Abstract
OBJECTIVE
Patients with a BMI <35 kg/m2 and patients with a BMI between 35 and 40 kg/m2 without comorbidities are noneligible by current eligibility criteria for bariatric surgery. We used Swedish obese subjects (SOS) to explore long-term outcomes in noneligible versus eligible patients.
RESEARCH DESIGN AND METHODS
The SOS study involved 2,010 obese patients who underwent bariatric surgery (68% vertical-banded gastroplasty, 19% banding, and 13% gastric bypass) and 2,037 contemporaneously matched obese controls receiving usual care. At inclusion, the participant age was 37–60 years and BMI was ≥34 kg/m2 in men and ≥38 kg/m2 in women. The effect of surgery was assessed in patients that do (n = 3,814) and do not (n = 233) meet current eligibility criteria. The date of analysis was 1 January 2012. The follow-up time was up to 20 years, with a median of 10 years.
RESULTS
Cardiovascular risk factors were significantly improved both in noneligible and eligible individuals after 10 years of follow-up. Surgery reduced the diabetes incidence in both the noneligible (adjusted hazard ratio 0.33 [95% CI 0.13–0.82], P = 0.017) and eligible (0.27 [0.22–0.33], P < 0.001) groups. We could not detect a difference in the effect of surgery between the groups (adjusted interaction P value = 0.713).
CONCLUSIONS
Bariatric surgery drastically reduced the incidence of type 2 diabetes both in noneligible and eligible patients and improved cardiovascular risk factors in both groups. Our results show that strict BMI cutoffs are of limited use for bariatric surgery prioritization if the aim is to prevent diabetes and improve cardiovascular risk factors.
The eligibility criteria for bariatric surgery established by the National Institutes of Health (NIH) in 1992 (1) are still the most widely used (2). According to these criteria, eligible individuals should have a BMI ≥40 kg/m2 or a BMI between 35 and 40 kg/m2 if they have high-risk comorbidities such as severe type 2 diabetes or cardiovascular risk factors. Therefore, individuals with a BMI between 35 and 40 kg/m2 without comorbidities or with a BMI <35 kg/m2 are noneligible for bariatric surgery (1).
In 1987, when the Swedish Obese Subjects (SOS) study was started, no official eligibility criteria for bariatric surgery existed. Thus, both noneligible and eligible subjects according to current eligibility guidelines were included. In the SOS study, we previously showed that bariatric surgery results in long-term weight loss and reduces mortality and the incidence of hard end points such as cardiovascular events, cancer, and type 2 diabetes (3–8). However, when patients were stratified by BMI, no difference in treatment effect with respect to mortality, cardiovascular disease, cancer, or diabetes prevention was found (3,5–7). Therefore, patients not considered eligible by current criteria could possibly benefit from bariatric surgery, through reduced risk for comorbidities or early death.
Indeed, several expert committees have recently made efforts to revise the NIH criteria (2,9–12) based on data from the SOS and other studies. These reports suggest that bariatric surgery is advisable for inadequately controlled type 2 diabetes in individuals with a BMI <35 kg/m2 (2,11,12) as diabetic patients with a wide range of BMIs can achieve type 2 diabetes remission by bariatric surgery treatment (4,13–19). Furthermore, it was recently proposed that bariatric surgery should be used for type 2 diabetes treatment also in the nonobese (11). In contrast, the reports only suggest small modifications for nondiabetic individuals, and no recommendations are given for nondiabetic individuals with a BMI <35 kg/m2 (2,9,11,12). Hence, the question remains of whether bariatric surgery can improve cardiovascular risk factors and prevent comorbidities, such as type 2 diabetes, in individuals who are noneligible according to current eligibility criteria.
We therefore explored whether the long-term effects of bariatric surgery on cardiovascular risk factors and incidence of type 2 diabetes differ between patients that do or do not meet current eligibility criteria. To answer this question, we analyzed data from the SOS study, a nonrandomized, prospective, controlled intervention study that compares the long-term effects of bariatric surgery with usual care in obese individuals.
RESEARCH DESIGN AND METHODS
Study design
The SOS intervention study is an ongoing, controlled trial that enrolled a total of 4,047 obese patients between 1987 and 2001. Of these patients, 2,010 underwent bariatric surgery and 2,037 contemporaneously matched obese controls received conventional care. The selection of these individuals has previously been described in detail (3–5). The inclusion criteria were 37–60 years of age and BMI ≥34 kg/m2 for men and ≥38 kg/m2 for women before or at a matching examination. Thus, individuals that were noneligible by current eligibility criteria for bariatric surgery could be included in the study.
The BMI inclusion criteria in SOS were based on a 100% increase in mortality as compared with BMI 20–25 kg/m2 in men and women of a Norwegian population study (20), representing the most valid Nordic data available at the start of the SOS study. The exclusion criteria were minimal and were aimed at obtaining operable individuals (21).
In the surgery group, 376 individuals underwent nonadjustable or adjustable banding, 1,369 underwent vertical banded gastroplasty, and 265 underwent gastric bypass. Control individuals were given the customary treatment for obesity at their primary healthcare centers, i.e., essentially the standard nonsurgical obesity treatment in Sweden.
Physical examinations were performed at matching and baseline and repeated after 0.5, 1, 2, 3, 4, 6, 8, 10, 15, and 20 years. Biochemical analyses were performed at matching and baseline examinations and after 2, 10, 15, and 20 years.
The cutoff date for the current analysis was 1 January 2012. Seven regional ethics review boards approved the study protocol, and informed consent was obtained from all participants.
Criteria for comorbidities
In this report, selected comorbidities based on laboratory and physical examinations and also on self-reported medications at the time of the matching examination were examined. These comorbidities included hypertension, type 2 diabetes, and dyslipidemia and were diagnosed based on cutoff values from international expert reports (22–24) or the use of medication for the specific condition. Patients were given the diagnosis of type 2 diabetes when having a fasting venous whole blood glucose of ≥6.1 mmol/L (corresponding to fasting plasma glucose ≥7.0 mmol/L) (22) and/or self-reported treatment with antidiabetic drugs, including insulin. The study was started before repeated measurements were routinely used for the diagnosis of type 2 diabetes, and blood glucose was therefore measured only once per study occasion. The criteria for hypertension were systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg (23) and/or presence of antihypertensive treatment. Dyslipidemia was defined as having serum cholesterol ≥5.2 mmol/L and/or serum triglycerides ≥1.7 mmol/L (24) and/or using lipid-lowering medication regularly.
Patient groups in SOS divided by surgery eligibility criteria
Based on current eligibility criteria, patients were divided into eligible and noneligible individuals using data from the matching examination: eligible, BMI ≥40 kg/m2 or BMI 35 to <40 kg/m2 and at least one of the comorbidities defined above; noneligible, BMI 35 to <40 kg/m2 with no comorbidities or BMI <35 kg/m2.
Statistics
To describe group characteristics at matching, mean values and SDs were used.
A general linear model was used to assess differences in the effect of treatment on outcome variables in criteria groups by inclusion of an interaction term (i.e., product of type of treatment [surgery or control] and criteria group [noneligible or eligible]). Δ values from baseline to 10 years were used, and the model was adjusted for sex and age.
Time to diagnosis of type 2 diabetes was calculated from the study inclusion date. Study patients with a type 2 diabetes diagnosis at the matching examination or at baseline were excluded from the incidence analysis. Study patients that were not diagnosed with type 2 diabetes during the study were treated as censored observations at the time of dropout from the study or at the end of follow-up. Time to diabetes in the two treatment groups was analyzed using Kaplan-Meier estimates of cumulative incidence rates. A Cox proportional hazards model was used to evaluate the effect of surgery on diabetes incidence in the different selection criteria groups, unadjusted or adjusting for sex and age at baseline.
The expected number of surgeries needed to prevent one diabetes event over 15 years (numbers needed to treat) was calculated in different groups as the reciprocal of the absolute risk difference (obtained from Kaplan-Meier estimates over 15 years) between surgery and control individuals.
All P values are two-sided, and P < 0.05 was considered as statistically significant. In all calculations, the intention-to-treat principle was applied in that each participant remained in the original treatment group. Statistical analyses were carried out using the Stata statistical package 10.1 (Stata Statistical Software: Release 10.1, StataCorp LP, College Station, TX).
RESULTS
Characteristics of the patients in the noneligible and eligible groups
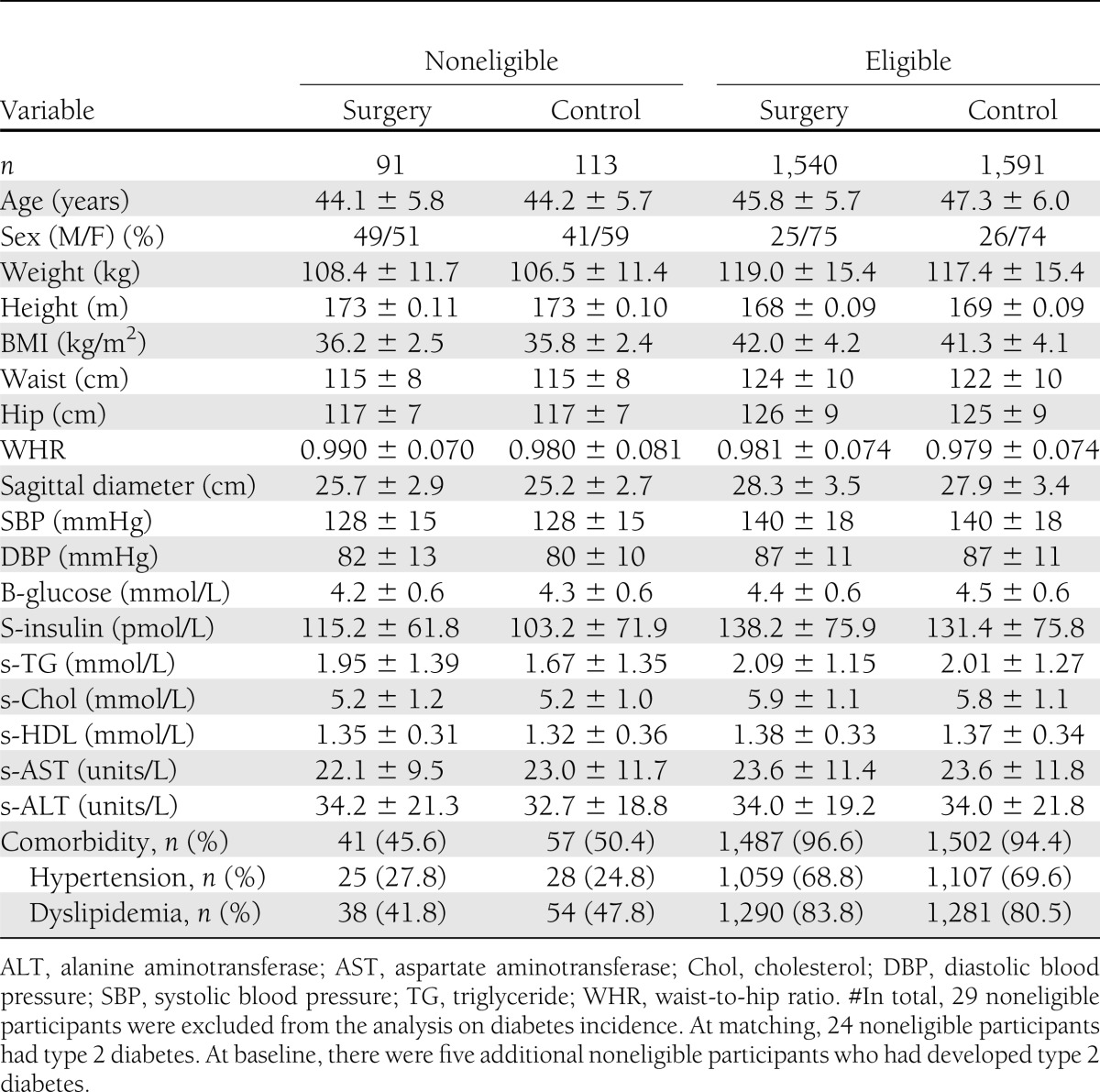
When the 4,047 SOS patients were divided by whether they fulfilled the current selection criteria for bariatric surgery, 233 patients were noneligible (Table 1). As expected, the noneligible group had lower rates of diabetes, hypertension, and/or dyslipidemia (Table 1). The noneligible patients had a median BMI of 35.7 (IQR 34.1–38.2) kg/m2, whereas the eligible group had a median BMI of 41.0 (IQR 38.5–44.0) kg/m2. Out of the 4,047 patients, 3,335 patients without diabetes at both matching and baseline were included in the analysis on diabetes incidence (Table 2). On the date of analysis (1 January 2012), the median follow-up time was 10 (range 0–20) years. The follow-up rates, after accounting for mortality, were 89% at 2 years, 73% at 10 years, and 53% at 15 years. In addition to dropout and mortality, the low number of participants at year 15 is explained by the fact that not all study participants had reached that follow-up point at the time of data analysis (7).
Table 1.
Characteristics and comorbidities in the SOS study at matching

Table 2.
Characteristics and comorbidities in noneligible and eligible SOS study participants without type 2 diabetes at study start#
Effect of bariatric surgery on risk factors in noneligible and eligible patients
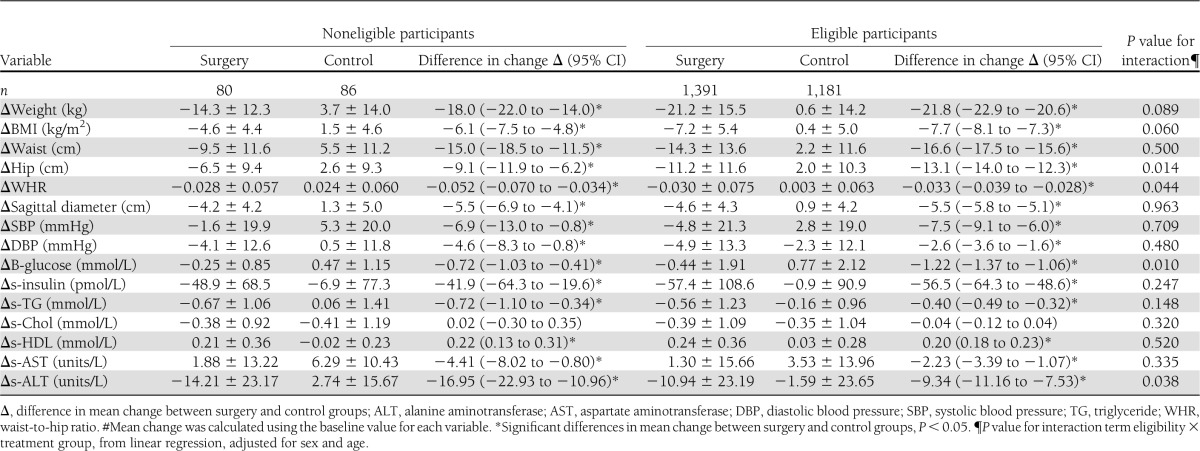
Body weight and cardiovascular risk factors, such as insulin, lipids, and blood pressure, were significantly improved in both noneligible and eligible patients after 10 years of follow-up (Table 3). Furthermore, using the analysis of the eligibility treatment interaction, we could not detect a difference in treatment effect between noneligible and eligible patients with respect to most cardiovascular risk factors and BMI. The exceptions were slightly smaller effects on blood glucose and hip circumference and a greater effect on waist-to-hip ratio and alanine aminotransferase (ALT) in the noneligible group (Table 3).
Table 3.
Mean change in clinical and biochemical measurements over 10 years in the SOS study#
Diabetes incidence in the noneligible and eligible groups
The effect of bariatric surgery on diabetes incidence was similar in noneligible and eligible patients. Out of the 3,335 patients without type 2 diabetes at study start, 204 were noneligible (Table 2). After 15 years of follow-up, bariatric surgery reduced the cumulative incidence of diabetes in both the noneligible (adjusted hazard ratio 0.33 [95% CI 0.13–0.82], P = 0.017) and eligible groups (0.27 [0.22–0.33], P < 0.001), and the treatment effect on diabetes incidence was not significantly different between the groups (adjusted interaction P value = 0.713) (Fig. 1). Of the nondiabetic participants in the noneligible group, 12% of surgery patients and 13% of controls had impaired fasting glucose at study start (7). In the eligible group, the percentages were 17 and 18, for surgery and control patients, respectively. The number needed to treat to prevent one case of type 2 diabetes over 15 years was not significantly different between noneligible (6.9 [3.5–296]) and eligible (4.1 [3.5–5.1]) groups.
Figure 1.
The Kaplan-Meier cumulative incidence of type 2 diabetes over 15 years by treatment in noneligible and eligible groups. Both unadjusted HR and HR adjusted for confounders (sex and age) are shown. Unadjusted interaction P value = 0.568 and adjusted interaction P value = 0.713, reflecting that we could not detect a difference in treatment effect between the noneligible and eligible group. The follow-up time in the figure is truncated at 15 years due to the low number of people at risk beyond this time point; however, all follow-up data up to 20 years have been used in the calculations of hazard ratios. Note that only patients without diabetes at matching and baseline were included in the analysis. NNT, number needed to treat.
CONCLUSIONS
In this explorative analysis, we investigated whether the long-term effects of bariatric surgery on the incidence of type 2 diabetes and changes in cardiovascular risk factors differ between patients that do or do not meet current eligibility criteria. Our results show that bariatric surgery reduces diabetes incidence by 73% in eligible SOS individuals after 15 years of follow-up. However, bariatric surgery reduced diabetes incidence by 67% in noneligible patients, and the number needed to treat to prevent one diabetes event over 15 years was low in both groups, reflecting the strong effect of surgical treatment. Improvements in body weight, lipids, blood pressure, glucose, and insulin were significant not only in the eligible group but also in the noneligible group after 10 years of follow-up. Hence, our results clearly show that noneligible patients may also benefit from bariatric surgery.
In this report, there was a marked reduction of diabetes incidence 15 years after the surgical intervention, both in noneligible and eligible patients. Previous studies have shown that type 2 diabetes may be preventable by changes in diet and/or exercise (25–28). In the China Da Qing Diabetes Prevention Study, the diabetes incidence was reduced by 51% after 6 years of active lifestyle intervention, and by 43% over a 20-year period in patients with impaired glucose tolerance at baseline (25). In the Diabetes Prevention Program, intensive lifestyle intervention reduced diabetes incidence by 58% after a mean follow-up time of 2.8 years in normal-weight to obese patients with impaired glucose tolerance (27). In the Finnish Diabetes Prevention Study, diabetes incidence was reduced by 58% after 4 years of active lifestyle intervention (26), and by 36% 3 years after the lifestyle counseling had stopped (28).
Currently, BMI cutoff levels are used to determine whether an individual is eligible for bariatric surgery (1). However, in the SOS study with obese people, we have found no evidence that high BMI is important for the effect of bariatric surgery on diabetes prevention (7), the incidence of cardiovascular events (6), cancer (3), or overall mortality (5), whereas glucose and/or insulin were useful predictors of the treatment effect for several of these end points. These results suggest that BMI is not the optimal basis for establishing eligibility for bariatric surgery, an idea that is also supported by a recently published statement from the International Diabetes Federation (2). Indeed, the BMI-independent Edmonton obesity staging system (29) has been proposed for the selection of high-risk individuals as it may better predict health improvement and mortality rates in obese patients (30,31).
In this study, surgery improved cardiovascular risk factors and prevented type 2 diabetes both in noneligible and eligible patients. Eighteen studies have demonstrated that bariatric surgery has a favorable effect on established type 2 diabetes also in subjects with BMI <35 kg/m2 (for review see 32), findings presumably supporting the concept that high BMI is not necessarily an important factor for treatment efficiency. One possibility would be to select patients with impaired fasting glucose (33) where the effect of bariatric surgery on diabetes prevention is high (7). It has also been suggested that surgical treatment could be more effective than conventional treatment for resolving the metabolic syndrome in individuals with mild obesity (BMI = 30–35 kg/m2) (34). These findings, together with the results from this report, suggest that guidelines for bariatric surgery eligibility should be refined and complemented with metabolic assessment in obese patients.
The main limitation of the SOS study is that participants could not be randomized due to the high mortality rates after bariatric surgery in the 1970s and 1980s (35). In addition, the number of patients in the noneligible subgroup in the SOS study is low, especially affecting the power of the treatment interaction analyses.
In conclusion, this report clearly shows that bariatric surgery can prevent the development of type 2 diabetes both in noneligible and eligible patients. Furthermore, cardiovascular risk factors are significantly improved in both noneligible and eligible patients. Our data indicate that, among obese individuals, strict BMI cutoffs are of limited use for bariatric surgery prioritization. Thus, as long as current eligibility criteria are used, some patients with high risk for future metabolic disease may not qualify for bariatric surgery.
Acknowledgments
This report was supported by grants from the Swedish Research Council (K2012-55X-22082-01, K2010-55X-11285-13, and K2008-65X-20753-01), the Swedish Foundation for Strategic Research to Sahlgrenska Centre for Cardiovascular and Metabolic Research, the Swedish federal government under the LUA/ALF agreement, the VINNOVA-VINNMER program, Hoffmann-La Roche, Cederroth, AstraZeneca, Sanofi, Ethicon, and Johnson & Johnson. L.S. has obtained lecture and consulting fees from AstraZeneca, Biovitrum, Bristol-Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Lenimen, Merck, Novo Nordisk, Hoffmann-La Roche, Sanofi, and Servier and holds stock in Lenimen and is chairman of its board. L.M.S.C. has served as a consultant for AstraZeneca and holds stock in Sahltech. K.S. holds stock in Pfizer. No other potential conflicts of interest relevant to this article were reported.
K.S. and Å.A. designed the analyses, interpreted the data, and wrote the manuscript. M.P. designed and conducted the analyses, interpreted the data, contributed to discussion, and edited the manuscript. P.J., S.R., and P.-A.S. contributed to discussion and reviewed the manuscript. L.S. and L.M.S.C. interpreted the data, contributed to discussion, and edited the manuscript. All the authors read and approved the final version of the manuscript. M.P., L.S., and L.M.S.C. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the staff members at the 480 primary healthcare centers and 25 surgical departments in Sweden that participated in the study. The authors acknowledge Gerd Bergmark, Christina Torefalk, and Lisbeth Eriksson (The Sahlgrenska Academy, University of Gothenburg) for invaluable administrative support.
Footnotes
Clinical trial reg. no. NCT01479452, clinicaltrials.gov.
A slide set summarizing this article is available online.
References
- 1.Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr 1992;55(2 Suppl.):615S–619S [DOI] [PubMed] [Google Scholar]
- 2.Dixon JB, Zimmet P, Alberti KG, Rubino F, International Diabetes Federation Taskforce on Epidemiology and Prevention Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med 2011;28:628–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sjöström L, Gummesson A, Sjöström CD, et al. Swedish Obese Subjects Study Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol 2009;10:653–662 [DOI] [PubMed] [Google Scholar]
- 4.Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 5.Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 6.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56–65 [DOI] [PubMed] [Google Scholar]
- 7.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704 [DOI] [PubMed] [Google Scholar]
- 8.Romeo S, Maglio C, Burza MA, et al. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care 2012;35:2613–2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchwald H. Consensus conference statement. Bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Relat Dis 2005;1:371–381 [DOI] [PubMed]
- 10.Fried M, Hainer V, Basdevant A, et al. Bariatric Scientific Collaborative Group Expert Panel Interdisciplinary European guidelines for surgery for severe (morbid) obesity. Obes Surg 2007;17:260–270 [DOI] [PubMed] [Google Scholar]
- 11.Rubino F, Kaplan LM, Schauer PR, Cummings DE, Diabetes Surgery Summit Delegates The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg 2010;251:399–405 [DOI] [PubMed] [Google Scholar]
- 12.Runkel N, Colombo-Benkmann M, Hüttl TP, et al. Evidence-based German guidelines for surgery for obesity. Int J Colorectal Dis 2011;26:397–404 [DOI] [PubMed] [Google Scholar]
- 13.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 14.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–323 [DOI] [PubMed] [Google Scholar]
- 15.Huang CK, Shabbir A, Lo CH, Tai CM, Chen YS, Houng JY. Laparoscopic Roux-en-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25-35. Obes Surg 2011;21:1344–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall TC, Pellen MG, Sedman PC, Jain PK. Preoperative factors predicting remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery for obesity. Obes Surg 2010;20:1245–1250 [DOI] [PubMed] [Google Scholar]
- 17.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 18.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein S, Ghosh A, Cremieux PY, Eapen S, McGavock TJ. Economic impact of the clinical benefits of bariatric surgery in diabetes patients with BMI ≥35 kg/m2. Obesity (Silver Spring) 2011;19:581–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waaler HT. Height, weight and mortality. The Norwegian experience. Acta Med Scand Suppl 1984;679:1–56 [DOI] [PubMed] [Google Scholar]
- 21.Sjöström L, Larsson B, Backman L, et al. Swedish obese subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obes Relat Metab Disord 1992;16:465–479 [PubMed] [Google Scholar]
- 22.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 23.Whitworth JA, World Health Organization, International Society of Hypertension Writing Group 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003;21:1983–1992 [DOI] [PubMed] [Google Scholar]
- 24.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–3421 [PubMed] [Google Scholar]
- 25.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 26.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 27.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Finnish Diabetes Prevention Study Group Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 29.Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes (Lond) 2009;33:289–295 [DOI] [PubMed] [Google Scholar]
- 30.Padwal RS, Pajewski NM, Allison DB, Sharma AM. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ 2011;183:E1059–E1066 [DOI] [PMC free article] [PubMed]
- 31.Kuk JL, Ardern CI, Church TS, et al. Edmonton Obesity Staging System: association with weight history and mortality risk. Appl Physiol Nutr Metab 2011;36:570–576 [DOI] [PubMed] [Google Scholar]
- 32.Shimizu H, Timratana P, Schauer PR, Rogula T. Review of metabolic surgery for type 2 diabetes in patients with a BMI <35 kg/m2 J Obes 2012;147256 [DOI] [PMC free article] [PubMed]
- 33.Pour OR, Dagogo-Jack S. Prediabetes as a therapeutic target. Clin Chem 2011;57:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien PE, Dixon JB, Laurie C, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med 2006;144:625–633 [DOI] [PubMed] [Google Scholar]
- 35.Brolin RE. Results of obesity surgery. Gastroenterol Clin North Am 1987;16:317–338 [PubMed] [Google Scholar]



