Abstract
OBJECTIVE
We sought to estimate the association between intimate partner violence, a prevalent psychosocial stressor, and the incidence of type 2 diabetes in women.
RESEARCH DESIGN AND METHODS
In 2001, 68,376 Nurses’ Health Study II participants answered questions on physical, sexual, and psychological intimate partner violence in adulthood (age ≥18 years) and reported the years in which any abuse occurred. We used Cox proportional hazards models to estimate the associations between intimate partner violence exposures and incidence of type 2 diabetes from 2001 to 2007. We also estimated effects of duration and time since intimate partner violence on type 2 diabetes incidence.
RESULTS
Of 68,376 respondents, 64,732 met inclusion criteria at the 2001 baseline; of these, 23% reported lifetime physical intimate partner violence, 11% reported lifetime sexual intimate partner violence, and 8% reported moderate and <2% reported severe psychological intimate partner violence. Hazard ratios (HRs) and 95% CIs for type 2 diabetes, adjusted for potential confounders, were 1.18 (1.00–1.39) and 1.08 (0.86–1.35) for more than one lifetime episode of physical and sexual intimate partner violence, respectively, and 1.78 (1.21–2.61) for severe psychological abuse. Addition of updated BMI and other diabetes risk factors reduced the physical intimate partner violence HR to 1.12 (0.94–1.33) and the psychological intimate partner violence HR to 1.61 (1.09–2.38).
CONCLUSIONS
Physical intimate partner violence is modestly associated with incidence of type 2 diabetes in this population. Severe psychological violence may substantially increase type 2 diabetes risk.
Although psychosocial stress and trauma are increasingly recognized as risk factors for cardiovascular disease (1–6), less is known about the ways common stressors may influence the development of type 2 diabetes, despite acknowledged overlap in cardiovascular disease and type 2 diabetes etiologies (7–10). Child abuse and intimate partner violence (IPV) are prevalent sources of stress for American women, with 43% of women reporting physical abuse in childhood or adolescence (11) and almost a quarter reporting physical abuse by an intimate partner as adults (11,12). Recent studies have indicated that child abuse and IPV are risk factors for hypertension and cardiovascular events (13,14). Child abuse and IPV may influence type 2 diabetes through depression (15–17) and/or through behavioral coping mechanisms that lead to increases in BMI (18–20). More direct endocrine mechanisms may involve hypercortisolemia and insulin resistance (21).
A handful of studies have been conducted on child abuse and type 2 diabetes and showed positive associations (19,20,22,23). In our own work, we have documented 25–70% increases in type 2 diabetes rates associated with exposure to moderate to severe physical or sexual abuse in childhood (19). Less is known about adult experiences of abuse and type 2 diabetes. To our knowledge, the association between IPV and type 2 diabetes has been examined only by a single, cross-sectional study, which examined the association between self-report of any lifetime IPV and diabetes among 42,566 women and found a statistically nonsignificant 11% increase in diabetes risk associated with IPV exposure (24).
In this report, we examine associations between IPV experienced in adulthood (age ≥18 years) and the incidence of type 2 diabetes in women in the longitudinal Nurses’ Health Study II (NHSII), currently the largest prospective cohort in the world with information on child abuse and IPV exposures.
RESEARCH DESIGN AND METHODS
Data sources
The NHSII comprises 116,430 female registered nurses recruited at age 25 to 42 years in 1989. Biennial questionnaires gather sociodemographic, behavioral, and medical data. In 2001, a supplemental Violence Questionnaire asking about experiences of interpersonal violence in childhood, adolescence, and adulthood was sent to 91,297 NHSII participants who had responded to the previous biennial questionnaire within three mailings. Questionnaires were returned by 68,376 (75%) of the Violence Questionnaire recipients. Violence Questionnaire respondents were more likely to be white than the rest of the NHSII cohort and reported more physical activity, lower rates of smoking, and a slightly lower average BMI in 2001 (see Supplementary Table 1). Respondents did not differ substantially in childhood socioeconomic status or childhood body size. A 2008 follow-up questionnaire asked 2001 Violence Questionnaire responders about their experiences of IPV since 2001 and was returned by 54,700 women.
Variables and variable definitions
Primary exposures.
We examined three primary IPV exposures: 1) adult lifetime physical IPV, 2) adult lifetime sexual IPV, and 3) psychological IPV in a 2001 intimate relationship.
Adult lifetime physical and sexual IPV. The 2001 Violence Questionnaire asked participants whether, in adulthood (age ≥18 years), they had “ever been hit, slapped, kicked, or otherwise physically hurt” by a spouse or significant other. Women were considered to have experienced physical IPV if they responded, “yes, this happened once” or “yes, this happened more than once.” Likewise, women were considered to have experienced sexual IPV if they responded, “yes, this happened once” or “yes, this happened more than once” to the question “has your spouse/significant other ever forced you to have sexual activities?” on the 2001 questionnaire. Physical and sexual IPV were defined as adult lifetime exposures and were categorized as no IPV, one episode of IPV, and more than one episode of IPV.
Recent psychological IPV. For intimate relationships that were ongoing at the time of the 2001 questionnaire, women were asked to complete the Women’s Experiences with Battering (WEB) scale, a measure of psychological IPV that captures the extent to which women feel disempowered, trapped, and threatened by their intimate relationships (25,26). The WEB score ranges from 10 to 60, with 60 indicating the most severe psychological abuse, and <20 considered nonabusive (25,26). The WEB has demonstrated high internal consistency, reliability, and high correlation with known abuse status (26). The WEB scale is provided in the Supplementary data. We coded the WEB as a continuous variable, for tests of linearity and linear trend, and as a categorical variable in three score levels of 10–19, 20–39, and 40–60. Tests of linearity used likelihood ratio tests to compare the linear term with the linear term plus cubic splines (27).
Secondary exposures.
Women who indicated that they had experienced adult lifetime physical or sexual IPV, or who answered affirmatively to the question “have you ever been emotionally abused by your spouse/significant other?” were asked to report the years in which they had experienced any of these types of IPV, from 1962 (the year the oldest women in the cohort turned 18) through the end of follow-up in 2007. We used these reported years of “any physical, sexual or emotional IPV” to construct two time-varying IPV exposures: 1) cumulative number of years of IPV exposure (ie, updated duration of IPV exposure) and 2) time-varying total number of years since most recent report of IPV (ie, updated “recency” of IPV exposure).
Duration of IPV exposure. For duration analyses, women entered the study at NHSII baseline (1989) with the total number of years before baseline in which they had reported an IPV occurrence. Additional years of IPV over 1989–2007 follow-up were added to this cumulative total. After examination of continuous and categorical duration of IPV exposure, we categorized the duration variable into never IPV (0 years), 1–4 years of total IPV exposure, and ≥5 years of total IPV exposure. There was no evidence that type 2 diabetes risk changed with additional total IPV years after the 5-year threshold.
Time since IPV exposure. For analyses of time since most recent IPV exposure (“recency of IPV”), women entered the study in 1989 with the number of years since their most recent report of IPV; for example, women reporting IPV in 1987 but not 1988 were considered to have had 1 full IPV-free year at NHSII baseline in 1989. We included only women who had reported at least 1 year of IPV before baseline, so that the number of years since IPV could be defined for all women. Women accumulated IPV-free years until they reported a year in which IPV occurred, at which time their number of IPV-free years was reset to 0. We categorized the recency variable into current IPV (0 years since IPV exposure), 1–4 years since IPV exposure, and ≥5 years since IPV exposure. There was no evidence that type 2 diabetes risk changed with additional IPV-free years after the 5-year threshold.
Outcome.
Biennial questionnaires asked women “Since [date of previous questionnaire], have you had any of these physician-diagnosed illnesses?” and provided a list of disease outcomes, including “diabetes mellitus.” Women who reported a diabetes diagnosis were sent a supplemental questionnaire to confirm the date of diagnosis and to identify the type of diabetes (type 1 or type 2). In a validation study among a random sample of nurses, the type 2 diabetes diagnosis was confirmed by medical record in 61 of 62 women self-reporting type 2 diabetes (28). In a substudy of undiagnosed diabetes, only 1 (0.5%) of a random sample of 200 nurse participants who had never reported diabetes had an elevated fasting plasma glucose level (29). More than 97% of our sample visited a physician for a screening examination between 1999 and 2001, and 57% had had a fasting blood glucose test in the past 2 years.
Covariates.
We included the following child and adolescent risk factors for type 2 diabetes, known to have occurred before onset of IPV exposure, as potential confounders in main adjusted models: child physical abuse (indicators for mild, moderate, and severe, as described in detail elsewhere [19]), child sexual abuse (indicators for sexual touching only, forced sex once, and forced sex more than once [19]), race (indicators for African American, Asian, Hispanic, and other, with non-Hispanic white as the referent), mother’s and father’s educational attainment (indicators for <9, 9–11, 12, and 13–15 years, with ≥16 years as the referent), continuous somatogram score at age 5 (the participant could choose one of nine female figures, ranging from very lean, a score of 1, to very obese, a score of 9, that best represented her body type at age 5 years [30]), BMI at age 18 years (indicators for <21, 25–29, and ≥30 kg/m2, with 21–24 kg/m2 as the referent), and parental history of diabetes.
We also present results further adjusted for updated adult lifestyle risk factors for type 2 diabetes that may operate as time-varying confounders and/or as mediators of the IPV–type 2 diabetes association, including physical activity, smoking, alcohol intake, oral contraceptive use, BMI, menopausal status, parity and age at first birth, history of hypertension, history of high cholesterol, marital status, living arrangement, total caloric intake, polyunsaturated-to-saturated fat intake ratio, trans fat intake, cereal fiber intake, and dietary glycemic load over follow-up. We handled missing covariates with missing indicators. We also ran complete case analyses to ensure that the use of missing indicators did not strongly affect our estimates. Results from complete case models and missing indicator models were similar.
Data analysis
Primary analyses: physical, sexual, and psychological IPV.
We used Cox proportional hazards regression to model the incidence of type 2 diabetes as a function of ever-adult physical IPV, ever-adult sexual IPV, and psychological IPV reported for a 2001 intimate partnership. Participants contributed study time from age in 2001, with return of the Violence Questionnaire, through age at the last returned questionnaire, diagnosis of type 2 diabetes, death, or June 2007. We began follow-up for primary analyses in 2001, rather than at 1989 NHSII baseline, because physical, sexual, and psychological IPV were not dated separately, and thus, the temporal relationship between exposure and outcome could not be established before exposure ascertainment in 2001. For each exposure, we ran an age-adjusted model, a child/adolescent confounder-adjusted model, and a model additionally adjusted for time-varying adult lifestyle factors.
Of 68,376 women who responded to the 2001 Violence Questionnaire, we excluded 2,216 who reported a diabetes diagnosis or insulin or oral hypoglycemic use in or before 2001 and 7 women who died before returning the questionnaire on which 2001 baseline diabetes status was assessed. We also excluded 1,421 women missing race, child physical abuse, or child sexual abuse covariates because sparse data resulted in unstable missing indicator estimates. Of the remaining sample of 64,732, 1,296 were missing data on physical IPV and 1,295 were missing data on sexual IPV, leaving 63,436 and 63,437 for physical and sexual IPV analyses, respectively. For analyses of 2001 psychological IPV, we included only those women in an intimate partnership in 2001(n = 53,074).
We examined the possibility of multiplicative interactions between physical, sexual, and psychological IPV exposures and child abuse (coded as any vs. no physical or sexual abuse at age ≤17 years) by using likelihood ratio tests to compare models with adult and child abuse main effect parameters to models with main effect parameters plus interaction terms. We also estimated type 2 diabetes incidence associated with exposure to abuse in childhood but not in adulthood, in adulthood (any physical or sexual IPV or WEB >20) but not in childhood, and in both childhood and adulthood, relative to no abuse in either childhood or adulthood.
Finally, to estimate the public health impact of exposure to physical, sexual, or psychological IPV on type 2 diabetes risk, we estimated the population-attributable risk (PAR), using a macro developed by Spiegelman and colleagues (31). The PAR macro calculates the number of cases in the cohort, given observed exposure and covariate prevalences, then estimates the number of cases expected if the exposure were removed. These quantities are used to estimate the percentage of type 2 diabetes that would be eliminated if IPV were eliminated in the cohort. For PAR analyses, we adjusted for the following subset of child/adolescent confounders: age, child abuse, family history of diabetes, and BMI at age 18 years. The PAR macro requires that relative risks be greater than 1 for all combinations of covariates, and using a subset of covariates allowed us to meet this condition. Other child/adolescent covariates were relatively unimportant confounders (combined, they influenced the exposure–outcome associations by <10%).
Secondary analyses: duration and timing of IPV.
We modeled incidence of type 2 diabetes as a function of time-varying duration of IPV and timing since IPV (“recency”) using Cox proportional hazards models. In contrast to lifetime physical, lifetime sexual, and current psychological IPV exposures, duration and recency of IPV were based on dated reports, allowing us to establish exposure-outcome temporality before 2001 exposure ascertainment. We therefore began follow-up at 1989 NHSII baseline and continued through age at last returned questionnaire, diagnosis of type 2 diabetes, death, or June 2007.
For analyses of duration and recency of IPV, we included the 54,700 participants who responded to both the 2001 and 2008 Violence Questionnaires (ie, those with complete time-varying exposure data through end of follow-up in 2007). We excluded 359 women reporting prevalent diabetes and/or use of hypoglycemics at the 1989 NHSII baseline. We also excluded 1,100 women missing race, child physical abuse, or child sexual abuse covariates, leaving 53,241 women for duration analyses. Recency analyses were additionally restricted to the 17,306 women who reported adult IPV exposure of 1 year or more before 1989, so that the exposure (number of years since last reported IPV) could be defined.
RESULTS
Of 64,732 women meeting 2001 baseline inclusion criteria for primary analyses, 63,436 were included in physical IPV analyses, of which 22% reported at least one incident of physical IPV during their adult life. Of the 63,437 women included in the sexual IPV analyses, 10% reported adult sexual IPV. Of the 53,074 women included in WEB analyses, 90% had a WEB score of less than 20 (considered to be nonabusive [26]), whereas 8% had WEB scores in the moderate psychological IPV range, and fewer than 2% had WEB scores indicating severe psychological IPV. Type 2 diabetes developed in ∼2% of the cohort during the 2001–2007 follow-up.
Of the 53,241 women included in secondary analyses of duration of IPV, 56% reported no IPV in any year from age 18 years through the end of follow-up. Among women reporting at least 1 year of IPV, mean IPV duration was 6 (SD, 7) years at the end of follow-up. Of the 17,030 women included in IPV recency analyses, 30% reported current IPV at the beginning of follow-up in 1989; on average, women entered the study with 5 (SD, 5) years since their most recent IPV report, and ended follow-up with a mean of 15 (SD, 11) years since their most recent IPV exposure.
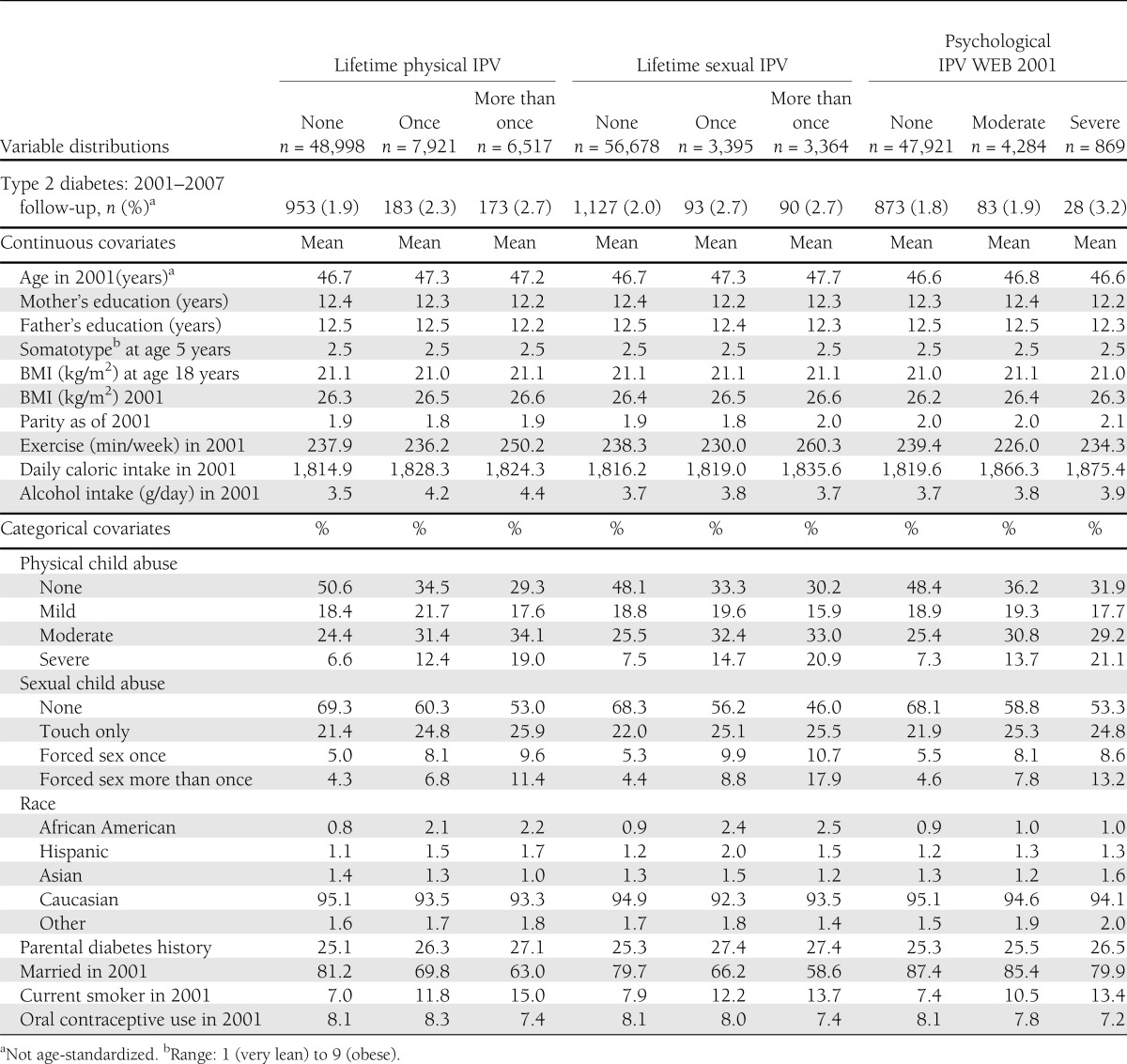
Table 1 presents the distribution of the outcome and covariates across physical, sexual, and psychological IPV exposure categories. Of the child and adolescent covariates examined, child abuse was most strongly related to adult IPV. Women exposed to IPV in adulthood were more likely to be current smokers and less likely to be married in 2001 than IPV-unexposed women.
Table 1.
Age-standardized distribution of covariates (mean value or percentage of sample in each level) and number of incident type 2 diabetes cases, across IPV exposure categories: NHSII, 2001
Physical and sexual IPV results
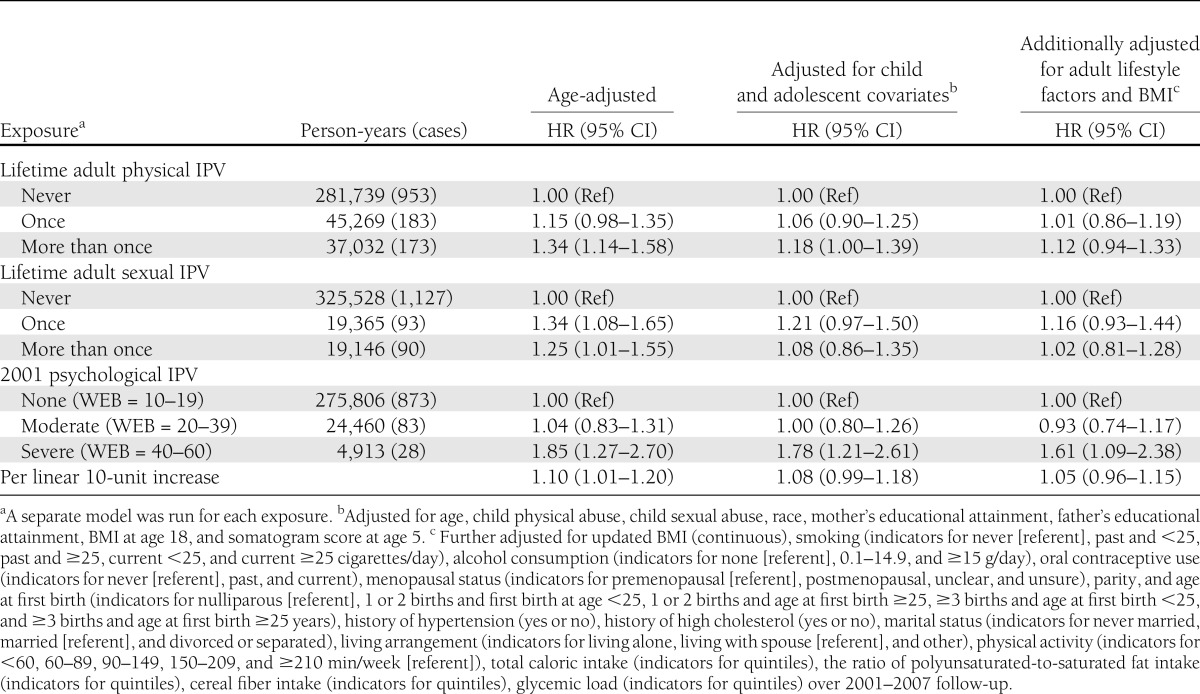
As reported in Table 2, physical and sexual IPV were associated with age-adjusted increases in type 2 diabetes incidence of 15–34%, depending on the type and frequency of IPV. After adjustment for child and adolescent confounders, physical IPV experienced more than once was associated with an 18% increase in type 2 diabetes increase (hazard ratio [HR] 1.18 [95% CI 1.00–1.39]). The estimate for a single sexual IPV experience was of a similar magnitude to physical IPV results, but was not statistically significant (1.21 [0.97–1.50]). Child abuse was the most important confounder in the models. Further adjustment for adult lifestyle factors, which may have been influenced by exposure and may therefore be mediators of the adult IPV–type 2 diabetes association, resulted in a slight additional attenuation of results for physical and sexual IPV. We saw no indication that experiencing both physical and sexual IPV put women at increased risk of type 2 diabetes when compared with experiencing a single IPV type alone (data not shown).
Table 2.
HR estimates for incident type 2 diabetes in relation to physical, sexual, and psychological adult IPV: NHSII with follow-up from 2001 through 2007
Psychological IPV results
In child/adolescent confounder-adjusted models, a 10-unit increase in the WEB score was linearly associated with a type 2 diabetes HR of 1.08 (95% CI 0.99–1.18; test for nonlinearity P = 0.13). Results using indicator variables suggested that only severe psychological IPV is related to type 2 diabetes incidence, with a HR of 1.00 (0.80–1.26) for moderate psychological IPV and 1.78 (1.21–2.61) for severe psychological IPV. Additional adjustment for adult lifestyle factors decreased the HR to 1.61 (1.09–2.38) for severe psychological IPV.
Duration of IPV results
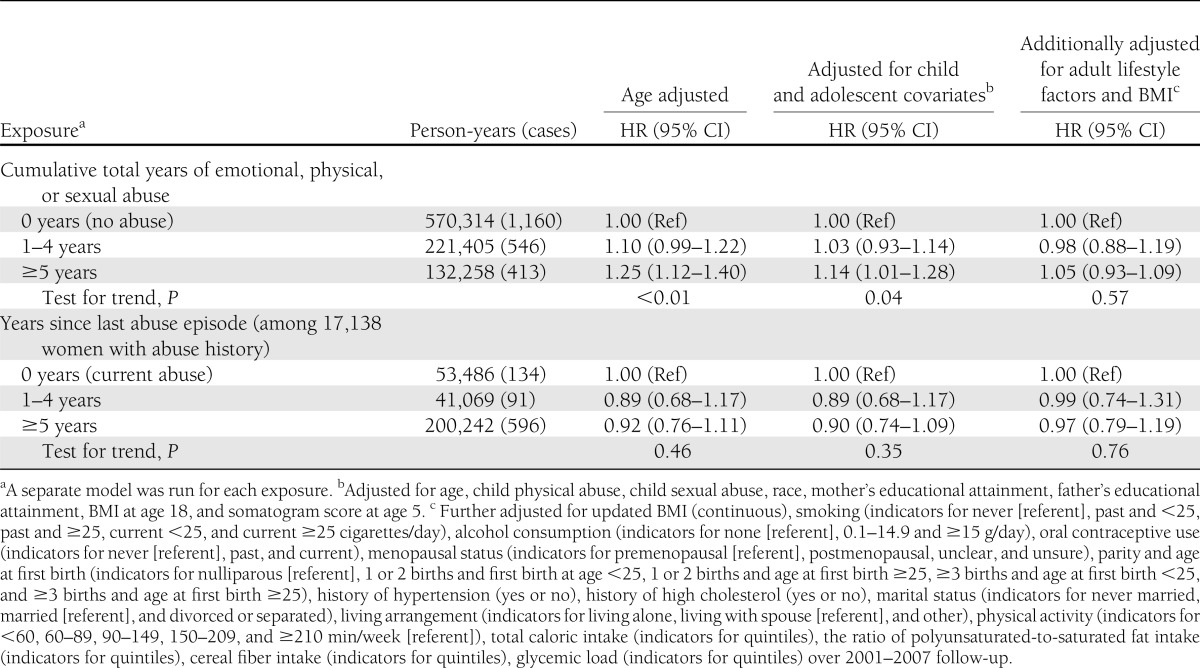
Estimates of the effect of IPV duration on type 2 diabetes incidence (Table 3) suggested that 5 or more cumulative years of exposure to physical, sexual, and/or emotional IPV in adulthood is associated with a modest increase in type 2 diabetes risk (child/adolescent confounder-adjusted HR 1.14 [95% CI 1.01–1.28]). Adjusting for adult lifestyle factors reduced this estimate to 1.05 (0.93–1.09). Once child and adolescent confounders were taken into account, 1 to 4 accumulated years of IPV did not appear to increase type 2 diabetes risk.
Table 3.
HR estimates for incident type 2 diabetes in relation to cumulative total number of years in which any physical, sexual, or emotional abuse was reported and number of abuse-free years since most recent abuse report: NHSII with follow-up from 1989 through 2007
Recency of IPV results
When compared with having experienced IPV in the past year, having been free of IPV for 1 or more years was associated with a suggestive but statistically nonsignificant ∼10% reduction in type 2 diabetes risk after adjustment for child and adolescent covariates (Table 3).
There was no statistical evidence for interactions between any of our adult IPV variables and child abuse. However, estimates of the association between simplified child and adult abuse combinations and type 2 diabetes suggest that exposure to abuse in both childhood and adulthood may be uniquely detrimental: adjusted HRs were 1.06 (95% CI 0.90–1.25) for child abuse alone, 1.00 (0.75–1.33) for adult IPV (physical IPV, sexual IPV, or WEB score >20) alone, and 1.34 (1.12–1.59) for child abuse plus adult IPV.
We estimated the proportion of type 2 diabetes risk in the study cohort attributable to lifetime exposure to physical, sexual, or psychological IPV to be 6% (95% CI 1–11%).
CONCLUSIONS
In this analysis, we found modest 15–20% increases in type 2 diabetes incidences associated with physical IPV in adulthood and with 5 or more years of any physical, sexual, or emotional IPV. Increases in risk associated with adult sexual IPV were of similar magnitude but were not statistically significant. Recent severe psychological IPV, as measured by the WEB score, was associated with an almost 80% increased risk of type 2 diabetes over follow-up when compared with no psychological IPV. This association is similar in magnitude to the 54% increase in type 2 diabetes incidence associated with severe child physical abuse and the 69% increase associated with severe child sexual abuse found previously in this cohort (19).
Our study shares certain limitations with most studies to date of health effects of abuse. In particular, we relied on women’s self-report of their IPV experiences, which could not be validated. Because IPV goes under-reported, it is unclear what gold standard should be used to validate self-reports of IPV. Using cases of IPV that are externally identified (e.g., by a clinician) would ensure more specificity of exposure classification but would likely identify only the most severe cases of abuse and would miss most of the exposed population. In addition, the strongest effect we observed was for severe psychological IPV, which may not be readily identified if it is not accompanied by physical violence. Although we could not validate self-reports of IPV, the prevalence in our study is similar to the IPV prevalence self-reported on national surveys. For example, the proportion of women reporting at least one episode of physical IPV in adulthood was 22% in our cohort, 22% in the 1995–1996 National Violence Against Women Survey (11), and 24% in the 2010 National Intimate Partner and Sexual Violence Survey (12).
We lacked specific information on the timing of physical, sexual, and psychological IPV separately. Our analyses of time-varying duration and recency of “any IPV” are strengths of this study; however, we were not able to investigate the effect of the duration and recency of a specific type of IPV on the risk of type 2 diabetes. The broad “any IPV” umbrella included any reported emotional, physical, and sexual IPV; if one type of abuse is less relevant to type 2 diabetes risk than the others, then its inclusion might have diluted our duration and recency of IPV results. Likewise, physical and sexual abuse were ascertained using single questions, which may not have captured the most important aspects of the abuse experience. Our null-to-modest results may therefore stem from measurement error rather than a lack of a true association between physical and sexual abuse and type 2 diabetes.
Finally, generalizability of our results may be limited. Assessments of generalizability suggest that results from our nurse population are similar to those in other white female samples (32). Additional studies in more ethnically diverse populations would enrich understanding of IPV effects on health.
To our knowledge, this is the first longitudinal study to examine IPV in adulthood as a risk factor for type 2 diabetes. The importance of stress in the development of chronic disease is garnering increasing attention, and the lack of rigorous study of interpersonal violence is a major gap in the literature, given the frequency with which women experience abuse in their intimate relationships. In addition to its substantive contribution, our study has several strengths, including a large prospective cohort and rich data on important covariates such as child abuse history. In addition, our outcome, type 2 diabetes, is accurately determined in the NHSII.
Our findings raise questions about the mechanisms by which IPV may influence type 2 diabetes risk. We hypothesized that adult BMI, which appears to be an important mediator of the child abuse–type 2 diabetes association (19), may play a role in the link between adult IPV and type 2 diabetes. Associations between IPV and BMI have been noted in at least two studies (33,34). However, we found no association between physical, sexual, or psychological IPV and adult BMI in our cohort, once child abuse was taken into account. Depression may also be a mediator of the IPV–type 2 diabetes association, but lack of data on the timing of depression incidence in our cohort prevented us from examining this hypothesis in detail. The strong association between psychological violence and type 2 diabetes may indicate that psychological appraisal of stress plays a role in the IPV–type 2 diabetes relationship. However, differences in timing of psychological IPV (ascertained for a current intimate partnership) and physical and sexual IPV (ascertained as lifetime variables) prevents a strong inference about the relationship between IPV type and type 2 diabetes risk. Other potential mechanisms that might be considered for future research include a link of abuse with hypercortisolemia and insulin resistance.
During the past several decades, type 2 diabetes has become a major contributor to disability and death in the U.S. (35). Our study results indicate that recent exposure to severe psychological IPV may have an important influence on type 2 diabetes incidence. These findings provide additional support for recent recommendations in favor of IPV screening in clinical care (36). Taken together, we estimate that exposure to physical, sexual, or psychological IPV accounts for ∼6% of the type 2 diabetes incidence in this population. The impact of IPV on long-term health may be even greater in populations where severe IPV is more prevalent (37). Understanding how IPV, experienced by more than a quarter of American women, influences the development of type 2 diabetes may shed light on the ways that stress influences health outcomes more generally and may help to characterize the unique factors important to women’s health and well-being.
Acknowledgments
This project was supported by National Institutes of Health Grants RO1-HL-081557 and RO1-HL-064108.
No potential conflicts of interest relevant to this article were reported.
S.M.M. conducted analyses, wrote the manuscript, and approved the final version of the manuscript. R.J.W., H.-J.J., and F.B.H. collected data, provided critical revisions, and approved the final version of the manuscript. E.N.H. conducted analyses, provided critical revisions, and approved the final version of the manuscript. D.S. provided guidance on statistical analysis, provided critical revisions, and approved the final version of the manuscript. J.W.R.-E. collected data, wrote the manuscript, and approved the final version of the manuscript. S.M.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1082/-/DC1.
References
- 1.Allan R, Scheidt S. Stress, anger, and psychosocial factors for coronary heart disease. In Prevention of Myocardial Infarction. Manson JE, Ridker PM, Gaziano JM, et al. , Eds. New York, Oxford University Press, 1996, p. 274–299 [Google Scholar]
- 2.Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med 1996;334:413–419 [DOI] [PubMed] [Google Scholar]
- 3.Rosengren A, Hawken S, Ounpuu S, et al. INTERHEART investigators Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:953–962 [DOI] [PubMed] [Google Scholar]
- 4.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999;99:2192–2217 [DOI] [PubMed] [Google Scholar]
- 5.House JS, Landis KR, Umberson D. Social relationships and health. Science 1988;241:540–545 [DOI] [PubMed] [Google Scholar]
- 6.Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation 2005;111:480–487 [DOI] [PubMed] [Google Scholar]
- 7.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 2006;113:1888–1904 [DOI] [PubMed] [Google Scholar]
- 8.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 2004;291:1978–1986 [DOI] [PubMed] [Google Scholar]
- 9.Krentz AJ. Type 2 diabetes and atherosclerotic cardiovascular disease: do they share common antecedents? Br J Diabetes Vasc Dis 2002;2:370–378 [Google Scholar]
- 10.Kumari M, Head J, Marmot M. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med 2004;164:1873–1880 [DOI] [PubMed] [Google Scholar]
- 11.Tjaden P, Thoennes N. Prevalence, Incidence, and Consequences of Violence Against Women: Findings From the National Violence Against Women Survey. Washington, DC, US Department of Justice, Office of Justice Programs, 2000 [Google Scholar]
- 12.Black MC, Basile KC, Breiding MJ, et al. The National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, 2011. [Google Scholar]
- 13.Riley EH, Wright RJ, Jun HJ, Hibert EN, Rich-Edwards JW. Hypertension in adult survivors of child abuse: observations from the Nurses’ Health Study II. J Epidemiol Community Health 2010;64:413–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med 2004;34:509–520 [DOI] [PubMed] [Google Scholar]
- 15.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008;31:2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engum A. The role of depression and anxiety in onset of diabetes in a large population-based study. J Psychosom Res 2007;62:31–38 [DOI] [PubMed] [Google Scholar]
- 17.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 2006;49:837–845 [DOI] [PubMed] [Google Scholar]
- 18.Noll JG, Zeller MH, Trickett PK, Putnam FW. Obesity risk for female victims of childhood sexual abuse: a prospective study. Pediatrics 2007;120:e61–e67 [DOI] [PubMed] [Google Scholar]
- 19.Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, et al. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med 2010;39:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong M, Giles WH, Felitti VJ, et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation 2004;110:1761–1766 [DOI] [PubMed] [Google Scholar]
- 21.Kyrou I, Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol 2009;9:787–793 [DOI] [PubMed] [Google Scholar]
- 22.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998;14:245–258 [DOI] [PubMed] [Google Scholar]
- 23.Kendall-Tackett KA, Marshall R. Victimization and diabetes: an exploratory study. Child Abuse Negl 1999;23:593–596 [DOI] [PubMed] [Google Scholar]
- 24.Breiding MJ, Black MC, Ryan GW. Chronic disease and health risk behaviors associated with intimate partner violence-18 U.S. states/territories, 2005. Ann Epidemiol 2008;18:538–544 [DOI] [PubMed] [Google Scholar]
- 25.Smith PH, Tessaro I, Earp JA. Women’s experiences with battering: a conceptualization from qualitative research. Womens Health Issues 1995;5:173–182 [DOI] [PubMed] [Google Scholar]
- 26.Smith PH, Earp JA, DeVellis R. Measuring battering: development of the Women’s Experience with Battering (WEB) Scale. Womens Health 1995;1:273–288 [PubMed] [Google Scholar]
- 27.Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. Harvard School of Public Health. The SAS LGTPHCURV9 Macro. Available from http://www.hsph.harvard.edu/faculty/donna-spiegelman/files/lgtphcurv9_7-3-2011.pdf Accessed 11 March 2012
- 28.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 29.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 2001;161:1581–1586 [DOI] [PubMed] [Google Scholar]
- 30.Stunkard AJ, Sorensen T, Schulsinger F. The Genetics of Neurological and Psychiatric Disorders. New York, Raven Press, 1983 [Google Scholar]
- 31.Hertzmark E, Wand H, Spiegelman D; Channing Laboratory, Harvard School of Public Health. The SAS PAR Macro. Available from http://www.hsph.harvard.edu/faculty/donna-spiegelman/files/par_documentation-_march_2012.pdf Accessed 11 March 2012
- 32.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6:49–62 [DOI] [PubMed] [Google Scholar]
- 33.Newton TL, Fernandez-Botran R, Miller JJ, Lorenz DJ, Burns VE, Fleming KN. Markers of inflammation in midlife women with intimate partner violence histories. J Womens Health (Larchmt) 2011;20:1871–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yount KM, Li L. Domestic violence and obesity in Egyptian women. J Biosoc Sci 2011;43:85–99 [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association. National Diabetes Fact Sheet, 2011. Available from http://www.diabetes.org/diabetes-basics/diabetes-statistics/ Accessed 13 January 2012
- 36.Nelson HD, Bougatsos C, Blazina I. Screening women for intimate partner violence: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med 2012;156:796–808 [DOI] [PubMed]
- 37.Hassan F, Sadowski LS, Bangdiwala SI, et al. Physical intimate partner violence in Chile, Egypt, India and the Philippines. Inj Control Saf Promot 2004;11:111–116 [DOI] [PubMed] [Google Scholar]


