Abstract
OBJECTIVE
To examine the BMI-stratified associations between diabetes and the risks of all-cause death, cardiovascular disease (CVD) death, and cancer death.
RESEARCH DESIGN AND METHODS
Using a prospective study with 12 rural Japanese general populations (n = 3,641, mean age, 53.7 years; 33.5% men), we examined the associations between diabetes and the risk of all-cause death, CVD death, and cancer death. We also examined the effects of BMI and age on such associations.
RESULTS
During an average duration of 10.2 years (37,278 person-years), 240 deaths occurred (54 deaths from CVD, 101 from cancer, and 85 from other causes). Cox regression analysis showed leanness (defined as the lowest quartile of entire BMI; mean, 19.5 kg/m2), but not obesity (BMI ≥25 kg/m2), and diabetes were independently associated with an increased risk of all-cause death (hazard ratio [HR] 1.70 and 1.65, respectively; both P < 0.01.). Stratification with cause-specific deaths showed that leanness and obesity were associated with CVD death (HR 3.77 and 2.94, respectively), whereas diabetes was associated with cancer death (HR 1.87; all P < 0.05). The increased risk of all-cause death in diabetes was substantially higher in lean subjects aged <65 years (HR 3.4) or those aged ≥65 years (HR 4.2), whereas the risk in obese diabetes patients was significant only in subjects aged <65 years (HR 2.32; all P < 0.05).
CONCLUSIONS
Among the Japanese general population, diabetes confers an increased risk of all-cause death. Particular attention must be paid to the pronounced high mortality in diabetes accompanied with leanness, regardless of age.
Diabetes is one of the fastest-growing public health problems in the world. Its prevalence is particularly advancing in Asia-Pacific regions, where more than half of diabetes patients reside and where nearly half of all deaths from cardiovascular disease (CVD) occur worldwide (1–4).
Diabetes increases the risk of premature death in the general population. Some studies have reported that the effect of diabetes on death can be augmented by obesity (5,6), whereas other studies have reported that the association is augmented by leanness (7–9). These data, however, have been derived from Western populations, and thus little evidence is available about the effects of BMI on the associations between diabetes and all-cause death in Asians.
Several distinctive features are apparent among the pathogenetic factors for diabetes and its complication in Asian populations compared with Western populations (2,3). Although most patients with diabetes are overweight or obese in Western populations, diabetes in lean patients is highly prevalent in Asian countries, where more than half of diabetic patients are considered normal weight (BMI <25 kg/m2). Moreover, in contrast to Western populations, in which obesity has been shown to have an adverse effect on mortality (10,11), a recent large pooled analysis of 19 cohorts in Asia showed that leanness (i.e., underweight), rather than obesity, was associated with a substantially increased risk of death in Asian populations (12). Accordingly, precise estimates of the association between diabetes and the risk of death in Japan, including data on whether the association is augmented by obesity and/or leanness, are a critical prerequisite for informed decisions about strategies for the prevention and control of diabetes-related mortality.
Accordingly, we used a 10-year prospective study among 12 rural Japanese general populations to examine the associations between diabetes and the risks of all-cause death, CVD death, and cancer death. We also examined the effects of BMI and age on the associations between the diabetes and these risks.
RESEARCH DESIGN AND METHODS
The Jichi Medical School (JMS) Cohort Study is a prospective, population-based study aimed at exploring the risk factors for CVD in 12 communities in Japan. Details regarding the JMS Cohort Study design and additional descriptive data are available in the Supplementary Data or in our previous reports (13–15). Enrollment into the JMS Cohort Study and baseline data collection were performed between April 1992 and November 1993. A total of 12,490 subjects (39.3% male [n = 4,911]), who were a mean ± SD age of 55.3 ± 11.6 years, participated in the current study. Of these, 12,393 (99.2%) gave us written informed consent to be prospectively followed up for study purposes and complete follow-up was achieved for 12,388 (99.9%).
Glucose parameters (i.e., plasma glucose levels and hemoglobin Alc [HbA1c]) and responses to a self-administered questionnaire documenting their medical history of diabetes were available for 3,727 subjects (33.2% male [n = 1,240]), who were a mean age of 53.8 ± 12.0 years. We excluded 86 participants who had insufficient data for at least one clinical parameter of age, sex, BMI, systolic or diastolic blood pressure (BP), habitual smoking, information on medical history of hypertension, myocardial infarction, stroke, and cancer, or data of circulating lipid parameters. Ultimately, data from 3,641 subjects were analyzed in the current study (Supplementary Fig. 1). The 3,641 subjects who were included in the present analysis were showed a younger age (53.7 ± 12.1 vs. 55.9 ± 11.4 years; P < 0.001) and a lower prevalence of men (33.5% [n = 1,220] vs. 41.7% [n = 3,649]; P < 0.001) compared with the 8,747 who were excluded from the analysis.
Measurements of baseline variables
To synchronize the methods of data collection, we established a central committee composed of the chief medical officers from the participating districts. This committee developed a detailed manual for data collection. Information about lifestyle and medical history was gathered by means of a written questionnaire. In some subjects, information about physical activity (n = 3,615 [99%]), educational level (n = 3,601 [99%]), and marital status (n = 3,616 [99%]) were also obtained (Supplementary Data). BMI was calculated as weight (kg)/height (m2). Systolic and diastolic BP were measured with a fully automated sphygmomanometer, BP203RV-II (Nippon Colin, Komaki, Japan), which was placed on the right arm of a subject who had rested in a sitting position for 5 min before measurement. Hypertension was defined as systolic BP/diastolic BP ≥140/90 mmHg or self-reported usage of antihypertensive medication.
Blood samples were drawn from the antecubital vein of seated subjects, with minimal tourniquet use (details are described in the Supplementary Data). Blood samples of 1,344 subjects (36.9%) were drawn after overnight fasting. Total cholesterol and triglycerides were measured using an enzymatic method (Wako; interassay coefficient of variation [CV], 1.5% for total cholesterol and 1.7% for triglyceride). HDL cholesterol was measured using the phosphotungstate precipitation method (Wako; interassay CV, 1.9%). Blood glucose was measured via an enzymatic method (Kanto Chemistry; interassay CV, 1.9%), and the value for HbA1c was estimated as a National Glycohemoglobin Standardization Program equivalent value calculated with the following formula (16): HbAlc (%) = HbAlc (Japan Diabetes Society) (%) + 0.4%. These laboratory data were measured concurrent with sample collection.
Definition of leanness, obesity, and diabetes
The Japan Society for the Study of Obesity defines obesity as a BMI ≥25 kg/m2 and leanness as a BMI <18.5 kg/m2 (2,17) therefore, in the current study, we defined obesity as BMI ≥25 kg/m2 (mean 27.0 ± 2.4 kg/m2; n = 1,196). However, if we had defined leanness as BMI <18.5 kg/m2, only 160 subjects (4% of total patients) would qualify. Thus, in the current study, we defined leanness as the lowest quartile of BMI (range 14.2–21.1; mean 19.5 ± 1.2 kg/m2; n = 910). As a consequence, we defined normal BMI as BMI ranging from the second quartile of BMI to 25 kg/m2 (mean 22.7 ± 1.0 kg/m2; n = 1,535).
Diabetes was defined in accordance with the American Diabetes Association guidelines (18) as a fasting glucose concentration of 126 mg/dL or higher, casual blood glucose concentration of 200 mg/dL or higher, HbA1c of 6.5% or higher, or self-reported use of antihyperglycemic drugs.
End point
As described in previous reports (13–15), mortality data from the date of entry to 31 December 2002 were collected from the Cause-of-Death Register at public health centers in each community with the permission of the Agency of General Affairs and the Ministry of Health, Labor, and Welfare. The follow-up period was 10.2 ± 2.1 years (37,278 person-years). Information on the cause of death was coded for participants who died using ICD-10 codes. Causes of death were classified as follows: 1) CVD death: heart disease including sudden death (I21–I23, I46, I48–I50, Q20–Q28), CVD (I60, I61, I63, I69), and other CVD (I71); 2) cancer death (C02, C10, C14–C20, C22–C26, C30, C34, C41, C50, C53, C54, C61, C64, C65, C71, C74, C76, C81–C85, C90–C93); and 3) other causes, such as infection and suicide (A41, B15–19, D65, G12, G21, G93, J10–J18, J43, J84, J96, K72, M62, N00–N08, R57, R54, R64, S06, T58, X60–X84, Y85–Y87, W75–W84).
Statistical analysis
All statistical analyses were performed with SPSS 18.0J software (SPSS Inc, Chicago, IL). Clinical parameters in subjects with or without death were compared using the unpaired t test, and categorical parameters were compared with the χ2 test. Next, we used Cox regression analysis to examine the independent effects of diabetes or BMI on the risk of all-cause death. After adjusting for significant covariates, such as age, sex, current smoking status, and systolic BP values, the hazard ratios (HR) and 95% CIs were calculated for all-cause death, CVD death, or cancer death in subjects with diabetes or leanness (obesity).
Finally, our population was subdivided into six categories according to BMI (leanness, normal BMI, and obesity) and the presence of diabetes, and the HR (95% CI) of all-cause deaths in each of the six categories was calculated. In that analysis, to examine whether the association among the six categories and all-cause death differed between middle-aged/younger individuals and older individuals, we used a Cox regression analysis separately in subjects aged <65 and ≥65 years. This analysis included significant covariates for adjusted variables, such as sex, current smoking status, systolic BP values, and pre-existing myocardial infarction, stroke, or cancer. We then performed additional adjustments for each of the following possible confounders: physical activity (n = 3,615), educational level (n = 3,601), and marital status (n = 3,616). Finally, using a Cox regression analysis, we examined whether there were any interactions between diabetes and BMI in the risk of all-cause death separately in those aged <65 years and those ≥65 years. A 2-sided P value <0.05 was defined as statistically significant.
RESULTS
Baseline clinical characteristics
The mean ± SD age of the 3,641 subjects was 53.7 ± 12.1 years, and 1,220 (33.5%) were men. At the time of study recruitment, there were 507 patients (13.9%) defined as having diabetes, 1,277 (35.1%) with hypertension, 58 (1.6%) with a pre-existing myocardial infarction or stroke, and 43 (1.2%) with a pre-existing cancer.
Leanness, diabetes, and all-cause deaths
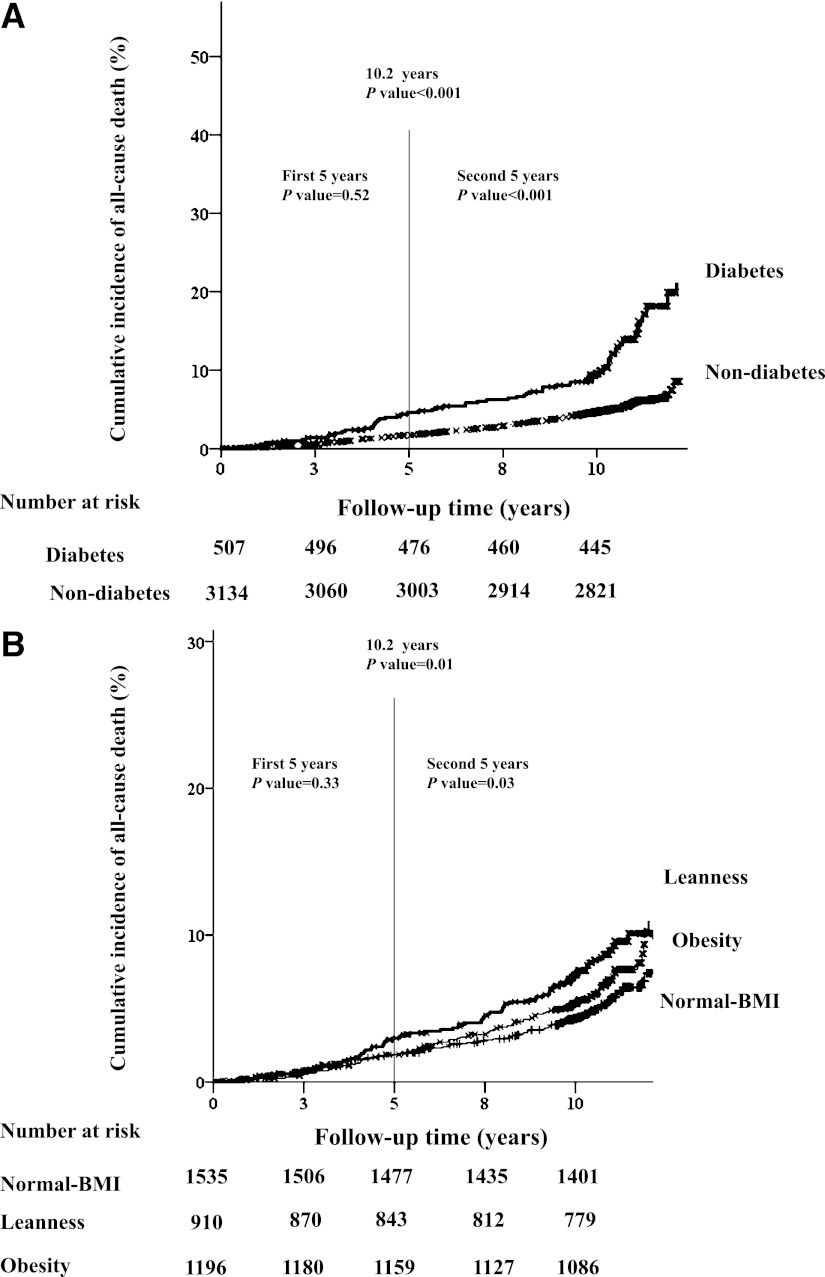
During an average duration of 10.2 ± 2.1 years (37,278 person-years), 240 deaths occurred (6.4 events/1,000 person-years), including 54 CVD deaths, 101 cancer deaths, and 85 other-cause deaths (e.g., infection, suicide, accident). The baseline clinical characteristics according to the incidence of all-cause death are reported in Table 1. The prevalence of leanness or diabetes was higher in subjects with death than in those without death. The crude incidence rate of all-cause death was 13.0 events/1,000 person-years for diabetes and 5.4 events/1,000 person-years for nondiabetes. By comparison, the crude incidence rate per 1,000 person-years of all-cause death was 8.4 events in lean subjects, 5.2 events in subjects with normal BMI, and 6.5 events in obese subjects. The prevalence of cause-specific death subdivided by the presence of diabetes or BMI is reported in Supplementary Tables 1 and 2. Kaplan-Meier curves showing cumulative all-cause death according to the presence of diabetes or the classification of BMI are shown in Fig. 1A and B. There was no significant difference in the rate of all-cause death among the subjects subdivided by the presence of diabetes or BMI in the first 5-year period, whereas the differences were significant for the second 5-year period (both P < 0.05 by log-rank test).
Table 1.
Baseline clinical characteristics of the study population according to the occurrence of death
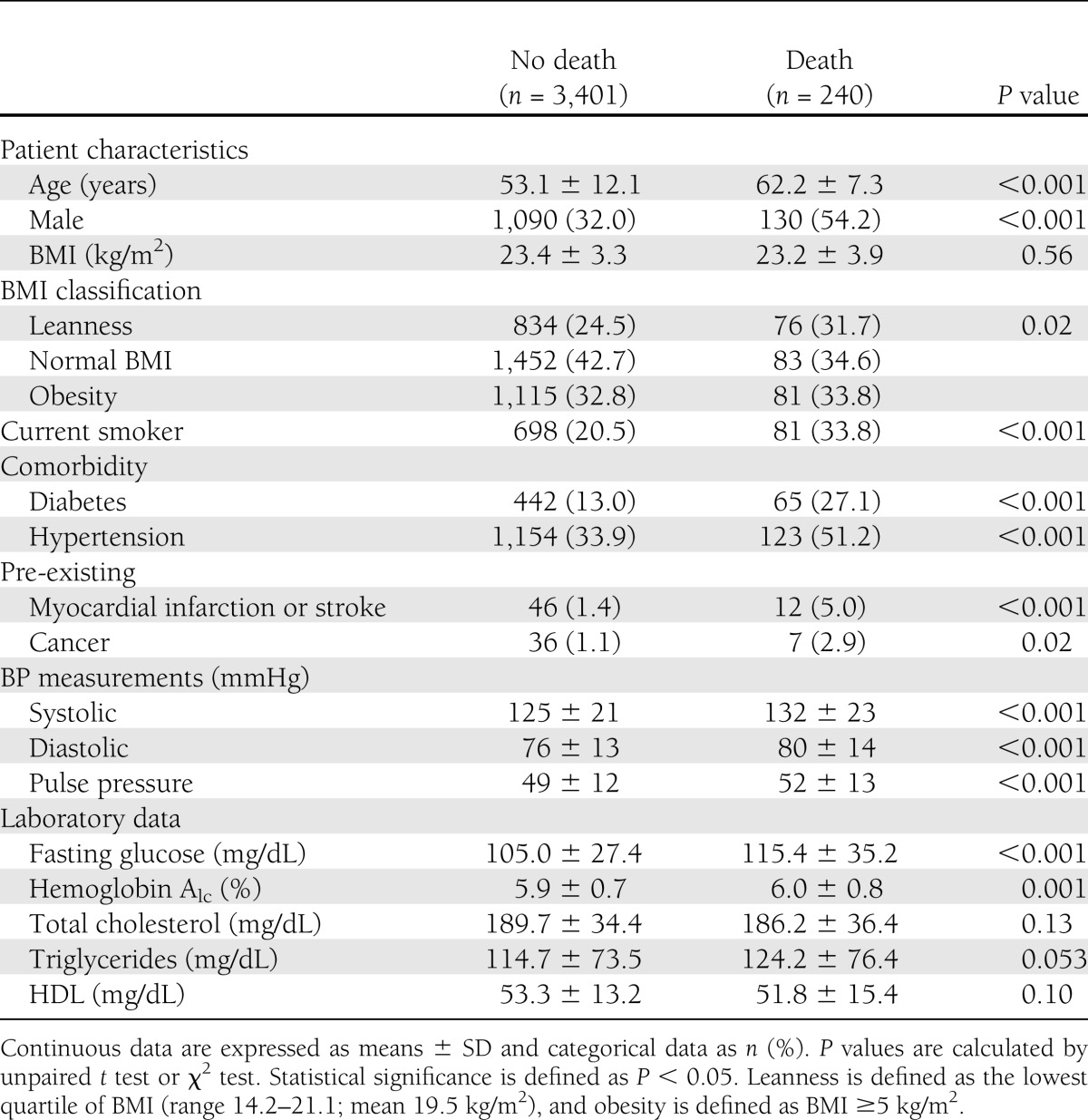
Figure 1.
Kaplan-Meier curves show the cumulative incidence of all-cause death by the presence of diabetes vs. nondiabetes (A), or the classification of BMI by leanness or obesity vs. normal BMI (B). P values were calculated using log-rank test.
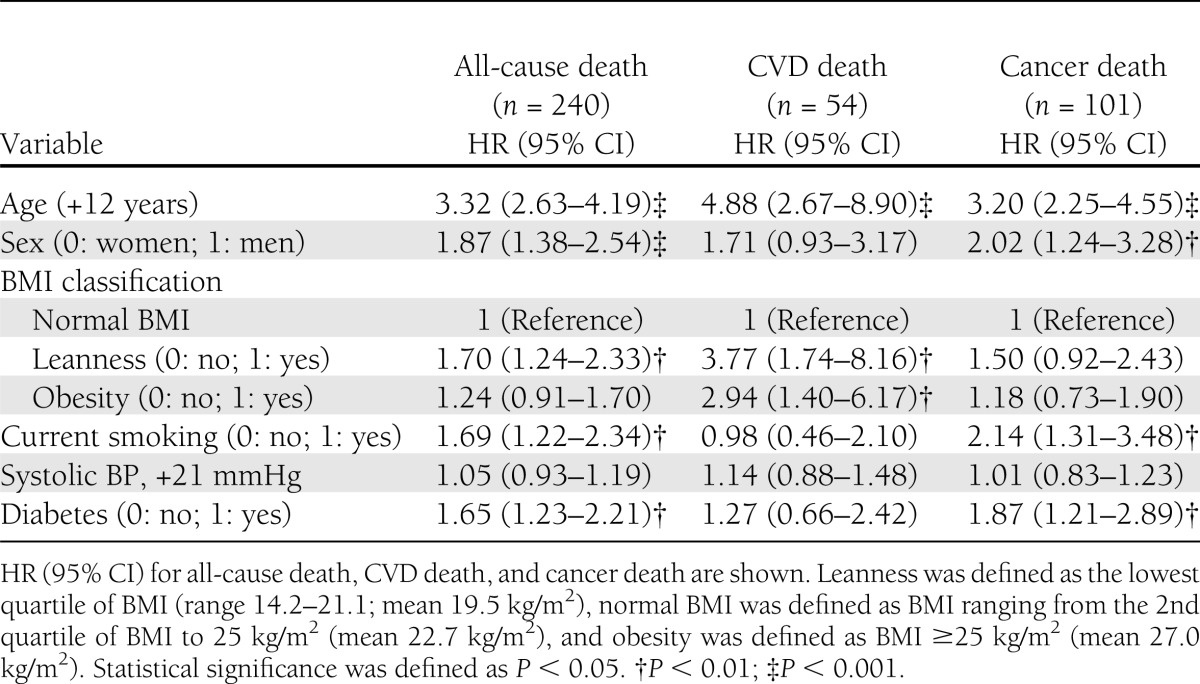
Next, the HR (95% CI) of all-cause death associated with leanness (obesity) or diabetes was calculated using Cox regression analysis (Table 2). The HR (95% CI) of all-cause death in subjects with leanness, but not obesity, and diabetes were significant, even after adjustment for significant covariates. Stratification with cause-specific deaths showed that leanness and obesity were both associated with an increased risk of CVD death, whereas diabetes was associated with an increased risk of cancer death. When we defined obesity as the highest quartile of BMI (≥25.3 kg/m2; mean 27.7 kg/m2, n = 910) instead of BMI ≥25 kg/m2, the risk of CVD death in obesity remained unchanged (data not shown). Furthermore, when we examined the male-to-female differences in the risk of all-cause death as well as cancer death in diabetes, we found that this conclusion remained unchanged (Supplementary Tables 3 and 4).
Table 2.
Cox regression analysis for all-cause death, CVD death, and cancer death in the total population (n = 3,641)
The increased risk of all-cause death in leanness or diabetes (Table 2) did not change when the 66 subjects who died within 2 years of follow-up were excluded (data not shown). After exclusion of 58 subjects with a pre-existing myocardial infarction or stroke and 43 with cancer at baseline, associations of leanness or diabetes with all-cause death remained significant (data not shown). When we adjusted for various confounding factors, such as physical activity, educational level, and marital status in the associations between diabetes or leanness and all-cause death, the risk of diabetes or leanness remained unchanged (data not shown).
Effects of BMI or age on the associations between diabetes and all-cause death
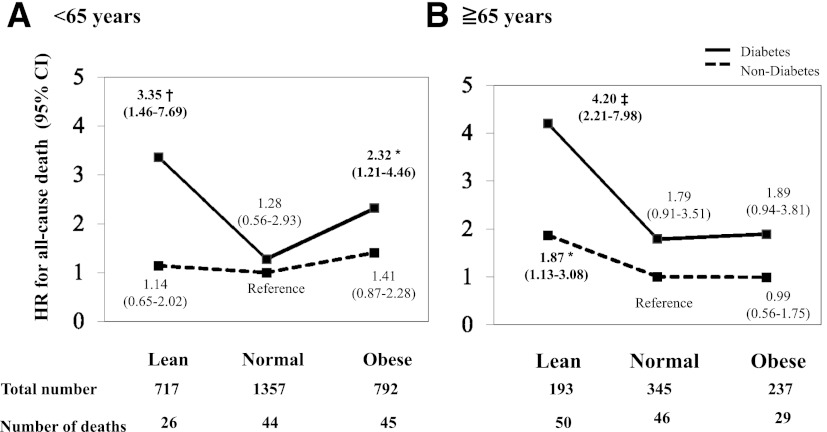
Among 2,866 subjects aged <65 years, the risk of all-cause death conferred by diabetes, compared with nondiabetes with normal BMI (reference), increased when diabetes was accompanied by leanness or obesity (Fig. 2A). In contrast, among the 775 subjects aged ≥65 years, the risk of all-cause death conferred by diabetes, compared with nondiabetes with normal BMI, increased when the diabetes was accompanied by leanness but not by obesity (Fig. 2B). We repeated the analysis after excluding subjects who died within the initial 2 years of follow-up or those who had a pre-existing myocardial infarction, stroke, or cancer at baseline, and the conclusions remained unchanged (data not shown). Furthermore, we performed additional adjustments for each of the following possible confounders in Fig. 2: physical activity, educational level, and marital status. The conclusions remained unchanged (data not shown).
Figure 2.
Cox regression analysis of all-cause death separately by BMI or age. The HR (95% CI) of all-cause deaths according to the presence of diabetes and BMI are shown separately in those aged <65 years (A) and those ≥65 years (B). The analysis was adjusted for sex, current smoking status, systolic BP values, and pre-existing myocardial infarction, stroke, or cancer. The reference group was defined as nondiabetes with normal BMI. Statistical significance was defined as P < 0.05. *P < 0.05 vs. reference group, †P < 0.01 vs. reference group, and ‡P < 0.001 vs. reference group.
Finally, using a Cox regression analysis (including sex, current smoking status, systolic BP values, and pre-existing myocardial infarction, stroke, or cancer), we examined whether there was an interaction between diabetes and BMI in the risk of all-cause death separately in those aged <65 years and those ≥65 years. As a result, we found a significant interaction between diabetes and leanness only in subjects aged <65 years (P = 0.047).
CONCLUSIONS
In the present 10-year prospective study in a rural Japanese general population, we have demonstrated for the first time the associations between diabetes, BMI, and the risk of death. The main findings of the current study are that 1) leanness, but not obesity, and diabetes were independently associated with an increased risk of all-cause death; 2) leanness and obesity were both associated with an increased risk of CVD death, whereas diabetes was associated with cancer death; and 3) lean diabetic subjects had a substantially high risk of all-cause death regardless of age, whereas obese diabetic subjects showed increased risk only in those aged <65 years. These findings provide opportunities for a careful evaluation of the risks accompanying diabetes that can be modified by BMI or age.
Excess mortality in diabetes
Most of the literature regarding diabetes and excess mortality has been derived from Western populations (19,20), and there are few prospective studies on mortality and cause of death from diabetes in Japan. The recent substantial increase in rates of diabetes in Asian countries (2,3) highlights the need to better understand the mortality related to diabetes and to identify high-risk subjects in order to prevent excess mortality from diabetes within each Asian country. In the current study, diabetes almost doubled the incidence of all-cause death compared with nondiabetes, and diabetes increased the risk of all-cause deaths by 65%, even after adjustment for significant covariates (Table 2).
Contrary to previous reports from Western populations (21,22), we observed that CVD mortality was not significantly greater in diabetes than nondiabetes. Because the number of CVD death was small, this issue merits further analysis. Nevertheless, a direct comparison between diabetes in Japanese in Caucasians showed that the CVD mortality is threefold higher in Caucasians (23). Moreover, a World Health Organization multinational study demonstrated that Japanese cohorts showed a very low excess mortality from CVD death compared with Western populations (24). These findings indicate that the association of diabetes with CVD mortality may differ between Japanese and Western populations; further studies on a large cohort from a Japanese general population will be required to clarify that issue.
Diabetes and cancer are common conditions, and their codiagnosis in the same individual is not infrequent (25). In a previous Japanese cohort study, diabetes was associated with an increase in the risk of total cancer incidence of 27% in men and 21% in women (26). We also demonstrated that cancer death was higher in diabetes compared with nondiabetes without sex differences (Supplementary Tables 3 and 4). It is not easy to differentiate whether diabetes causes cancer or whether risk factors for diabetes, such as obesity and physical inactivity, are associated with cancer. However, our data showed that an increased risk of cancer mortality in diabetes remained unchanged even after adjustment for significant covariates, including obesity or physical activity. By site, emerging data support higher risks of death from cancers of the liver, pancreas, and colon among adults with diabetes, whereas the evidence with other malignancies is equivocal (25–27). There were not enough cancer deaths in the current study to analyze the association between diabetes and cancer death by tumor site (Supplementary Tables 1 and 2).
The biological plausibility of the associations between diabetes and cancer has tended to be site-specific but may partly be the result of diabetic metabolic and hormonal alternations as well as their underlying common biological mechanisms such as hyperinsulinemia, abnormal production of adipocytokines and growth factors, and epigenetic changes (25). Because our data showed only cancer deaths, but not cancer incidences, we cannot exclude the possibility that a diagnosis of diabetes or poor glycemic control can lead to poor outcomes among subjects who developed cancer during the follow-up period (28). Further studies are warranted to quantify the specific impact of diabetes on cancer incidence versus cancer survival.
Effects of age and BMI on the association between diabetes and all-cause death
A recent meta-analysis from Western populations demonstrated the “obesity paradox” in diabetes and the importance of assessing nonobese (BMI <25 kg/m2) diabetes as being high-mortality cases (9). We demonstrated that, compared with nondiabetes with normal BMI, lean diabetes showed a substantially higher risk of all-cause death in subjects aged <65 years and also those aged ≥65 years. Especially in subjects aged <65 years, a significant interaction was found between diabetes and leanness in the risk of all-cause death. Obese diabetes, by contrast, showed an elevated risk only in subjects aged <65 years (Fig. 2), a finding similar to the previous findings of Nilsson et al. (29) that obese diabetes in older persons (all subjects were 75 years old) presented no mortality risk. This may be partly explained by a survival bias; that is, the detrimental effects of obesity-related metabolic abnormalities are generally less prominent among older subjects than they are in middle-aged individuals (30,31).
The reasons for pronounced mortality in lean diabetes in the current study are not clear. The pathophysiology of lean diabetes is heterogeneous, including impaired insulin secretion, increased endogenous glucose production, high inflammatory state, and higher perceived stress hormones (32,33). The contrasting general clinical phenotypes of lean diabetes compared with obese diabetes have recently been discussed (32,33), although we cannot contribute to that discussion. Incomplete control for confounding or reverse causation bias could also explain the substantial risk in lean diabetes. In an effort to control for reverse causation, we repeated the analysis after excluding subjects who died within the initial 2 years of follow-up and those who had a pre-existing myocardial infarction, stroke, or cancer at baseline; the conclusions remained unchanged. However, that the leanness associated with higher mortality during the follow-up period was a result of occult diseases involving muscle or fat wasting cannot be excluded.
The strengths of the current study include 1) this is a population-based study in an Asian (Japanese) population where there are limited data available addressing the association between diabetes, leanness, and mortality; and 2) complete follow-up was achieved in almost the entire cohort. There were also several limitations, however. A number of people from the original cohort (13–15) were excluded (71%); thus, there is a potential bias in the study participants chosen for the study analysis. Data on diabetes type, duration (i.e., no distinction between subjects with new-onset diabetes and those with longer-duration diabetes), disease severity, and the presence of diabetes complications at baseline would have been informative and would have extended the knowledge achieved in the current study. Second, we could not separate diabetes into type 1 or type 2 diabetes, although the incidence of type 1 diabetes is extremely low (approximately 2 cases/year/100,000 individuals) in Japan (34). Lastly, information regarding BMI and glucose values was available only at baseline, making it impossible to evaluate the effects of changes during the follow-up period in either or both of these factors.
In conclusion, among the Japanese general population, a diagnosis of diabetes confers an increased risk of all-cause death. Particular attention must be paid to the pronounced high mortality for lean diabetes. Taken together with the evidence of a rapidly increasing number of diabetes cases in Asia, the findings from the current study suggest that an integrated strategy combining preventive actions against diabetes, improvement of care for diabetes that includes early detection of diabetes complications as well as undiagnosed chronic disease, and multidisciplinary care programs is needed.
Acknowledgments
This study was supported by Grant 10470113 to K.K. and Grants 13470096, 15390209, and 18390198 to S.I. (http://kaken.nii.ac.jp/en/) from the Ministry of Education, Culture, Sports, Science and Technology in Japan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
No potential conflicts of interest relevant to this article were reported.
Y.Y. designed the study, collected and analyzed the data, and wrote the manuscript. K.Kar. analyzed the data and wrote the manuscript. S.I., T.O., T.G., K.Kay., A.T., K.S., Y.N., and E.K. designed and supervised the study, collected the data, and revised the manuscript. S.I. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1736/-/DC1.
References
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997;349:1269–1276 [DOI] [PubMed] [Google Scholar]
- 2.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129–2140 [DOI] [PubMed] [Google Scholar]
- 3.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011;34:1249–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yano Y, Sato Y, Fujimoto S, et al. Association of high pulse pressure with proteinuria in subjects with diabetes, prediabetes, or normal glucose tolerance in a large Japanese general population sample. Diabetes Care 2012;35:1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998;316:823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009;52:65–73 [DOI] [PubMed] [Google Scholar]
- 7.McEwen LN, Kim C, Karter AJ, et al. Risk factors for mortality among patients with diabetes: the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care 2007;30:1736–1741 [DOI] [PubMed] [Google Scholar]
- 8.Kokkinos P, Myers J, Faselis C, Doumas M, Kheirbek R, Nylen E. BMI-mortality paradox and fitness in African American and Caucasian men with type 2 diabetes. Diabetes Care 2012;35:1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA 2012;308:581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 2006;355:763–778 [DOI] [PubMed] [Google Scholar]
- 11.Bogers RP, Bemelmans WJ, Hoogenveen RT, et al. for the BMI-CHD Collaboration Investigators Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med 2007;167:1720–1728 [DOI] [PubMed] [Google Scholar]
- 12.Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med 2011;364:719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirokawa K, Tsutusmi A, Kayaba K. Impacts of educational level and employment status on mortality for Japanese women and men: the Jichi Medical School cohort study. Eur J Epidemiol 2006;21:641–651 [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa S, Gotoh T, Nago N, Kayaba K, Jichi Medical School (JMS) Cohort Study Group The Jichi Medical School (JMS) Cohort Study: design, baseline data and standardized mortality ratios. J Epidemiol 2002;12:408–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsutsumi A, Kayaba K, Kario K, Ishikawa S. Prospective study on occupational stress and risk of stroke. Arch Intern Med 2009;169:56–61 [DOI] [PubMed] [Google Scholar]
- 16.The Committee of Japan Diabetes Society on the diagnostic criteria of diabetes mellitus Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J Japan Diab Soc 2010;53:450–467 (in Japanese) [Google Scholar]
- 17.Examination Committee of Criteria for ‘Obesity Disease' in Japan; Japan Society for the Study of Obesity New criteria for ‘obesity disease' in Japan. Circ J 2002;66:987–992 [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roglic G, Unwin N, Bennett PH, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care 2005;28:2130–2135 [DOI] [PubMed] [Google Scholar]
- 20.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med 2007;147:149–155 [DOI] [PubMed] [Google Scholar]
- 21.Preis SR, Hwang SJ, Coady S, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 2009;119:1728–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995-2005: a population-based study. Lancet 2007;369:750–756 [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto T, Ohashi Y, Yamada N, Kikuchi M. Coronary heart disease mortality is actually low in diabetic Japanese by direct comparison with the Joslin cohort. Diabetes Care 1994;17:1062–1063 [DOI] [PubMed] [Google Scholar]
- 24.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001;44(Suppl. 2):S14–S21 [DOI] [PubMed] [Google Scholar]
- 25.Chowdhury TA. Diabetes and cancer. QJM 2010;103:905–915 [DOI] [PubMed] [Google Scholar]
- 26.Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med 2006;166:1871–1877 [DOI] [PubMed] [Google Scholar]
- 27.Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care 2012;35:1835–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barone BB, Yeh HC, Snyder CF, et al. Postoperative mortality in cancer patients with preexisting diabetes: systematic review and meta-analysis. Diabetes Care 2010;33:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsson G, Hedberg P, Öhrvik J. Survival of the fattest: unexpected findings about hyperglycaemia and obesity in a population based study of 75-year-olds. BMJ Open 2011;1:e000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inelmen EM, Sergi G, Coin A, Miotto F, Peruzza S, Enzi G. Can obesity be a risk factor in elderly people? Obes Rev 2003;4:147–155 [DOI] [PubMed] [Google Scholar]
- 31.Oreopoulos A, Kalantar-Zadeh K, Sharma AM, Fonarow GC. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med 2009;25:643–659, viii [DOI] [PubMed] [Google Scholar]
- 32.Prato SD, LaSalle J, Matthaei S, Bailey CJ, Global Partnership for Effective Diabetes Management Tailoring treatment to the individual in type 2 diabetes practical guidance from the Global Partnership for Effective Diabetes Management. Int J Clin Pract 2010;64:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahara M, Kaneto H, Katakami N, Matsuoka TA, Matsuhisa M, Shimomura I. Impaired suppression of endogenous glucose production in lean Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2011;93:e1–e2 [DOI] [PubMed] [Google Scholar]
- 34.Neville SE, Boye KS, Montgomery WS, Iwamoto K, Okamura M, Hayes RP. Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev 2009;25:705–716 [DOI] [PubMed] [Google Scholar]