≥50 years and the patient used oral hypoglycemic treatment for at least 1 year, the case was classified as type 2 diabetes (8–10). A previous THIN study with a similar diabetes ascertainment algorithm estimated a diabetes prevalence that closely matched the prevalence in the Health Survey of England, which is a national population survey (9).
We also examined the effect of statins on peptic ulcer as a negative outcome control (11) because there is no plausible mechanism that statins would affect risk of peptic ulcer, and all of the observed association between statin use and incident peptic ulcer should be due to bias. A validation study of uncomplicated peptic ulcer cases in THIN found a positive predictive value of 94% (12).
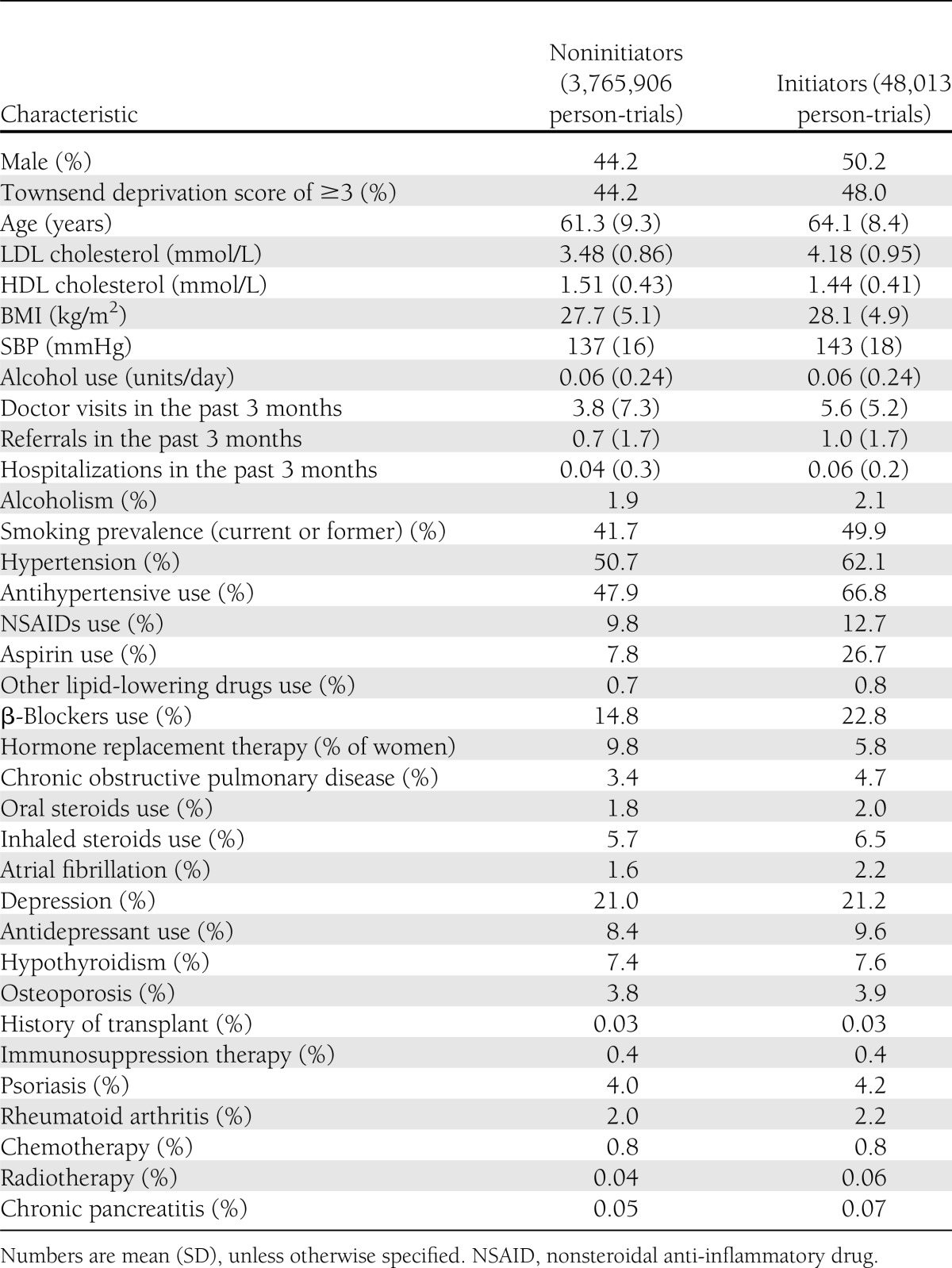
Potential confounders included age, sex, Townsend deprivation index (a measure of neighborhood deprivation), tobacco smoking (never, former, or current), alcohol use, test results (LDL and HDL cholesterol, serum triglycerides), physical examination results [BMI, systolic blood pressure (SBP)], history of diabetes in first-degree relatives, concurrent chronic diseases, use of other drugs, and number of visits, referrals, and hospitalizations in the past 3 months. See Table 1 for the complete list of variables. We truncated the distribution of risk factors at a threshold based on expert opinion to prevent implausible values (0.2%) from affecting the results. We carried the last observation forward for a maximum of 12 months for LDL, HDL, and SBP and of 24 months for alcohol use, smoking, and BMI.
Table 1.
Characteristics of initiators and noninitiators of statin therapy at the start of the trial’s follow-up: THIN trials 2000–2010
Statistical analyses
The details of the methods are described elsewhere (6). Briefly, we generated a set of emulated trials in which the first trial recruited participants for the duration of the first month of the study (in this study, January 2000). Eligibility criteria were applied, and patients who were eligible were categorized as either initiators (i.e., those who started using statins in January 2000) or noninitiators (i.e., those who did not start statin therapy in January 2000). Noninitiators were eligible for the emulated trial recruiting in February 2000, whereas initiators should wait for at least 24 months after they discontinued statin therapy to become eligible again. We constructed 131 emulated trials using THIN, pooled the data from all trials, and followed patients until occurrence of diabetes, death, or administrative end of follow-up, whichever occurred earlier.
We estimated the observational analog of the intention-to-treat hazard ratio (HR) of diabetes for initiators versus noninitiators by fitting a Cox model that included the baseline confounders for each trial. We used a robust variance estimator because each individual may participate in multiple trials.
To evaluate the sensitivity of our estimates to differential survival, we used inverse probability weighting to account for censoring by death. In each trial, we estimated the monthly probability of death from any cause using a logistic regression conditional on common risk factors of death and diabetes. We carried forward the last observed value of the selected covariates (listed in Table 1) indefinitely and added a set of additional variables that indicated the length of time passed since last measurement of SBP and LDL and HDL cholesterol levels. The denominator of the stabilized weight for month m was calculated as the estimated probability of surviving from baseline until month m (6). We truncated the distribution of weights at 50 (which is larger than the 99th percentile) to prevent the influence of observations with very large weights (6).
Informally, the weights create a pseudopopulation in which censoring by death is independent of the measured risk factors. Although such pseudopopulation does not exist in practice, this approach assesses the sensitivity of the effect estimates to differences in risk factor profiles between the surviving treated and untreated. If the weighted analysis yields similar estimates for the effect of statins on diabetes risk as the unweighted analysis, strong bias due to differential survival is unlikely. We hypothesized that any bias due to differential survival would be larger in the older subgroups and conducted an analysis restricted to patients ≥62 years of age (the median age at baseline). We also examined the effect of each statin drug compared with no statins and the effect of hydrophilic statins (pravastatin, rosuvastatin, or fluvastatin) and lipophilic statins (simvastatin or atorvastatin) compared with no statins separately.
RESULTS
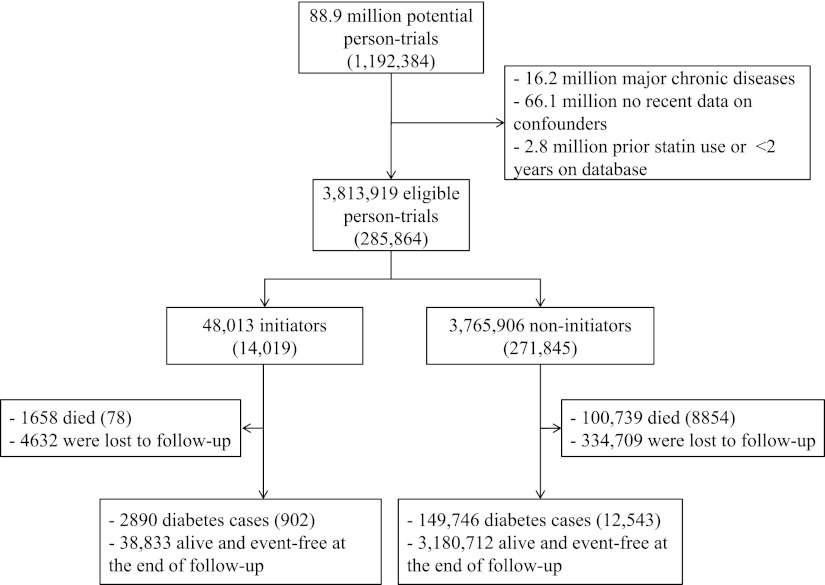
Figure 2 shows a flow chart of the process for selecting eligible study participants, and Table 1 presents their characteristics by statin initiation status. We included data from 285,864 patients who did not have diabetes at baseline. On average, the 48,013 statin initiators were 2.8 years older, had 0.4 kg/m2 higher BMI, and 0.7 mmol/L higher LDL than the 3,765,906 noninitiators. A larger proportion of statin initiators used antihypertensive medications and aspirin, and they had on average two more doctor visits in the past 3 months compared with noninitiators.
Figure 2.
Flow chart of person-trials in the analysis: THIN database 2000–2010. Numbers in parentheses indicate unique individuals.
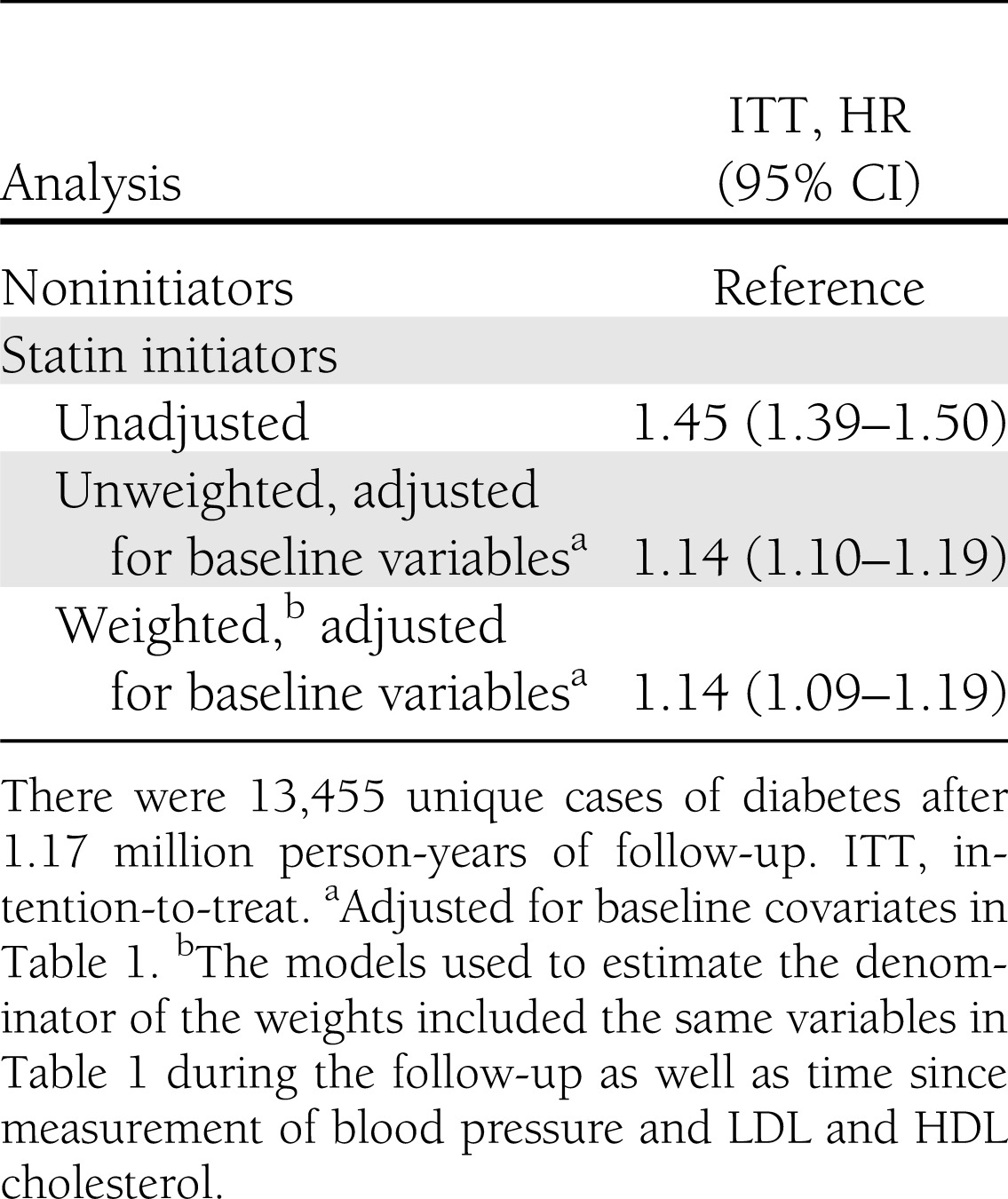
During 1.17 million person-years of follow-up, there were 13,455 new cases of diabetes and 8,932 deaths. Median time of follow-up was 28 months among initiators and 29 months among noninitiators. The crude incidence rate of type 2 diabetes was 15.9 per thousand person-years among initiators and 11.3 among noninitiators. The results of the intention-to-treat analysis are presented in Table 2. The unadjusted HR (95% CI) was 1.45 (1.39–1.50). Adjustment for confounding by indication reduced the HR to 1.14 (1.10–1.19). The adjusted HR was 1.24 (1.14–1.34) in the first year of follow-up and 1.12 (1.06–1.17) after excluding the first year.
Table 2.
HR (95% CI) for the analog of the intention-to-treat effect of statins on type 2 diabetes: THIN database 2000–2010
By the end of follow-up, there were 78 unique deaths among 14,019 statin initiators and 8,854 among 271,845 noninitiators. Initiating statin therapy reduced the risk of death significantly. The adjusted intention-to-treat HR for mortality was 0.88 (0.84–0.93). The estimated inverse-probability weights had a mean (SD) of 1.00 (0.23). The HRs of diabetes in the weighted analyses were almost identical to the unweighted ones both in the main analysis and in the analysis restricted to older patients.
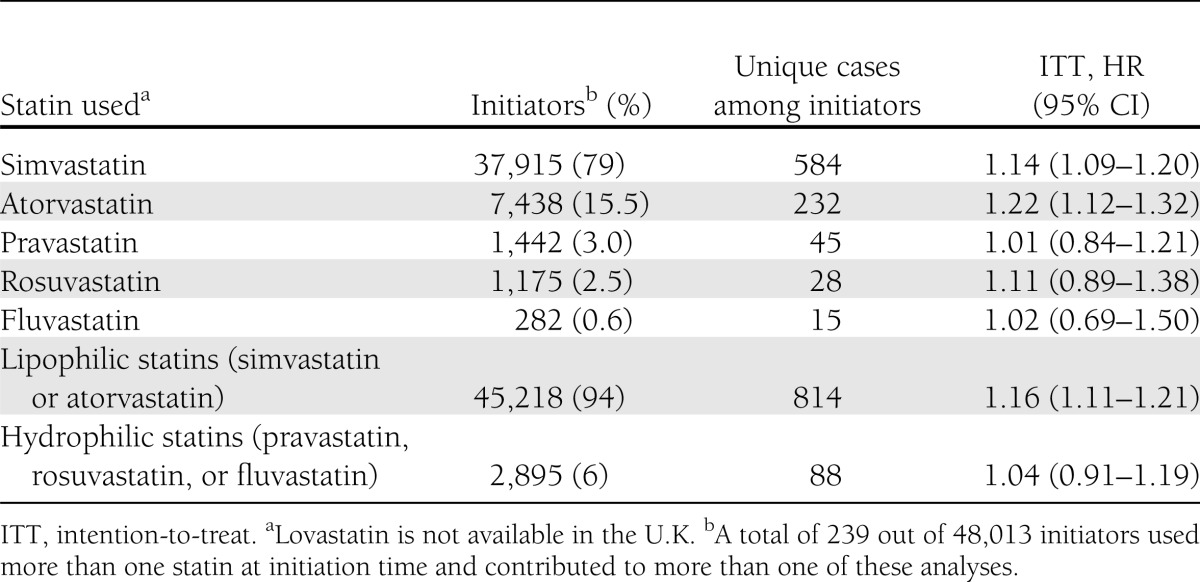
Of all statin initiators, 79.0% used simvastatin and 15.5% atorvastatin. Table 3 presents the adjusted and weighted HR of diabetes for initiation of each statin or a group of statins versus no statin therapy. Lipophilic statins (i.e., simvastatin and atorvastatin) were associated with an increased risk of diabetes. The HR was 1.14 (1.09–1.20) for simvastatin and 1.22 (1.12–1.32) for atorvastatin. There were not enough events to conduct a meaningful comparison of initiation of other statins. The intention-to-treat analysis among those ≥62 years of age gave an HR of 1.16 (1.10–1.23). Finally, the HR of peptic ulcer comparing statin initiation versus no initiation was 0.98 (0.91–1.06).
Table 3.
The number (proportion) of initiators for each statin or group of statins and adjusted and weighted intention-to-treat HRs (95% CI) for type 2 diabetes: THIN database 2000–2010
CONCLUSIONS
We estimated that statin initiators had a 14% increased incidence of diabetes compared with noninitiators in this general population in the U.K. The increased risk was observed with simvastatin and atorvastatin, the most commonly used statins in this population. We found no evidence that the increased risk may be explained by differential survival of people treated with statins. Our estimates did not change after inverse-probability weighting for death or after restricting the analysis to older individuals.
Our study is observational, and therefore, our estimates may be affected by unmeasured confounding. However, the magnitude of our estimate of the effect of statins on diabetes and total mortality are similar to those reported in meta-analyses of randomized trials (1,13). Also, our results cannot be fully explained by confounding by undiagnosed diabetes as the increased risk was detected even after the first year of follow-up. Our estimates might also be biased if the association between statins and diabetes were explained by better monitoring of statin users. However, we found the expected null association between statin initiation and peptic ulcer, another condition that would be more likely to be diagnosed among better-monitored patients.
Imperfect adherence to statin therapy could have attenuated our intention-to-treat effect estimate. However, the HR of diabetes in an as-treated analysis (6) for 26 months of statin use, which was the average duration of treatment among initiators, versus no statin use was 1.15 (results not shown).
Our inverse-probability weighted estimates suggest that the differential survival of statin users does not explain their increased risk of diabetes. However, we may not have been able to adjust for all common risk factors of mortality and diabetes. For example, the THIN database does not include any information on diet or physical activity. Furthermore, the data on smoking and alcohol use are not frequently updated which will result in measurement error and residual confounding by these factors.
There are several proposed biological mechanisms for the harmful effects of statins on diabetes. Simvastatin and atorvastatin may increase insulin resistance (14–16), although fluvastatin may decrease it (17). Other potential pathways are through reduction in synthesis of various metabolites such as isoprenoid, which leads to lower glucose uptake in adipocytes or reduced insulin secretion by blocking the calcium channels in the β-cells (18). Further research is warranted to identify the role of various molecular pathways.
It is worth noting that even with the slight increase in the risk of diabetes among statin initiators, the cardiovascular and mortality risk reduction outweighs this additional risk in patients with moderate or high CVD risk and even those who are at higher risk of diabetes (19).
In summary, our results support a slightly increased risk of diabetes due to statin therapy in this general population in the U.K. We did not find any evidence of bias due to differential survival. Similar analyses could be conducted on the randomized trials to examine the possibility of this type of bias.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
G.D. and M.A.H. developed the concept of the study. G.D. conducted the analyses and wrote the first draft of the manuscript. M.A.H., L.A.G.R., and O.F.C. contributed to the design of the study, preparation of the data, and revision of the manuscript. M.A.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–742 [DOI] [PubMed] [Google Scholar]
- 2.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011;305:2556–2564 [DOI] [PubMed] [Google Scholar]
- 3.Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med 2012;172:144–152 [DOI] [PubMed] [Google Scholar]
- 4.Ma T, Chang MH, Tien L, Liou YS, Jong GP. The long-term effect of statins on the risk of new-onset diabetes mellitus in elderly Taiwanese patients with hypertension and dyslipidaemia: a retrospective longitudinal cohort study. Drugs Aging 2012;29:45–51 [DOI] [PubMed] [Google Scholar]
- 5.Mills EJ, Wu P, Chong G, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170 255 patients from 76 randomized trials. QJM 2011;104:109–124 [DOI] [PubMed] [Google Scholar]
- 6.Danaei G, García Rodríguez LA, Cantero OF, Logan R, Hernán MA. Observational data for comparative effectiveness research: An emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res. 19 October 2011 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koh KK, Sakuma I, Quon MJ. Differential metabolic effects of distinct statins. Atherosclerosis 2011;215:1–8 [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez G, Soriano LC, Choi HK. Impact of diabetes against the future risk of developing gout. Ann Rheum Dis 2010;69:2090–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González EL, Johansson S, Wallander MA, Rodríguez LA. Trends in the prevalence and incidence of diabetes in the UK: 1996-2005. J Epidemiol Community Health 2009;63:332–336 [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Perez A, Schlienger RG, Rodríguez LA. Acute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort study. Diabetes Care 2010;33:2580–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margulis AV, García Rodríguez LA, Hernández-Díaz S. Positive predictive value of computerized medical records for uncomplicated and complicated upper gastrointestinal ulcer. Pharmacoepidemiol Drug Saf 2009;18:900–909 [DOI] [PubMed] [Google Scholar]
- 13.Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2011;1:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moutzouri E, Liberopoulos E, Mikhailidis DP, et al. Comparison of the effects of simvastatin vs. rosuvastatin vs. simvastatin/ezetimibe on parameters of insulin resistance. Int J Clin Pract 2011;65:1141–1148 [DOI] [PubMed] [Google Scholar]
- 15.Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol 2010;55:1209–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kater AL, Batista MC, Ferreira SR. Improved endothelial function with simvastatin but unchanged insulin sensitivity with simvastatin or ezetimibe. Metabolism 2010;59:921–926 [DOI] [PubMed] [Google Scholar]
- 17.Teixeira AA, Buffani A, Tavares A, et al. Effects of fluvastatin on insulin resistance and cardiac morphology in hypertensive patients. J Hum Hypertens 2011;25:492–499 [DOI] [PubMed] [Google Scholar]
- 18.Sasaki J, Iwashita M, Kono S. Statins: beneficial or adverse for glucose metabolism. J Atheroscler Thromb 2006;13:123–129 [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012;380:565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]