Abstract
OBJECTIVE
To study the association between vitamin D status and the risk of incident impaired fasting glucose (IFG) and diabetes in a population-based cohort of diabetes-free subjects.
RESEARCH DESIGN AND METHODS
In a historical prospective cohort study of subjects from the Clalit Health Services database, which includes information on nearly 4 million people, diabetes-free subjects aged 40–70 years with serum 25-hydroxycholecalciferol (25-OHD) measurements available were followed for 2 years to assess the development of IFG and diabetes in five 25-OHD subgroups: ≥25, 25.1–37.5, 37.6–50, 50.1–75, and >75 nmol/L.
RESULTS
The baseline cohort included 117,960 adults: 83,526 normoglycemic subjects and 34,434 subjects with IFG. During follow-up, 8,629 subjects (10.3% of the normoglycemic group) developed IFG, and 2,162 subjects (1.8% of the total cohort) progressed to diabetes. A multivariable model adjusted for age, sex, population group, immigrant status, BMI, season of vitamin D measurement, LDL and HDL cholesterol, triglycerides, estimated glomerular filtration rate, history of hypertension or cardiovascular disease, Charlson comorbidity index, smoking, and socioeconomic status revealed an inverse association between 25-OHD and the risk of progression to IFG and diabetes. The odds of transitioning from normoglycemia to IFG, from normoglycemia to diabetes, and from IFG to diabetes in subjects with a 25-OHD level ≤25 nmol/L were greater than those of subjects with a 25-OHD level >75 nmol/L [odds ratio 1.13 (95% CI 1.03–1.24), 1.77 (1.11–2.83), and 1.43 (1.16–1.76), respectively].
CONCLUSIONS
Vitamin D deficiency appears to be an independent risk factor for the development of IFG and diabetes.
The incidence of type 2 diabetes is increasing worldwide at an alarming rate (1). Although healthy diet, weight control, and physical activity remain the core of diabetes prevention and treatment (2,3), emerging modifiable risk factors are attracting more attention. Recent studies suggested that hypovitaminosis D could be a risk factor for the metabolic syndrome and type 2 diabetes (4–10). In a prospective study, Mattila et al. (11) found an inverse association between serum 25-hydroxycholecalciferol (25-OHD) levels and the risk of incident type 2 diabetes; however, this association became nonsignificant after adjustment for confounders. Kayaniyil et al. (12) did find, however, that low serum 25-OHD was a risk factor for the metabolic syndrome, a risk that remained significant after adjustment for confounders. A prospective population-based study of 552 people by Forouhi et al. (6) found increased 10-year risks for incident diabetes and insulin resistance in subjects with lower baseline 25-OHD levels; vitamin D was also an independent risk factor for diabetes. In contrast, others found no correlation between 25-OHD levels and the risk of the metabolic syndrome or diabetes (13–16). Notably, most studies were observational or cross-sectional, and a causal relationship between vitamin D status and development of diabetes therefore could not be determined. In addition, some studies were underpowered or lacked adequate covariate adjustment. Consequently, the impact of vitamin D on glucose tolerance and the risk of diabetes is not clear. Indeed, the 2011 Institute of Medicine Dietary Reference Intake for Calcium and Vitamin D and the Endocrine Society Task Force guideline concluded that the evidence for a causal relationship between vitamin D deficiency and cardiovascular disease or diabetes is inconclusive (17,18).
The objective of the current study was to assess the impact of vitamin D status, as measured by serum 25-OHD (19), on the risk of developing impaired fasting glucose (IFG) or diabetes. We hypothesized that subjects with low 25-OHD levels are at increased risk of progression to impaired glucose metabolism. We performed a large, population-based cohort study including adults free of diabetes with 25-OHD measurements and analyzed the progression to IFG or diabetes. The results were adjusted for a wide range of potential confounders to contribute convincing findings to the debate on whether low vitamin D is an independent risk factor for IFG and diabetes.
RESEARCH DESIGN AND METHODS
The study was a prospective historical study analyzing data from the Clalit Health Services (CHS) electronic database of nearly 4 million people in Israel.
CHS is one of the four not-for-profit health maintenance organizations that provide hospital- and community-based medical services, including diagnostic procedures, medical treatments, and hospitalizations, for the Israeli population. All Israeli residents are required by law to choose to be covered by one of the health maintenance organizations, of which CHS is the largest, providing medical services to more than 4 million people. On average, CHS members are slightly older than members of the other health maintenance organizations and somewhat lower in socioeconomic status (SES). Electronic medical records have been available for all CHS members since 1998. The electronic database includes detailed demographic and clinical data and sociodemographic information derived from Israel’s Central Bureau of Statistics and the National Insurance Institute (Social Security). The data are compiled into a centralized data warehouse from electronic records from primary care and specialist clinics, hospitals, pharmacies, and laboratories. CHS also coordinates a chronic disease registry, and all affiliated primary care physicians provide clinical information on all subjects aged >20 years with any of 110 chronic diseases. Member records are comprehensive within the data warehouse because members receive most of their care and treatments within CHS. The data warehouse was made accessible for this study after approval by the Clalit ethics committee.
Inclusion criteria
The study population included all CHS members aged 40–70 years who were continuous members between July 2008 and June 2012, had at least one record of a 25-OHD measurement between July 2008 and June 2010 (baseline period), and had at least two blood glucose measurements, with the first being during the baseline period and the second from July 2010 until June 2012 (follow-up period). If more than one 25-OHD test was available, the first recorded result was selected as the baseline measurement.
Exclusion criteria
Subjects were excluded if during the baseline period they had a diagnosis of diabetes entered in their medical records, they purchased diabetes medications, or they had blood glucose or HbA1c measurements compatible with diabetes (blood glucose level ≥7 mmol/L or HbA1c ≥6.5%). A prolonged baseline period was used to accumulate sufficient data for definitive exclusion of subjects with diabetes. Other exclusion criteria were serum creatinine >133.0 μmol/L, serum calcium >2.7 mmol/L, diagnosis of hyperparathyroidism, renal or liver failure, and steroid treatment for longer than 3 months. A total of 200,492 people had at least one 25-OHD test performed between July 2008 and June 2010. After exclusion of subjects who were not continuous members (n = 7,800), those who had fewer than 2 glucose tests (n = 12,707), members with diagnosis of diabetes (n = 49,269), and those with the aforementioned comorbidities (n = 12,756), 117,960 subjects finally fulfilled the study criteria. The cohort was then divided into two groups according to the glycemic status during the baseline period (July 2008–June 2010). The normal glucose (NG) group (n = 83,526) had all glucose measurements <5.6 mmol/L, and the IFG group (n = 34,434) had one or more glucose measurements ≥5.6 mmol/L and <7.0 mmol/L (20).
Independent variables
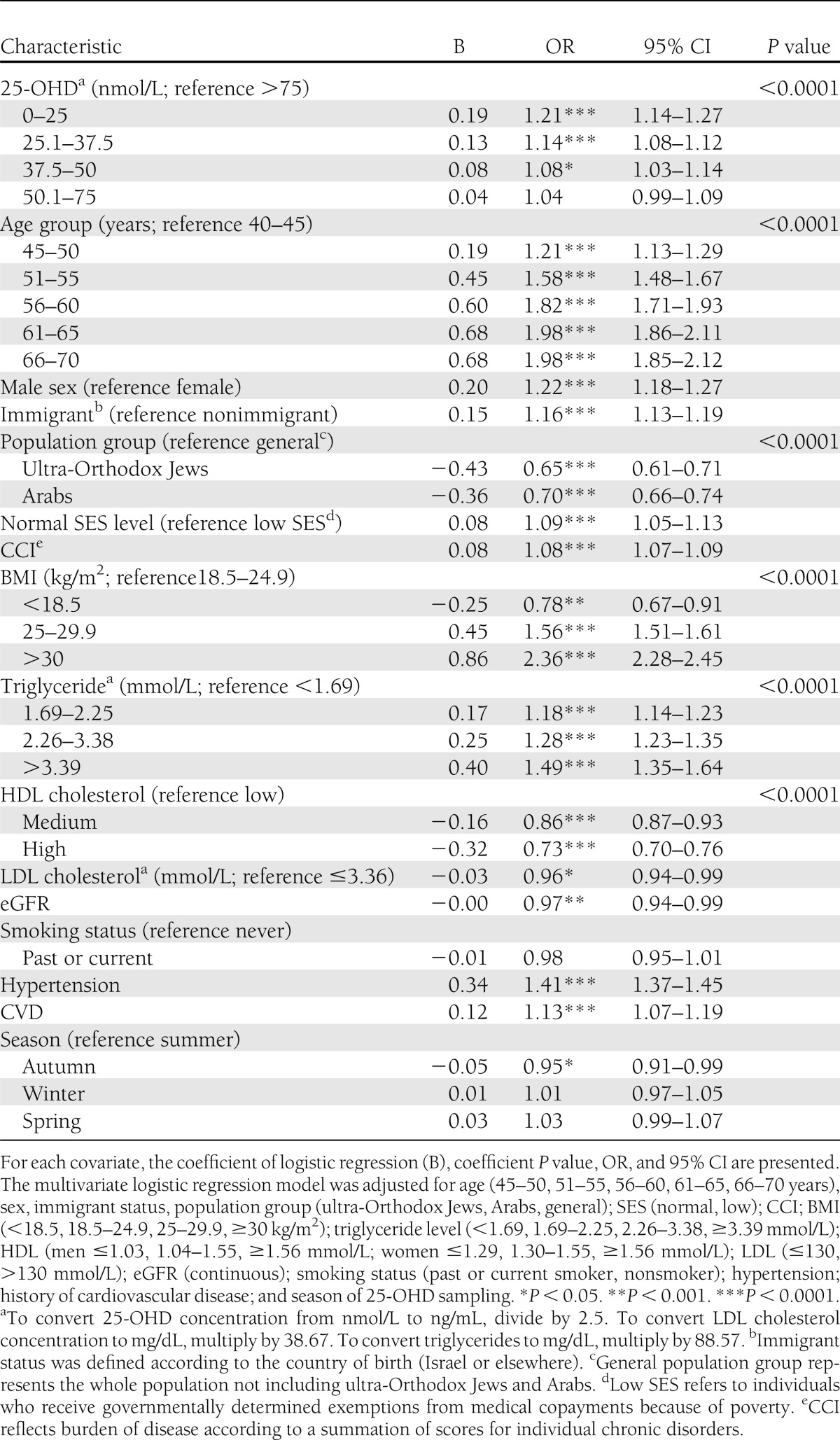
Demographic variables that were recorded closest to the index date (July 2010) were included in the multivariable analysis: age, sex, population group, immigrant status, and SES. Low SES was the designation for individuals who received National Insurance payment exemption because of low income. The following independent predictors were also included: BMI (<18.5, 18.5–24.9, 25–29.9, and ≥30 kg/m2), season of vitamin D measurement, triglyceride level (<1.69, 1.69–2.25, 2.26–3.38, and ≥3.39 mmol/L), HDL cholesterol (for men, ≤1.03, 1.04–1.55, and ≥1.56 mmol/L; for women, ≤1.29, 1.30–1.55, and ≥1.56 mmol/L), LDL cholesterol (≤3.36 and >3.36 mmol/L), hypertension, smoking status, estimated glomerular filtration rate (eGFR), cardiovascular disease (a diagnosis of ischemic heart disease, previous myocardial infarction, or coronary artery bypass grafting before index date), and Charlson comorbidity index (CCI) (21). There was no linear association between most potential variables included in our model and the outcome; we therefore divided these variables into clinically meaningful categories rather than expressing them as continuous variables.
Main predictor
Baseline level of 25-OHD (the first laboratory test recorded) was the primary independent variable in the model. Levels were divided into five 25-OHD subgroups: ≤25, 25.1–37.5, 37.6–50, 50.1–75, and >75 nmol/L. We analyzed 25-OHD levels by these five strata to test for the impact of very low vitamin D levels, which are less common, on the risk of incident IFG or diabetes.
Dependent variables
Progression to IFG or diabetes during the follow-up period was the primary study outcome. Diabetes in the follow-up period was diagnosed by at least one of the following criteria: 1) fasting plasma glucose level ≥7.0 mmol/L at least twice (n = 725), 2) glucose level ≥11.1 mmol/L 120 min after an oral glucose tolerance test (n = 156), 3) one blood glucose measurement ≥7.0 mmol/L and HbA1c ≥6.5% (n = 314), 4) diagnosis of diabetes in the medical records and one glucose measurement ≥7.0 mmol/L, (n = 432), and 5) diagnosis of diabetes in medical records and HbA1c ≥6.5% (n = 535). IFG was diagnosed if at least one blood glucose measurement was >5.6 mmol/L without fulfilling the diagnostic criteria for diabetes.
Laboratory methods
Biochemical analyses were performed at CHS laboratories with routine standardized methodologies on fresh samples of blood obtained after an overnight fast. Glucose was measured in plasma, and all other biochemical analyses were performed on serum. The laboratories are authorized to perform tests according to the international quality standard ISO 9001. Periodic assessment of quality control is performed on a regular basis. The accuracy of the measurements in the individual laboratory is confirmed by in-house daily quality control monitoring (OLIMPUS and BIO-RAD) and by monthly external quality control program (NEQAS). The 25-OHD level was tested by the LIAISON 25-OH Vitamin D TOTAL Assay (DiaSorine), a competitive two-step chemiluminescence assay that measures both the D2 and D3 25-OHD metabolites. The measuring range is 10–375 nmol/L; the analytical sensitivity is <2.5 nmol/L, and the functional sensitivity is <10.0 nmol/L. The intra-assay coefficients of variation for low (18 nmol/L) and high (320 nmol/L) 25-OHD concentrations were 5.5% and 4.8%, respectively. The interassay coefficients of variation were 12.7% and 7.9% for low and high 25-OHD concentrations, respectively. Performance characteristics of the vitamin D assay were evaluated by the CHS laboratory and were comparable to the manufacturer’s specifications.
Statistical analyses
The cohort at baseline was divided into five 25-OHD subgroups. Descriptive statistics were then assessed for both the NG and IFG groups across the vitamin D subgroups. Evidence of a trend across vitamin D groups was assessed with univariate linear regression for each covariate. Multivariable logistic regression assessed the association between IFG (relative to NG) and vitamin D levels while controlling for the covariates. All covariates were checked against one another for collinearity.
The proportions of members at baseline who underwent one of the three glycemic transitions (NG to IFG, NG to diabetes, and IFG to diabetes) were tested across the five baseline 25-OHD groups. Differences across groups were assessed by χ2, Student t, and rank sum tests for categorical, normally distributed, and nonnormally distributed parameters, respectively. Three logistic regression models were conducted to assess the association between 25-OHD groups and the odds of progression across the three glycemic categories while controlling for age, sex, population group, immigrant status, BMI, season of vitamin D measurement, LDL and HDL cholesterol, triglycerides, estimated GFR, history of hypertension or cardiovascular disease, CCI, smoking, and SES. A subgroup analysis was performed among subjects with low 25-OHD levels at baseline (≤37.5 nmol/L) who had at least two 25-OHD measurements during the study period to evaluate the impact of changes in vitamin D levels on the risk of development of diabetes. For this analysis, we included subjects of the two lowest vitamin D categories (≤25 and 25.1–37.5 nmol/L) and pooled the baseline IFG and NG groups to increase the number of participants. The first 25-OHD measurement was used as the baseline indicator, and the subsequent measurements were averaged to create the follow-up indicator. The odds of developing diabetes were assessed. Statistical analysis was performed with SPSS17 software (IBM Corporation, Armonk, NY). Data are expressed as mean ± SD. Significance was set at P = 0.05 level.
RESULTS
Baseline analysis
Our study included a cohort of 117,960 members free of diabetes on as of July 2010, with 71% in the NG group and 29% in the IFG group. Baseline characteristics of the cohort are shown in Table 1. The mean age of the subjects included in the cohort was 56.6 years, 76.6% were females, and the mean BMI was 27 kg/m2. IFG subjects were more likely to be men than NG subjects and were also older. A multivariable analysis showed that IFG was also associated with high BMI [odds ratio (OR) 2.36 (95% CI 2.28–2.45)], hypertension [1.41 (1.37–1.45)], low HDL cholesterol [1.37 (1.32–1.43)], and high triglyceride levels [1.49 (1.35–1.64)] (Table 2).
Table 1.
Baseline characteristics of 117,960 CHS members by glycemic status
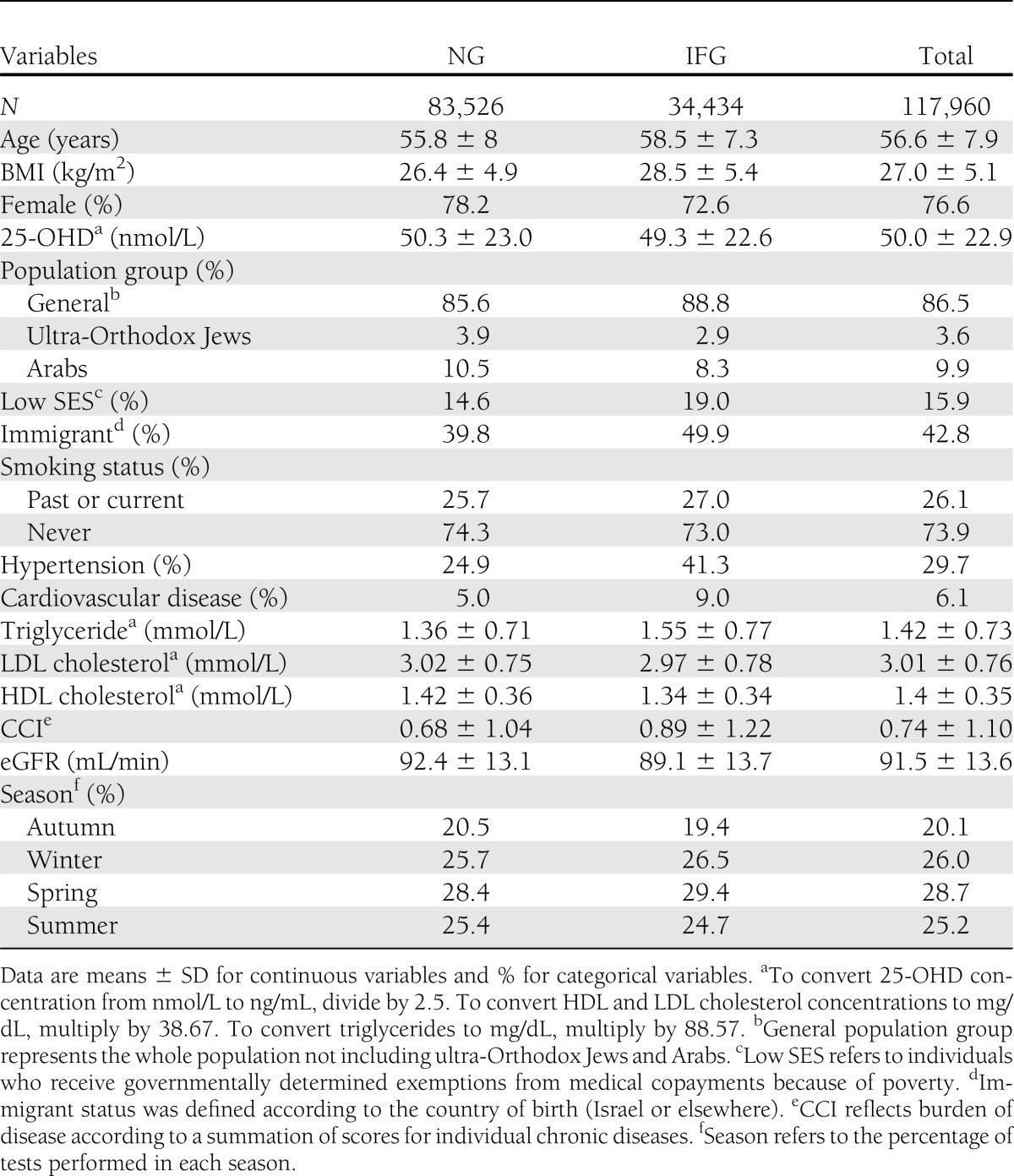
Table 2.
Multivariable logistic regression for IFG group compared with NG group at baseline
One-third of the subjects had 25-OHD level ≤37.5 nmol/L, and only 13% had levels >75 nmol/L. Subjects with low 25-OHD levels were younger and had lower SES and CCI. In addition, these subjects were more likely to have IFG, higher BMI, and decreased HDL cholesterol (not shown). The 25-OHD levels were inversely associated with IFG. The odds of IFG among subjects with 25-OHD levels ≤25 nmol/L were 1.21 (95% CI 1.14–1.27) relative to those with 25-OHD levels >75 nmol/L (P < 0.001) (Table 2).
Follow-up analysis
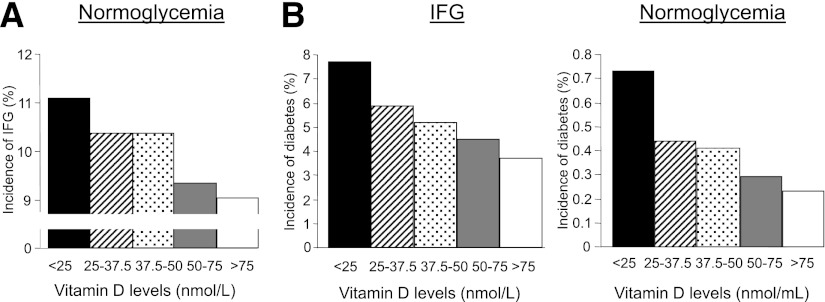
During the 2 years of follow-up, 8,629 subjects (10.3%) developed IFG and 2,162 subjects developed diabetes; among these, 334 (0.4%) were from the NG group and 1828 (5.3%) were from the IFG group. The intervals between initial 25-OHD measurement and diagnosis of IFG or diabetes were 16.5 ± 7.7 and 25.1 ± 8.5 months, respectively. Progression to IFG or diabetes occurred 23.4 ± 6.7 and 32.4 ± 8.1 months, respectively, after initial blood glucose measurement. The percentage of subjects progressing to diabetes or to IFG continuously decreased with increasing 25-OHD levels (Fig. 1). Univariate logistic regression to assess the association between 25-OHD and transition from NG to IFG yielded an OR of 1.16 (95% CI 1.06–1.27) when comparing the ≥25 nmol/L group with the >75 nmol/L 25-OHD group, with an OR of 2.17 (1.79–2.64) among those transitioning from IFG to diabetes and an OR of 2.88 (1.87–4.41) among those transitioning from NG to diabetes.
Figure 1.
Incidences of transitions between glycemic states by 25-OHD level. Graphs show unadjusted incidences of transitions from NG to IFG (A) and from NG or IFG to diabetes (B) according to 25-OHD level at baseline divided into five subgroups: ≥25, 25.1–37.5, 37.6–50, 50.1–75, and >75 nmol/L. The incidences of IFG and diabetes are expressed as percentages of the NG and IFG groups, as indicated.
After adjustment for all covariates, including BMI, the association between low 25-OHD levels and the risk of progression to IFG or diabetes was attenuated but remained significant for 25-OHD levels ≥25 nmol/L (NG to IFG or diabetes) and for 25-OHD levels ≥37.5 nmol/L (IFG to diabetes) (Table 3). The progression of NG to IFG had an OR of 1.13 (95% CI 1.03–1.24), NG to diabetes had an OR of 1.77 (1.11–2.83), and IFG to diabetes had an OR of 1.43 (1.16–1.76) when comparing subjects with 25-OHD ≥25 nmol/L with those with 25-OHD >75 nmol/L.
Table 3.
Adjusted odds of progression between glycemic states by 25-OHD level
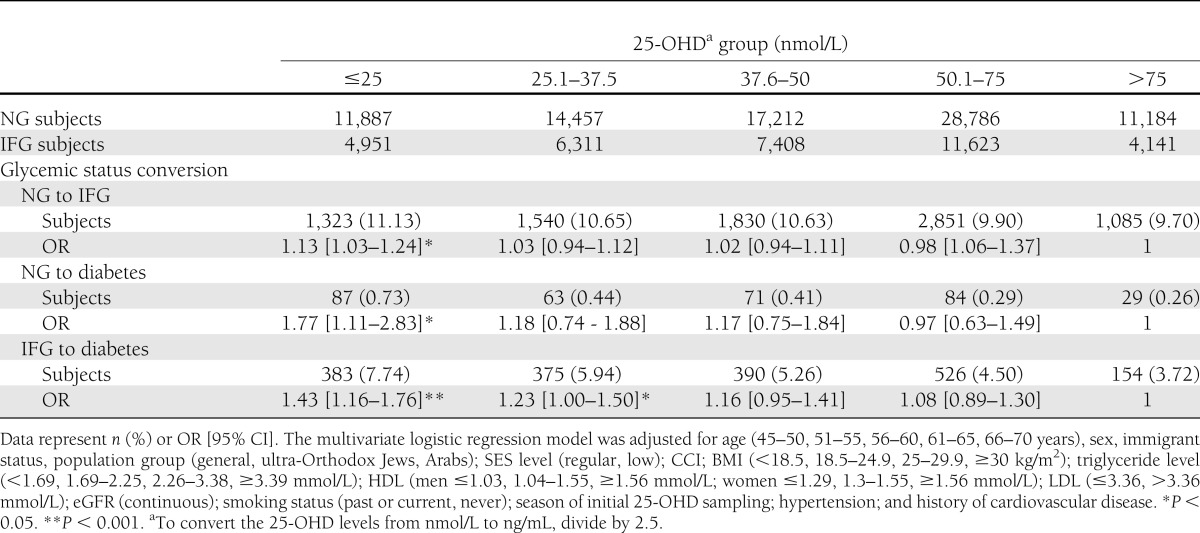
The majority of subjects included in our cohort were females, which may have strongly influenced the findings in the whole cohort. Stratification of the data according to sex showed that low vitamin D was also associated with a higher risk of progression from IFG to diabetes in males [OR 1.82 (95% CI 1.21–2.73); P < 0.01] when comparing subjects with 25-OHD ≥25 nmol/L versus those with 25-OHD ≥75 nmol/L.
The subgroup analysis examining whether a change in 25-OHD levels affected the progression to diabetes included 19,664 subjects with a baseline 25-OHD ≤37.5 nmol/L and average subsequent follow-up measurements ≤37.5 nmol/L (persistent low vitamin D) or ≥50 nmol/L (corrected vitamin D). In 53.6%, 25-OHD levels increased to ≥50 nmol/L during follow-up. In the corrected vitamin D group, average vitamin D level increased by 41.3 ± 18.5 nmol/L (P < 0.001). This correction was associated with a 40% reduction in the odds of incident diabetes relative to subjects whose low 25-OHD levels persisted (P < 0.001). After covariate adjustment, the reduction in the odds was 26% and remained statistically significant (P < 0.005).
CONCLUSIONS
Previous studies suggested that low vitamin D levels are associated with diabetes and the metabolic syndrome (4–12); however, the question of whether low vitamin D is an independent risk factor for diabetes remains open. To our knowledge, this is the largest longitudinal study published to date on the link between vitamin D and glycemic status. A large database of real-life data on close to 4 million individuals allowed assessment with reduced bias of the association between vitamin D concentrations and the risk of diabetes, with rigorous adjustment for multiple confounders. The main findings of this large-scale cohort of Israeli adults are as follows: 1) 25-OHD levels are inversely associated with an increased risk of the presence and future development of IFG, 2) low 25-OHD levels are associated with an increased risk of future development of diabetes, and 3) increasing 25-OHD levels in subjects with vitamin D deficiency may reduce the risk of diabetes.
Corroborating previous studies, we found that low 25-OHD levels at baseline were associated with a lower SES and with a higher prevalence of different components of the metabolic syndrome, including IFG, higher BMI, elevated triglycerides, and low HDL (7,12,22–27). During follow-up, low serum 25-OHD concentrations were associated with increased risk of progression to IFG and diabetes, an association that remained significant even after adjustment for various socioeconomic, anthropometric and metabolic confounders. Low vitamin D thus seems to be an independent risk factor for prediabetes and diabetes.
This is the first study to demonstrate that vitamin D concentrations are significantly associated with incident IFG. In quantitative terms, the impact of vitamin D deficiency on the risk of IFG was relatively small but significant. In the Framingham Offspring Study cohort, a higher predicted 25-OHD score was associated with a lesser increase in fasting plasma glucose concentration during 7 years among subjects who were diabetes free at baseline, further suggesting that vitamin D might be an important determinant for change in fasting glucose in NG subjects (28).
In our cohort, increased incidence of diabetes among subjects with low vitamin D is remarkable considering the relatively short follow-up period. The risk of progression to diabetes was higher among subjects with 25-OHD levels ≤25 nmol/L (NG to diabetes) and 37.5 nmol/L (IFG to diabetes) but not among those with higher 25-OHD levels. This is most likely explained by the short follow-up, which may not reveal the deleterious effects of mild vitamin D insufficiency, thus leading to underestimation of the impact of vitamin D on the risk of diabetes; however, the presence of a vitamin D level threshold above which there is no impact on the development of diabetes cannot be excluded.
In the Diabetes Prevention Program, the association between vitamin D levels and the risk of diabetes was linear in subjects at risk for diabetes (23). We maintain that future studies are needed to clarify whether our finding that vitamin D levels influence the risk of IFG or diabetes only below certain thresholds in fact holds true in other contexts.
Vitamin D insufficiency has been implicated in β-cell dysfunction and insulin resistance (8), which may increase blood glucose at all stages of diabetes. The precise mechanisms involved are currently not known. Further studies are required to shed light on the role of vitamin D in the pathophysiology of diabetes.
Despite the mounting evidence supporting the relationship between vitamin D and the risk of diabetes, the results of clinical trials and post hoc analyses on the effect of vitamin D supplementation on glycemic outcomes have been inconclusive (4,29–33). Notably, in our cohort the risk of diabetes was reduced in subjects with initial low serum 25-OHD who had a documented increase in 25-OHD level relative to those with persistent low levels. In this retrospective population-based study, no information was available on how vitamin D deficiency was corrected in this group, precluding definitive conclusions as to the impact of vitamin D supplementation on the risk of diabetes. Collectively, our findings suggest that vitamin D is an important determinant of glycemia along the whole spectrum of glucose metabolism and that increasing vitamin D level may reduce the risk of diabetes. Large-scale, randomized, controlled trials targeting different 25-OHD levels are required to assess the impact of vitamin D supplementation on diabetes prevention.
This study has several limitations that warrant consideration. First, the analysis is retrospective, and therefore a causal relationship between vitamin D deficiency and diabetes cannot be definitively determined. Nevertheless, the longitudinal collection of data and the adjustment for multiple confounders reduce the impact of potential biases and strongly suggest that vitamin D status affects the risk of diabetes. Second, the analysis could be overly influenced by the findings among females, who comprised the majority of our cohort. We believe that the dominance of female sex in our sample is explained by physicians’ awareness of poorer vitamin D status among females. Stratification of the data according to sex showed that low vitamin D was also associated with a higher risk of progression to diabetes in males, and we therefore believe that the impact of vitamin D status on the risk of diabetes is not limited to females. Third, no information is available on the indication for measuring vitamin D in a given subject. During the last few years, primary care physicians, specialists, and the general public became aware of the high prevalence of vitamin D deficiency and its potential implications for public health. In addition, 25-OHD testing became more accessible; we therefore assume that the majority of tests were performed as screening and not for any specific medical indication. We cannot rule out, however, the possibility that subjects selected for vitamin D and repeated blood glucose testing were those already at increased risk of diabetes. Future studies will clarify whether vitamin D deficiency increases the risk of diabetes in the general population or only in subjects more likely to develop diabetes. Fourth, CHS members are slightly older and have somewhat lower SES relative to the general population in Israel. Still, our analyses have controlled for these variables and should reduce potential confounding effects, thus allowing conclusions that are largely generalizable. Fifth, we did not have information on calcium and vitamin D intake and on physical exercise; however, the large number of subjects in this cohort minimizes the likelihood that the results were biased by differences in these parameters. Seasonality is an important predictor of vitamin D status. In our cohort, the number of tests increased steadily with time; however, there was no seasonal variation in the frequency of blood sampling, and the impact of vitamin D status on the risk of IFG or diabetes was not affected by the season in which blood samples were obtained.
In conclusion, vitamin D concentrations are inversely associated with future incidence of IFG and diabetes. Hypovitaminosis D, especially at low levels (≤37.5 nmol/L) is an independent risk factor for diabetes, and increasing 25-OHD may partially minimize the risk of diabetes. Confirmation of these results in large-scale randomized, controlled trials will have important implications for diabetes prevention and public health, because vitamin D supplementation is easy to implement, inexpensive, and safe. Further studies are required to assess the link between 25-OHD and other metabolic anomalies that are common in diabetes (e.g., hyperlipidemia) and to determine the optimal vitamin D target in the general population and among those at risk for diabetes.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
A.T. designed the study and wrote the manuscript. B.S.F. was involved in the design of the study, research of the data, and discussion. I.F. and M.B.H. researched data. G.L. was involved in the interpretation and discussions of the data and wrote part of the manuscript. R.D.B. was involved in the design of the study and discussion of the data. B.S.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Centers for Disease Control and Prevention National Diabetes Fact Sheet. Atlanta, GA, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 4.Pittas AG, Chung M, Trikalinos T, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med 2010;152:307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scragg R, Sowers M, Bell C, Third National Health and Nutrition Examination Survey Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2813–2818 [DOI] [PubMed] [Google Scholar]
- 6.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990-2000. Diabetes 2008;57:2619–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar A, Sabanayagam C, Kalidindi S. Serum 25-hydroxyvitamin D levels and prediabetes among subjects free of diabetes. Diabetes Care 2011;34:1114–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta AK, Brashear MM, Johnson WD. Prediabetes and prehypertension in healthy adults are associated with low vitamin D levels. Diabetes Care 2011;34:658–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281 [DOI] [PubMed] [Google Scholar]
- 11.Mattila C, Knekt P, Männistö S, et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care 2007;30:2569–2570 [DOI] [PubMed] [Google Scholar]
- 12.Kayaniyil S, Vieth R, Harris SB, et al. Association of 25(OH)D and PTH with metabolic syndrome and its traditional and nontraditional components. J Clin Endocrinol Metab 2011;96:168–175 [DOI] [PubMed] [Google Scholar]
- 13.Kim MK, Il Kang M, Won Oh K, et al. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clin Endocrinol (Oxf) 2010;73:330–338 [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care 2005;28:2926–2932 [DOI] [PubMed] [Google Scholar]
- 15.Rueda S, Fernández-Fernández C, Romero F, Martínez de Osaba J, Vidal J. Vitamin D, PTH, and the metabolic syndrome in severely obese subjects. Obes Surg 2008;18:151–154 [DOI] [PubMed] [Google Scholar]
- 16.Knekt P, Laaksonen M, Mattila C, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 2008;19:666–671 [DOI] [PubMed] [Google Scholar]
- 17.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 19.Henry HL, Bouillon R, Norman AW, et al. 14th Vitamin D Workshop consensus on vitamin D nutritional guidelines. J Steroid Biochem Mol Biol 2010;121:4–6 [DOI] [PubMed] [Google Scholar]
- 20.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 22.Hyppönen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes 2008;57:298–305 [DOI] [PubMed] [Google Scholar]
- 23.Pittas AG, Nelson J, Mitri J, et al. Diabetes Prevention Program Research Group Plasma 25-hydroxyvitamin D and progression to diabetes in patients at risk for diabetes: an ancillary analysis in the Diabetes Prevention Program. Diabetes Care 2012;35:565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Need AG, O’Loughlin PD, Horowitz M, Nordin BE. Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clin Endocrinol (Oxf) 2005;62:738–741 [DOI] [PubMed] [Google Scholar]
- 25.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care 2005;28:1228–1230 [DOI] [PubMed] [Google Scholar]
- 26.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2007;167:1159–1165 [DOI] [PubMed] [Google Scholar]
- 27.Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes 2010;59:242–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu E, McKeown NM, Pittas AG, et al. Predicted 25-hydroxyvitamin D score and change in fasting plasma glucose in the Framingham offspring study. Eur J Clin Nutr 2012;66:139–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007;30:980–986 [DOI] [PubMed] [Google Scholar]
- 30.de Boer IH, Tinker LF, Connelly S, et al. Women’s Health Initiative Investigators Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care 2008;31:701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr 2009;48:349–354 [DOI] [PubMed] [Google Scholar]
- 32.Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011;96:1931–1942 [DOI] [PubMed] [Google Scholar]
- 33.Nikooyeh B, Neyestani TR, Farvid M, et al. Daily consumption of vitamin D- or vitamin D + calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr 2011;93:764–771 [DOI] [PubMed] [Google Scholar]